Abstract
Objective:
To evaluate the efficacy and tolerability of quetiapine monotherapy for anxiety symptoms in patients with bipolar disorder experiencing depression in the BipOLar DEpRession (BOLDER I and II) studies.
Method:
A post hoc analysis of anxiety symptoms in 1,051 acutely depressed patients with bipolar I or II disorder (DSM-IV) from 2 double-blind, randomized, placebo-controlled 8-week studies of quetiapine (300 or 600 mg once daily) was conducted. Anxiety symptoms were assessed using Hamilton Anxiety Rating Scale (HARS) total and psychic (items 1–6, 14) and somatic (items 7–13) anxiety subscale scores (mixed-model repeated measure and last-observation-carried-forward analysis of change from baseline at each assessment). The BOLDER I study was conducted between September 2002 and October 2003, and the BOLDER II study was conducted between June 2004 and August 2005.
Results:
Mean baseline HARS total scores were similar across the treatment groups (300 mg/d: 18.9, 600 mg/d and placebo: both 18.6). There was a significantly greater improvement from baseline in mean HARS total scores at the first evaluation (week 1) in both quetiapine groups compared with placebo (300 mg/d: −4.6, P < .001 and 600 mg/d: −4.1, P = .003 vs placebo: −2.8). These improvements were sustained through week 8 with both quetiapine doses (300 mg/d: −10.1, P < .001 and 600 mg/d: −10.5, P < .001 vs placebo: −6.9). At week 8, there was also significant improvement from baseline in HARS psychic and somatic anxiety subscale scores compared with placebo (P < .001). The baseline severity of anxiety did not impact the improvement in depressive symptoms. Common adverse events included dry mouth, sedation, somnolence, and dizziness.
Conclusions:
In this pooled analysis, quetiapine monotherapy was more effective than placebo and generally well tolerated for the treatment of both depressive and anxiety symptoms in patients with bipolar disorder.
Trial Registration:
clinicaltrials.gov Identifiers: NCT00060489 (BOLDER I) and NCT00083954 (BOLDER II)
Bipolar disorder is a complex and chronic psychiatric condition characterized by episodes of mania (bipolar type I) or the less severe hypomania (bipolar type II) and recurrent episodes of depression.1 Taken together, bipolar I and II disorders and other bipolar spectrum disorders affect an estimated 3% of the population.2 Bipolar disorder is associated with considerable functional impairment and substantially increased risk for completed suicide.3–5 There is increasing awareness of the debilitating effects of bipolar disorder on physical health, which likely include both known risk factors (smoking, diabetes, obesity) and excess inflammatory activity inherent to the disorder itself.6 Both the short-term treatment and long-term management remain significant challenges for clinicians treating patients with bipolar disorder.4 The staggering health and economic7 costs associated with this disorder also underscore the crucial need for developing effective treatments.
While significant advances in the treatment of acute mania have been made in the past 10 years,8 there is still a dearth of empirically derived information on optimal treatment of bipolar depression.9,10 Currently, only a combination of olanzapine with fluoxetine and quetiapine monotherapy are approved by the US Food and Drug Administration for the treatment of bipolar depression. The approval of quetiapine was based on its efficacy as monotherapy in treating depressive episodes in patients with bipolar disorder in 2 similarly designed, randomized, placebo-controlled BipOLar DEpRession (BOLDER I [Trial 049] and II [Trial 135]) studies.11,12 Quetiapine is also effective for the treatment of acute mania both as monotherapy and in combination with lithium or divalproex.13–16 Two studies have also now shown the effectiveness of an extended-release formulation of quetiapine fumarate for the treatment of symptoms of anxiety in patients with generalized anxiety disorder (GAD).17,18
Clinical Points
♦ Quetiapine monotherapy led to substantial improvement of anxiety symptoms within the first week for acutely depressed patients with either bipolar I or II disorder.
♦ The number needed to treat to attain remission for depressive symptoms in patients with bipolar I or II disorder with quetiapine was 5.1 for 300 mg/d and 5.0 for 600 mg/d.
♦ Anxiety symptom severity at baseline did not alter response to depressive symptom treatment with quetiapine in those patients with bipolar I or II disorder.
♦ The results of this analysis support the efficacy of quetiapine treatment for both depressive and anxiety symptoms in patients with bipolar I or II disorder.
In addition to the debilitating effects of depressive episodes, many patients have comorbid anxiety disorders,19–21 or clinically significant anxiety symptoms.22 Comorbid anxiety is associated with earlier onset of bipolar disorder, more severe depression, decreased response to therapy resulting in poorer outcomes, increased time to remission, and increased risk of suicide.19,22–26 Associations between anxiety and bipolar disorder have frequently been observed, and because of this, it has been suggested that there may be some genetic linkage and shared biologic underpinnings.24
Given the importance of anxiety in bipolar depression, an initial post hoc analysis of the first bipolar depression study (BOLDER I) was undertaken to determine the effectiveness of quetiapine monotherapy for the treatment of coexisting anxiety symptoms in patients with bipolar I and II depression.27 Quetiapine was associated with significant (P < .001) improvement in Hamilton Anxiety Rating Scale (HARS)28 total scores compared with placebo. Effect sizes for the difference from placebo were 0.53 for quetiapine 300 mg/d and 0.68 for quetiapine 600 mg/d. This improvement in anxiety appeared to be independent of baseline severity of depressed symptoms of depression, as there were similar significant improvements over placebo (P < .001) in anxiety (as measured by HARS total scores) in 2 subgroups of patients categorized by baseline Montgomery-Asberg Depression Rating Scale (MADRS)29 total scores ≤ 30 and > 30.
This report presents the results of an analysis of the pooled patient samples from the 2 bipolar depression (BOLDER I and II) studies, which was conducted to further examine the pattern of response of anxiety symptoms in patients with bipolar disorder. This pooled analysis provides better precision due to the larger sample (N = 978) for a more in-depth evaluation of the relationship between depression response and improvement in anxiety symptoms, including improvement in individual HARS items, the impact of the severity of baseline anxiety, and predictors of response, than the analysis of data from each study separately.
METHOD
Study Design
Both of the bipolar depression studies were 8-week, multicenter, double-blind, randomized, placebo- controlled studies intended to evaluate the efficacy and safety of quetiapine monotherapy (fixed doses of 300 mg/d and 600 mg/d) compared with placebo for the treatment of a current major depressive episode in adult patients with bipolar I or II disorder.11,12 Both study protocols were approved by institutional review boards at each site and were in accordance with the most recent amendment of the Declaration of Helsinki as well as the International Conference on Harmonization/Good Clinical Practice guidelines. All patients gave written consent prior to participation. The BOLDER I study was conducted between September 2002 and October 2003, and the BOLDER II study was conducted between June 2004 and August 2005.
