Abstract
Asymmetric cell divisions generate sibling cells of distinct fates (‘A’, ‘B’) and constitute a fundamental mechanism that creates cell-type diversity in multicellular organisms. Antagonistic interactions between the Notch pathway and the intrinsic cell-fate determinant Numb appear to regulate asymmetric divisions in flies and vertebrates. During these divisions, productive Notch signaling requires sanpodo, which encodes a novel transmembrane protein. Here, we demonstrate that Drosophila sanpodo plays a dual role to regulate Notch signaling during asymmetric divisions — amplifying Notch signaling in the absence of Numb in the ‘A’ daughter cell and inhibiting Notch signaling in the presence of Numb in the ‘B’ daughter cell. In so doing, sanpodo ensures the asymmetry in Notch signaling levels necessary for the acquisition of distinct fates by the two daughter cells. These findings answer long-standing questions about the restricted ability of Numb and Sanpodo to inhibit and to promote, respectively, Notch signaling during asymmetric divisions.
Keywords: Notch, Numb, Sanpodo, Asymmetric divisions, Drosophila
INTRODUCTION
Asymmetric divisions — the fundamental process through which precursor cells create daughter cells of distinct fates (‘A’, ‘B’) — generate cell-type diversity throughout the animal and plant kingdoms. Antagonistic interactions between the Notch signaling pathway and the phosphotyrosine-binding (PTB)-domain-containing protein Numb regulate asymmetric divisions in higher metazoans (reviewed by Gonczy, 2008; Matsuzaki, 2000; Posakony, 1994; Schweisguth, 2004). During these divisions Numb segregates into one daughter cell, where it inhibits Notch signaling (Rhyu et al., 1994). The absence of Notch signaling in this cell permits it to adopt the default ‘B’ fate. In the other daughter cell, the absence of Numb allows high-level Notch signaling, which induces this cell to acquire the Notch-dependent ‘A’ fate.
In Drosophila the Notch pathway regulates many other developmental decisions, including wing blade formation, wing vein formation and lateral inhibition, during which individual precursor cells are selected from groups of equivalent cells (reviewed by Simpson, 1998). Cells undergoing these Notch-dependent events also co-express Notch and numb (Fehon et al., 1991; Matsuzaki, 2000). However, loss-of-function studies reveal that numb inhibits Notch signaling only during asymmetric divisions (Dye et al., 1998; Lear et al., 1999; Salzberg et al., 1994; Skeath and Doe, 1998; Uemura et al., 1989), suggesting that additional factors restrict the inhibitory action of Numb on Notch signaling to ‘B’ daughter cells during asymmetric divisions.
sanpodo (spdo) encodes a novel transmembrane protein, and like numb, regulates Notch signaling only during asymmetric divisions (Hutterer and Knoblich, 2005; O'Connor-Giles and Skeath, 2003). However, in contrast to numb, spdo promotes the Notch-dependent ‘A’ fate, as both daughter cells adopt the default ‘B’ fate in the absence of spdo (Dye et al., 1998; Salzberg et al., 1994; Skeath and Doe, 1998). spdo is expressed exclusively in asymmetrically dividing cells, and physically associates with Notch and Numb (Hutterer and Knoblich, 2005; O'Connor-Giles and Skeath, 2003). Numb inhibits the ability of Spdo to localize to the cell membrane, leading to differential subcellular localization of Spdo between the daughter cells: in the ‘A’ cell Spdo localizes to the cell membrane, whereas in the ‘B’ cell Spdo localizes primarily to cytoplasmic vesicles (Hutterer and Knoblich, 2005; Langevin et al., 2005; O'Connor-Giles and Skeath, 2003). The ability of Numb to regulate Spdo localization, together with the requirement for spdo to promote productive Notch signaling during asymmetric divisions, led to the model that Numb acts through Spdo to inhibit Notch signaling in the ‘B’ daughter cell (O'Connor-Giles and Skeath, 2003; Hutterer and Knoblich, 2005).
The abilities of Numb and Spdo to regulate Notch signaling specifically during asymmetric divisions raise two important questions. Why does numb inhibit Notch signaling only during asymmetric divisions? And, why does productive Notch signaling require spdo function during asymmetric divisions, but no other Notch-dependent event? Here, through loss-of-function and gene-misexpression studies on spdo, we demonstrate that Numb converts spdo from an activator to an inhibitor of Notch signaling: in cells that lack Numb protein, Spdo expression potentiates Notch signaling, whereas in cells that contain Numb, Spdo expression dampens Notch signaling. During normal development spdo is only expressed in asymmetrically dividing cells, and within asymmetrically dividing cells Numb segregates exclusively into the ‘B’ daughter cell. Thus, under wild-type conditions, Notch-dependent ‘A’ daughter cells are the only cells that express both Notch and Spdo, but not Numb, whereas ‘B’ daughter cells are the only cells that express Notch, Numb and Spdo. By exerting opposite effects on Notch signaling in a Numb-dependent manner, Spdo then simultaneously directs Notch signaling to exceed threshold levels in the ‘A’ daughter cell and to remain well below such levels in the ‘B’ daughter cell, thus ensuring the faithful execution of asymmetric divisions.
MATERIALS AND METHODS
Fly strains
Wild type is Oregon R or w1118, spdoG104, spdoAC81, spdoZZ27, mastermindNN46 (O'Connor-Giles and Skeath, 2003; Skeath and Doe, 1998); numb1, numb2 (Uemura et al., 1989); Dl7, Dl3 (Lindsley, 1992; Micchelli et al., 1997); DlRevF10 SerVX82 (Micchelli et al., 1997); neur9L119 (Jurgens, 1984); Su(H)SF8 (Schweisguth and Posakony, 1992); E(spl)rv1 (Lindsley, 1992); groE48 (Preiss et al., 1988); N81K1 (Lindsley, 1992), svp-lacZ (Mlodzik et al., 1990).
Gene-misexpression studies were carried out using the GAL4/UAS system (Brand and Perrimon, 1993). GAL4 lines: prospero-GAL4 (Shiga, 1996), scabrous-GAL4 (Nakao and Campos-Ortega, 1996), twist-GAL4 (Greig and Akam, 1993), patched-GAL4 (Wilder and Perrimon, 1995), nubbin-GAL4 (Calleja et al., 1996).
UAS transgenes: UAS-SpdoG14, UAS-SpdoG1, UAS-SpdoG14 UAS-SpdoG1 (O'Connor-Giles and Skeath, 2003); UAS-Notch (Giniger, 1998); UAS-Fringe (Okajima and Irvine, 2002); UAS-Delta and UAS-GFP (Bloomington Stock Center), UAS-numbRNAi (Tang et al., 2005), UAS-neuralized (Lai et al., 2001); UAS-neuralized UAS-Delta (Pavlopoulos et al., 2001); UAS-NotchΔECN (Struhl et al., 1993).
Genotypes generated for the study: twist-GAL4; svp-lacZ spdoZZ27/TM3, twistGAL4; spdoZZ27/TM3, numb2 twist-GAL4/CyO, prospero-GAL4 spdoG104/TM3, scabrous-GAL4; spdoZZ27/TM3, numb2; prospero-GAL4 spdoG104/TM3, numb2 twist-GAL4; spdoG104/TM3, scabrous-GAL4; Dl7/TM6, twist-GAL4; DlRevF10 SerVX82/TM6, UAS-spdoG14; spdoG104/TM3, UAS-Notch; spdoG104/TM3, UAS-Notch spdoG104/TM3, UAS-fringe; spdoG104/TM3, UAS-Delta; spdoG104/TM3, UAS-neuralized; spdoG104/TM3, UAS-neuralized UAS-Delta; spdoG104/TM3, UAS-spdoG14; spdoG104/TM3, numb2 UAS-spdoG14/CyO; UAS-spdoG1, numb2 UAS-Notch/CyO; spdoG104/TM3, numb2 UAS-fringe/CyO; spdoG104/TM3, UAS-Delta; Dl7/TM6, UAS-fringe;Dl7/TM6, UAS-Notch; Dl7/TM6, UAS-Delta; DlRevF10SerVX82/TM6, UAS-fringe; DlRevF10 SerVX82/TM6, UAS-Notch; DlRevF10 SerVX82/TM6, numb2 UAS-spdoG14/CyO; Dl7 UAS-spdoG1/TM6 and numb2 twist-GAL4/CyO; Dl7/TM6.
