Abstract
Objective:
To investigate the efficacy of duloxetine in the treatment of pain and improvement in functional impairment and quality of life in patients with fibromyalgia from a pooled analysis of 4 placebo-controlled, double-blind, randomized trials.
Method:
Patients were eligible for inclusion in the studies if they were at least 18 years of age, met criteria for fibromyalgia as defined by the American College of Rheumatology, and had specified minimum pain severity scores. Across all studies, 797 patients received duloxetine 60–120 mg/d and 535 patients received placebo. Pain was assessed by the Brief Pain Inventory (BPI) 24-hour average pain severity score; other efficacy measures included the Clinical Global Impressions-Severity of Illness scale (CGI-S), Patient Global Impressions-Improvement scale (PGI-I), 17-item Hamilton Depression Rating Scale (HDRS-17), Fibromyalgia Impact Questionnaire (FIQ) total score, BPI pain interference items, Sheehan Disability Scale (SDS), and Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) mental and physical components. Changes from baseline to endpoint (last observation carried forward) for most of the above efficacy measures were analyzed using an analysis-of-covariance model.
Results:
After 12 weeks of treatment, pain was significantly reduced in patients treated with duloxetine (P < .001) compared with placebo. In addition, duloxetine was superior to placebo in improving CGI-S (P < .001); PGI-I (P < .001); FIQ total (P < .001); HDRS-17 total (P = .003); SDS global functioning (P < .001), work/school (P = .018), and family life (P < .001); SF-36 mental (P < .001) and physical (P = .026) component; and BPI pain interference (P < .001) scores. Treatment-by-subgroup interactions were not significant for sex (P = .320), age (P = .362), or race (P = .180).
Conclusions:
This pooled analysis provides evidence that 12 weeks of treatment with duloxetine 60–120 mg/d effectively improves fibromyalgia symptoms and may offer benefits beyond pain relief.
Fibromyalgia is a chronic disorder characterized by widespread pain and tenderness and is commonly associated with other symptoms, including physical and mental fatigue, nonrestorative sleep, and mood disturbance.1–3 Fibromyalgia occurs in about 5%–6% of patients in primary care clinics and 10%–20% of rheumatology outpatients.4,5 Patients with fibromyalgia experience significant impairment in quality of life6 and disability7 and have high levels of health care utilization and costs.8
Fibromyalgia is thought to be associated with abnormal pain processing in the central nervous system.9 Dysfunction of the serotonin- and norepinephrine-mediated descending pain inhibitory pathways is one of the potential mechanisms for the pain associated with fibromyalgia and other chronic pain disorders.10,11 Serotonin-norepinephrine reuptake inhibitors (SNRIs), which increase serotonin and norepinephrine transmission, are effective in the treatment of a variety of chronic pain conditions.12
Duloxetine hydrochloride is a potent SNRI that is relatively balanced with similar affinity for both serotonin and norepinephrine reuptake inhibition.13 The efficacy of duloxetine in the treatment of chronic pain was demonstrated in preclinical studies in rodent models,14 in patients with diabetes with neuropathic pain,15–17 and most recently in fibromyalgia.
The efficacy of duloxetine in the treatment of fibromyalgia was investigated in 4 randomized, double-blind, placebo-controlled trials.18–21 These trials differed with respect to dosing regimens, primary measures of pain, and treatment duration, and there were inconsistent results for pain efficacy and functional outcomes. In addition, most of the fibromyalgia patients enrolled in these trials were middle-aged white women. An analysis of the efficacy of duloxetine in men, nonwhites, and older patients was limited by the small number of patients in these subgroups in the individual trials, one of which did not include men.19
The goal of the present study was to gain a better understanding of the efficacy of duloxetine after approximately 3 months' treatment in patients with fibromyalgia by pooling the data across 4 studies. Pooling the data provides a larger sample size, which increases the statistical power to analyze secondary functional outcomes and to examine efficacy outcomes in underrepresented patient subgroups.
Clinical Points
♦ Fibromyalgia is a complex disorder with multidimensional features, including pain, functional impairment, and impaired quality of life.
♦ Treating fibromyalgia patients with agents with dual mechanisms of action, like duloxetine, may offer benefits beyond pain relief.