Study Population
Patients were enrolled from 39 centers in the United States in the first study and 41 centers in the United States in the second study. Eligible patients were adult male and female outpatients (aged 18 to 65 years) who met lifetime Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)1 criteria for mania (bipolar I) or hypomania (bipolar II) and were experiencing a current major depressive episode of at least 4 weeks and less than 12 months. Eligibility also required the patients to have a Hamilton Depression Rating Scale (HDRS)30 17-item total score ≥ 20, a HDRS item 1 (depressed mood) score ≥ 2, and a Young Mania Rating Scale (YMRS)31 total score ≤ 12 at both screening and randomization. The MADRS was used as the primary efficacy measure for bipolar depression.
Patients meeting the following criteria were not included in either study: nonresponse of the current depressive episode to adequate treatment (approximately 6 or more weeks) with more than 2 classes of antidepressants, a current Axis I disorder other than bipolar disorder that was the primary focus of treatment within 6 months of screening, a current or history of clinically significant medical illness, and fulfillment of DSM-IV criteria for substance dependence (excluding nicotine) within 12 months of screening.
Study Medication
In both studies, patients were randomly assigned to 1 of the following 3 treatment groups: quetiapine 300 mg/d, quetiapine 600 mg/d, or placebo.
Quetiapine or placebo, identical in appearance and number of tablets, were administered orally once per day at bedtime. Quetiapine was initiated at 50 mg/d on day 1 and increased to 100 mg/d on day 2, to 200 mg/d on day 3, and to 300 mg/d by day 4 or 600 mg/d by the end of week 1. At the discretion of the investigator, a 1-time dose reduction was permitted of 100 mg/d in all active treatment groups for intolerability after week 1 (to 200 mg/d and 500 mg/d). The patients stayed on the reduced dose for the remainder of the study.
The study protocols allowed continuation of nonpsychotropic medication taken before entry into the study, but prohibited the concomitant use of psychoactive drugs and medications with potent effects on cytochrome P450 3A4 activity. Use of zolpidem tartrate and lorazepam was allowed for the first 3 weeks of the study at the discretion of the investigator. All other psychoactive drugs were discontinued 7–28 days (depending on the medication) prior to randomization.
Efficacy Measures
Patients were assessed by investigators who were blinded to treatment at baseline and then weekly through week 8 (day 57). The primary efficacy endpoint of the 2 individual bipolar depression studies was the mean change in MADRS total score from baselineto week 8.
One of the secondary endpoints of the original studies, the HARS, was assessed at baseline and weekly in the first study (BOLDER I), and at baseline and weeks 1, 4, and 8 in the second study (BOLDER II). For this post hoc analysis, data from baseline and weeks 1, 4, and 8 were used. The mean HARS change from baseline was used to determine the effect of quetiapine on symptoms of anxiety in depressed patients with bipolar disorder.
The data from this pooled sample were evaluated to determine the mean change from baseline in MADRS and HARS total scores, HARS psychic and somatic anxiety subscale scores, and individual MADRS and HARS items. Additional evaluations of the pooled data included the percentage of patients who responded to treatment, defined as a ≥ 50% reduction in MADRS total score compared with baseline, and patients who met remission criteria, defined as a MADRS total score ≤ 12, both of which were used to determine the number needed to treat (NNT).
Safety Measures
In both studies, the incidence of adverse events and discontinuations from the studies were assessed. The proportion of patients with treatment-emergent mania/ hypomania, defined as those with a YMRS total score ≥ 16 at 2 consecutive assessments or at final assessment, or adverse event reports of mania or hypomania, was evaluated. Other safety measures included change in weight, vital signs, 12-lead electrocardiography, routine hematology, and laboratory tests.
Statistical Methods
Pooled analyses were performed using the intent-to-treat sample population, which was defined as all randomly assigned patients who took at least 1 dose of study medication and had at least 1 postbaseline efficacy assessment. Most analyses used the mixed-model repeated-measure (MMRM) methods with the baseline value as the covariate; treatment, bipolar type, visit, and treatment-visit interaction as fixed effects; and center as random effect and repeated over visit. The banded Toeplitz 8 covariance structure was used to model within-patient variability. Some subpopulation analyses used an analysis of covariance (ANCOVA) with the baseline value as the covariate, treatment and bipolar type as fixed effect, and center as random effect.
Therapeutic effect size was used to determine the magnitude of improvement resulting from quetiapine treatment compared with placebo. This was calculated using the MMRM analysis model as the least squares mean (LSM) difference between quetiapine and placebo divided by the estimated pooled standard deviation. The higher the effect size, the stronger the effect of treatment on outcome, with 0.2 signifying a small clinical effect, 0.5 signifying a moderate clinical effect, and 0.8 signifying a large clinical effect.32 Descriptive statistics are presented for all safety variables.
RESULTS
Patient Population
Data included in these analyses are from a combined total of 1,051 patients randomly assigned to receive quetiapine 300 mg/d (n = 353), quetiapine 600 mg/d (n = 349), or placebo (n = 349). The combined intent- to-treat population included 978 patients (quetiapine 300 mg, n = 327; quetiapine 600 mg, n = 321; placebo, n = 330).
The combined patient population, like those of the individual bipolar depression studies, showed similar patient demographics and baseline disease characteristics across the treatment groups (Table 1), and these were judged not to invalidate the results of the efficacy or safety analyses. In each treatment sample, approximately two-thirds had bipolar I and one-third had bipolar II disorder. Mean baseline MADRS total scores for the 3 treatment groups were comparable and were consistent with moderate to severe depression: quetiapine 300 mg/d, 30.7 (SD = 5.4); quetiapine 600 mg/d, 30.1 (SD = 5.4); and placebo, 30.1 (SD = 5.4). Mean baseline HARS total scores were similar between the treatment groups and indicative of mild to moderate anxiety: quetiapine 300 mg/d, 18.9 (SD = 6.7); quetiapine 600 mg/d, 18.6 (SD = 6.6); and placebo, 18.6 (SD = 6.5). The use of lorazepam, which was permitted during the first 3 weeks of the study to treat severe anxiety, was similar across the groups: 5.4% of the patients in the quetiapine 300-mg/d group and 4.6% in the quetiapine 600-mg/d group versus 5.8% in the placebo group. Similarly, the use of zolpidem to treat insomnia was 2.9% and 4.3% versus 5.8%, respectively.
Table 1.
