Abstract
Activating transcription factor 4 (Atf4) is a leucine-zipper-containing protein of the cAMP response element-binding protein (CREB) family. Ablation of Atf4 (Atf4−/−) in mice leads to severe skeletal defects, including delayed ossification and low bone mass, short stature and short limbs. Atf4 is expressed in proliferative and prehypertrophic growth plate chondrocytes, suggesting an autonomous function of Atf4 in chondrocytes during endochondral ossification. In Atf4−/− growth plate, the typical columnar structure of proliferative chondrocytes is disturbed. The proliferative zone is shortened, whereas the hypertrophic zone is transiently expanded. The expression of Indian hedgehog (Ihh) is markedly decreased, whereas the expression of other chondrocyte marker genes, such as type II collagen (Col2a1), PTH/PTHrP receptor (Pth1r) and type X collagen (Col10a1), is normal. Furthermore, forced expression of Atf4 in chondrocytes induces endogenous Ihh mRNA, and Atf4 directly binds to the Ihh promoter and activates its transcription. Supporting these findings, reactivation of Hh signaling pharmacologically in mouse limb explants corrects the Atf4−/− chondrocyte proliferation and short limb phenotypes. This study thus identifies Atf4 as a novel transcriptional activator of Ihh in chondrocytes that paces longitudinal bone growth by controlling growth plate chondrocyte proliferation and differentiation.
Keywords: Atf4, Ihh, Growth plate chondrocytes, Endochondral ossification, Gene transcription, Mouse
INTRODUCTION
The mammalian skeleton is formed through intramembranous and endochondral ossification. The initial step of skeletal development is patterning. At 10 days post-coitum (dpc), during mouse embryogenesis, a group of homogenous cells gives rise to mesenchymal structures and functions in a precise spatial and temporal pattern. Condensation of mesenchymal progenitor cells into a mass that provides the mold of the future skeleton follows this initial patterning (Kaufman, 1992). The condensed mesenchymal cells then differentiate into osteoblasts directly during intramembranous ossification, or into chondrocytes to form the cartilage anlagen, which is eventually replaced by bone during endochondral ossification (Karsenty and Wagner, 2002). Endochondral ossification is crucial for skeletal growth in the developing vertebrate, as well as for skeletal repair in adults. It involves slowly proliferating, rounded, resting chondrocytes in the reserve zone acquiring cues to become rapidly dividing cells that are flattened and packed into columnar chondrocytes in the proliferating zone. Rapidly proliferating chondrocytes then stop dividing to progress to a transition stage of prehypertrophic chondrocytes, which quickly undergo differentiation (hypertrophy). Mature hypertrophic chondrocytes eventually die, allowing vascular invasion, a process that involves the entry of osteoclast and osteoblast precursors. Osteoclasts assist in the removal of cartilage matrix and osteoblasts use the remnants of cartilage matrix as a scaffold for the deposition of new bone matrix to form calcified bone. Therefore, endochondral ossification is a dynamic event that relies on chondrocyte proliferation and differentiation and is tightly regulated by systemic factors, locally secreted factors and transcription factors (reviewed by Day and Yang, 2008; de Crombrugghe et al., 1991; Kronenberg, 2003; Mackie et al., 2008; Nilsson et al., 2005; Ornitz, 2005; Zuscik et al., 2008).