METHOD
Data were pooled from 4 randomized, double-blind, placebo-controlled, multicenter studies of the efficacy of duloxetine in patients with fibromyalgia.18–21 The studies differed with respect to dosage and administration, duration of treatment, and primary outcomes (Table 1). The study investigators included clinicians with specialties in rheumatology, primary care, chronic pain, and psychiatry. Specific details of the studies have been reported previously and will be briefly summarized here. For this analysis, only 3-month data were included.
Table 1.
Summary of 4 Randomized, Double-Blind, Placebo-Controlled Studies of Duloxetine for the Treatment of Fibromyalgia
| Study | Treatment Duration | Dose | Duloxetine (n) | Placebo (n) | Primary Efficacy Measures |
| 118 | 12 wk | 60 mg bid | 104 | 103 | FIQ total score |
| FIQ pain score | |||||
| 219 | 12 wk | 60 mg qd | 118 | 120 | BPI 24-h average pain score |
| 60 mg bid | 116 | ||||
| 320 | 28 wk | 20 mg qd | 79 | 144 | BPI 24-h average pain score |
| 60 mg qd | 150 | PGI-I score | |||
| 120 mg qd | 147 | ||||
| 421 | 28 wk | 60/120 mg qd | 162 | 168 | BPI 24-h average pain score |
| PGI-I score |
Abbreviations: bid = twice daily, BPI = Brief Pain Inventory FIQ = Fibromyalgia Impact Questionnaire, PGI-I = Patient Global Impressions-Improvement scale, qd = once daily.
Entry Criteria
Both male and female patients were considered for entry into studies 1, 3, and 4; study 2 included only women. Patients were eligible for inclusion in the studies if they were at least 18 years of age, met criteria for fibromyalgia as defined by the American College of Rheumatology,2 and had specified minimum pain severity scores. In study 1, patients were required to have a pain score of at least 4 on the pain item of the Fibromyalgia Impact Questionnaire (FIQ) (score range of 0–10, with 10 indicating very severe pain).22 In studies 2–4, patients were required to have a pain score of at least 4 on the 24-hour average pain severity item of the Brief Pain Inventory (BPI) (score range of 0–10, with 10 indicating pain as bad as you can imagine).23
The following major exclusion criteria were common to all 4 studies: unstable medical or psychiatric illness, current primary psychiatric diagnosis other than major depressive disorder (MDD), a primary diagnosis of anxiety disorder within the prior year, pain from traumatic injury or structural or regional rheumatic disease, rheumatoid arthritis, inflammatory arthritis, or autoimmune disease. Concomitant medication exclusions included use of medications that might interfere with the evaluation of pain improvement, including analgesics (with the exception of acetaminophen up to 2 g/d and aspirin up to 325 mg/d for cardiac prophylaxis), antidepressants, anticonvulsants, or other medication taken for fibromyalgia or pain. Sedating antihistamines and episodic use of chloral hydrate, zolpidem, zolpiclone, and zaleplon were allowed for sleep. Patients were encouraged to not initiate or alter unconventional or alternative therapies.
Outcome Measures
Pain assessment was the protocol-defined primary outcome measure for each study (Table 1). Study 1 used the FIQ item for pain, and studies 2–4 used the BPI 24-hour average pain severity score. Three of the studies had coprimaries: FIQ total score in study 1 and Patient Global Impressions-Improvement scale (PGI-I)24 in studies 3 and 4. The FIQ is a patient self-reported instrument that assesses the impact of fibromyalgia symptoms and functional impairment. The FIQ total score ranges from 0 (no impact) to 80 (maximum impact). The PGI-I is a patient-rated global assessment of response to treatment, with scores ranging from 1 (very much better) to 7 (very much worse).
The PGI-I was also a secondary outcome in studies 1 and 2. Secondary outcomes also included the BPI items for severity of worst pain and least pain during the past 24 hours, pain right now, and pain interference (from 0, does not interfere, to 10, completely interferes) with general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life. The interference item scores were averaged to produce a global interference score that ranged from 0–10. Response to treatment was defined as ≥ 50% or ≥ 30% reduction in the BPI 24-hour average pain severity score and PGI-I scores of 1 (very much improved) or 2 (much improved).