Patient Demographics and Baseline Disease Characteristics From Pooled Studies of Patients With Bipolar I or II Disorder Experiencing a Depressive Episode (intent-to-treat population)
| Variable | Quetiapine 300 mg/d (n = 327) | Quetiapine 600 mg/d (n = 321) | Placebo (n = 330) |
| Gender, n (%) | |||
| Female | 179 (54.7) | 182 (56.7) | 202 (61.2) |
| Male | 148 (45.3) | 139 (43.3) | 128 (38.8) |
| Age, mean (SD), y | 36.8 (10.9) | 37.7 (11.2) | 38.0 (11.4) |
| Weight, mean (SD), kg | 86.8 (21.4) | 86.2 (22.6) | 83.2 (21.7) |
| DSM-IV diagnosis, n (%) | |||
| Bipolar I | 220 (67.3) | 215 (67.0) | 222 (67.3) |
| Bipolar II | 107 (32.7) | 106 (33.0) | 108 (32.7) |
Improvement in Depression
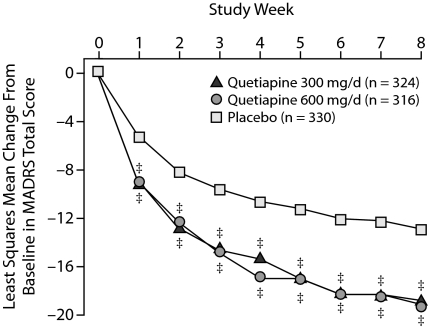
The change from baseline in MADRS total score, used to assess overall improvement in depression, was statistically significantly greater for the quetiapine 300-mg/d and 600-mg/d treatment groups compared with the placebo group (P < .001) at every assessment from week 1 through week 8 (Figure 1). Mean change in MADRS total score from baseline to week 8 was −18.8 and −19.2 for the quetiapine 300-mg/d and 600-mg/d groups, respectively, compared with –12.9 for the placebo group. The effect sizes for quetiapine treatment compared with placebo were 0.65 for the quetiapine 300-mg/d group and 0.69 for the quetiapine 600-mg/d group after 8 weeks of treatment. The results of MMRM analysis reflect those using last-observation-carried-forward (LOCF) ANCOVA.
Figure 1.
Least Squares Mean Change From Baseline in MADRS Total Score at Each Assessment From Pooled Studies of Patients With Bipolar I or II Disorder Experiencing a Depressive Episode (MMRM)
‡P < .001.
Abbreviations: MADRS = Montgomery-Asberg Depression Rating Scale, MMRM = mixed-model repeated measure.
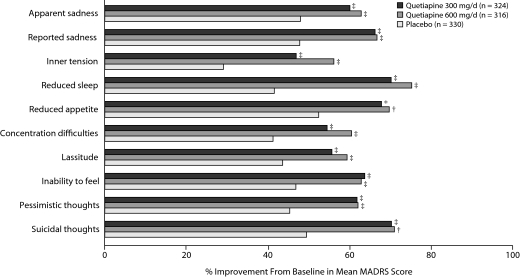
Both doses of quetiapine were associated with statistically significant improvements from baseline compared with placebo for all 10 individual MADRS items (P < .05, Figure 2).
Figure 2.
Mean Percent Improvement From Baseline in Individual MADRS Items From Pooled Studies of Patients With Bipolar I and II Disorder Experiencing a Depressive Episode (MMRM)a
aP values based on change from baseline MMRM analyses.
*P < .05 versus placebo; †P < .01; ‡P < .001.
Abbreviations: MADRS = Montgomery-Asberg Depression Rating Scale, MMRM = mixed-model repeated measure.
Improvement in Anxiety
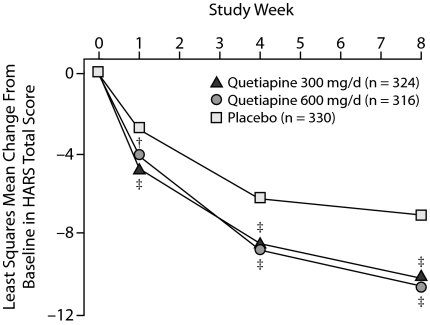
Change from baseline in HARS total scores, a measure of improvement in overall anxiety, was statistically significantly greater in the quetiapine 300-mg/d and 600-mg/d groups than in the placebo group starting at week 1 (Figure 3). Mean change in HARS total scores at week 8 was –10.1 and –10.5 for the quetiapine 300-mg/d and 600-mg/d groups, respectively, compared with –6.9 for the placebo group (P < .001 vs placebo for both groups). Effect sizes for quetiapine compared with placebo with regard to anxiety symptoms were 0.56 for the quetiapine 300-mg/d group and 0.62 for the quetiapine 600-mg/d group after 8 weeks of treatment. The results of the MMRM analysis match those obtained using LOCF ANCOVA.
Figure 3.
Least Squares Mean Change From Baseline in HARS Total Score at Each Assessment From Pooled Studies of Patients With Bipolar I or II Disorder Experiencing a Depressive Episode (MMRM)
†P < .01.
‡P < .001.
Abbreviations: HARS = Hamilton Rating Scale for Anxiety, MMRM = mixed-model repeated measure.
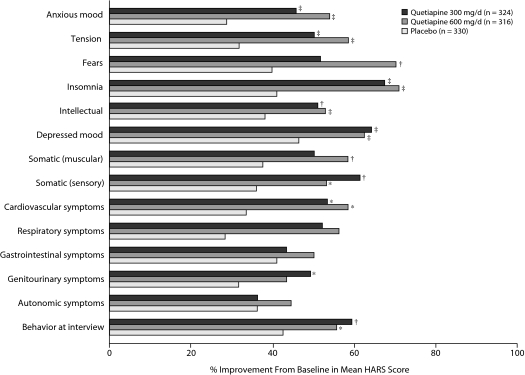
Quetiapine treatment at 300 mg/d and 600 mg/d significantly improved HARS psychic and somatic anxiety subscale scores at week 8 (P < .01, Table 2). Analyses of individual HARS items (Figure 4) found statistically significant improvements in 11 of the 14 items after 8 weeks of quetiapine treatment. Notably, significant reductions were observed in the core items of anxious mood and tension following quetiapine treatment (P < .001 vs placebo for both groups). Other psychic items with statistically significant improvements included fears (P < .001, quetiapine 600-mg/d group), insomnia (P < .001, both groups), intellectual symptoms (P < .01, both groups), and depressed symptoms (P < .001, both groups).
Table 2.
Least Squares Mean Change in MADRS and HARS Total and Anxiety Subscale Scores From Pooled Studies of Quetiapine Treatment in Patients With Bipolar I or II Disorder Experiencing a Depressive Episode (MMRM)
| Scale | na | Baseline Score, Mean (SD) | Least Squares Mean Change at Week 8 | P Value |
| MADRS total score | ||||
| Quetiapine 300 mg/d | 216 | 30.7 (5.4) | –18.8 | < .001 |
| Quetiapine 600 mg/d | 183 | 30.1 (5.4) | –19.2 | < .001 |
| Placebo | 202 | 30.1 (5.4) | –12.9 | |
| HARS score | ||||
| Total | ||||
| Quetiapine 300 mg/d | 217 | 18.9 (6.7) | –10.1 | < .001 |
| Quetiapine 600 mg/d | 189 | 18.6 (6.6) | –10.5 | < .001 |
| Placebo | 207 | 18.6 (6.5) | –6.9 | |
| Psychic anxiety subscale | ||||
| Quetiapine 300 mg/d | 217 | 12.7 (3.6) | –7.2 | < .001 |
| Quetiapine 600 mg/d | 189 | 12.3 (3.6) | –7.5 | < .001 |
| Placebo | 207 | 12.2 (3.5) | –4.8 | |
| Somatic anxiety subscale | ||||
| Quetiapine 300 mg/d | 217 | 5.9 (3.8) | –2.9 | .003 |
| Quetiapine 600 mg/d | 189 | 5.7 (3.8) | –3.0 | .001 |
| Placebo | 207 | 5.8 (3.7) | –2.1 | |
Intent-to-treat population at week 8.
