Abstract
Background
Cellular reactions to alloplastic bone substitute materials (BSM) are a subject of interest in basic research. In regenerative dentistry, these bone grafting materials are routinely combined with enamel matrix derivatives (EMD) in order to additionally enhance tissue regeneration.
Materials and methods
The aim of this study was to evaluate the proliferative activity of human osteogenic cells after incubation over a period of seven days with commercial BSM of various origin and chemical composition. Special focus was placed on the potential additional benefit of EMD on cellular proliferation.
Results
Except for PerioGlas®, osteogenic cell proliferation was significantly promoted by the investigated BSM. The application of EMD alone also resulted in significantly increased cellular proliferation. However, a combination of BSM and EMD resulted in only a moderate additional enhancement of osteogenic cell proliferation.
Conclusion
The application of most BSM, as well as the exclusive application of EMD demonstrated a positive impact on the proliferation of human osteogenic cells in vitro. In order to increase the benefit from substrate combination (BSM + EMD), further studies on the interactions between BSM and EMD are needed.
Background
The treatment of quantitative and qualitative defects of supporting bone tissue is one major aspect of modern dentoalveolar surgery and periodontology. In this context, alloplastic bone substitute materials (BSM) are well documented as alternatives to autogenous bone grafts for certain indications in the management of hard tissue deficiencies [1-5].
Various commercial BSM of different origin, chemical composition, and micro or macro-structural properties have been introduced and investigated in recent years [6-8]. Today, a large percentage of these BSM are based on calcium phosphate composites, such as hydroxyapatite (HA) and tricalcium phosphate (TCP), as well as bioactive glass (silicate: SiO2) [9]. In addition to the various well-employed substitutes with rather homogenous chemical compositions, such as Bio-Oss® (HA), Cerasorb® (β-TCP), and PerioGlas® (SiO2), recent developments have focused on "composites" with different chemical phases, such as Straumann® BoneCeramic (HA + β-TCP) [10], NanoBone® (SiO2 + HA), and BONIT®matrix (SiO2 + HA + β-TCP) [11]. The latter biomaterials have been designed to combine the biological advantages of calcium phosphate and bioactive glass. Hence, the various BSM feature different biological behaviour in vitro and in vivo [12-14]. In a recent in vitro comparison of five commercial bone substitutes, Kuebler et al. [13] demonstrated significant differences among the investigated specimens with regard to osteogenic cell proliferation, pointing out the need for further research.
Emdogain®, a commercial mixture of porcine derived enamel matrix derivatives (EMD), is an evidence-based option for the treatment of bony defects in periodontal therapy [15-17]. Biologically active EMD ingredients are ligands, such as amelogenin, ameloblastin, enamelin, and tuftelin, that play a crucial role in the development of teeth and supporting structures [18,19]. A recent systematic review summarised the effect of EMD on relevant cell populations in the periodontal region, such as epithelial cells, gingival fibroblasts, periodontal ligament cells, osteogenic cells, and cementoblasts as stimulatory rather than inhibitory [18]. For osteogenic cells specifically, EMD have been shown to support cell viability and proliferation in a dose dependent manner [20,21], as well as encourage cell attachment [22], cell motility [23], and cell differentiation [22,24,25]. Recent studies of periodontal regeneration focused on the augmentation of BSM with EMD [5,26]. However, up to now, no significant clinical benefit could be measured, making further research on this approach desirable.
The application of either BSM or EMD into the hard tissue defect should ideally initiate and support tissue regeneration. For osteogenic cells, cell recruitment and migration into the defect (osteoconduction), and cell proliferation precede osteogenic cell differentiation [27], while cell proliferation plays a pivotal role for further successful regeneration. In cellular research, many biological assays focus on cell proliferation. The toxic or radioactive properties of assays like the H3-thymidin or BrDU assay are disadvantageous. The Alamar Blue® assay is a well established, non-toxic, and non-radioactive method for continuously quantifying cellular proliferation over a long time interval [28].
The aim of this study was to compare the impact of various bone substitute materials on the proliferation of human osteogenic cells in vitro, employing the Alamar Blue® assay over 7 days. Furthermore, the impact of the additional application of EMD on osteogenic cell proliferation activity was investigated.