The severity of depressive symptoms was measured by the patient-reported Beck Depression Inventory-II25 (score range from 0, not at all depressed, to 63, severely depressed) in study 1 and by the clinician-rated 17-item Hamilton Depression Rating Scale (HDRS-17)26 (score range from 0, not at all depressed, to 52, severely depressed) in studies 2–4. The Clinical Global Impressions-Severity of Illness scale (CGI-S), completed by the physician investigators,24 was used to provide a clinician-rated global assessment of symptom severity, with scores ranging from 1 (normal, not at all ill) to 7 (among the most extremely ill patients). In all of the studies, the impact of duloxetine compared with placebo on health and functional outcomes was measured by the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36)27 and the Sheehan Disability Scale (SDS).28 The SF-36 includes 8 health status domains that are each scored 1–100, with higher scores indicating better health. Results are summarized into component scores measuring overall mental health (mental component summary) and physical health (physical component summary). The SDS evaluates the disruption in work, social life/leisure activities, and family life and is scored on a scale of 0 (not at all) to 10 (very severely), with a total (global) score of 0–30. The safety and tolerability of duloxetine were assessed in each of these studies, and a pooled analysis will be reported separately.
Statistical Analysis
Duloxetine data reported during the acute treatment phase from all 4 studies were pooled for this analysis. Endpoint for acute treatment was at week 12 for studies 1 and 2, week 15 for study 3, and week 13 for study 4. For this analysis, changes from baseline to a 3-month (12 weeks) endpoint were estimated for studies 3 and 4. Patients receiving duloxetine were combined into 1 treatment group regardless of the dosing regimen employed in their study because previous analyses found no differences in efficacy outcomes between 60 mg/d or 120 mg/d.19 However, in study 3, one treatment group received duloxetine 20 mg/d, and these data were not included in this analysis because this dose was used as a subtherapeutic control.20
All analyses were conducted on the basis of intent-to-treat principles. Treatment group differences in change from baseline to endpoint in continuous measures were analyzed using an analysis of covariance (ANCOVA) model with missing values imputed via last observation carried forward. The ANCOVA model included terms for baseline, treatment, and study. Continuous efficacy measures with longitudinal observations were evaluated by a likelihood-based mixed-effects model repeated-measure analysis that included terms for treatment, study, baseline, week, treatment by week, week*week, and treatment by week*week. The covariance was chosen based on Akaike's information criterion. Categorical outcomes were compared using the Cochran-Mantel-Haenszel method. Treatment comparisons were based on 2-sided tests of significance at the .05 level.
Subgroup analyses comparing efficacy outcomes were conducted with ANCOVA models containing terms for treatment, study, and subgroup, and the treatment-by-subgroup interaction was implemented with the baseline value included as a covariate. The subgroups included strata for sex (male and female), race (white and other, which included Hispanic and black), and age category (< 65 and ≥ 65 years). The consistency of the treatment effect between subgroups was evaluated by the significance of treatment-by-subgroup interaction, which was considered to be significant when P ≤ .10. A subgroup analysis of patients with and without MDD was not included in this study because it will be reported separately.
RESULTS
Demographics and Baseline Characteristics
A total of 1,411 patients were randomly assigned to treatment across the 4 studies. There were 79 patients excluded from this analysis because they received duloxetine 20 mg/d, which was found to be a suboptimal dose in 1 study. Of the remaining 1,332 patients, 797 received duloxetine 60–120 mg/d and 535 received placebo. The majority of the patients were middle aged (mean = 50 years), female (95%), and white (88%), and 26% had a current diagnosis of MDD (Table 2). On average, pain severity and pain interference with daily activities were moderately severe (Table 3), as were the CGI-S ratings, patient-reported impact of fibromyalgia (Table 3), and global functional impairment (Table 4). In addition, both SF-36 mental and physical component summary scores were well below the norms reported for healthy individuals (Table 4).27
Table 2.
Demographic and Baseline Characteristics of Patients From 4 Studies of Duloxetine for the Treatment of Fibromyalgia
| Characteristic | Duloxetine (n = 797) | Placebo (n = 535) | Total (N = 1,332) |
| Age, mean (SD), y | 50.6 (10.7) | 49.6 (11.3) | 50.2 (11.0) |
| Female, n (%) | 754 (94.6) | 508 (95.0) | 1,262 (94.7) |
| Male, n (%)a | 43 (5.4) | 27 (5.1) | 70 (5.3) |
| White, n (%) | 705 (88.5) | 464 (86.7) | 1,169 (87.8) |
| Hispanic, n (%) | 67 (8.4) | 51 (9.5) | 118 (8.9) |
| Black, n (%) | 16 (2.0) | 13 (2.4) | 29 (2.2) |
| Major depressive disorder, n (%) | 203 (25.5) | 147 (27.5) | 350 (26.3) |
Percent based on the 3 studies that included male patients.