Abbreviations: HARS = Hamilton Anxiety Rating Scale, MADRS = Montgomery-Asberg Depression Rating Scale, MMRM = mixed-model repeated measures.
Figure 4.
Mean Percent Improvement From Baseline in Individual HARS Items From Pooled Studies of Patients With Bipolar I and II Disorder Experiencing a Depressive Episode (MMRM)a
aP values based on change from baseline MMRM analyses.
*P < .05 placebo; †P < .01; ‡P < .001.
Abbreviations: HARS = Hamilton Rating Scale for Anxiety, MMRM = mixed-model repeated measure.
Somatic (sensory) and cardiovascular symptoms significantly improved with quetiapine treatment at 300 mg/d and 600 mg/d (P < .05). Genitourinary symptoms showed significant improvements with quetiapine 300 mg/d, whereas somatic (muscular) symptoms were significantly improved with quetiapine 600 mg/d (P < .05). For those somatic items that did not show significant improvements in 1 or both quetiapine treatment groups, such as somatic (muscular, quetiapine 300 mg/d), respiratory (both doses), gastrointestinal (both doses), and autonomic (both doses) symptoms, mean scores for all 3 treatment groups were low at baseline (0.5–1.1).
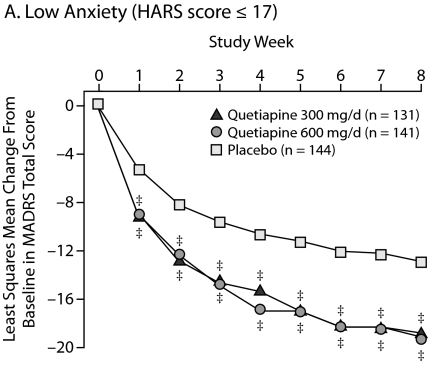
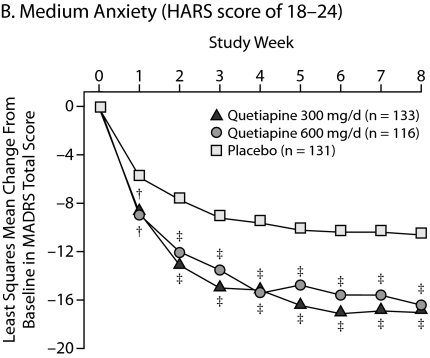
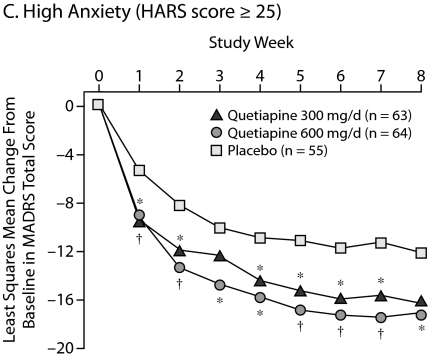
Improvements in High Versus Low Anxiety Subpopulations
To determine the effect of the severity of baseline anxiety on treatment efficacy, patients were divided into those with low anxiety (HARS total score ≤ 17), moderate anxiety (HARS total score between 18 and 24), and severe anxiety (HARS total score ≥ 25). Consistent improvements in HARS scores for quetiapine over placebo were seen across the severity categories with statistical significance achieved in the categories of low to moderate anxiety. In patients with severe anxiety, the quetiapine treatment groups showed a numerical (–13.3 for quetiapine 300 mg/d and –13.9 for quetiapine 600 mg/d) but not a statistically significant improvement over placebo (–10.8) in HARS total score, given the low patient numbers in this subgroup and the magnitude of the placebo response in the other subgroups (Table 3).
Table 3.
Least Squares Mean Changes in HARS Total Score Stratified According to Baseline Anxiety Level From Pooled Studies of Quetiapine Treatment in Patients With Bipolar I or II Disorder Experiencing a Depressive Episode (LOCF ANCOVA)
| Anxiety Level | na | Baseline Score, Mean (SD) | Least Squares Mean Change in HARS Total Score at Week 8 | P Value |
| Low anxiety (HARS score ≤ 17 at baseline) | ||||
| Quetiapine 300 mg/d | 131 | 12.4 (3.6) | – 5.47 | < .001 |
| Quetiapine 600 mg/d | 140 | 12.6 (4.0) | – 5.08 | .003 |
| Placebo | 144 | 12.8 (3.7) | – 3.10 | |
| Moderate anxiety (HARS score = 18–24 at baseline) | ||||
| Quetiapine 300 mg/d | 133 | 20.7 (1.9) | – 10.11 | < .001 |
| Quetiapine 600 mg/d | 116 | 20.6 (1.8) | – 9.77 | < .001 |
| Placebo | 130 | 20.8 (2.0) | – 6.19 | |
| Severe anxiety (HARS score ≥ 25 at baseline) | ||||
| Quetiapine 300 mg/d | 63 | 28.4 (3.6) | – 13.33 | .113 |
| Quetiapine 600 mg/d | 63 | 27.9 (2.7) | – 13.87 | .057 |
| Placebo | 55 | 28.5 (3.5) | – 10.76 | |
Intent-to-treat population at week 8.
Abbreviations: HARS = Hamilton Anxiety Rating Scale, LOCF ANCOVA = last-observation-carried-forward analysis of covariance.
In addition, the impact of baseline severity of anxiety on depressive symptoms was assessed (Figure 5). Patients with low and moderate anxiety at baseline responded similarly, with significant and sustained improvements in depressive symptoms with both doses of quetiapine compared with placebo from week 1 through week 8. For patients with high baseline anxiety, there were similar improvements in depressive symptoms with both doses of quetiapine from week 1 through week 7; however, only the quetiapine 600-mg/d group reached significance at week 8 (P = .026).
Figure 5.
Least Squares Mean Change From Baseline in MADRS Total Score Stratified According to Baseline Anxiety Level From Pooled Studies of Patients With Bipolar I and II Disorder Experiencing a Depressive Episode (ITT, LOCF ANCOVA)
*P < .05 versus placebo.
†P < .01.
‡P < .001.
Abbreviations: HARS = Hamilton Rating Scale for Anxiety, ITT = intent-to-treat, LOCF ANCOVA = last-observation-carried-forward analysis of covariance, MADRS = Montgomery-Asberg Depression Rating Scale.
Improvements in HARS Scores in MADRS Remitters, Nonremitters, Responders, and Nonresponders
An analysis (descriptive only) was undertaken to determine the mean changes in HARS total scores in those patients classified as remitters (n = 448, 45.8%) defined as a final MADRS total score ≤ 12, in nonremitters (n = 530, 54.2%), in responders (n = 512, 52.4%) defined as a reduction of ≥ 50% in MADRS total score, and nonresponders (n = 466, 47.6%). In patients who met the remission criterion, the change from baseline to week 8 in HARS total score was –12.8 for quetiapine 300 mg/d, –12.5 for quetiapine 600 mg/d, and –11.4 for placebo. In patients who were nonremitters, mean change from baseline at week 8 was –4.5, –3.9, and –2.9 in quetiapine 300 mg/d, 600 mg/d, and placebo groups, respectively. In patients who met the response criterion, the change from baseline to week 8 in HARS total score was –12.5 for quetiapine 300 mg/d, –12.1 for quetiapine 600 mg/d, and –10.8 for placebo, while in patients who were nonresponders, mean change from baseline at week 8 was –3.8, –3.4, and –2.3.