Materials and methods
Cell Line
A commercial hip bone derived osteoblastic cell line (HHOBc, PromoCell, Heidelberg, Germany) was utilised. Cells were cultivated using a standard osteoblast cultivation medium, consisting of fetal calf serum (FCS, Gibco Invitrogen, Karlsruhe, Germany), Dulbecco's modified Eagle's medium (DMEM, Gibco Invitrogen), dexamethasone (100 nmol/l, Serva Bioproducts, Heidelberg, Germany), L-glutamine (Gibco Invitrogen), and streptomycin (100 mg/ml, Gibco Invitrogen). Cultivation was carried out at 37°C in a constant, humidified atmosphere with 95% room air and 5% CO2.
Prior to our experiments, the cell line was qualitatively characterised by the immunohistochemical expression of alkaline phosphatase (AP) and osteocalcin (labelled streptavidin-biotin/horseradish peroxidase). Cells were passaged at regular intervals, depending on their growth characteristics, using 0.25% trypsin (Seromed Biochrom KG, Berlin, Germany).
All trials were carried out at the 4th cell passage. Osteogenic cells were detached and seeded on the different test substrates.
Test Substrates and Incubation
Seven different commercial alloplastic BSM were investigated. Except for the biological sample derived from bovine bone (Bio-Oss®), all other samples were synthetic, composed of pure β-tricalcium phosphate (Cerasorb® M, Bioresorb® Macro Pore), pure bioactive glass (PerioGlas®), biphasic BSM (β-tricalcium phosphate + hydroxyapatite: Straumann® BoneCeramic; silicon dioxide + hydroxyapatite: NanoBone®) or triphasic BSM (silicon dioxide + β-tricalcium phosphate + hydroxyapatite: Bonit®matrix). Table 1 provides a synopsis.
Table 1.
Bone substitute materials investigated
| Chemical composition and origin | Abbr. | Commercial name, manufacturer | Investigated particle size, manufacturer's data |
|
|---|---|---|---|---|
| tricalcium phosphate: β-TCP |
synthetic | CBM | Cerasorb® M, Curasan | 500-1000 μm |
| BRE | Bioresorb® Macro Pore, Oraltronics® |
500-1000 μm | ||
| biological apatite: HA | bovine | BIO | Bio-Oss®, Geistlich | 250-1000 μm |
| silicate: SiO2 |
synthetic | PGL | PerioGlas®, Sunstar Butler | 90-710 μm |
| biphasic: β- TCP, HA |
synthetic | BOC | Straumann® BoneCeramic, Straumann |
500-1000 μm |
| biphasic: SiO2, HA |
synthetic | NBO | NanoBone®, Artoss | mean particle size: 600 μm |
| triphasic: SiO2, gβ-TCP, HA |
synthetic | BIM | Bonit® matrix, DOT | 300 x 600 μm |
(β-TCP: β-tricalcium phosphate, HA: hydroxyapatite, SiO2: silicon dioxide). Abbr.: Abbreviation. Manufacturers: Cursan AG (Kleinostheim, Germany), Oraltronics® Dental Implant Technology GmbH (Bremen, Germany), Geistlich Biomaterials (Baden, Germany), John O. Butler GmbH (Kriftel, Germany), Straumann GmbH (Freiburg, Germany), Artoss GmbH (Rostock, Germany), DOT GmbH (Rostock, Germany).
The porcine derived protein mixture Emdogain® (Straumann, Freiburg, Germany) was utilised as a commercial EMD.
In our investigation, 100 mg of the respective BSM were loosely placed into black 24 well plates (Thermo Fisher Scientific, Langenselbold, Germany), ensuring complete coverage of the well surface. Wells without BSM served as a control group. For those wells incubated additionally with EMD, an emulsion of 100 μg Emdogain®/ml was prepared and added to the respective wells. Osteogenic cells were added to the respective compositions at a density of 1*104 cells per well, and further cultivated at 37°C in a constant, humidified atmosphere of 95% room air and 5% CO2.