Table 3.
Baseline Efficacy Measures From 4 Studies of Duloxetine for the Treatment of Fibromyalgia
| Duloxetine |
Placebo |
|||
| Efficacy Measure (score range) | n | Mean (SD) | n | Mean (SD) |
| Brief Pain Inventory score (0–10) | ||||
| 24-h Average pain severity | 774 | 6.4 (1.6) | 526 | 6.4 (1.6) |
| Least pain severity | 774 | 7.5 (1.7) | 526 | 7.5 (1.7) |
| Worst pain severity | 775 | 4.8 (2.1) | 526 | 4.9 (2.1) |
| Pain severity right now | 775 | 6.3 (2.1) | 526 | 6.3 (2.1) |
| Pain interference | 775 | 5.7 (2.2) | 526 | 5.7 (2.1) |
| FIQ total score (0–80) | 756 | 50.9 (12.8) | 513 | 51.6 (12.2) |
| CGI-S score (0–7) | 744 | 4.1 (0.9) | 506 | 4.1 (1.1) |
| HDRS-17 score (0–52) | 620 | 10.4 (6.0) | 390 | 10.3 (5.9) |
Abbreviations: CGI-S = Clinical Global Impressions-Severity of Illness scale, FIQ = Fibromyalgia Impact Questionnaire, HDRS-17 = 17-item Hamilton Depression Rating Scale.
Table 4.
Baseline Scores for the SF-36 and Sheehan Disability Scale From 4 Studies of Duloxetine for the Treatment of Fibromyalgia
| Duloxetine |
Placebo |
|||
| Measure (score range) | n | Mean (SD) | n | Mean (SD) |
| SF-36 score (0–100) | ||||
| Mental component summary | 717 | 44.5 (12.0) | 489 | 44.2 (11.3) |
| Physical component summary | 717 | 28.6 (7.9) | 489 | 28.4 (7.6) |
| Bodily pain | 723 | 30.2 (14.0) | 489 | 29.9 (14.1) |
| General health perception | 720 | 46.2 (21.2) | 489 | 44.3 (20.6) |
| Mental health | 723 | 63.6 (20.7) | 489 | 62.8 (19.2) |
| Physical functioning | 723 | 41.6 (22.1) | 489 | 42.4 (21.5) |
| Role limit, emotional | 720 | 53.1 (43.6) | 489 | 54.6 (42.7) |
| Role limit, physical | 721 | 16.3 (28.0) | 489 | 16.0 (26.4) |
| Social functioning | 722 | 56.0 (25.8) | 489 | 54.8 (24.4) |
| Vitality | 723 | 24.1 (19.2) | 489 | 22.8 (17.5) |
| Sheehan Disability Scale score | ||||
| Global impairment (0–30) | 718 | 16.5 (7.5) | 487 | 17.1 (7.0) |
| Work/school (0–10) | 628 | 5.6 (2.8) | 417 | 5.9 (2.5) |
| Family life (0–10) | 725 | 5.5 (2.7) | 489 | 5.7 (2.6) |
| Social life (0–10) | 724 | 5.4 (2.7) | 489 | 5.5 (2.6) |
Abbreviation: SF-36 = Medical Outcomes Study 36-Item Short-Form Health Survey.
Efficacy
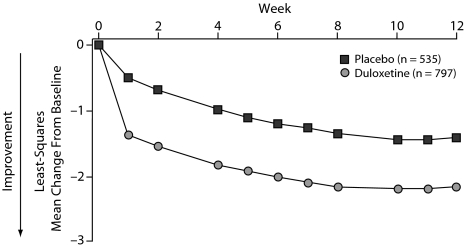
Changes in the BPI 24-hour average pain severity scores over time demonstrated significantly greater improvement in patients treated with duloxetine versus placebo beginning at week 1 and continuing through week 12 (all assessments P < .001) (Figure 1). Duloxetine also demonstrated significantly greater improvement compared with placebo on the BPI severity scores for least pain, worst pain, and pain right now and on the mean of the pain interference scores (Table 5).
Figure 1.
Visitwise Least Squares Mean Changes From Baseline in BPI 24-Hour Average Pain Scores in Fibromyalgia Patients Treated With Duloxetine 60–120 mg/da,b
aData pooled from 4 clinical trials.
bAll P < .001 vs placebo.
Abbreviation: BPI = Brief Pain Inventory.
Table 5.