Indian hedgehog (Ihh) belongs to the hedgehog (Hh) family and is one of the aforementioned locally secreted factors required for mammalian skeletal development. By binding to its receptor patched, Hh ligands induce the release of patched inhibition of smoothened, which allows activation of signaling events that promote cell proliferation (Alcedo and Noll, 1997; Day and Yang, 2008). In the growth plate, Ihh is secreted by prehypertrophic chondrocytes and acts as a paracrine factor to promote adjacent chondrocyte proliferation. Ihh can also diffuse and reach cells in the articular perichondrium, where it induces the expression of parathyroid hormone related protein (PTHrP; Pthlh — Mouse Genome Informatics), which in turn inhibits chondrocyte hypertrophy and maintains the pool of proliferative chondrocytes (Chung et al., 2001; Guo et al., 2006; Karp et al., 2000; Minina et al., 2001; St-Jacques et al., 1999). Although the mechanism is not yet fully understood, this action of PTHrP to inhibit hypertrophy forms a negative-feedback regulatory loop on the production of Ihh, which controls the coordination between proliferation and differentiation of participating chondrocytes. Using genetic mouse models, Mak et al. demonstrated that Ihh also promotes chondrocyte hypertrophy in a PTHrP-independent manner (Mak et al., 2008). Given the crucial role that Hh signaling plays in the regulation of skeletal development, it is of interest to understand upstream signaling pathways that regulate Ihh at the transcriptional level.
Recent studies have identified several transcription factors that activate Ihh transcription. Runx proteins are a group of cell-specific transcription factors belonging to the Runt family (see Karsenty, 2001). Using genetic mouse models, Yoshida et al. found that Runx2, with the assistance of Runx3, binds directly to the Ihh promoter and activates its expression (Yoshida et al., 2004). This was the first and thus far only characterization of a transcriptional mechanism involved in the regulation of chondrocyte proliferation and hypertrophy in vivo. Msx2, a homeodomain-containing protein that regulates cellular development in many tissues, including bone, teeth and neurons, has been shown to activate Ihh transcription in vitro (Amano et al., 2008); however, whether Msx2 is a transcriptional regulator of Ihh in vivo remains to be determined.
Atf4 is a leucine-zipper-containing transcription factor of the CREB family (Shaywitz and Greenberg, 1999). Using biochemical and genetic approaches, we have found that inactivation of Atf4 in mice results in severe osteopenia, which is caused by a failure of Atf4−/− osteoblasts to achieve terminal differentiation and to synthesize type I collagen, the main constituent of bone matrix (Yang et al., 2004). In addition, Atf4−/− mice display dwarfism, suggesting a role of Atf4 in development of the growth plate chondrocyte. In this study, we identified Atf4 as a direct transcriptional activator of Ihh and thus a regulator of chondrocyte proliferation and differentiation.
MATERIALS AND METHODS
Animals
Wild-type (WT) and Atf4−/− embryos and mice were obtained by crossing Atf4+/− mice. Zero dpc (E0) was defined by the morning the vaginal plug was found. Atf4 genotyping was performed by PCR using tail DNA (Masuoka and Townes, 2002). For each genotype, at least three embryos or mice were analyzed.
Atf4 expression
Primary chondrocytes were isolated by sequential digestion of rib cage cartilage from E14 embryos with collagenase D. Nuclear extracts isolated from the indicated sources were subjected to western blot analysis using an antibody against Atf4 (N127) (Yang and Karsenty, 2004). Immunohistochemistry was performed on paraffin-embedded sections (5 μm) of WT and Atf4−/− humeri. After deparaffinization and rehydration, antigens were retrieved by heating at 100°C for 10 minutes in Tris/EDTA buffer (pH 9.0) and immunostained with antibody N127. Sections were counterstained with Hematoxylin.
Skeletal preparation and histology
Skeletal preparation was according to standard protocols. For histology, embryos and P0 mice were fixed in 4% paraformaldehyde (PFA), embedded in Paraplast, and sectioned at 5 μm. Slides were stained with Hematoxylin for nuclei, Alcian Blue for cartilage matrix, and Alizarin Red for bone matrix.
Microcomputed tomography (μCT) analysis
WT and Atf4−/− tibiae were collected and fixed overnight in 4% PFA (pH 7.4) and then 70% ethanol. Samples were scanned using a μCT system (Scanco μCT 40; Bassersdorf, Switzerland). Tomographic cross-sectional images of the proximal tibia were acquired at 55 kV and 145 mA, at an isotropic voxel size of 12 μm and an integration time of 250 milliseconds. Contours were fitted to the outer perimeter of the tibia beginning immediately distal to the growth plate and extending 1.2 mm distally using the auto-contouring feature in the Scanco Software with the threshold of 300 mg hydroxyapatite/cm3.