Alamar Blue® proliferation assay
The Alamar Blue® (AB) assay (Biozol, Echingen, Germany) was performed according to manufacturer's guidelines for the quantification of cellular proliferation. The AB assay is based on the incorporation of a fluorogenic redox indicator of cell growth in culture. The turnover of AB is a reflection of cell proliferation, and is quantified by measuring the fluorescence in Relative Flourescence Units (RFU). Fluorescence was detected using a fluorescence reader (FLx800 Microplate Fluorescence Reader, BIO-TEK Instruments, Vinooski, Vermont, USA) at 560/20 nm and 620/40 nm at the following time points: immediately after the addition of AB (0 h), then at 3 h, 6 h, 12 h, 24 h, 2 d, 3 d, 4 d and 7 d. Uncultured wells served as a reference. Assays were run in triplicate for each BSM and BSM/EMD composition, and at each time point.
Statistics
Statistical analysis was performed using the statistical software SigmaStat (Version 3.1.; Systat Software, Inc., Richmond, USA). Means and standard deviations were calculated for each group. Results are shown graphically in a plot (abscissa: point of time, ordinate: RFU values). In order to identify the BSM or BSM/EMD composition showing the greatest proliferation after both 24 h and 7 d, all groups were compared using Bonferroni's t-test. Furthermore, the groups were compared against pure EMD. To verify the differences between BSM without EMD and BSM with EMD, a separate t-test was performed. The outcome each statistical test was considered to be significant with p < 0.05 and highly significant with p < 0.001.
Results
In general, all of the investigated BSM and BSM/EMD compositions revealed continuous cell proliferation over the observation period, with some significant differences.
After 24 h, the mean values (standard deviation in parentheses) for AB reduction of osteogenic cells cultivated on the various BSM without EMD were: control 832 (± 25) RFU, Cerasorb® M 963 (± 16) RFU, Bioresorb® 1073 (± 19) RFU, Bio-Oss® 863 (± 18) RFU, PerioGlas® 705 (± 8) RFU, Straumann® BoneCeramic 963 (± 45) RFU, NanoBone® 1088 (± 6) RFU, BONIT®matrix 1184 (± 32) RFU.
After seven days, the values for AB reduction of osteogenic cells cultivated on the various BSM without EMD were: control 1447 (± 20) RFU, BONIT®matrix 2450 (± 48) RFU, Straumann® BoneCeramic 2508 (± 100) RFU, PerioGlas® 1396 (± 31) RFU, Cerasorb® M 2494 (± 61) RFU, Bioresorb® 2921 (± 69) RFU, NanoBone® 2733 (± 34) RFU, Bio-Oss® 1714 (± 23) RFU. After 7 days, a significant increase in AB reduction, compared to the negative control, was found in decreasing order for Bioresorb® > NanoBone® > Straumann® BoneCeramic > Cerasorb® M > BONIT®matrix > Bio-Oss®. Furthermore, a slight, but not significant decrease in AB reduction was documented for PerioGlas® (figure 1, table 2).
Figure 1.
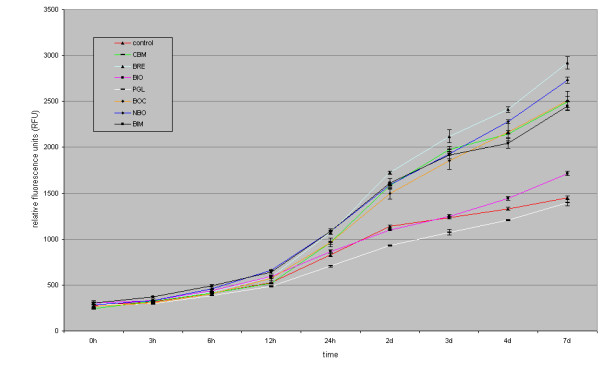
figure illustrating Bonferroni's t-test for AB reduction of osteogenic cells cultivated on the various BSM compared to the control after 7 d.
Table 2.