Summary of Efficacy Results From 4 Studies of Duloxetine for the Treatment of Fibromyalgia
| Duloxetine |
Placebo |
|||||
| Measure | n | Least Squares Change, Mean (SE) | n | Least Squares Change, Mean (SE) | Between-Group Difference (95% CI at endpoint) | P Value |
| Brief Pain Inventory score | ||||||
| 24-h Average pain severity | 774 | −1.88 (0.09) | 526 | −1.12 (0.10) | 0.76 (0.50–1.02) | < .001 |
| Least pain severity | 774 | −1.99 (0.09) | 526 | −1.31 (0.11) | 0.68 (0.40–0.97) | < .001 |
| Worst pain severity | 775 | −1.36 (0.08) | 526 | −0.67 (0.10) | 0.69 (0.44–0.94) | < .001 |
| Pain right now | 775 | −1.90 (0.09) | 526 | −1.20 (0.11) | 0.69 (0.42–0.97) | < .001 |
| Pain interference | 775 | −2.01 (0.09) | 526 | −1.18 (0.10) | 0.83 (0.57–1.08) | < .001 |
| CGI-S score | 744 | −0.77 (0.04) | 506 | −0.44 (0.05) | 0.34 (0.21–0.46) | < .001 |
| FIQ total score | 756 | −12.62 (0.61) | 513 | −8.20 (0.69) | 4.43 (2.62–6.23) | < .001 |
| HDRS-17 total score | 620 | −3.04 (0.19) | 390 | −2.11 (0.24) | 0.93 (0.32–1.54) | < .01 |
| PGI-I score | 764 | 3.19 (0.06) | 516 | 3.60 (0.07) | 0.42 (0.24–0.59) | < .001 |
Abbreviations: CGI-S = Clinical Global Impressions-Severity of Illness scale, FIQ = Fibromyalgia Impact Questionnaire, HDRS-17 = 17-item Hamilton Depression Rating Scale, PGI-I = Patient Global Impressions-Improvement scale.
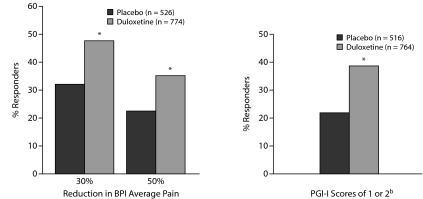
Duloxetine was also statistically superior to placebo with respect to improvement on all other efficacy measures, including CGI-S, FIQ total scores, HDRS-17 total score, and PGI-I (Table 5). In addition, a significantly greater proportion of patients treated with duloxetine versus placebo were responders, with a 30% or 50% reduction from baseline in the BPI 24-hour average pain score and PGI-I scores of 1 or 2 (Figure 2). Almost half (47.7%) of the patients treated with duloxetine experienced a 30% reduction in BPI 24-hour average pain score, and over one third (35.3%) had a 50% reduction. By contrast, fewer than one third (32.1%) of placebo-treated patients had a 30% reduction in BPI 24-hour average pain score, and less that one fourth (22.2%) had a 50% reduction. Over one third (38.4%) of duloxetine-treated patients reported feeling much improved, and less than one fourth (21.7%) of the placebo-treated patients reported feeling much improved.
Figure 2.
Percent of Patients Who Responded With 30% and 50% Reductions in BPI Average Pain Scores and PGI-I Scores of 1 or 2a,b
aData pooled from 4 clinical trials.
b1 = very much better and 2 = much better.
*P < .001 vs placebo.
Abbreviations: BPI = Brief Pain Inventory, PGI-I = Patient Global Impressions-Improvement scale.
The results of the subgroup analyses are summarized in Table 6. In female patients, there were statistically significant differences in mean changes in pain reduction in the duloxetine group as compared with the placebo group. However, for male patients, mean changes from baseline were nearly the same magnitude in both treatment groups.
Table 6.