Immunohistochemistry
Immunohistochemistry and immunofluorescence analysis were performed essentially as described (Skeath and Carroll, 1992) using the following antibodies: anti-Eve (Frasch et al., 1987), anti-Odd (Ward and Coulter, 2000), 22C10 (1:20; Developmental Hybridoma Studies Bank), anti-Nmr1 (1:2000) (Leal et al., 2009), anti-Spdo (1:1000) and anti-Numb (1:500) (O'Connor-Giles and Skeath, 2003), anti-β-gal (1:1000; Promega and ICN), anti-Senseless (1:800) (Nolo et al., 2000) and anti-Mef-2 (1:500) (Lilly et al., 1995).
RESULTS
Only during asymmetric divisions does the Notch signaling pathway require spdo, and respond to numb function. The genetic and molecular mechanisms that underlie the specificity of these interactions remain unclear. In this paper, we assess the potential of spdo function to influence Notch pathway activity in the presence and absence of numb in two ways. We used misexpression approaches to investigate spdo function during Notch-dependent events that do not normally require spdo, including lateral inhibition and wing formation. We used loss-of-function approaches, combined with overexpression of Notch pathway members, to assess spdo function in ‘A’ and ‘B’ daughter cells during asymmetric divisions.
Misexpression of spdo dampens Notch signaling activity during lateral inhibition
To determine the effect of expressing spdo in cells that normally express Notch and numb but not spdo, we focused on the process of lateral inhibition. During lateral inhibition, the Notch pathway restricts the ability of cells within equivalence groups (gray circles, Fig. 1A) from adopting the precursor fate (black circles, Fig. 1A), such that only one precursor normally arises from a given equivalence group (Fig. 1A). Reduction of Notch signaling during lateral inhibition results in the formation of additional precursors, whereas heightened Notch activity results in the failure of precursors to segregate from equivalence groups (Simpson, 1998). To test the hypothesis that Spdo enables Numb to inhibit Notch signaling, we focused on two such processes — the formation of sensory organ precursors and heart precursors in the Drosophila peripheral nervous system and mesoderm, respectively. Specifically, we followed the formation of these precursors and the structures and cell types to which they give rise to determine the effect of spdo misexpression on Notch signaling levels during lateral inhibition.
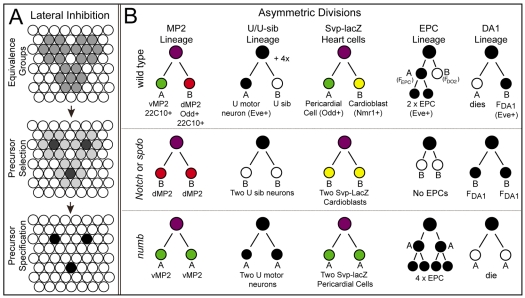
Fig. 1.
Schematics of Notch-mediated lateral inhibition and the lineages of five pairs of sibling cells. (A) Initially all cells within three equivalence groups acquire the potential (gray) to adopt the precursor fate. One cell (gray) is singled out as the presumptive precursor from each cluster. This cell then acts through the Notch pathway to inhibit all other cells in the group (light gray) from adopting the precursor fate, such that individual precursors cells (black) segregate from each group (right). (B) Cell lineages of the five sibling pairs assayed in this paper shown for one hemisegment of wild type, Notch/spdo or numb mutant embryos. Lineages shown in color represent those in which we follow the fate of both sibling cells. Lineages shown in black/white are those in which we use eve expression (black) to follow the fate of one of the two sibling cells in each lineage.
Cells undergoing lateral inhibition normally express both Notch and numb, but not spdo. However, loss of numb function does not affect Notch signaling during lateral inhibition (Dye et al., 1998; Lear et al., 1999; Salzberg et al., 1994; Skeath and Doe, 1998; Uemura et al., 1989), indicating that Numb does not inhibit Notch signaling in this context. We took advantage of these circumstances to ask what happens when we express spdo in cells undergoing lateral inhibition. We reasoned that if numb acts through spdo to inhibit Notch pathway function, then expressing spdo in these cells should inhibit Notch signaling in a numb-dependent manner, and result in the formation of ectopic precursors and the structures/cells to which they give rise. In support of this model, ectopic spdo expression in the scutellar region of the wing imaginal disc led to an approximate doubling of scutellar bristles and their sensory organ precursors relative to wild type (compare Fig. 2A,C with 2F,G).
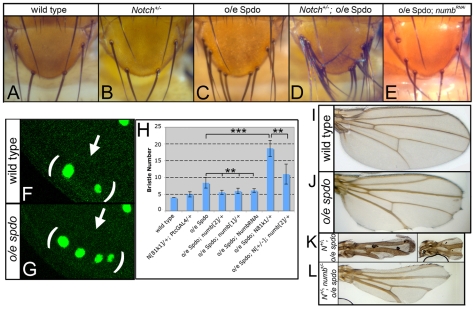
Fig. 2.
sanpodo misexpression inhibits Notch signaling during lateral inhibition and wing development in a numb-dependent manner. (A) Four scutellar bristles arise in wild type. (B) Removing one copy of Notch has little effect on bristle number. (C-E) Expression of spdo by patched-GAL4 (o/e spdo) in the scutellar region promotes ectopic bristle formation (C), a phenotype enhanced by reducing Notch function 50% (D), and suppressed by co-expression of a numb-RNAi transgene (E) (Tang et al., 2005). (F) In wild type two sensory organ precursors, as labeled by anti-senseless (Nolo et al., 2000), develop in the scutellar region (arrows, brackets) of the late third instar wing imaginal disc. (G) spdo overexpression in this region promotes the formation of ectopic sensory organ precursors (arrow). (H) Average number of scutellar bristles per indicated genotype. Error bars indicate s.d. **P<10−4; ***P<10−10. (I) nubbin-GAL4-mediated expression of spdo in the wing (o/e spdo) leads to wing notching, vein thickening and reduced wing size. (J) Reduction of Notch function by 50% (N+/−; o/e spdo), using the Notch81k1 allele, enhanced these phenotypes. (K,L) Simultaneously reducing numb function by 50% (N+/−; numb+/−; o/e spdo, L) suppressed the phenotypes observed in N+/−; o/e spdo flies (K). Images in I-K are shown at identical magnification.
To determine whether this finding was specific to the nervous system or generalizable to lateral inhibition throughout development, we expressed spdo ubiquitously in the embryonic mesoderm and assayed cardioblast development. As observed for sensory organ precursors, the cardioblast precursors are selected from equivalence groups via Notch-mediated lateral inhibition, and reduction of Notch signaling function leads to excess cardioblast precursors and cardioblasts (Carmena et al., 1998; Hartenstein et al., 1992). In this experiment, we focused on Seven-up-lacZ+ (Svp-lacZ+) cardioblast precursors and their resultant progeny. In wild-type embryos, two Svp-lacZ+ heart precursors develop per hemisegment (arrows, Fig. 3B); each precursor then divides asymmetrically to produce an Svp-lacZ+ cardioblast and an Svp-lacZ+ pericardial cell (Fig. 3A; arrows, Fig. 3B′) (Ward and Skeath, 2000). The remaining four cardioblasts per hemisegment derive from two Svp-lacZ− precursors; each of which produces two cardioblasts (Fig. 3A,B′) (Ward and Skeath, 2000). As observed for bristles and their precursors, generalized spdo expression in the mesoderm led to the formation of extra Svp-lacZ+ precursors and their progeny (arrowheads, Fig. 3C,C′). Although we did not assay the development of Svp-lacZ− cardioblast precursors, the formation of extra Svp-lacZ− cardioblasts (arrow, Fig. 3C′) indicated that their numbers also increased in this background. Thus, misexpression of spdo is also sufficient to inhibit Notch signaling during lateral inhibition in the mesoderm. Note that essentially all Svp-lacZ+ precursors divided asymmetrically in this experiment (99.8%, n=829; see Table S3 in the supplementary material). Thus, generalized spdo expression in the mesoderm did not alter the ability of precursors to divide asymmetrically during Notch-mediated asymmetric divisions.