Bonferroni's t-test for AB reduction of osteogenic cells cultivated on the various BSM compared to the control after 7 d
| Comparison | Diff of Means | t | p |
|---|---|---|---|
| control vs. BRE | 1473 | 26.8 | <0.001** |
| control vs. NBO | 1286 | 23.4 | <0.001** |
| control vs. BOC | 1061 | 19.3 | <0.001** |
| control vs. CBM | 1047 | 19.1 | <0.001** |
| control vs. BIM | 1002 | 18.2 | <0.001** |
| control vs. BIO | 267 | 4.8 | 0.001* |
| control vs. PGL | 51 | 0.9 | 0.936 |
(BIO = Bio-Oss®, NBO = NanoBone®, BRE = Bioresorb®, CBM = Cerasorb® M, PGL = PerioGlas®, BOC = Straumann® BoneCeramic, BIM = BONIT®matrix; t = probability; p = p-value; *significant, ** highly significant).
After 24 h, AB reduction values for osteogenic cells cultivated on the various BSM with EMD were: control 1055 (± 16) RFU, Cerasorb® M 1034 (± 40) RFU, Bioresorb® 1166 (± 13) RFU, Bio-Oss® 918 (± 24) RFU, PerioGlas® 701 (± 12) RFU, Straumann® BoneCeramic 1045 (± 24) RFU, NanoBone® 1181 (± 37) RFU, BONIT®matrix 1182 (± 93) RFU.
After 7 days, AB reduction values for osteogenic cells cultivated on the various BSM with EMD were: control 1447 (± 80) RFU, EMD 2212 (± 80) RFU, BONIT®matrix 2660 (± 206) RFU, Straumann® BoneCeramic 2538 (± 105) RFU, PerioGlas® 1399 (± 30) RFU, Cerasorb® M 2456 (± 98) RFU, Bioresorb® 2998 (± 83) RFU, NanoBone® 2781 (± 162) RFU, Bio-Oss® 1854 (± 54) RFU. Compared to the untreated control group, the AB reduction showed a significant increase in descending order for Bioresorb® > NanoBone® > BONIT®matrix > Straumann® BoneCeramic > Cerasorb® M > Emdogain® > Bio-Oss®. A slight, but not significant decrease in AB reduction was documented for PerioGlas® (figure 2, table 3). Table 3 also provides a comparison between EMD and BSM enriched with EMD.
Figure 2.
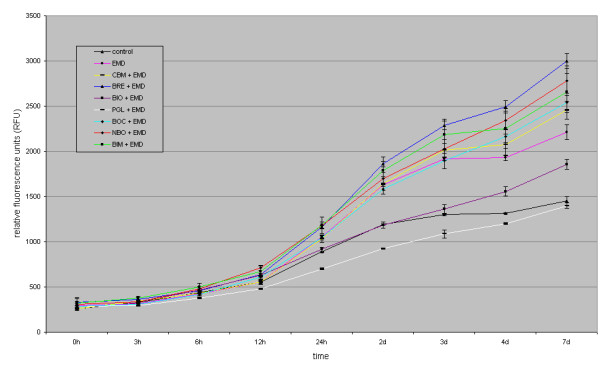
figure illustrating Bonferroni's t-test for AB reduction of osteogenic cells cultivated on the various BSM compared to the untreated control or EMD after 7 d.
Table 3.
Bonferroni's t-test for AB reduction of osteogenic cells cultivated on the various BSM compared to the untreated control or EMD after 7 d
| Comparison | Diff of Means | t | p |
|---|---|---|---|
| control vs. BRE + EMD | 1544 | 14.0 | <0.001** |
| control vs. NBO + EMD | 1327 | 12.0 | <0.001** |
| control vs. BIM + EMD | 1206 | 10.9 | <0.001** |
| control vs. BOC + EMD | 1084 | 9.8 | <0.001** |
| control vs. CBM + EMD | 1002 | 9.1 | <0.001** |
| control vs. EMD | 758 | 6.9 | <0.001** |
| control vs. BIO + EMD | 400 | 3.6 | 0.015* |
| control vs. PGL + EMD | 54 | 0.4 | >1.0 |
| EMD vs. PGL + EMD | 812 | 7.0 | <0.001** |
| EMD vs. BRE + EMD | 786 | 6.8 | <0.001** |
| EMD vs. NBO + EMD | 569 | 4.9 | 0.001* |
| EMD vs. BIM + EMD | 448 | 3.8 | 0.009* |
| EMD vs. BIO + EMD | 358 | 3.1 | 0.048* |
| EMD vs. BOC + EMD | 326 | 2.8 | 0.085 |
| EMD vs. CBM + EMD | 244 | 2.1 | 0.352 |
(BIO = Bio-Oss®, NBO = NanoBone®, BRE = Bioresorb®, CBM = Cerasorb® M, PGL = PerioGlas®, BOC = Straumann® BoneCeramic, BIM = BONIT®matrix; t = probability; p = p-value; *significant, ** highly significant).