Baseline and Endpoint Changes in BPI 24-Hour Pain Measures in Demographic Subgroups for Age, Gender, and Ethnicity
| Duloxetine |
Placebo |
Treatment-by-Subgroup Interaction P Value a | ||||||
| Subgroup | n | Baseline, Mean (SD) | Least Squares Change, Mean (SE) | n | Baseline, Mean (SD) | Least Squares Change, Mean (SE) | Duloxetine vs Placebo P Value | |
| Womenb | 500 | 6.46 (1.6) | −1.74 (0.1) | 382 | 6.41 (1.6) | −1.10 (0.1) | < .001 | .320 |
| Men | 44 | 6.07 (1.4) | −1.28 (0.4) | 26 | 6.27 (1.6) | −1.25 (0.5) | .969 | |
| < 65 y | 707 | 6.39 (1.5) | −1.90 (0.1) | 483 | 6.46 (1.6) | −1.11 (0.1) | < .001 | .362 |
| ≥ 65 y | 67 | 6.60 (1.9) | −1.92 (0.3) | 43 | 6.02 (1.8) | −1.50 (0.4) | .374 | |
| White | 683 | 6.33 (1.5) | −1.92 (0.1) | 455 | 6.32 (1.5) | −1.12 (0.1) | < .001 | .180 |
| Other | 91 | 6.97 (1.8) | −1.70 (0.3) | 71 | 7.04 (1.8) | −1.37 (0.3) | .386 | |
Treatment-by-subgroup interaction is significant at P ≤ .10.
Study 2 was not included in the subgroup analysis by sex, because only female patients were enrolled.
Abbreviation: BPI = Brief Pain Inventory.
Older patients (≥ 65 years) had changes that were similar to younger patients, but between-treatment differences were not significant. Nonwhite patients had changes that were similar to those of white patients, but differences between treatment groups were not significant. The treatment-by-subgroup interaction on the mean pain severity scores for sex (P = .320), age (< 65 and ≥ 65 years, P = .362), or ethnicity (P = .180) were not significant, suggesting that the effect of duloxetine on pain reduction was similar in patients regardless of their gender, age, or ethnicity. The duloxetine-treated group was statistically superior to placebo with regard to improvement on all SF-36 domains and the SDS scores (Table 7).
Table 7.
Summary of Endpoint Changes for the SF-36 and Sheehan Disability Scale From 4 Studies of Duloxetine for the Treatment of Fibromyalgia
| Duloxetine |
Placebo |
Between-Group Difference (95% CI at endpoint) | ||||
| Measure | n | Least Squares Change, Mean (SE) | n | Least Squares Change, Mean (SE) | P Value | |
| SF-36 | ||||||
| Mental component summary | 717 | 4.60 (0.39) | 489 | 1.63 (0.45) | −2.97 (–4.14 to –1.81) | < .001 |
| Physical component summary | 717 | 4.09 (0.32) | 489 | 3.01 (0.37) | −1.08 (–2.03 to –0.12) | < .05 |
| Bodily pain | 723 | 14.1 (0.73) | 489 | 7.95 (0.84) | −6.19 (–8.39 to –4.00) | < .001 |
| General health | 720 | 7.02 (0.59) | 489 | 4.31 (0.69) | −2.71 (–4.47 to –0.95) | < .01 |
| Mental health | 723 | 8.85 (0.64) | 489 | 3.03 (0.75) | −5.82 (–7.73 to –3.91) | < .001 |
| Physical functioning | 723 | 9.28 (0.71) | 489 | 5.96 (0.83) | −3.32 (–5.45 to –1.20) | < .01 |
| Role limit, emotional | 720 | 13.0 (1.54) | 489 | 4.53 (1.76) | −8.43 (–13.0 to –3.85) | < .001 |
| Role limit, physical | 721 | 12.6 (1.34) | 489 | 7.74 (1.53) | −4.81 (–8.80 to –0.83) | < .05 |
| Social functioning | 722 | 10.4 (0.83) | 489 | 7.01 (0.97) | −3.43 (–5.93 to –0.93) | < .01 |
| Vitality | 723 | 10.5 (0.79) | 489 | 5.84 (0.93) | −4.66 (–7.03 to –2.28) | < .001 |
| Sheehan Disability Scale | ||||||
| Global impairment | 718 | −4.37 (0.27) | 487 | −2.88 (0.31) | 1.49 (0.69–2.29) | < .001 |
| Work/school | 628 | −1.46 (0.10) | 417 | −1.09 (0.12) | 0.37 (0.06–0.67) | < .05 |
| Family life | 725 | −1.40 (0.10) | 489 | −0.89 (0.11) | 0.51 (0.22–0.79) | < .001 |
| Social life | 724 | −1.53 (0.10) | 489 | −0.97 (0.11) | 0.56 (0.27–0.85) | < .001 |
Abbreviation: SF-36 = Medical Outcomes Study 36-Item Short-Form Health Survey.