In vivo proliferation assay
For embryo limbs, pregnant mice received intraperitoneal injections of 0.1 mg 5-bromo-2′-deoxyuridine (BrdU)/g body weight and were sacrificed 2 hours later. For P0 pups, BrdU (0.1 mg/g body weight) was injected under the skin on the back of the neck 2 hours prior to sacrifice. In organ cultures, BrdU was added 1 hour prior to sample harvesting. Limbs were dissected and fixed in 4% PFA overnight at 4°C. After embedding and sectioning, BrdU was detected with a BrdU Staining Kit (Zymed Laboratories) following the manufacturer's procedure.
TUNEL assay
Apoptotic cells in the growth plate of WT and Atf4−/− humeri were detected by in situ terminal deoxynucleotidyltransferase deoxyuridine triphosphate nick end labeling (TUNEL) assay using the In Situ Cell Death Detection Kit (Roche) following the manufacturer's instructions.
In situ hybridization
Alternate sections used for histological analysis were in situ hybridized for chondrocytic marker genes. Probes for type II collagen (Col2a1), Ihh and type X collagen Col10a1 were as described previously (Ducy et al., 1997; Takeda et al., 2001). The probe for PPR was from Dr T. J. Martin (University of Melbourne, Australia). The probe for Gli1 was a mouse cDNA fragment (bp 968 to 1437), which was generated by RT-PCR using primers: forward, 5′-GAAGGAATTCGTGTGCCATT-3′ and reverse, 5′-TCCAAGCTGGACAAGTCCTC-3′. Antisense cDNAs were used for riboprobe synthesis with RNA polymerases (Invitrogen) and [35S]uridine triphosphate (Perkin Elmer).
Establishment of Atf4-overexpressing chondrocytes
TMC23 chondrocytic cells (Xu et al., 1998) at 90% confluence were transfected with 50 ng Atf4 or Runx2 expression plasmid (pcDNA3.1-Atf4 or pcDNA3.1-Runx2) using Lipofectamine (Invitrogen). When cells reached 100% confluence, they were trypsinized and replated in αMEM containing G418 (400 μg/ml). G418-resistant clones were selected and maintained in G418-containing αMEM.
Electrophoretic mobility shift assay (EMSA)
Oligonucleotides of OSE1 in the osteocalcin gene 2 (Bglap2) promoter and of OSE1-like sequences (A1 to A9) in the Ihh promoter were synthesized. Annealed double-stranded oligonucleotides were labeled with [32P]dCTP and [32P]ATP and used as probes in EMSA, which was performed as described previously (Ducy and Karsenty, 1995; Schinke and Karsenty, 1999) using purified recombinant Atf4 or nuclear extracts of primary chondrocytes.
Northern hybridization and real-time quantitative RT-PCR (qRT-PCR)
Total RNA was isolated using TRIzol (Invitrogen) and 10 μg from each sample was resolved in a 1% agarose gel, transferred onto nylon membrane, and hybridized with Ihh, PTHrP or Gapdh cDNA probes following standard protocols. qRT-PCR was performed using a standard TaqMan PCR Kit protocol on an Applied Biosystems 7300 machine. After treatment with DNase I, total RNA (2 μg) was reverse-transcribed with reverse transcriptase (Invitrogen) using 100 μM random hexamer primers. Specific oligonucleotide primers were from Applied Biosystems (Ihh, Mm00439613 m1; PTHrP, Mm00436056_g1; Gli1, Mm00494645_m1; PPR, Mm00441046_m1).