In a comparison of pure BSM and the BSM/EMD composition, all of the BSM, except for PerioGlas® and BONIT®matrix, showed an increase in AB reduction values at 24 h with the addition of EMD. For NanoBone® and Bioresorb®, the addition of EMD resulted in significantly increased AB reduction values. After 7 days, The only BSM to show a decrease in the AB reduction value with EMD as compared to without EMD was Cerasorb® M. For Bio-Oss®, the addition of EMD resulted in a significantly increased AB reduction value (figures 3 and 4, table 4).
Figure 3.
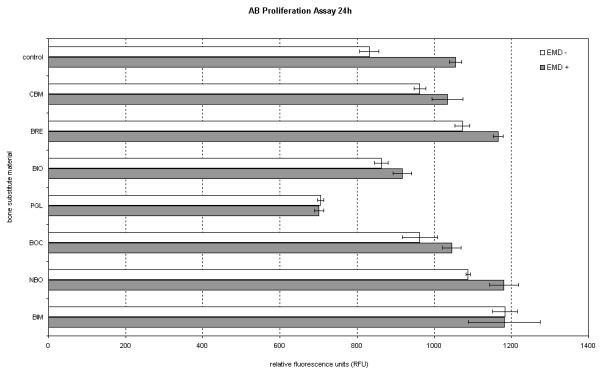
figure illustrating Comparison of BSM without EMD to BSM + EMD on osteogenic cell proliferation after 24 h and 7 d using the t-test.
Figure 4.
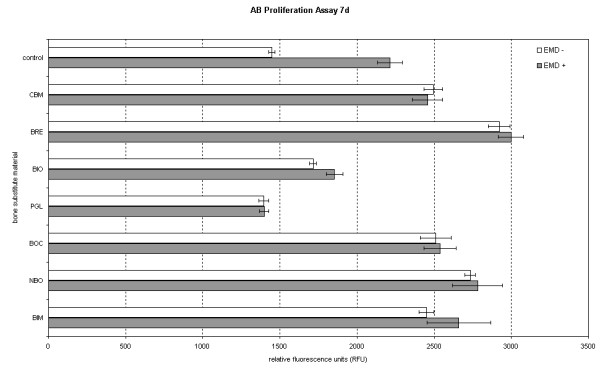
figure illustrating Comparison of BSM without EMD to BSM + EMD on osteogenic cell proliferation after 24 h and 7 d using the t-test.
Table 4.
Comparison of BSM without EMD to BSM + EMD on osteogenic cell proliferation after 24 h and 7 d using the t-test
| 24 h | |||
|---|---|---|---|
| Comparison | Diff of Means | t | p |
| BIO vs. BIO + EMD | -54 | -2.5 | 0,06 |
| NBO vs. NBO + EMD | -93 | -3.4 | 0,025 * |
| BRE vs. BRE + EMD | -93 | -5.7 | 0,004 * |
| CBM vs. CBM + EMD | -71 | -2.3 | 0,078 |
| PGL vs. PGL + EMD | +4 | 0.4 | 0,686 |
| BOC vs. BOC + EMD | -82 | -2.2 | 0,086 |
| BIM vs. BIM + EMD | +2 | -1.4 | 0,231 |
| 7d | |||
| Comparison | Diff of Means | T | p |
| BIO vs. BIO + EMD | -139 | -3.3 | 0.028 * |
| NBO vs. NBO + EMD | -48 | -0.4 | 0.701 |
| BRE vs. BRE + EMD | -83 | -0.3 | 0.400 |
| CBM vs. CBM + EMD | +38 | -0.4 | 0.663 |
| PGL vs. PGL + EMD | -3 | -0.1 | 0.911 |
| BOC vs. BOC + EMD | -29 | -0.2 | 0.787 |
| BIM vs. BIM + EMD | -210 | -1.4 | 0.231 |
(BIO = Bio-Oss®, NBO = NanoBone®, BRE = Bioresorb®, CBM = Cerasorb® M, PGL = PerioGlas®, BOC = Straumann® BoneCeramic, BIM = BONIT®matrix; t = probability; p = p-value; *significant).