DISCUSSION
In this pooled analysis of the acute treatment phases of 4 randomized, double-blind, placebo-controlled trials in patients with fibromyalgia, duloxetine 60–120 mg/d significantly reduced pain as compared with placebo beginning in the first week of treatment and continuing at each subsequent week throughout the 12 weeks of therapy. In previous reports, studies 219 and 320 reported significantly greater improvement in the primary measure of pain with duloxetine treatment at weeks 12 and 15, respectively; but studies 118 and 421 reported no significant between-treatment differences after 12–13 weeks. It is not clear why duloxetine did not separate from placebo in these 2 studies. However, study 1 used the FIQ pain item as the primary pain measure, which might be problematic because patients retrospectively rate their pain over the prior week rather than over the past 24 hours. In study 4, the BPI 24-hour average pain item was used to assess pain as a coprimary measure with the PGI-I, and there were significant improvements in both of these measures at each assessment through week 8 and at week 18 but not at week 12.
Duloxetine-treated patients compared with patients taking placebo had significantly greater reduction in the total impact of fibromyalgia symptoms and improvement in mood, quality of life, and function. Improvement on each of the 8 SF-36 health domains and both of the component summaries was significant in the duloxetine-treated group compared with the placebo-treated group. Although the clinical relevance of statistically significant improvements in the SF-36 domains has not been definitively established in fibromyalgia, duloxetine treatment was associated with scores that increased from baseline by 7 to 14 points as compared with an increase of 3 to 8 points with placebo treatment. These improvements suggest that duloxetine may offer benefits that extend beyond pain relief in patients with fibromyalgia.
The subgroup analyses of sex, race, and age found no significant treatment-by-subgroup interaction for mean changes in the BPI 24-hour average pain scores. For race and age, these analyses support initial findings in all 3 primary evaluations of these subgroups. However, the results of the sex subgroup analysis differ from the findings in study 1, which reported a significant interaction of treatment with sex for the BPI 24-hour average pain score.18 Even though studies 3 and 4 reported no significant treatment-by-sex interaction for BPI 24-hour average pain score, which is supported by the current analyses, conclusions regarding the effect of duloxetine in male patients remain unclear. Additional studies are needed to better understand fibromyalgia and treatment response in male patients, nonwhites, and adult patients of all ages.29–31
Several limitations of this study should be considered. First, the results are based on the acute phase of 4 clinical trials, and the results may not generalize to treatment with duloxetine beyond 12 weeks. Longer-term studies are needed to evaluate the efficacy of duloxetine as a maintenance treatment in this chronic disorder.
Second, most of the patients included in these studies were middle-aged white women, which may limit generalization of these results to other individuals with fibromyalgia. The American College of Rheumatology criteria for fibromyalgia used in this study to identify potential patients may have excluded some men from participating in the trials, because men have been reported to have fewer tender points than women.29,30 These criteria may also inadvertently exclude nonwhite individuals because of potential racial differences in pain thresholds and tender point count, as suggested in a recent study of differences in widespread pain and tenderness in black and white women.31
The location of the study site could potentially affect the recruitment of minority patients if racial diversity in the surrounding community is low.32 The enrollment of older patients in these clinical trials may have been influenced by the increased likelihood of exclusionary medical comorbidity in the older population.29 Additional studies that include more diverse patient populations are needed to better understand the efficacy of duloxetine in all patients with fibromyalgia. Finally, because clinicians often recommend combination medication treatments for fibromyalgia as well as nonpharmacologic therapies, such as cognitive-behavioral therapy and exercise,33 future studies of duloxetine use in multidisciplinary treatment regimens are needed.
In conclusion, this pooled analysis of 4 randomized, placebo-controlled studies provides evidence that 12 weeks of treatment with duloxetine 60–120 mg/d effectively improves fibromyalgia symptoms and may offer benefits beyond pain relief.
Drug names: duloxetine (Cymbalta), zaleplon (Sonata and others), zolpidem (Ambien and others).
Financial disclosure: Dr Arnold has received grant/research support from Eli Lilly, Pfizer, Cypress Biosciences, Wyeth, Sanofi-Aventis, Boehringer Ingelheim, Allergan, and Forest; is a consultant to Eli Lilly, Pfizer, Cypress Biosciences, Wyeth, Sanofi-Aventis, Boehringer Ingelheim, Sepracor, Forest, Allergan, Vivus, Organon, and Takeda; and serves on the speakers bureaus of Eli Lilly and Pfizer. Dr Clauw has received grant/research support from Cypress Biosciences and has served as a consultant to Cypress Biosciences, Forest, Pierre Fabre, Eli Lilly, Pfizer, and Wyeth. Drs Wohlreich, Wang, Ahl, Gaynor, and Chappell are all employees of Eli Lilly and Company and/or one of its subsidiaries.