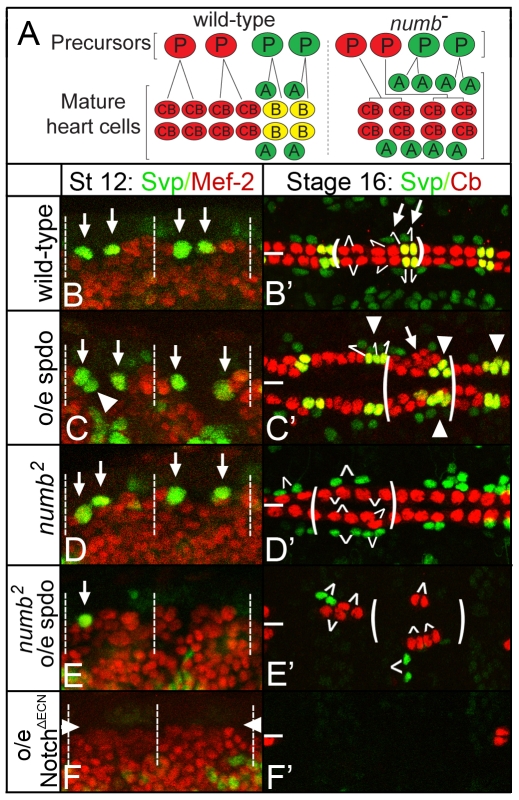
Fig. 3.
sanpodo exerts a context-dependent effect on Notch signaling during lateral inhibition. (A) Model of heart precursors and differentiated (mature) heart cells. Left, in a wild-type hemisegment two cardioblast precursors (red cells labeled ‘P’) each divide to produce two cardioblasts (red cells labeled ‘CB’), and two Svp-lacZ+ heart precursors (green cells labeled ‘P’) each divide to produce one Svp-lacZ+ pericardial cell (green, ‘A’ daughter cell) and one Svp-lacZ+ cardioblast (red, ‘B’ cell). Right, in numb mutant embryos heart precursors form normally but Svp-lacZ+ heart precursors produce only pericardial cells (green, ‘A’ cells). Precursors are shown for one hemisegment, whereas mature heart cells are shown for one full segment. (B-F) High-magnification views of two hemisegments of the dorsal mesoderm of embryos of indicated genotype labeled for Svp-lacZ+ (green) or Mef-2 (red), which labels mesodermal cells. (B′-F′) High-magnification views of three segments of the heart of embryos of indicated genotype labeled for Svp-lacZ+ (green) and Nmr-1 (red), Nmr-1 specifically labels cardioblasts (Leal et al., 2009). (B) In wild-type stage 12 embryos, two Svp-lacZ+ heart precursors arise per hemisegment (arrows; dotted lines demarcate segment-sized regions in B-F). (B′) By stage 16, two rows of cardioblasts (red; yellow for Svp-lacZ+ cardioblasts) and Svp-lacZ+ pericardial cells (green) align on either side of the dorsal midline (thick white line). In B′-E′ one segment is bracketed and white lines indicate defined or inferred sibling relationships. (C) twist-GAL4-mediated expression of spdo in a wild-type embryo generates extra Svp-lacZ+ heart precursors (arrowhead) by stage 12, and extra Svp-lacZ+ pericardial cells and cardioblasts (arrowheads, C′) as well as extra Svp-lacZ− cardioblasts (arrow, C′) by stage 16. (D) In numb mutant embryos two Svp-lacZ+ precursors arise normally (arrows), but each divides to produce two pericardial cells (green cells, D′), no Svp-lacZ+ cardioblasts develop in this background. (E) Expressing spdo throughout the mesoderm of a numb mutant embryo inhibits the formation of Svp-lacZ+ precursors (arrow), which leads to fewer Svp-lacZ+ pericardial cells (green cells, E′); note also the reduction in cardioblasts (red cells, E′). (F,F′) In wild-type mesodermal expression of a constitutively active form of Notch (NotchΔECN) leads to a near complete loss of Svp-lacZ+ heart precursors (arrowheads, F) and heart cells (F′). Anterior, left.
To determine whether expressing spdo during other Notch-dependent events also compromised Notch signaling activity, we expressed spdo throughout the wing blade, and assayed for defects in three additional Notch-mediated events: wing blade growth, wing patterning and wing vein formation (reviewed by Blair, 2000; De Celis, 2003). spdo misexpression in the wing resulted in reduced wing size, notched wings and vein thickening (Fig. 2J) — phenotypes indicative of reduced Notch signaling (see Blair, 2000; De Celis, 2003). In agreement, when Notch function is reduced by 50% the wing and lateral inhibition phenotypes observed upon spdo misexpression were dramatically enhanced (Fig. 2D,H,K). We conclude that superimposing spdo expression on cells undergoing these four Notch-dependent events dampens Notch signaling levels, consistent with the model that Numb normally fails to inhibit Notch signaling during these events owing to the absence of Spdo.
Misexpression of spdo amplifies Notch signaling in the absence of Numb
To test directly whether spdo-mediated inhibition of Notch signaling depends on numb, we misexpressed spdo in backgrounds with reduced numb function. We found that reducing numb function by 50% genetically (Fig. 2H, Table 1), or by co-expression of a numb-RNAi transgene (Fig. 2E,H), suppressed the ability of spdo to inhibit Notch signaling during lateral inhibition. For example, expression of spdo alone increased the number of scutellar bristles from four in wild type to just over eight (Fig. 2H), whereas expressing spdo in backgrounds compromised for numb function yielded an average of six scutellar bristles per fly (Fig. 2E,H). Similarly, reducing numb function by 50% in embryos that express spdo generally throughout the mesoderm restored heart cell numbers to near wild-type levels (Table 1). Thus, the ability of spdo to inhibit Notch signaling is numb dependent.
Table 1.
spdo regulates Notch pathway activity in a numb-dependent manner
| Genotype | Number of Svp-lacZ+ heart cells | P-value Number of Svp-lacZ− cardioblasts | P-value | Number of Eve+ pericardial cells | P-value | |
| Wild type | 28.1±0.5 (n=15) | 37.9±0.9 (n=15) | - | - | ||
| o/e spdo | 31.2±2.2 (n=15) | <0.0001* | 47.9±4.9 (n=15) | <0.0001* | - | - |
| o/e spdo; numb2/+ | 27.6±2.2 (n=15) | <0.001† | 39.2±1.8 (n=15) | <0.0001† | - | - |
| numb2 | 22.9±3.5 (n=20) | 33.9±1.7 (n=20) | 44.2±2.4 (n=20) | |||
| o/e spdo; numb2 | 8.6±3.1 (n=20) | <1×10−10‡ | 27.4±3.8 (n=40) | <1×10−5‡ | 18.7±9.0 (n=20) | <1x10−10‡ |
Numbers are given per bilateral side of an embryo; n refers to the number of embryos scored. The P-value is derived by t-test. Values are given ± s.d. o/e spdo refers to twist-GAL4-mediated expression of UAS-SpdoG14; UAS-SpdoG1 transgenes.
Relative to wild-type embryos.
Relative to o/e Spdo embryos.
Relative to numb mutant embryos.