Discussion
When employing alloplastic bone substitute materials (BSM) for guided bone regeneration, the biocompatibility and biological activity of the material used plays an essential role, alongside the distinct physical properties of the graft, like stiffness and stability, for the overall therapeutic success. In this context, the development of an "ideal" synthetic bone graft that fulfils the attributes "biocompatible", "degradable", "osteoconductive", and "osteoinductive" is the focus of recent research. A major issue for the clinical practitioner is whether a bone graft acts as a plain defect filler, or has additional osteoconductive or osteoinductive capacities [29]. The pore size of the BSM plays a crucial role in enhancing the osteoconductive potential of the BSM. Current literature postulates a minimum pore size of between 200-400 μm as necessary for osteoconduction, vascularisation, and formation of mineralised tissue within a scaffold [30-32]. Furthermore, it is known that an increasing number of interconnective pores raises the internal surface area of a BSM, with promotion of the growth of regenerative cells [33].
The assessment of cell proliferation in vitro provides valuable clues about substrate biocompatibility. Furthermore, proliferating cells are a precondition for osteoconductivity and osteoinductivity. The BSM investigated in our study represent a cross-section of the currently commercially available grafting materials, reflecting the most popular and well-documented chemical compositions (HA, TCP, bioactive glasses). The sample size of 100 mg of BSM was chosen in order to completely cover the floor of a well in a 24 well plate. This ensured that the majority of the cultivated cells was in close contact with the BSM particles. Our results suggest that none of the grafting materials used in this study has a significantly negative influence on cellular proliferation, as compared to the control. In fact, all but one of the BSM tested led to an increased AB reduction over the observation period of 7d. Only PerioGlas® showed a slight, but not significant decrease in AB reduction, compared to the control. Our findings are, to a certain extent, contrary to former studies [13,34]. Possible explanations might be dissimilarities in the experimental set-up. Furthermore, it should be kept in mind that in vitro studies only give a limited reflection of the complex in vivo situation.
Although the biomaterial Bio-Oss® showed very good results in various clinical trials [26,35], our in vitro investigation showed weaker results for cell proliferation as compared to the other test materials, with the exception of PerioGlas®. These findings for Bio-Oss® are in agreement with other in vitro studies [13]. In our study, all of the other investigated BSM clearly promoted osteogenic cell proliferation, with the highest values after 24 h for BONIT®matrix, and after 7 d for Bioresorb® Macro Pore. Nanocrystalline HA (NanoBone®) has been shown to promote other cell lines with osteogenic potential, in a fashion similar to that observed in our study [36].
Conclusion
In our study, the addition of EMD resulted in an increase in AB reduction for almost all test groups, but significantly for the control, NanoBone®, and Bioresorb® Macro Pore after 24 h, as well as for the control, and Bio-Oss® after 7 d. We observed a minimal, EMD-dependent decrease in AB reduction for PerioGlas® after 24 h, and for Cerasorb® M after 7 d. Schwarz et al. observed a benefit in the functionalisation of titanium surfaces with EMD [21]. Altogether, the addition of EMD seems to promote osteogenic cell proliferation to a certain degree. In the routine clinical situation, the benefit of combining BSM and EMD is well established, and scientifically documented [15,16,26].
In our study, we found no clear correlation between the BSM chemical composition or structural properties, and osteogenic cell proliferation - regardless of the addition of EMD. Further research must be conducted to understand the exact modus of interaction between EMD and BSM, e.g. studies of protein release kinetics from BSM with different chemical and structural properties. We could identify promising BSM candidates for enhancing osteogenic cell activity.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
The study design was established by MOK, CR and BA. CR and MOK carried out the in vitro experiments and wrote the manuscript. RS performed the data management and data analysis. AK and WG carried out the manuscript editing and manuscript review. All authors read and approved the final version of the manuscript.