Construction of an Ihh luciferase reporter construct and mutagenesis
A 4.5 kb Ihh promoter fragment isolated from a bacterial artificial chromosomal clone, RP24-317G11 (Children's Hospital of Oakland Research Institute, Oakland, CA, USA) was inserted to a luciferase (Luc)-containing reporter vector. A construct containing a 1.3 kb Ihh promoter fragment was generated by removal of the 5′ end of the 4.5 kb fragment by restriction digestion. Constructs containing 742 bp and 715 bp Ihh promoter fragments were cloned by PCR using the High Fidelity PCR System (Roche) or Pfu DNA polymerase (Stratagene) with the following primer sequences: for the 742 bp fragment, forward 5′-CTGAGAAAGGGAATGTTGCC-3′ and reverse 5′-GCGTGCTGTCCCCCTCGGCG-3′; for the 707 bp fragment, forward 5′-AACTCGAGCACCAGGTTATGAATGACCT-3′ and reverse 5′-GCGTGCTGTCCCCCTCGGCG-3′.
Multimers (five copies) of the WT or mutated A9 oligonucleotides were made by ligation of BglII- and BamHI-linked double-stranded oligos and digestion with BglII and BamHI. For reporter constructs, ligated products were inserted (SmaI site) upstream of the TATA box (a 16 bp sequence) from the mouse osteocalcin gene 2 promoter (OG2-TATA box), which is followed by the Luc gene. All inserts were confirmed by DNA sequencing.
DNA transfection assay and mutagenesis
COS1 cells were plated at 5×104 cells/well in 24-well plates. After 18 hours, the cells were transfected with Lipofectamine. Each transfection contained per well, 0.25 μg of Ihh-Luc, 0.25 μg of Atf4 and 0.025 μg of β-galactosidase (β-gal) plasmids. Luc and β-gal assays were performed 24 hours post-transfection. Data presented are ratios of Luc/β-gal activity from at least three different experiments and each experiment was performed in triplicate for each DNA sample.
Organ culture and purmorphamine treatment
E14 limbs were freed of skin and muscles and cultured in BGJ-B medium with antibiotic/antimycotic (Life Technologies) and 0.5% BSA in a 24-well cell culture plate (Minina et al., 2001) for 4 days at 37°C in 5% CO2 and humidified atmosphere. Right limbs of each embryo were supplemented with 10 μM purmorphamine (Calbiochem) and left limbs of the same embryo were cultured with DMSO as a control. The experiments were repeated three times. Limb explants were photographed before and after culture and then fixed and embedded in paraffin and sectioned at 5 μm for histological and in situ analyses.
RESULTS
Atf4 is expressed in chondrocytes
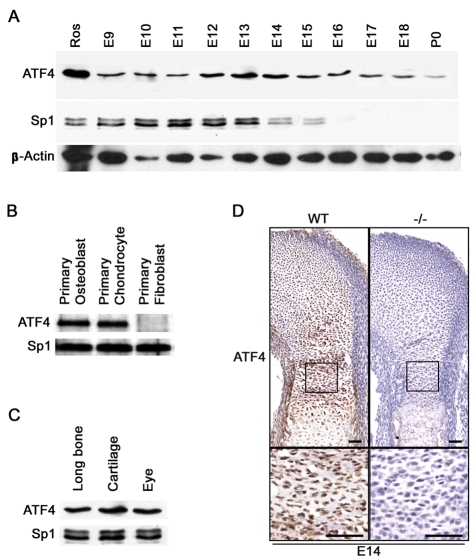
To address the function of Atf4 during chondrogenesis, we first analyzed its expression pattern. In whole embryo nuclear extracts, Atf4 protein was detected from embryonic day (E) 9 (9 dpc) to E11. Atf4 was expressed at high levels from E12 to E14 and at low levels from E15 to birth in nuclear extracts of isolated limbs (Fig. 1A). To further confirm that Atf4 was expressed in chondrocytes, nuclear extracts of primary chondrocytes isolated from pup ribs at post-natal day (P) 3 were examined. Atf4 was present at the same level as in primary osteoblasts isolated from P3 pup calvariae. Atf4 was not detected in primary fibroblasts of the same developmental stage (Fig. 1B). These results, together with the fact that Atf4 is not detectable in any other adult tissues except bone (Yang and Karsenty, 2004), eye and cartilage (Fig. 1C), suggest that Atf4 expression is broad in embryos during early development but becomes more restricted to skeletal tissues from E14 onward. Immunohistochemistry revealed that Atf4 was present in all growth plate chondrocytes, with high levels of expression in perichondrium, proliferative and prehypertrophic chondrocytes (Fig. 1D and see Fig. S1A in the supplementary material). Therefore, the expression pattern of Atf4 is consistent with a role in the regulation of chondrocyte biology.