If spdo acts through numb to inhibit Notch signaling, then misexpression of spdo in the absence of numb during lateral inhibition might have little effect on Notch signaling activity. To test this prediction, we expressed spdo throughout the mesoderm of embryos homozygous mutant for numb, and assayed the resulting effect on the development of differentiated heart cells and their precursors. Surprisingly, we observed a clear decrease in the formation of heart precursors and their progeny (Fig. 3D, Fig. 4C) — a phenotype indicative of increased Notch signaling. For example, in wild-type or numb mutant embryos, two Svp-lacZ+ heart precursors arise per hemisegment (Fig. 3B,D). In contrast, when spdo was overexpressed throughout the mesoderm of otherwise numb mutant embryos less than half the normal number of Svp-lacZ+ precursors and their progeny arose (Fig. 3E,E′; Table 1). Fewer Svp-lacZ− cardioblasts also developed in this background (Fig. 3E′; Table 1), suggesting that a fraction of their precursors also failed to develop. These phenotypes are similar to but less severe than observed upon expression of a constitutively active form of Notch throughout the mesoderm of wild-type embryos, which blocks the formation of essentially all cardioblasts and their precursors (Fig. 3F,F′). We conclude that expressing spdo in the absence of numb during lateral inhibition increased Notch signaling levels, leading to the segregation of fewer precursors than normal from equivalence groups.
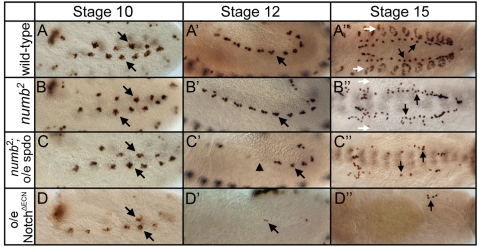
Fig. 4.
spdo potentiates Notch signaling in the absence of numb. Left and middle panels show lateral view; right panel shows dorsal view of eve expression in the mesoderm. (A-A″) In wild type, mesodermal Eve+ equivalence groups (arrows, A) generate Eve+ precursor cells (arrows, A′) that produce the EPCs (arrows, A″), and the Eve+ DA1 muscles (white arrows, A″). (B-B″) In numb mutant embryos, equivalence group formation and precursor selection occurs normally (arrows, B,B′); however, defects in asymmetric divisions lead to a doubling of EPC numbers (arrows, B″) and a loss of DA1 muscles (white arrows, B″). (C-C″) Eve+ equivalence groups form normally in numb mutant embryos that misexpress spdo in the mesoderm under the control of twist-GAL4 (arrows); however, Eve+ precursors fail to segregate from half of these groups (arrowhead, C′), resulting in fewer EPCs (arrows, C″). (D-D″) Eve+ equivalence groups form, albeit with reduced eve expression, upon mesodermal expression of a constitutively active form of Notch (NotchΔECN) in wild-type embryos; however, few precursors and EPCs arise in this background (arrows, D′,D″). Anterior, left.
To test directly whether the decrease in heart cells arose owing to a failure of precursors to segregate reliably from equivalence groups, rather than from a defect in establishing equivalence groups, we followed the dynamics of even skipped (eve) expression in the mesoderm (Fig. 4). In wild type, the Eve+ precursors of the Eve pericardial cells (EPCs) and the Eve+ DA1 muscles (arrow, Fig. 4A′) are selected via lateral inhibition from adjacent eve-expressing equivalence groups (arrows, Fig. 4A) (Carmena et al., 1998). In their respective lineages, EPCs represent ‘A’ daughter cells (arrows, Fig. 4A″), and Eve+ DA1 muscles represent ‘B’ daughter cells (white arrows, Fig. 4A″; see also Fig. 1B). In numb mutant embryos, the formation of eve-expressing equivalence groups, and the segregation of Eve+ precursors from them both occurred normally (arrows, Fig. 4B,B′). However, defects in asymmetric divisions led to a doubling of EPCs (‘A’ cells) and a loss of DA1 muscles (‘B’ cells) (Fig. 4B; see also Fig. 1B). Eve+ equivalence groups also formed normally in numb mutant embryos that express spdo throughout the mesoderm (arrows, Fig. 4C). However, in this background Eve+ precursors failed to segregate from roughly half of the equivalence groups (arrowhead, Fig. 4C′), resulting in the formation of far fewer EPCs than in numb mutant embryos (compare Fig. 4B″ with 4C″; Table 1). This phenotype is similar to but less severe than observed upon generalized mesodermal expression of a constitutively active form of Notch, where essentially no precursors segregate from Eve+ equivalence groups (Fig. 4D-D″). We conclude that expressing spdo in the absence of numb amplifies Notch signaling levels during lateral inhibition, resulting in the subsequent failure of precursors to segregate from equivalence groups. Conversely, expressing spdo in the presence of Numb decreases Notch signaling levels during lateral inhibition, resulting in the segregation of extra precursors.
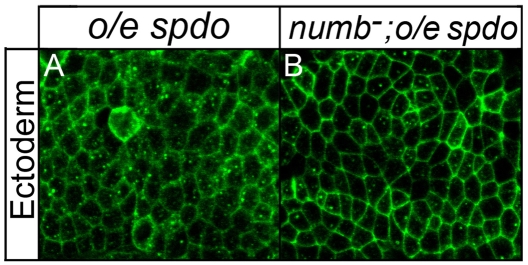
During asymmetric divisions Numb inhibits Spdo from localizing to the cell membrane of the ‘B’ daughter cell; in the ‘A’ daughter cell, which lacks Numb, Spdo localizes to the cell membrane (O'Connor-Giles and Skeath 2003; Hutterer and Knoblich 2005; Langevin et al., 2005). To see if numb maintains its ability to regulate the localization of Spdo in cells that do not normally express spdo, we misexpressed spdo throughout the ectoderm, a tissue in which spdo is never expressed, and then visualized Spdo localization in wild-type and numb mutant embryos. Spdo localized primarily to the cytoplasm in a diffuse and punctate pattern in ectodermal cells in otherwise wild-type embryos (Fig. 5A). By contrast, in numb mutant embryos Spdo exhibited increased localization to the cell membrane (Fig. 5B). Thus, numb maintained its competence to regulate the subcellular localization of Spdo in ectodermal cells, even though spdo is not normally expressed in these cells. Taken together, we find that in the presence of Numb Spdo localizes predominately to the cytoplasm and decreases Notch signaling activity, whereas in the absence of Numb, Spdo exhibits increased localization to the cell membrane and increases Notch signaling.
Fig. 5.
numb regulates the subcellular localization of Spdo. Spdo localization (green) in ectodermal cells of otherwise wild-type (A) or numb mutant (B) embryos. (A) Spdo exhibits predominately diffuse and punctate localization in ectodermal cells of wild-type embryos. (B) Spdo exhibits increased localization to the cell membrane of ectodermal cells in numb mutant embryos. Each panel shows a single z-section of a stage 12 embryo taken at similar apical basal positions; both embryos were processed in parallel and imaged on a Leica SPII confocal microscope using identical parameters. Spdo misexpression mediated by scabrous-GAL4. Anterior, left.
spdo potentiates Notch signaling in the ‘A’ daughter cell
During wild-type development, spdo expression is restricted to asymmetrically dividing cells. Thus, the only cells that normally express Notch and Spdo but not Numb are the Notch-dependent ‘A’ daughter cells, and the only cells that express Notch, Spdo and Numb are ‘B’ daughter cells (Fehon et al., 1991; O'Connor-Giles and Skeath, 2003; Rhyu et al., 1994). Given the above results, we set out to test the hypotheses that: (1) Spdo functions in ‘A’ cells to amplify Notch signaling during asymmetric divisions; whereas (2) in ‘B’ cells, Spdo enables Numb to inhibit Notch signaling.