NNT Analysis for Improvement in Depressive Symptoms
The NNTs were calculated in patients classified as responders (defined as ≥ 50% reduction in MADRS total score) and remitters (defined as a MADRS total score ≤ 12). The NNT at week 8 was 5.4 (95% CI, 3.9–9.2) and 5.6 (95% CI, 3.9–9.6) for quetiapine 300 and 600 mg/d, respectively, in responders, and 5.1 (95% CI, 3.7–8.2) and 5.0 (95% CI, 3.6–8.0) for quetiapine 300 and 600 mg/d, respectively, in remitters.
Subpopulation Analysis: Impact of Anxiety
An analysis was undertaken to determine whether there was a correlation between age and time of onset of bipolar disorder and anxiety. The age at onset and years since first bipolar episode appeared to be similar in patients with low (HARS score ≤ 17), moderate (HARS score 18–24), or high (HARS score ≥ 25) anxiety at baseline (19.9, 19.1, and 19.3 years for mean age at onset and 19.1, 19.5, and 20.5 years since first bipolar episode, respectively).
In addition, to determine whether patients with anxiety at baseline had an increased likelihood of having medical comorbidities, the following were assessed by examining the patient's medical history: fibromyalgia, migraine, chronic fatigue, and irritable bowel syndrome. While the prevalence of chronic fatigue and fibromyalgia appeared similar in all 3 anxiety groups (between 0% and 1.3%), there was a greater incidence as indicated by medical history of irritable bowel syndrome and migraine in patients with moderate (4.7% and 14.5%) and severe (6.0% and 15.9%) anxiety at baseline compared with patients with low anxiety at baseline (1.9% and 8.4%).
Safety/Tolerability Analysis
Detailed safety results from the 2 individual bipolar depression studies and some combined safety results are reported elsewhere.11,12,33 In the combined studies, at the end of 8 weeks of treatment, the mean weight change was +1.2 kg and +1.5 kg in the quetiapine 300-mg/d and 600-mg/d groups, respectively, versus +0.2 kg in the placebo group. The proportion of patients with a ≥ 7% weight gain was 7.1%, 10.0%, and 2.4% in quetiapine 300-mg/d, 600-mg/d, and placebo groups, respectively; most individuals who gained this weight were classified in the lower BMI category at baseline (18 to < 25 kg/m2) in all 3 treatment groups.
In the combined study population, 36.6%, 46.0%, and 37.5% of patients in the quetiapine 300-mg/d, 600-mg/d, and placebo groups, respectively, discontinued treatment (Table 4). Most discontinuations in the quetiapine groups were due to adverse events compared with a lack of efficacy in the placebo group. The most common adverse events in the quetiapine groups were dry mouth, somnolence, sedation, dizziness, and constipation (Table 5). The proportion of patients reporting adverse events was similar in those treated with quetiapine but higher than those treated with placebo in the subgroups with high (94.0%, quetiapine 300 mg/d; 93.1%, quetiapine 600 mg/d; 84.8%, placebo), moderate (92.8%, quetiapine 300 mg/d; 89.8%, quetiapine 600 mg/d; 86.0%, placebo), or low (89.6%, quetiapine 300 mg/d; 90.5%, quetiapine 600 mg/d, 78.3%, placebo) anxiety at baseline.
Table 4.
Study Discontinuations: Pooled Results From 2 Studies (safety population)
| Variable, n (%) | Quetiapine 300 mg/d (n = 350) | Quetiapine 600 mg/d (n = 348) | Placebo (n = 347) |
| Total withdrawals | 128 (36.6) | 160 (46.0) | 130 (37.5) |
| Discontinued from treatment | |||
| Eligibility criteria not fulfilled | 0 (0.0) | 0 (0.0) | 2 (0.6) |
| Adverse event | 43 (12.3) | 66 (19.0) | 18 (5.2) |
| Lack of therapeutic response | 7 (2.0) | 6 (1.7) | 37 (10.7) |
| Protocol noncompliance | 15 (4.3) | 10 (2.9) | 18 (5.2) |
| Not willing to continue study | 30 (8.6) | 35 (10.1) | 30 (8.6) |
| Lost to follow-up | 33 (9.4) | 40 (11.5) | 25 (7.2) |
| Other | 0 (0.0) | 3 (0.9) | 0 (0.0) |
| Completed study | 222 (63.4) | 188 (54.0) | 217 (62.5) |
Table 5.
Common Adverse Events (≥ 5% in any quetiapine group and at least twice that of placebo) Associated With Quetiapine Treatment: Pooled Results From 2 Studies (safety population)
| Adverse event, n (%)a | Quetiapine 300 mg/d (n = 350) | Quetiapine 600 mg/d (n = 348) | Placebo (n = 347) |
| Dry mouth | 152 (43.4) | 152 (43.7) | 44 (12.7) |
| Sedation | 108 (30.9) | 104 (29.9) | 28 (8.1) |
| Somnolence | 100 (28.6) | 94 (27.0) | 23 (6.6) |
| Dizziness | 54 (15.4) | 68 (19.5) | 24 (6.9) |
| Constipation | 35 (10.0) | 37 (10.6) | 13 (3.7) |
| Lethargy | 20 (5.7) | 18 (5.2) | 6 (1.7) |
| Nasal congestion | 15 (4.3) | 22 (6.3) | 9 (2.6) |
| Vision blurred | 12 (3.4) | 18 (5.2) | 7 (2.0) |
| Weight increased | 10 (2.9) | 20 (5.7) | 4 (1.2) |
Based on Medical Dictionary for Regulatory Activities; patients with multiple events in the same category are counted only once.
No deaths occurred in either study. The proportion of patients with adverse events potentially associated with suicidality (suicidal ideation/suicide attempt) was 1.4% for quetiapine 300 mg/d, 1.7% for quetiapine 600 mg/d, and 0.9% for placebo. The rate of treatment-emergent mania/hypomania was 2.9% in both quetiapine groups compared with 5.2% in the placebo group.
DISCUSSION
This secondary analysis of combined data from 2 large bipolar depression studies (BOLDER I and II) demonstrated that quetiapine monotherapy significantly reduces the anxiety symptoms associated with major depressive disorder in patients with bipolar I or II disorder. Quetiapine monotherapy improved anxiety symptoms within the first week of treatment with sustained improvement throughout the study to week 8. It should be noted that patients meeting the DSM-IV criteria for comorbid anxiety disorders were excluded, and only anxiety associated with depression or subdiagnostic levels of anxiety were measured in this trial.