Fig. 1.
Atf4 is expressed in chondrocytes. (A) Western blot of nuclear extracts of mouse embryos (E9-11) and limbs (E12-P0) for Atf4. Sp1 and β-actin were used as a loading control. (B) Western blot of nuclear extracts from the indicated primary cells. (C) Western blot of nuclear extracts from the indicated tissues. (D) Immunohistochemistry of growth plate sections of E14 wild-type (WT) and mutant (Atf4−/−) humeri. The boxed regions are magnified beneath. Scale bars: 50 μm.
Dwarfism in Atf4−/− mice
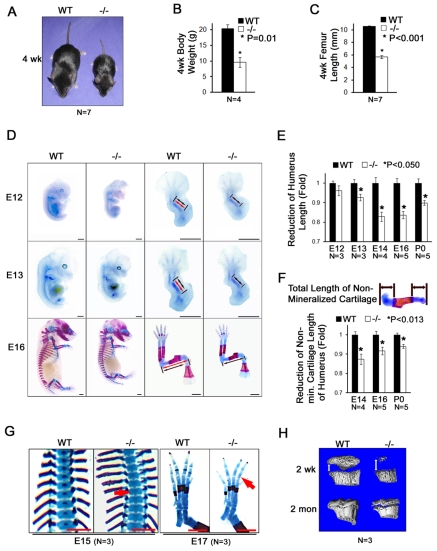
In analyzing Atf4−/− mouse phenotypes we noticed a striking reduction in body size compared with their wild-type (WT) littermates (Fig. 2A). Quantification revealed a 50% reduction in body weight (9.53±1.55 g versus 20.25±1.33 g, P=0.014 by paired Student's t-test, n=4) and in femoral bone length (5.66±0.23 mm versus 10.55±0.08 mm, P=3×10−7, n=7) in 1-month-old mice (Fig. 2B,C), indicating severe limb dwarfism. To determine the onset of this phenotype, we examined embryos stained with Alizarin Red and Alcian Blue and measured their sizes from E9 to E18. The gross sizes of WT and Atf4−/− embryos were indistinguishable from E9 to E11 (data not shown), indicating that deletion of Atf4 did not cause a general reduction in body size prior to the developmental stages during which the first bone elements are shaped. However, Atf4−/− embryos appeared smaller than WT littermates at E12, an early stage of chondrogenesis (Fig. 2D), and the small stature was persistent with an increasing penetrance from 55.6% at E12 and 85.6% at E14 to 100% at E16 and birth (Table 1).
Fig. 2.