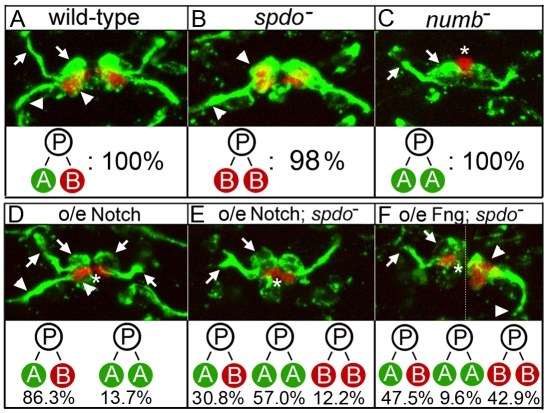
We reasoned that if Spdo amplifies Notch signaling levels in ‘A’ daughter cells, then increasing Notch signaling levels through other means might bypass the requirement for spdo in these cells. Thus, we overexpressed wild-type forms of Notch pathway members in embryos homozygous mutant for spdo and assayed asymmetric divisions in four distinct cell lineages in the embryonic central nervous system (CNS) and mesoderm (see Fig. 1B). In all cases, we found that overexpression of the full-length forms of either the Notch receptor or Fringe [which has been shown to modify Notch to enhance Notch-Delta signaling during wing development (Irvine, 1999; Moloney et al., 2000)] largely or completely restored ‘A’ cell development in spdo mutant embryos. For example, in each hemisegment of the CNS, the MP2 precursor divides asymmetrically to yield vMP2 (arrows, Fig. 6A), which projects an axon anteriorly, and dMP2 (arrowheads, Fig. 6B), which expresses Odd skipped and extends an axon posteriorly (see also Fig. 1B). Similarly, each of the first five ganglion mother cells generated by neuroblast 7-1 divides asymmetrically to produce an Eve+ U motoneuron and an Eve+ U sibling neuron (Fig. 1B; arrowheads in Fig. S1A in the supplementary material) (Pearson and Doe, 2003). vMP2 and the Eve+ U neurons are ‘A’ daughter cells, and adopt the fate of their respective siblings at nearly 100% frequencies in the absence of spdo (arrowheads, Fig. 6B; see Fig. S1B and Tables S1 and S2 in the supplementary material). However, overexpression of either Notch or Fringe throughout the CNS of spdo mutant embryos restored vMP2 development (‘A’ fate) in 87.8 and 57.1% of hemisegments, respectively (arrows, Fig. 6E,F; see Table S1 in the supplementary material), and Eve+ U neuron development in most or all hemisegments (arrowheads, see Fig. S1F,G and Table S2 in the supplementary material). Note that a significant fraction of ‘B’ daughter cells also adopted the Notch-dependent ‘A’ fate in the MP2 lineage upon Notch or Fringe overexpression in spdo mutant embryos (see below).
Fig. 6.
spdo facilitates Numb-mediated inhibition of Notch signaling in the ‘B’ daughter cell during asymmetric divisions in the CNS. (A) In wild-type embryos vMP2 (arrows, ‘A’ cell) extends an axon anteriorly, while its sibling dMP2 (arrowheads, ‘B’ cell) expresses Odd (red) and extends an axon posteriorly. (B) In spdo mutant embryos both siblings adopt the Odd+ dMP2 fate (arrowheads) and extend axons posteriorly. (C) In numb mutant embryos both siblings adopt the vMP2 fate (arrows) and extend axons anteriorly. In C-F, an asterisk denotes the unrelated Odd+ MP1 neurons (red), which form in all genotypes shown but are obscured in some panels. (D) Single segment of wild-type embryo in which Notch has been overexpressed in the CNS. Left, daughter cells adopt normal vMP2 (arrows) and dMP2 (arrowheads) fates. Right, both daughter cells adopt the vMP2 fate and extend axons anteriorly (arrows). (E) Notch overexpression in spdo mutant embryos induces both daughter cells to adopt the vMP2 fate (arrows) and extend axons anteriorly in most hemisegments. (F) Fringe overexpression in spdo mutant embryos. Left: both daughter cells adopt the vMP2 fate (arrows) and extend axons anteriorly. Right: both daughter cells adopt the dMP2 fate (arrowheads) and extend axons posteriorly. (see Table S3 in the supplementary material). The dotted line in F indicates use of different focal planes from the same segment. Anterior is up; N>200 hemisegments per genotype. prospero-GAL4 was used for gene overexpression. See also Table S3 in the supplementary material.
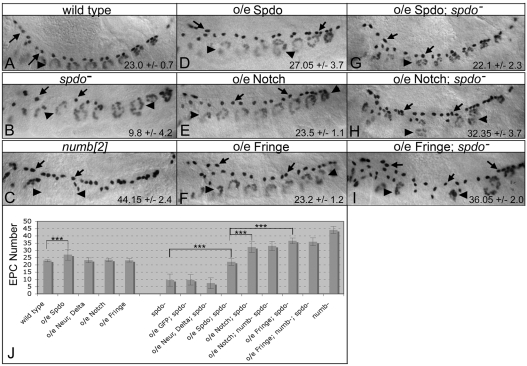
Overexpression of Notch or Fringe can also bypass the requirement for spdo function during asymmetric divisions in the mesoderm. As noted, Svp-lacZ+ heart precursors divide asymmetrically to produce sibling pericardial cells (‘A’ cells) and cardioblasts (‘B’ cells), and within the EPC lineage the Eve+ EPCs represent ‘A’ daughter cells (Fig. 1B; arrows, Fig. 7A; Fig. 8A). In these lineages the absence of spdo function causes both daughter cells to adopt the ‘B’ fate about 75% of the time (Fig. 7B, Fig. 8B; see Table S3 in the supplementary material) (Park et al., 1998; Ward and Skeath, 2000). However, overexpression of Notch or Fringe throughout the mesoderm of spdo mutant embryos restored the ‘A’ daughter cell fate in both lineages to wild-type or near wild-type levels (arrows, Fig. 7H,I; arrowheads, Fig. 8G,I; see Table S3 in the supplementary material). As observed in the CNS, ‘B’ daughter cells in both lineages also adopted the ‘A’ fate at appreciable frequencies in these backgrounds (see below). Thus, increasing the level or activity of the Notch receptor largely or completely bypassed the requirement for spdo to promote the ‘A’ fate, indicating that Spdo is not absolutely required for Notch signal transduction during asymmetric divisions, but rather acts to amplify pathway activity above the level required to induce the ‘A’ fate. Our prior finding that Spdo enhances Notch signaling activity during lateral inhibition in the absence of numb supports this conclusion. Note, that the ability of Notch or Fringe overexpression to restore ‘A’ cell development in spdo mutant embryos is ligand dependent, as overexpression of either transgene in the neuroectoderm/CNS or mesoderm of Delta mutant embryos failed to rescue Notch-dependent cell fates (see Fig. S2 in the supplementary material).
Fig. 7.
Overexpression of Notch or Fringe bypasses the requirement for spdo function during EPC development. Each panel shows a lateral view of eve expression in the dorsal mesoderm. (A) Wild-type stage 14 embryo showing EPCs (arrows) and Eve+ DA1 muscles (arrowheads). (B) spdo mutant embryos exhibit a decrease in EPC number (arrows). (C) In numb mutant embryos EPC numbers double (arrows) and Eve+ DA1 muscles are lost (arrowheads). (D) twist-GAL4-mediated overexpression of Spdo (o/e spdo) in the mesoderm of wild-type embryos increased EPC numbers (arrows). (E,F) Identical experiments with either Notch (E) or Fringe (F) had little effect on EPCs (arrows) or DA1 muscles (arrowheads). (G-I) Mesodermal expression of spdo in a spdo mutant embryo rescued the spdo mutant phenotype (compare to B), whereas mesodermal overexpression of Notch (H) or Fringe (I) in spdo mutant embryos increased EPC numbers (arrows) and decreased DA1 muscle numbers (arrowheads), phenotypes similar to those of numb mutant embryos (compare with C). Numbers indicate average number of EPCs ±s.d. per bilateral side of an embryo. n≥20 embryo sides, except for o/e spdo; spdo− (n=14). (J) Chart shows average number of EPCs per indicated genotype.