Quetiapine monotherapy demonstrated efficacy in treating both major depressive disorder and anxiety symptoms. There were significant improvements not only in MADRS and HARS total scores from week 1 and sustained through week 8 but also in individual items of the 2 scales (all 10 items of the MADRS and 11 of 14 items of the HARS) and the HARS psychic and somatic anxiety subscales scores. Quetiapine monotherapy was found to be effective in both patients with bipolar I and bipolar II disorder and in those with and without a rapid-cycling disease course (data not shown). These results are similar to those found in an initial secondary analysis of the first (BOLDER I) study data.27
Few studies have examined whether treatment with atypical antipsychotics can lessen the severity of bipolar disorder and improve anxiety symptoms or disorders. In a post hoc analysis of an 8-week, randomized, double-blind, placebo-controlled trial of patients with bipolar I depression in which subjects with comorbid anxiety symptoms were included, Tohen et al34 reported that olanzapine and olanzapine-fluoxetine combination therapy showed statistically significant improvement in HARS scores compared with placebo. In a further post hoc analysis of this study, patients were split into 2 subgroups, those with and without comorbid anxiety, according to their symptomatic presentation at baseline. The subgroup classified as having comorbid anxiety (HARS total score ≥ 18 at baseline) was less likely to respond to treatment with olanzapine and olanzapine-fluoxetine combination than patients who were classified as noncomorbid (HARS total score < 18).35 Olanzapine and olanzapine-fluoxetine were both shown to improve depressive and anxiety symptoms in patients with bipolar I disorder in both subgroups.35
In the present study, the patients were categorized by baseline HARS scores into 3 subgroups: low (HARS score ≤ 17), moderate (HARS score of 18–24), or high (HARS score ≥ 25) anxiety. The results from this analysis indicate that quetiapine is effective for bipolar I or II depression regardless of the severity of baseline anxiety. The lack of statistical significance in the high-anxiety subgroup may be due to the smaller patient numbers in this subgroup, which can further limit the power to detect any difference between the treatment groups. Overall, the majority of patients in this study had no greater than mild to moderate anxiety at baseline, which is to be expected given the exclusion of coexisting anxiety disorders in these 2 studies.
The effect size and NNT analyses conducted in this study provide clinicians with a means to assess the magnitude of the active treatment effect compared with placebo in order to make informed clinical decisions. Comparison of the results with other studies is also possible with these approaches as long as the patient populations are similar. An effect size of 0.2 is considered to be of low clinical benefit, 0.5 to be of moderate clinical benefit, and 0.8 to be of large clinical benefit.32 In this combined analysis from the 2 BOLDER studies, based on improvement in HARS total score, the effect sizes were 0.56 and 0.62 for patients treated with quetiapine 300 mg/d and 600 mg/d, respectively, indicating at least a moderate beneficial effect on anxiety symptoms. Similarly, the effect sizes based on the MADRS were 0.65 and 0.69 for patients treated with quetiapine 300 mg/d and 600 mg/d, respectively. The NNT results also indicate that quetiapine demonstrates a clinical benefit in terms of response (≥ 50% reduction in MADRS total score, NNT = 5.4 and 5.6 for quetiapine 300 mg/d and 600 mg/d, respectively) and remission (MADRS total score ≤ 12, NNT = 5.1 and 5.0, respectively). The NNT results are similar to those reported in an analysis of the BOLDER I study only,36 which showed an NNT of approximately 5 for both doses of quetiapine in patients classified as responders and remitters. In the Tohen et al study,34 the NNTs for responders at week 8 were 12 (95% CI, 7–62) for olanzapine monotherapy and 4 (95% CI, 3–8) for olanzapine-fluoxetine combination, although the study included only patients with bipolar I disorder experiencing depressive episodes.
Quetiapine monotherapy was generally well tolerated, with dry mouth, sedation, somnolence, constipation, and dizziness the most commonly reported adverse events. The safety and tolerability results from the 2 individual bipolar depression studies are published elsewhere.11,12 In the combined patient population, the use of the sedative lorazepam to treat severe anxiety was limited and similar across all 3 treatment groups and is therefore unlikely to have influenced the findings of this study. Generally, the patients receiving quetiapine treatment had an overall higher weight gain than those in the placebo group.
Neither of the 2 bipolar depression studies was powered to detect any confounding effects of treatment, thus a potential correlation may exist between the observed improvements in anxiety and depressive symptoms, but this needs to be confirmed by further investigation. All of the results from the subgroup analyses must also be treated with caution owing to the small patient numbers, particularly in the high-anxiety-at-baseline subgroup, and no firm conclusions can be made with regard to potential differences in the efficacy of quetiapine treatment in these subgroups.
When managing patients with bipolar disorder, the majority of treatment guidelines recommend that coexisting anxiety disorders also be treated concurrently.37,38 To date, no single medication has been approved to treat the symptoms of both bipolar disorder and comorbid anxiety disorders. It has been observed that a large proportion of patients with bipolar disorder experience comorbid anxiety and consequently poorer treatment responses and outcomes and an increased rate of suicide in these patients.25,39,40 Therefore, there is a real clinical need for new strategies and treatments to help manage patients with bipolar disorder and coexisting anxiety. The results of this analysis raise the possibility that quetiapine monotherapy could potentially play a role in the treatment of depressed patients with bipolar disorder and comorbid anxiety disorder. Further studies in patients with both bipolar disorder and comorbid anxiety disorders are warranted to test this hypothesis.
The results from the combined bipolar depression studies indicate that, overall, quetiapine monotherapy was generally well tolerated and demonstrated significantly greater improvement compared with placebo in treating patients with bipolar depression and coexisting anxiety symptoms.
Drug names: divalproex (Depakote and others), fluoxetine (Prozac and others), lithium (Eskalith, Lithobid, and others), lorazepam (Ativan and others), olanzapine (Zyprexa), olanzapine/fluoxetine combination (Symbyax), quetiapine (Seroquel), zolpidem (Ambien and others).
