Abstract
Shockman, Gerald D. (Temple University School of Medicine, Philadelphia, Pa.), Joseph J. Kolb, Bohdan Bakay, Margaret J. Conover, and Gerrit Toennies. Protoplast membrane of Streptococcus faecalis. J. Bacteriol. 85:168–176. 1963.—The membrane fraction of Streptococcus faecalis (ATCC 9790) was isolated and purified, by a variety of procedures, from cultures that were grown under closely controlled conditions of physiological age and nutrition. The most satisfactory method required the use of lysozyme-to-cell ratios below 0.01 and the intermediate formation of protoplasts in osmotically protective media. Amino acid analyses of three of the membrane preparations indicated a characteristic and constant, but not unusual, pattern; 42% of the membranes from threonine-depleted and 49 to 55% of the membranes from log-phase cultures were accounted for as protein. Significant quantities of d-alanine or d-aspartic acid were not detected, indicating the absence of contaminating cell-wall substance. Essentially, all of the nitrogen was accounted for as amino acids. The lipid content of membranes from stationary-phase threonine-depleted (36%) and valine-depleted (40%) cultures was significantly higher than the corresponding fraction of exponential-phase cultures (28%). The phosphorus content of the membrane lipid was relatively constant (2.8 to 3.0%), and the nitrogen content was extremely low (0.12 to 0.26%). Thus, changes in the composition of the membrane fraction occurred during the transition of log-phase cells into threonine- or valine-depleted cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBY A. R., FEW A. V., McQUILLEN K. The chemical composition of the protoplast membrane of Micrococcus lysodeikticus. Biochim Biophys Acta. 1958 Jul;29(1):21–29. doi: 10.1016/0006-3002(58)90141-0. [DOI] [PubMed] [Google Scholar]
- GODSON G. N., HUNTER G. D., BUTLER J. A. Cellular components of Bacillus megaterium and their role in protein biosynthesis. Biochem J. 1961 Oct;81:59–68. doi: 10.1042/bj0810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLB J. J., WEIDNER M. A., TOENNIES G. Microdetermination of lipid phosphorus as a measure of bacterial membrane substance. Anal Biochem. 1963 Jan;5:78–82. doi: 10.1016/0003-2697(63)90061-7. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- McQuillen K., Roberts R. B., Britten R. J. SYNTHESIS OF NASCENT PROTEIN BY RIBOSOMES IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1437–1447. doi: 10.1073/pnas.45.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D. Bacterial cell wall synthesis: the effect of threonine depletion. J Biol Chem. 1959 Sep;234:2340–2342. [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. Relations between bacterial cell wall synthesis, growth phase, and autolysis. J Biol Chem. 1958 Feb;230(2):961–977. [PubMed] [Google Scholar]
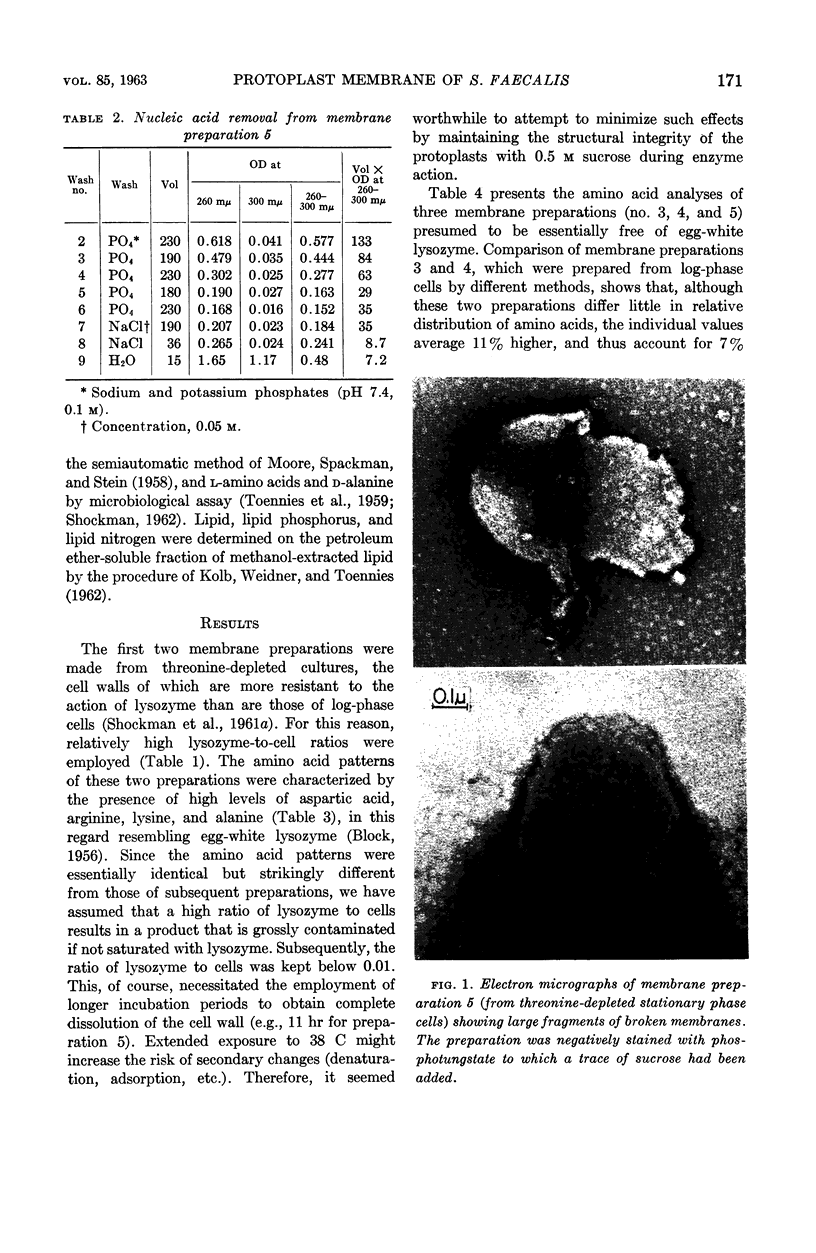
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Riley L. S., Toennies G. NUTRITIONAL REQUIREMENTS FOR BACTERIAL CELL WALL SYNTHESIS. J Bacteriol. 1961 Jan;81(1):44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOENNIES G., BAKAY B., SHOCKMAN G. D. Bacterial composition and growth phase. J Biol Chem. 1959 Dec;234:3269–3275. [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D. Quantitative amino acid assimilation in homofermentative metabolism. Arch Biochem Biophys. 1953 Aug;45(2):447–458. doi: 10.1016/s0003-9861(53)80021-4. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., BERGSTROM L. The chemical nature of the cytoplasmic membrane and cell wall of Bacillus megaterium, strain M. Biochim Biophys Acta. 1958 Nov;30(2):340–351. doi: 10.1016/0006-3002(58)90059-3. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., ZACHARIAS B., BECKMAN H. Affinity of lyzyme to structural elements of the bacterial cell as studied with enzyme labelled with radioactive iodine. Nature. 1959 Nov 28;184(Suppl 22):1744–1745. doi: 10.1038/1841744a0. [DOI] [PubMed] [Google Scholar]