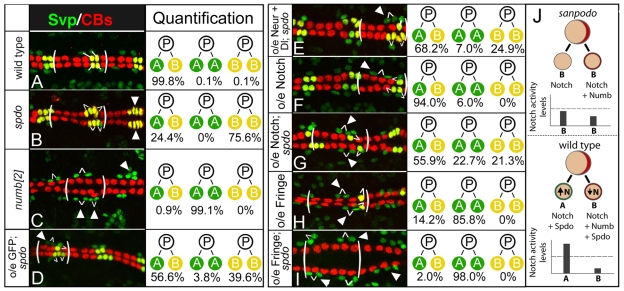
Fig. 8.
spdo facilitates Numb-mediated inhibition of Notch signaling during asymmetric divisions in the heart. (A) Wild-type pattern of sibling Svp-lacZ+ pericardial cells (green, ‘A’ cell) and cardioblasts (CBs; yellow, ‘B’ cell) in the stage 16 heart. In all panels one segment is bracketed, and white lines identify sibling relationships. (B) In spdo mutant embryos both Svp-lacZ+ daughter cells normally adopt the cardioblast or ‘B’ fate (yellow, arrowheads). (C) In numb mutant embryos both daughter cells adopt the pericardial or ‘A’ fate (green, arrowheads). (D,E) twist-GAL4-mediated expression of GFP (D), or Neuralized and Delta (E), in spdo mutant embryos causes both daughter cells to adopt the ‘A’ fate at low frequency (arrowhead), and increases the frequency of both daughter cells adopting alternate fates (‘A/B’). Note GFP overexpression has no effect on any other lineage tested (Fig. 3, see Tables S2 and S3 in the supplementary material). (F) Notch overexpression in wild type induces both daughter cells to adopt the ‘A’ fate at low frequency (arrowhead). (G) Notch overexpression in spdo mutant embryos increases the frequency at which both daughter cells adopt the ‘A’ fate (arrowheads). (H) Fringe overexpression in wild type directs both daughter cells to adopt the ‘A’ fate most of the time (arrowheads). (I) Fringe overexpression in spdo mutant embryos directs both daughter cells to adopt the ‘A’ fate (green, arrowheads) essentially all the time (arrowheads). Anterior, left. Quantification of sibling fates. n>800 sibling pairs assayed per genotype, except for numb (n=200). See also Table S3 in the supplementary material. (J) Model of spdo function during asymmetric divisions. Top, in spdo mutant embryos Notch signaling activity remains below the threshold level (dotted line) required to induce the ‘A’ fate, and both daughter cells adopt the ‘B’ fate. Bottom, in the absence of Numb, Spdo amplifies Notch signaling activity above the threshold required to induce the ‘A’ fate. In the presence of Numb, Spdo facilitates the ability of Numb to inhibit Notch signaling ‘B’ daughter cell, thereby reducing signaling activity below that observed in the absence of spdo.
To test whether increasing ligand levels was also sufficient to restore ‘A’ daughter cell development in the absence of spdo, we co-overexpressed Delta and Neuralized — which has been shown to increase Notch signaling (Pavlopoulos et al., 2001; Wang and Struhl, 2004) — in spdo mutant backgrounds. Co-expression of both factors failed to restore ‘A’ cell development in the EPC lineage (Fig. 7J), and provided only modest rescuing activity in the other three lineages (Fig. 8E; see Fig. S1D,E and Tables S1-S3 in the supplementary material). Given this result, the ability of Notch or Fringe overexpression to restore ‘A’ cell development to near wild-type levels suggests that the levels and/or activity of the Notch receptor are limiting in the absence of spdo for productive Notch signaling in the ‘A’ daughter cell.
spdo facilitates the ability of Numb to inhibit Notch signaling in ‘B’ cells
Next, we focused on spdo function in ‘B’ daughter cells, and asked whether spdo enables Numb to inhibit Notch signaling in this cell. Normally one cannot assess a role for spdo in ‘B’ daughter cells, as both daughter cells adopt the ‘B’ fate in the absence of spdo regardless of the presence of numb (Dye et al., 1998; Skeath and Doe, 1998). However, the ability of Notch or Fringe overexpression to bypass the requirement for spdo function in ‘A’ daughter cells allowed us to test whether spdo facilitates Numb-mediated inhibition of Notch signaling in ‘B’ daughter cells.
If Spdo enables Numb to inhibit Notch signaling in the ‘B’ daughter cell, then the ability of Notch or Fringe overexpression to convert ‘B’ cells to the ‘A’ fate should be greater in the absence rather than the presence of spdo. In agreement, Notch or Fringe overexpression was more effective at converting ‘B’ daughter cells to the ‘A’ fate in spdo mutant embryos than in wild-type embryos for all lineages tested. For example, in wild-type embryos the frequency of fate changes in the MP2 lineage (dMP2/‘B’ cell to vMP2/‘A’ cell) was 13.7% for Notch overexpression and 0% for Fringe overexpression (Fig. 6D; see Table S1 in the supplementary material). In spdo mutant embryos, the frequency of fate changes increased to 57.0% for Notch overexpression and 9.6% for Fringe overexpression (Fig. 6E,F, see Table S1 in the supplementary material). Similarly, the frequency of ‘B’ to ‘A’ fate changes in the Svp-lacZ+ heart lineage increased from 85.8% (n=910) in wild-type to 98.0% (n=895) in spdo mutant embryos for Fringe overexpression (compare Fig. 8H with 8I), and from 6.0% (n=949) in wild-type to 22.7% (n=942) in spdo mutant embryos for Notch overexpression (compare Fig. 8F with 8G; see Table S3 in the supplementary material).
The absence of spdo also rendered Notch or Fringe overexpression more effective at converting ‘B’ daughter cells to the ‘A’ fate in the EPC and Eve+ DA1 lineages. In their respective lineages, EPCs represent ‘A’ daughter cells, and Eve+ DA1 muscles represent ‘B’ daughter cells (see Fig. 1B). In wild-type embryos Notch or Fringe overexpression has little if any effect on EPC or DA1 development (arrows, Fig. 7E,F). By contrast, in the absence of spdo function the same treatment significantly increased EPC numbers (‘A’ cells) and decreased Eve+ DA1 muscle numbers (‘B’ cells) (arrows, Fig. 7H,I), consistent with ‘B’ daughter cells adopting the ‘A’ fate at appreciable frequencies in both lineages. In fact, the asymmetric division phenotypes observed upon Notch/Fringe overexpression in otherwise spdo mutant embryos often closely resembled those of numb mutant embryos, wherein essentially all ‘B’ daughter cells adopt the Notch-dependent ‘A’ fate (compare Fig. 6C with 6E, Fig. 7C with 7I, Fig. 8C with 8I). We conclude that Spdo enables Numb to inhibit Notch signaling in the ‘B’ cell during asymmetric divisions. Interestingly, simultaneous removal of numb and spdo did not significantly increase the conversion of ‘B’ daughter cells to the ‘A’ fate above that observed upon Notch or Fringe overexpression in the absence of spdo alone (Fig. 7J; see Table S2 in the supplementary material). Thus, our data suggest that numb acts primarily through spdo to block Notch signaling in the ‘B’ daughter cell. Together with the finding that superimposing spdo expression during lateral inhibition enables Numb to inhibit Notch signaling, these results explain why numb can only inhibit Notch signaling during asymmetric divisions — this is the only context in which Spdo is present.
DISCUSSION
Work from many labs indicates that the state of Notch signaling determines daughter cell fate during asymmetric divisions — high-level Notch signaling induces the ‘A’ fate; low-level Notch signaling permits the ‘B’ fate (Gonczy, 2008; Matsuzaki, 2000; Schweisguth, 2004). In this context, our work demonstrates that spdo acts in both daughter cells to accentuate the difference between Notch signaling levels in the two cells — amplifying Notch signaling in the absence of Numb in the ‘A’ cell, and enabling Numb to inhibit Notch signaling in the ‘B’ cell (Fig. 8J). By exerting opposite effects on Notch signaling in a Numb-dependent manner, Spdo simultaneously ensures that Notch signaling exceeds threshold levels in the ‘A’ cell, yet remains well below such levels in the ‘B’ cell, thus enabling the faithful execution of asymmetric divisions.