Study participants:
The Trial 049 (BOLDER I) Study Group
Mohammed Alam, American Med Research, Oak Brook, Illinois; Valerie Arnold, Clinical Neuroscience Solutions, Memphis, Tennessee; Charles Bailey, Clinical Neuroscience Solutions, Orlando, Florida; Guy Brannon, Brentwood Research Institute, Shreveport, Louisiana (affiliated with: Brentwood Hospital); David Brown, Community Clinical Research, Austin, Texas; Joseph Calabrese, University Hospitals of Cleveland/Case University School of Medicine, Ohio; John Carman, Carman Research, Smyrna, Georgia; Andrew Cutler, CORE Research, Winter Park, Florida; Bernadette D'Souza, Midwest Clinical Research, Dayton, Ohio (affiliated with Veterans Affairs Medical Center); Naresh Emmanuel, Carolina Clinical Research Services, Columbia, South Carolina; Lawrence Ginsberg, Red Oak Psychiatry Associates, Houston, Texas; Ram Gopalan, Comprehensive Neuroscience of Northern Virginia, Falls Church; William Granger, Research Strategies Inc, Reno, Nevada; Laszlo Gyulai, University of Pennsylvania Bipolar Disorder, Philadelphia; Howard Hassman, Comprehensive Clinical Research, Clementon, New Jersey; Saul Helfing, Oregon Center for Clinical Investigators Inc, Lake Oswego; George Joseph, Clinical Neuroscience Solutions, Jacksonville, Florida; Paul Keck, University of Cincinnati, Ohio; Terrence Ketter, Stanford University Bipolar Disorder Clinic, Palo Alto, California; Arif Khan, Northwest Medical Research Center, Bellevue, Washington; Ari Kiev, Social Psychiatry Research Institute, New York, New York; James Knutson, BHC Fairfax Hospital, Kirkland, Washington; Irving Kolin, Kolin Research Group, Winter Park, Florida (affiliated with University of Florida); Michael Levy, Behavioral Medical Research of Staten Island, New York; H. E. Logue, Birmingham Psychiatry Pharmaceutical Services Inc, Alabama; David Marks, Optimum Health Services, La Mesa, California; Greg Mattingly, St. Charles Psychiatric Association, Missouri; Charles Merideth, Affiliated Research Institute, San Diego, California; Janice Miller, Clinical Neuroscience Solutions, West Palm Beach, Florida; Dennis Munjack, Southwestern Research Inc, Beverly Hills, California; William Privitera, Future Search Trials, Austin, Texas; Fred Reimherr, University of Utah Medical Center, Salt Lake City; Robert Riesenberg, Atlanta Center for Medical Research, Georgia; Leon Rosenberg, Center for Emotional Fitness, Moorestown, New Jersey; Leon Rubenfaer, Pioneer Pharmaceutical Research, New Baltimore, Michigan; David Sack, Comprehensive Neuroscience, Cerritos, California; Abbey Strauss, Comprehensive Neuroscience Inc, Boynton Beach, Florida; David Walling, CNS Network, Garden Grove, California; and Richard Weisler, Richard H. Weisler, MD & Associates, Raleigh, North Carolina (affiliated with University of North Carolina, Chapel Hill, and Duke University Medical Center, Durham, North Carolina).
The Trial 135 (BOLDER II) Study Group
Mohammed Alam, American Medical Research, Oak Brook, Illinois; Daniel D. Anderson, Avi Clinical Research, Torrance, California; Valerie Arnold, CNS Healthcare, Memphis, Tennessee; Charles Bailey, Clinical Neuroscience Solutions, Orlando, Florida; Louise Beckett, IPS Research, Oklahoma City, Oklahoma (affiliated with University of Oklahoma Health Sciences Center); Grant Belnap, Mountain West Clinical Trials, Boise, Idaho; Guy Brannon, Brentwood Research Institute, Shreveport, Louisiana (affiliated with Brentwood Hospital); Richard W. Brown, Hartford Research, Cincinnati, Ohio; G. Michael Dempsey, Albuquerque Neuroscience Inc, New Mexico (affiliated with Memorial Hospital); Bernadette D'Souza, Midwest Clinical Research Center, Dayton, Ohio (affiliated with Veterans Affairs Medical Center); Steven Eisen, CNS Research Institute, PC, Philadelphia, Pennsylvania; Beal G. Essink, Oregon Center for Clinical Investigations Inc, Portland (affiliated with Oregon Health & Science University); Joseph Fanelli, Midwest Center for Neurobehavioral Medicine, Oakbrook Terrace, Illinois; Donald J. Garcia, Future Search Trials, Austin, Texas; Lawrence Ginsberg, Red Oak Psychiatry Associates, Houston, Texas; Steve Glass, CNS Research Institute, Clementon, New Jersey; Alan Jonas, Pharmasite Research Inc, Baltimore, Maryland; George Joseph, Clinical NeuroScience Solutions, Jacksonville, Florida; Arifulla Khan, Northwest Medical Research Center, Bellevue, Washington; Ari Kiev, Social Psychiatry Research Institute, Brooklyn, New York; James Knutson, Eastside Therapeutic Resource, Kirkland, Washington; Irving Kolin, Kolin Research Group, Winter Park, Florida (affiliated with University of Florida); Michael Levy, Behavioral Medical Research of Staten Island, New York; H. E. Logue, Birmingham Psychiatry Pharmaceutical Studies Inc, Alabama; R. Bruce Lydiard, Southeast Health Consultants, Charleston, South Carolina (affiliated with University of South Carolina); Charles Merideth, Affiliated Research Institute, San Diego, California; Jairo Nunez, CORE Research, Maitland, Florida; Fred Reimherr, University of Utah Medical Center, Salt Lake City; Leon Rosenberg, Center for Emotional Fitness, Moorestown, New Jersey; David Sack, Comprehensive Neuroscience, Cerritos, California; Jerry Steiert, Summit Research Network LLC, Seattle, Washington; Michael E. Thase, Western Psychiatric Institute and Clinic, Pittsburgh, Pennsylvania (affiliated with University of Pittsburgh); Nick Vatakis, Social Psychiatry Research Institute, New York, New York; David Walling, CNS Network, Garden Grove, California; Richard Weisler, Richard H. Weisler, MD & Associates, Raleigh, North Carolina (affiliated with University of North Carolina, Chapel Hill, and Duke University Medical Center, Durham, North Carolina); and Jill Zweig, Meadowbrook Research Inc, Scottsdale, Arizona.
Financial disclosure: Dr Lydiard has served as a consultant to Eli Lilly, Novartis, and Pfizer and has received research grants from Abbott, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, Jazz, Medicinova, Neurocrine, Pfizer, Sanofi-Aventis, UCB Pharma, and Wyeth. Dr Culpepper has served on the speakers’ bureaus of Forest, Pfizer, and Eli Lilly and as a consultant or member of the advisory boards for AstraZeneca, Forest, Eli Lilly, Pfizer, Somaxon, Takeda, and Wyeth. Drs Gustafsson and Paulsson and Ms Schiöler are employees of AstraZeneca Pharmaceuticals LP.
Funding/support: Supported by AstraZeneca Pharmaceuticals LP (5077US/0049, BOLDER I and D1447C00135, BOLDER II).
Previous presentations: These data were previously presented at the 159th Annual Meeting of the American Psychiatric Association, May 20–25, 2006, Toronto, Ontario, Canada; the 25th Biennial Congress of the Collegium Internationale Neuro-Psychopharmacologicum, July 9–13, 2006, Chicago, Illinois; the 58th Annual Meeting of the Institute on Psychiatric Services, October 5–8, 2006, New York, New York; the 5th European Stanley Conference on Bipolar Disorder, October 5–7, 2006, Barcelona, Spain; and the 45th Annual Meeting of the American College of Neuropsychopharmacology, December 3–7, 2006, Hollywood, Florida.