Funding/support: Funding support for the studies in the pooled analysis was provided by Eli Lilly and Company. The pooled analysis was funded by Lilly USA, LLC.
Previous presentation: Data included in this article were presented as a poster at the 27th Annual Meeting of the American Pain Society; May 8–10, 2008; Tampa, Florida.
REFERENCES
- 1.Yunus M, Masi AT, Calabro JJ, et al. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arthritis Rheum. 1981;11(1):151–171. doi: 10.1016/0049-0172(81)90096-2. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 3.Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl. 2005;75:6–21. [PubMed] [Google Scholar]
- 4.Goldenberg DL, Simms RW, Geiger A, et al. High frequency of fibromyalgia in patients with chronic fatigue seen in primary care practice. Arthritis Rheum. 1990;33(3):381–387. doi: 10.1002/art.1780330311. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe F, Ross K, Anderson J. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 6.Burckhardt CS, Clark SR, Bennett RM. Fibromyalgia and quality of life: a comparative analysis. J Rheumatol. 1993;20(3):475–479. [PubMed] [Google Scholar]
- 7.Bennett RM. Fibromyalgia and the disability dilemma: a new era in understanding a complex, multidimensional pain syndrome. Arthritis Rheum. 1996;39(10):1627–1634. doi: 10.1002/art.1780391004. [DOI] [PubMed] [Google Scholar]
- 8.Berger A, Dukes E, Martin S, et al. Characteristics and healthcare costs of patients with fibromyalgia syndrome. Int J Clin Pract. 2007;61(9):1498–1508. doi: 10.1111/j.1742-1241.2007.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4(4):299–305. doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- 10.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 11.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 12.Fishbain D. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305–316. doi: 10.3109/07853890008995932. [DOI] [PubMed] [Google Scholar]
- 13.Wong DT, Bymaster FP, Mayle DA, et al. LY248686: a new inhibitor of serotonin and norepinephrine uptake. Neuropsychopharmacology. 1993;8(1):23–33. doi: 10.1038/npp.1993.4. [DOI] [PubMed] [Google Scholar]
- 14.Iyengar S, Webster AA, Hemrick-Luecke SK, et al. Efficacy of duloxetine: a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311(2):576–584. doi: 10.1124/jpet.104.070656. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DJ, Lu Y, Detke MJ, et al. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346–356. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 17.Wernicke JF, Pritchett YL, D'Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 18.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50(9):2974–2984. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 19.Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. 2005;119(1–3):5–15. doi: 10.1016/j.pain.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Russell IJ, Mease P, Smith T, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136(3):432–444. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Chappell AS, Bradley LA, Wiltse C, et al. A 27-week, placebo-controlled clinical trial of duloxetine for the treatment of fibromyalgia. Int J Gen Med. 2008;1:91–102. doi: 10.2147/ijgm.s3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burckhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire: development and validation. J Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 23.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 24.Guy W. Washington, DC: US Department of Health, Education, and Welfare; 1976. Clinical Global Impressions. (ed) ECDEU Assessment Manual for Psychopharmacology, Revised. Publication Adm 76-338. [Google Scholar]
- 25.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 28.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe F, Ross K, Anderson J, et al. Aspects of fibromyalgia in the general population: sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22(1):151–156. [PubMed] [Google Scholar]
- 30.Yunus MB, Inanici F, Aldag JC, et al. Fibromyalgia in men: comparison of clinical features with women. J Rheumatol. 2000;27(2):485–490. [PubMed] [Google Scholar]
- 31.Gansky SA, Plesh O. Widespread pain and fibromyalgia in a biracial cohort of young women. J Rheumatol. 2007;34(4):810–817. [PubMed] [Google Scholar]
- 32.Raphael KG, Janal MN, Nayak S, et al. Psychiatric comorbidities in a community sample of women with fibromyalgia. Pain. 2006;124(1–2):117–125. doi: 10.1016/j.pain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Arnold LM. Biology and therapy of fibromyalgia: new therapies in fibromyalgia. Arthritis Res Ther. 2006;8(4):212. doi: 10.1186/ar1971. [DOI] [PMC free article] [PubMed] [Google Scholar]