Why Numb can inhibit Notch signaling during asymmetric divisions but no other Notch-dependent event has long remained unclear. Our genetic data demonstrate that numb acts through spdo to inhibit Notch signaling. As spdo is expressed exclusively in asymmetrically dividing cells, and Numb segregates exclusively into the ‘B’ daughter cell during asymmetric divisions, these results account for the specific ability of Numb to inhibit Notch signaling in ‘B’ daughter cells — the only cell type in Drosophila that co-expresses spdo and numb. spdo does not appear to enable Numb to inhibit Notch signaling by regulating the localization of Numb, as Numb localization is grossly normal in spdo mutant embryos (see Fig. S3 in the supplementary material).
Why does productive Notch signaling require spdo function in ‘A’ daughter cells during asymmetric divisions, but not during any other Notch-dependent event in Drosophila? We find that in the absence of Numb, Spdo amplifies but is not obligately required for transduction of Notch signaling. Thus, while Notch signaling can occur in ‘A’ daughter cells in the absence of spdo, spdo function is normally required to enable signaling levels to exceed the threshold required to induce the ‘A’ fate.
Our results indicate that limiting levels or activity of the Notch receptor probably underlies the sub-threshold nature of Notch signaling in ‘A’ daughter cells in the absence of spdo. Notch levels or activity may be limiting in ‘A’ daughter cells owing to the downregulation of proteins that localize to adherens junctions in asymmetrically dividing cells. Notch has been shown to localize preferentially to adherens junctions in epithelial cells, and asymmetrically dividing cells display reduced levels of Notch as well as other proteins that normally localize to adherens junctions (Fehon et al., 1991; Tepass et al., 1996; Uemura et al., 1996). Some of these other proteins, such as Echinoid, are known to facilitate Notch signaling during lateral inhibition and other Notch-dependent events (Ahmed et al., 2003; Fehon et al., 1991; Rawlins et al., 2003; Spencer and Cagan, 2003). Thus, reduced levels of Notch and facilitators of Notch signaling in asymmetrically dividing cells may account for the specific requirement for Spdo to amplify Notch signaling levels during asymmetric divisions.
Consistent with a role for spdo in simply amplifying Notch signaling levels in the absence of Numb, the Notch-dependent ‘A’ fate develops at low frequency in some lineages in the absence of spdo (Fig. 7B,J and Fig. 8B, see Table S3 in the supplementary material) (Jafar-Nejad et al., 2005; Park et al., 1998; Ward and Skeath, 2000). Thus, in the absence of spdo, Notch signaling levels appear close to, but usually below, the threshold required to induce the ‘A’ fate (Fig. 8J). Surprisingly, we also observe rare instances where Numb-dependent ‘B’ daughter cells adopt the ‘A’ fate in spdo mutant embryos, specifically in the development of Svp+ heart cells at 18°C (see Table S3 in the supplementary material). Such events have not been observed in wild type, and indicate that Numb requires Spdo in the ‘B’ cell to maintain Notch signaling levels reliably below the threshold required for the ‘A’ fate. Thus, the dual and opposing roles of spdo in the regulation of Notch signaling levels during asymmetric divisions are crucial for the unerring ability of the two daughter cells to adopt distinct fates.
What is the molecular mechanism through which spdo regulates Notch signaling during asymmetric divisions? Our results indicate that any mechanistic model for spdo function must account for the ability of spdo to boost Notch signaling in the absence of Numb and to reduce Notch signaling in the presence of Numb. Present models of spdo function, such as a postulated role for Spdo in promoting recycling of Delta in the ‘B’ cell (Emery et al., 2005; Jafar-Nejad et al., 2005), do not fully address the duality of spdo function in the two daughter cells. Rather our genetic data, together with prior work on Spdo physical interactions and Numb-dependent localization, lead us to postulate that in the absence of Numb, Spdo localizes to the cell membrane of the ‘A’ cell, where it increases Notch association with effectors, and in so doing boosts Notch signaling levels.
How could Numb convert Spdo from an activator to an inhibitor of Notch signaling? Numb binds directly to Spdo and regulates its subcellular localization, preventing Spdo from localizing to the cell membrane (Hutterer and Knoblich, 2005; Langevin et al., 2005; O’Connor-Giles and Skeath, 2003). If either Notch or an effector is internalized with Spdo by Numb, a quantitative decrease in Notch signaling would result. However, the levels of Notch at the cell membrane appear roughly equivalent between the two daughter cells (Berdnik et al., 2002), suggesting that if numb functions in this manner it may do so by targeting a Notch effector rather than Notch itself along with Spdo. Alternatively, small changes in Notch receptor levels may be sufficient to decrease signaling levels below the threshold required to induce the ‘A’ fate. The elucidation of the precise mechanism through which Spdo exerts opposite effects on Notch pathway activity in the two daughter cells probably awaits the systematic identification of the factors that physically interact with Spdo during asymmetric divisions.
Supplementary Material
Acknowledgements
We thank Hugo Bellen, Manfred Frasch, Ed Giniger, Gary Struhl, Alan Michelson, Weimin Zhong, the Developmental Studies Hybridoma Bank (Iowa) and as always Kathy Matthews, Kevin Cook and the Bloomington Stock Center for stocks and reagents. We thank Swathi Arur, Sarah Bray and Haluk Lacin for critical review of the manuscript. This work was supported by an NIGMS grant (R01-GM068048) to J.B.S. and an NINDS grant (K99/R00-NS060985) to K.M.O-G. A.B.B. was a trainee of the Lucille Markey Pathway in Human Pathobiology at Washington University. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/24/4089/DC1
References
- Ahmed A., Chandra S., Magarinos M., Vaessin H. (2003). Echinoid mutants exhibit neurogenic phenotypes and show synergistic interactions with the Notch signaling pathway. Development 130, 6295-6304 [DOI] [PubMed] [Google Scholar]
- Berdnik D., Torok T., Gonzalez-Gaitan M., Knoblich J. A. (2002). The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell 3, 221-231 [DOI] [PubMed] [Google Scholar]
- Blair S. S. (2000). Notch signaling: Fringe really is a glycosyltransferase. Curr. Biol. 10, R608-R612 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415 [DOI] [PubMed] [Google Scholar]
- Calleja M., Moreno E., Pelaz S., Morata G. (1996). Visualization of gene expression in living adult Drosophila. Science 274, 252-255 [DOI] [PubMed] [Google Scholar]
- Carmena A., Gisselbrecht S., Harrison J., Jimenez F., Michelson A. M. (1998). Combinatorial signaling codes for the progressive determination of cell fates in the Drosophila embryonic mesoderm. Genes Dev. 12, 3910-3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Celis J. F. (2003). Pattern formation in the Drosophila wing: The development of the veins. BioEssays 25, 443-451 [DOI] [PubMed] [Google Scholar]
- Dye C. A., Lee J. K., Atkinson R. C., Brewster R., Han P. L., Bellen H. J. (1998). The Drosophila sanpodo gene controls sibling cell fate and encodes a tropomodulin homolog, an actin/tropomyosin-associated protein. Development 125, 1845-1856 [DOI] [PubMed] [Google Scholar]
- Emery G., Hutterer A., Berdnik D., Mayer B., Wirtz-Peitz F., Gaitan M. G., Knoblich J. A. (2005). Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122, 763-773 [DOI] [PubMed] [Google Scholar]
- Fehon R. G., Johansen K., Rebay I., Artavanis-Tsakonas S. (1991). Complex cellular and subcellular regulation of notch expression during embryonic and imaginal development of Drosophila: implications for notch function. J. Cell Biol. 113, 657-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M., Hoey T., Rushlow C., Doyle H., Levine M. (1987). Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 6, 749-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E. (1998). A role for Abl in Notch signaling. Neuron 20, 667-681 [DOI] [PubMed] [Google Scholar]