Acknowledgments
The authors thank Clare Wheatcroft, PhD, from PAREXEL MMS, who provided medical writing support funded by AstraZeneca.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Arlington, VA: American Psychiatric Press, Inc; 2004. Revised. [Google Scholar]
- 2.Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293(20):2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 3.Angst J, Stassen HH, Clayton PJ, et al. Mortality of patients with mood disorders: follow-up over 34-38 years. J Affect Disord. 2002;68(2–3):167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 4.Baldessarini RJ, Tondo L. Suicide risk and treatments for patients with bipolar disorder. JAMA. 2003;290(11):1517–1519. doi: 10.1001/jama.290.11.1517. [DOI] [PubMed] [Google Scholar]
- 5.Osby U, Brandt L, Corria N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 6.Kim YK, Jung HG, Myint AM, et al. Imbalance between pro- inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104(1–3):91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Peele PB, Xu Y, Kupfer DJ. Insurance expenditures on bipolar disorder: clinical and parity implications. Am J Psychiatry. 2003;160(7):1286–1290. doi: 10.1176/appi.ajp.160.7.1286. [DOI] [PubMed] [Google Scholar]
- 8.Keck PE., Jr The role of second-generation antipsychotic monotherapy in the rapid control of acute bipolar mania. J Clin Psychiatry. 2005;66(suppl 3):5–11. [PubMed] [Google Scholar]
- 9.Baldassano CF, Datto SM, Littman L, et al. What drugs are best for bipolar depression? Ann Clin Psychiatry. 2003;15(3–4):225–232. doi: 10.1023/b:acli.0000008176.32677.32. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell PB, Malhi GS. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord. 2004;6(6):530–539. doi: 10.1111/j.1399-5618.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese JR, Keck PE, Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351–1360. doi: 10.1176/appi.ajp.162.7.1351. [DOI] [PubMed] [Google Scholar]
- 12.Thase ME, Macfadden W, Weisler RH, et al. Efficacy of quetiapine monotherapy in bipolar I and II depression. A double-blind, placebo-controlled study (the BOLDER II study) J Clin Psychopharmacol. 2006;26(6):600–609. doi: 10.1097/01.jcp.0000248603.76231.b7. [DOI] [PubMed] [Google Scholar]
- 13.Bowden CL, Grunze H, Mullen J, et al. A randomized, double-blind, placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. J Clin Psychiatry. 2005;66(1):111–121. doi: 10.4088/jcp.v66n0116. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre RS, Brecher M, Paulsson B, et al. Quetiapine or haloperidol as monotherapy for bipolar mania—a 12-week, double-blind, randomised, parallel-group, placebo-controlled trial. Eur Neuropsychopharmacol. 2005;15(5):573–585. doi: 10.1016/j.euroneuro.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Sachs G, Chengappa KN, Suppes T, et al. Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2004;6(3):213–223. doi: 10.1111/j.1399-5618.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 16.Yatham LN, Vieta E, Young AH, et al. A double-blind, randomized, placebo-controlled trial of quetiapine as an add-on therapy to lithium or divalproex for the treatment of bipolar mania. Int Clin Psychopharmacol. 2007;22(4):212–220. doi: 10.1097/YIC.0b013e328080ca57. [DOI] [PubMed] [Google Scholar]
- 17.Khan A, Joyce M, Eggens I. Extended release quetiapine fumarate (quetiapine XR) monotherapy in the treatment of patients with generalized anxiety disorder (GAD) Paper presented at: Anxiety Disorders Association of America Congress, March 6–9, 2008; Savannah, GA. [Google Scholar]
- 18.Chouinard G, Ahokas A, Bandelow B. Once-daily extended release quetiapine fumarate (quetiapine XR) monotherapy in generalized anxiety disorder (GAD): a placebo-controlled study with active-comparator paroxetine. Paper presented at: Anxiety Disorders Association of America Congress, March 6–9, 2008; Savannah, GA. [Google Scholar]
- 19.Boylan KR, Bieling PJ, Marriott M, et al. Impact of comorbid anxiety disorders on outcome in a cohort of patients with bipolar disorder. J Clin Psychiatry. 2004;65(8):1106–1113. doi: 10.4088/jcp.v65n0813. [DOI] [PubMed] [Google Scholar]
- 20.Kessler R. Comorbidity of unipolar and bipolar depression with other psychiatric disorders in a general population survey. In: Tohen M, editor. Comorbidity in Affective Disorders. New York, NY: Marcel Dekker, Inc.; 1999. pp. 1–25. [Google Scholar]
- 21.Pini S, Cassano GB, Simonini E, et al. Prevalence of anxiety disorders comorbidity in bipolar depression, unipolar depression and dysthymia. J Affect Disord. 1997;42(2–3):145–153. doi: 10.1016/s0165-0327(96)01405-x. [DOI] [PubMed] [Google Scholar]
- 22.Feske U, Frank E, Mallinger AG, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry. 2000;157(6):956–962. doi: 10.1176/appi.ajp.157.6.956. [DOI] [PubMed] [Google Scholar]
- 23.McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158(3):420–426. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- 24.McIntyre R, Katzman M. The role of atypical antipsychotics in bipolar depression and anxiety disorders. Bipolar Disord. 2003;5(suppl 2):20–35. doi: 10.1111/j.1399-2406.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 25.Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2004;161(12):2222–2229. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- 26.Vieta E, Colom F, Martinez-Aran A, et al. Bipolar II disorder and comorbidity. Compr Psychiatry. 2000;41(5):339–343. doi: 10.1053/comp.2000.9011. [DOI] [PubMed] [Google Scholar]
- 27.Hirschfeld RMA, Weisler RH, Raines S, et al. for the BOLDER Study Group. Quetiapine in the treatment of anxiety in patients with bipolar I or II depression: a secondary analysis from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(3):355–362. doi: 10.4088/jcp.v67n0304. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences: 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 33.Weisler R, Arvekvist R, Paulsson B. Quetiapine monotherapy for treating depressive episodes of bipolar disorder. Paper presented at: 59th Institute of Psychiatric Services Conference, October 11–14, 2007; New Orleans, LA. [Google Scholar]
- 34.Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60(11):1079–1108. doi: 10.1001/archpsyc.60.11.1079. [DOI] [PubMed] [Google Scholar]
- 35.Tohen M, Calabrese J, Vieta E, et al. Effect of comorbid anxiety on treatment response in bipolar depression. J Affect Disord. 2007;104(1–3):137–146. doi: 10.1016/j.jad.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Cookson J, Keck PE, Jr, Ketter TA, et al. Number needed to treat and time to response/remission for quetiapine monotherapy efficacy in acute bipolar depression: evidence from a large, randomized, placebo-controlled study. Int Clin Psychopharmacol. 2007;22(2):93–100. doi: 10.1097/YIC.0b013e3280119dfb. [DOI] [PubMed] [Google Scholar]
- 37.Suppes T, Dennehy EB, Hirschfeld RMA, et al. The Texas Implementation of Medication Algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870–886. doi: 10.4088/jcp.v66n0710. [DOI] [PubMed] [Google Scholar]
- 38.Perlis RH. The role of pharmacologic treatment guidelines for bipolar disorder. J Clin Psychiatry. 2005;66(suppl 3):37–47. [PubMed] [Google Scholar]
- 39.Frank E, Cyranowski JM, Rucci P, et al. Clinical significance of lifetime panic spectrum symptoms in the treatment of patients with bipolar I disorder. Arch Gen Psychiatry. 2002;59(10):905–911. doi: 10.1001/archpsyc.59.10.905. [DOI] [PubMed] [Google Scholar]
- 40.Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol. 2004;24(5):512–520. doi: 10.1097/01.jcp.0000138772.40515.70. [DOI] [PubMed] [Google Scholar]