Contributor Information
Christoph Reichert, Email: c_reichert@web.de.
Bilal Al-Nawas, Email: al-nawas@mkg.klinik.uni-mainz.de.
Ralf Smeets, Email: rasmeets@ukaachen.de.
Adrian Kasaj, Email: kasaj@gmx.de.
Werner Götz, Email: wgoetz@uni-bonn.de.
Marcus O Klein, Email: klein@mkg.klinik.uni-mainz.de.
Acknowledgements
This project is supported by a grant (MAIFOR 135/2007) from the University Mainz, medical section, for the promotion of medical research, Germany.
The authors thank the respective companies for providing the bone substitute materials.
References
- Eppley BL, Pietrzak WS, Blanton MW. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16(6):981–9. doi: 10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- Esposito M. The efficiancy of various bone augmentation procedures for dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2006;21(5):696–710. [PubMed] [Google Scholar]
- Orsini M. Long-term clinical results on the use of bone-replacement grafts in the treatment of intrabony periodontal defects. Comparison of the use of autogenous bone graft plus calcium sulfate to autogenous bone graft covered with a bioabsorbable membrane. J Peridontol. 2008;79(9):1630–1637. doi: 10.1902/jop.2008.070282. [DOI] [PubMed] [Google Scholar]
- Sculean A. Healing of human intrabony defects following regenerative periodontal therapy with a bovine-derived xenograft and guided tissue regeneration. Clin Oral Investig. 2004;8(2):70–74. doi: 10.1007/s00784-004-0254-7. [DOI] [PubMed] [Google Scholar]
- Sculean A. Clinical and histologic evaluation of an enamel matrix derivative combined with a biphasic calcium phosphate for the treatment of human intrabony periodontal defects. J Peridontol. 2008;79(10):1991–1999. doi: 10.1902/jop.2008.080009. [DOI] [PubMed] [Google Scholar]
- Klein MO. Pore characteristics of bone subsitute materials assessed by microcomputed tomography. Clin Oral Implants Res. 2009;20:67–74. doi: 10.1111/j.1600-0501.2008.01605.x. [DOI] [PubMed] [Google Scholar]
- Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25(6):987–94. doi: 10.1016/S0142-9612(03)00621-5. [DOI] [PubMed] [Google Scholar]
- Weibrich G. [Determining the size of the specific surface of bone substitutes with gas adsorption] Mund Kiefer Gesichtschir. 2000;4(3):148–52. doi: 10.1007/s100060050187. [DOI] [PubMed] [Google Scholar]
- Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am. 2007;19(4):513–21. doi: 10.1016/j.coms.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Dietze S. The ultrastructure and processing properties of Straumann Bone Ceramic and NanoBone. Folia Morphol (Warsz) 2006;65(1):63–5. [PubMed] [Google Scholar]
- Thimm BW. Biocompatibility studies of endothelial cells on a novel calcium phosphate/SiO2-xerogel composite for bone tissue engineering. Biomed Mater. 2008;3(1):15007. doi: 10.1088/1748-6041/3/1/015007. [DOI] [PubMed] [Google Scholar]
- Henkel KO. Macroscopical, histological, and morphometric studies of porous bone-replacement materials in minipigs 8 months after implantation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):606–13. doi: 10.1016/j.tripleo.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Kübler A. Growth and proliferation of human osteoblasts on different bone graft substitutes: an in vitro study. Implant Dent. 2004;13(2):171–179. doi: 10.1097/01.id.0000127522.14067.11. [DOI] [PubMed] [Google Scholar]
- Schwartz Z. Differential effects of bone graft substitutes on regeneration of bone marrow. Clin Oral Implants Res. 2008;19(12):1233–1245. doi: 10.1111/j.1600-0501.2008.01582.x. [DOI] [PubMed] [Google Scholar]
- Sculean A. Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J Clin Periodontol. 2008;35(9):817–24. doi: 10.1111/j.1600-051X.2008.01295.x. [DOI] [PubMed] [Google Scholar]
- Sculean A. The application of an enamel matrix protein derivative (Emdogain) in regenerative periodontal therapy: a review. Med Princ Pract. 2007;16(3):167–80. doi: 10.1159/000100386. [DOI] [PubMed] [Google Scholar]