- Gonczy P. (2008). Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355-366 [DOI] [PubMed] [Google Scholar]
- Greig S., Akam M. (1993). Homeotic genes autonomously specify one aspect of pattern in the Drosophila mesoderm. Nature 362, 630-632 [DOI] [PubMed] [Google Scholar]
- Hartenstein A. Y., Rugendorff A., Tepass U., Hartenstein V. (1992). The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development 116, 1203-1220 [DOI] [PubMed] [Google Scholar]
- Hutterer A., Knoblich J. A. (2005). Numb and alpha-Adaptin regulate Sanpodo endocytosis to specify cell fate in Drosophila external sensory organs. EMBO Rep. 6, 836-842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K. D. (1999). Fringe, Notch, and making developmental boundaries. Curr. Opin. Genet. Dev. 9, 434-441 [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H., Andrews H. K., Acar M., Bayat V., Wirtz-Peitz F., Mehta S. Q., Knoblich J. A., Bellen H. J. (2005). Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell 9, 351-363 [DOI] [PubMed] [Google Scholar]
- Jurgens G., Wieschaus E,, Nusslein-Volhard C., Kluding H. (1984). Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster II. Zygotic loci on the third chromosome. Roux's Arch. Dev. Biol. 193, 283-295 [DOI] [PubMed] [Google Scholar]
- Lai E. C., Deblandre G. A., Kintner C., Rubin G. M. (2001). Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev. Cell 1, 783-794 [DOI] [PubMed] [Google Scholar]
- Langevin J., Le Borgne R., Rosenfeld F., Gho M., Schweisguth F., Bellaiche Y. (2005). Lethal giant larvae controls the localization of notch-signaling regulators numb, neuralized, and Sanpodo in Drosophila sensory-organ precursor cells. Curr. Biol. 15, 955-962 [DOI] [PubMed] [Google Scholar]
- Leal S. M., Qian L., Lacin H., Bodmer R., Skeath J. B. (2009). Neuromancer1 and Neuromancer2 regulate cell fate specification in the developing embryonic CNS of Drosophila melanogaster. Dev. Biol. 325, 138-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear B. C., Skeath J. B., Patel N. H. (1999). Neural cell fate in rca1 and cycA mutants: the roles of intrinsic and extrinsic factors in asymmetric division in the Drosophila central nervous system. Mech. Dev. 88, 207-219 [DOI] [PubMed] [Google Scholar]
- Lilly B., Zhao B., Ranganayakulu G., Paterson B. M., Schulz R. A., Olson E. N. (1995). Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science 267, 688-693 [DOI] [PubMed] [Google Scholar]
- Lindsley D. a. Z. G. (1992). The Genome of Drosophila melanogaster New York: Academic Press; [Google Scholar]
- Matsuzaki F. (2000). Asymmetric division of Drosophila neural stem cells: a basis for neural diversity. Curr. Opin. Neurobiol. 10, 38-44 [DOI] [PubMed] [Google Scholar]
- Micchelli C. A., Rulifson E. J., Blair S. S. (1997). The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124, 1485-1495 [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Hiromi Y., Weber U., Goodman C. S., Rubin G. M. (1990). The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell 60, 211-224 [DOI] [PubMed] [Google Scholar]
- Moloney D. J., Panin V. M., Johnston S. H., Chen J., Shao L., Wilson R., Wang Y., Stanley P., Irvine K. D., Haltiwanger R. S., et al. (2000). Fringe is a glycosyltransferase that modifies Notch. Nature 406, 369-375 [DOI] [PubMed] [Google Scholar]
- Nakao K., Campos-Ortega J. A. (1996). Persistent expression of genes of the enhancer of split complex suppresses neural development in Drosophila. Neuron 16, 275-286 [DOI] [PubMed] [Google Scholar]
- Nolo R., Abbott L. A., Bellen H. J. (2000). Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349-362 [DOI] [PubMed] [Google Scholar]
- O'Connor-Giles K. M., Skeath J. B. (2003). Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev. Cell 5, 231-243 [DOI] [PubMed] [Google Scholar]
- Okajima T., Irvine K. D. (2002). Regulation of notch signaling by o-linked fucose. Cell 111, 893-904 [DOI] [PubMed] [Google Scholar]
- Park M., Yaich L. E., Bodmer R. (1998). Mesodermal cell fate decisions in Drosophila are under the control of the lineage genes numb, Notch, and sanpodo. Mech. Dev. 75, 117-126 [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E., Pitsouli C., Klueg K. M., Muskavitch M. A., Moschonas N. K., Delidakis C. (2001). neuralized Encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev. Cell 1, 807-816 [DOI] [PubMed] [Google Scholar]
- Pearson B. J., Doe C. Q. (2003). Regulation of neuroblast competence in Drosophila. Nature 425, 624-628 [DOI] [PubMed] [Google Scholar]
- Posakony J. W. (1994). Nature versus nurture: asymmetric cell divisions in Drosophila bristle development. Cell 76, 415-418 [DOI] [PubMed] [Google Scholar]
- Preiss A., Hartley D. A., Artavanis-Tsakonas S. (1988). The molecular genetics of Enhancer of split, a gene required for embryonic neural development in Drosophila. EMBO J. 7, 3917-3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins E. L., Lovegrove B., Jarman A. P. (2003). Echinoid facilitates Notch pathway signalling during Drosophila neurogenesis through functional interaction with Delta. Development 130, 6475-6484 [DOI] [PubMed] [Google Scholar]
- Rhyu M. S., Jan L. Y., Jan Y. N. (1994). Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76, 477-491 [DOI] [PubMed] [Google Scholar]
- Salzberg A., D'Evelyn D., Schulze K. L., Lee J. K., Strumpf D., Tsai L., Bellen H. J. (1994). Mutations affecting the pattern of the PNS in Drosophila reveal novel aspects of neuronal development. Neuron 13, 269-287 [DOI] [PubMed] [Google Scholar]
- Schweisguth F. (2004). Regulation of notch signaling activity. Curr. Biol. 14, R129-R138 [PubMed] [Google Scholar]
- Schweisguth F., Posakony J. W. (1992). Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell 69, 1199-1212 [DOI] [PubMed] [Google Scholar]
- Shiga Y., Tanaka-Matakatsu M., Hayashi S. (1996). A nuclear GFP/ beta-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Dev. Growth Differ. 38, 99-106 [Google Scholar]
- Simpson P. (1998). Introduction: Notch signalling and choice of cell fates in development. Semin. Cell Dev. Biol. 9, 581-582 [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. (1992). Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development 114, 939-946 [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Doe C. Q. (1998). Sanpodo and Notch act in opposition to Numb to distinguish sibling neuron fates in the Drosophila CNS. Development 125, 1857-1865 [DOI] [PubMed] [Google Scholar]
- Spencer S. A., Cagan R. L. (2003). Echinoid is essential for regulation of Egfr signaling and R8 formation during Drosophila eye development. Development 130, 3725-3733 [DOI] [PubMed] [Google Scholar]
- Struhl G., Fitzgerald K., Greenwald I. (1993). Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 74, 331-345 [DOI] [PubMed] [Google Scholar]
- Tang H., Rompani S. B., Atkins J. B., Zhou Y., Osterwalder T., Zhong W. (2005). Numb proteins specify asymmetric cell fates via an endocytosis- and proteasome-independent pathway. Mol. Cell. Biol. 25, 2899-2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Gruszynski-DeFeo E., Haag T. A., Omatyar L., Torok T., Hartenstein V. (1996). shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 10, 672-685 [DOI] [PubMed] [Google Scholar]
- Uemura T., Shepherd S., Ackerman L., Jan L. Y., Jan Y. N. (1989). numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 58, 349-360 [DOI] [PubMed] [Google Scholar]
- Uemura T., Oda H., Kraut R., Hayashi S., Kotaoka Y., Takeichi M. (1996). Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10, 659-671 [DOI] [PubMed] [Google Scholar]
- Wang W., Struhl G. (2004). Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131, 5367-5380 [DOI] [PubMed] [Google Scholar]
- Ward E. J., Coulter D. E. (2000). odd-skipped is expressed in multiple tissues during Drosophila embryogenesis. Mech. Dev. 96, 233-236 [DOI] [PubMed] [Google Scholar]
- Ward E. J., Skeath J. B. (2000). Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development 127, 4959-4969 [DOI] [PubMed] [Google Scholar]
- Wilder E. L., Perrimon N. (1995). Dual functions of wingless in the Drosophila leg imaginal disc. Development 121, 477-488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.