- Trombelli L, Farina R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J Clin Periodontol. 2008;35(s8):117–135. doi: 10.1111/j.1600-051X.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD. Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol. 2008;35(s8):87–105. doi: 10.1111/j.1600-051X.2008.01264.x. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Nanci A. Hertwig's epithelial root sheath, enamel matrix proteins, and initiation of cementogenesis in porcine teeth. J Clin Periodontol. 2004;31(8):184–192. doi: 10.1111/j.0303-6979.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- Guida L. In vitro biologic response of human bone marrow stromal cells to enamel matrix derivative. J Periodontol. 2007;78:2190–2196. doi: 10.1902/jop.2007.070185. [DOI] [PubMed] [Google Scholar]
- Schwarz F. Effect of enamel matrix protein derivative on the attachment, proliferation, and viability of human SaOs(2) osteoblasts on titanium implants. Clin Oral Investig. 2004;8(3):165–71. doi: 10.1007/s00784-004-0259-2. [DOI] [PubMed] [Google Scholar]
- Rincon JC. Enhanced proliferation, attachment and osteopontin expression by porcine periodontal cells exposed to Emdogain. Arch Oral Biol. 2005;50:1047–1054. doi: 10.1016/j.archoralbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Klein MO. In vitro assessment of motility and proliferation of human osteogenic cells on different isolated extracellular matrix components compared with enamel matrix derivative by continuous single-cell observation. Clin Oral Implants Res. 2007;18(1):40–5. doi: 10.1111/j.1600-0501.2006.01279.x. [DOI] [PubMed] [Google Scholar]
- Schwartz Z. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J Periodontol. 2000;71:1287–1296. doi: 10.1902/jop.2000.71.8.1287. [DOI] [PubMed] [Google Scholar]
- He J. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(2):239–45. doi: 10.1016/j.tripleo.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Sculean A. Clinical evaluation of an enamel matrix protein derivative (Emdogain) combined with a bovine-derived xenograft (Bio-Oss) for the treatment of intrabony periodontal defects in humans. Int J Periodontics Restorative Dent. 2002;22(3):259–67. [PubMed] [Google Scholar]
- Aubin JE, Heersche JM. Osteoprogenitor cell differentiation to mature bone-forming osteoblasts. Drug Development Research. 2000;49(3):206–215. doi: 10.1002/(SICI)1098-2299(200003)49:3<206::AID-DDR11>3.0.CO;2-G. [DOI] [Google Scholar]
- Larson EM. A new, simple, nonradioactive, nontoxic in vitro assay to monitor corneal endothelial cell viability. Invest Ophthalmol Vis Sci. 1997;38(10):1929–33. [PubMed] [Google Scholar]
- Albrektsson TJC. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001. pp. 96–101. [DOI] [PMC free article] [PubMed]
- Tsuruga E. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem. 1997;121(2):317–24. doi: 10.1093/oxfordjournals.jbchem.a021589. [DOI] [PubMed] [Google Scholar]
- Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Dong J. Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials. 2002;23(23):4493–502. doi: 10.1016/S0142-9612(02)00193-X. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Ahn ES. Nanostructured biomaterials for tissue engineering bone. Adv Biochem Eng Biotechnol. 2007;103:275–308. doi: 10.1007/10_021. [DOI] [PubMed] [Google Scholar]
- Xynos ID. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67(4):321–9. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- Traini T. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J Periodontol. 2007;78(5):955–61. doi: 10.1902/jop.2007.060308. [DOI] [PubMed] [Google Scholar]
- Kasaj A. Human periodontal fibroblast response to a nanostructured hydroxyapatite bone replacement graft in vitro. Arch Oral Biol. 2008;53(7):683–9. doi: 10.1016/j.archoralbio.2008.01.009. [DOI] [PubMed] [Google Scholar]