Atf4−/− embryos and mice exhibit dwarfism. (A) One-month-old WT and Atf4−/− mice. (B) Quantification of body weight of 1-month-old WT and Atf4−/− mice. Error bars indicate s.e.m.; n=4 mice of each genotype. P=0.01 by paired Student's t-test. (C) Quantification of femur length of 1-month-old WT and Atf4−/− mice. (D) Alizarin Red S and Alcian Blue stained skeletons of embryos and limbs at E12 (12 dpc), E13 and E16. Red and black double-headed arrows represent the length of WT and Atf4−/− humeri, respectively. (E) Quantification of humerus length during development for WT and Atf4−/− mice. Error bars indicate s.e.m. of Atf4−/− humerus length normalized to WT humerus length. (F) Quantification of non-mineralized cartilage length (top panel, double-headed arrows) in WT and Atf4−/− humerus during development. Error bars indicate s.e.m. of Atf4−/− normalized to WT humerus non-hypertrophic zone length. (G) Alizarin Red S and Alcian Blue stained skeletons of embryos showing a delay in the formation of the primary ossification center in E15 Atf4−/− thoracic vertebrae (left, arrow) and E17 hindlimb digits (right, arrow). (H) Microtomographic image showing a delay in formation of the secondary ossification center in Atf4−/− mice (as indicated by the double-headed arrow). Scale bars: 1 mm.
Table 1.
Penetrance of Atf4−/− dwarfism
| Developmental stage (dpc) | ||||||
| Atf4 genotype | 12 | 14 | 16 | Newborn | Total (%) | |
| +/+ | 17 | 7 | 10 | 14 | 48 (25.8) | |
| +/− | 30 | 27 | 10 | 34 | 101 (54.3) | |
| −/− | 9 | 7 | 9 | 12 | 37 (19.9) | |
| Dwarfism in −/− | 5 | 6 | 9 | 12 | ||
| Penetrance (%) | 55.6 | 85.7 | 100 | 100 | ||
The number of embryos/pups assayed at each developmental stage is indicated, together with the number of Atf4−/− that show dwarfism.
Given the severe decrease in limb length at adulthood (Fig. 2C), we focused our analysis on the long bones. At E12, the initial cartilaginous primordia of Atf4−/− humeri (black double-headed arrows in Fig. 2D) were smaller compared with WT littermates (red double-headed arrows in Fig. 2D), although the difference was not statistically significant (Table 2). From E13 to birth, the length of Atf4−/− humeri decreased by 7% at E13, 17% at E14, 16% at E16 and 10% at birth; this decrease was small but reproducible (Fig. 2D,E and Table 2). To assess the contribution of abnormal Atf4−/− chondrocyte function to the short limb phenotype, the length of non-mineralized cartilage in the humerus was measured. A small but significant decrease of 13% at E14, 7% at E16 and 6% at birth was found (Fig. 2F and Table 2). Together, these results suggest that Atf4 is required for chondrocyte function during skeletal development, but that the early condensation of mesenchymal cells to form the anlagen of the future bones and their differentiation into chondrocytes are independent of Atf4.
Table 2.
Affect of Atf4 mutation on total and non-mineralized cartilage lengths of humerus
| Length (mm) | ||||
| Stage | n | WT | Atf4−/− | P-value |
| Total humerus | ||||
| E12 | 4 | 0.645±0.006 | 0.620±0.015 | 0.095 |
| E13 | 4 | 0.743±0.011 | 0.688±0.023 | 0.050* |
| E14 | 4 | 1.765±0.054 | 1.463±0.041 | 0.023* |
| E16 | 5 | 3.186±0.078 | 2.664±0.059 | 0.000079** |
| P0 | 5 | 4.164±0.092 | 3.744±0.052 | 0.002** |
| Non-mineralized humerus | ||||
| E14 | 4 | 1.307±0.030 | 1.142±0.048 | 0.013* |
| E16 | 5 | 1.756±0.061 | 1.629±0.058 | 0.012** |
| P0 | 5 | 1.515±0.035 | 1.424±0.042 | 0.003** |
Length is shown as mean ± s.e.
P-value by Student's t-test. *Significant; **strongly significant.
Delayed hypertrophic mineralization in Atf4−/− growth plates
Upon further detailed analysis of skeletal preparations, we found that the mineralization of chondrocytes was also affected by Atf4 ablation. The first signs of mineralization, which appeared as a single focus of Alizarin Red staining in the center of cartilaginous elements, were detected at E14 in every skeletal element of WT hindlimbs, except for digit bones. However, this staining was only detected in the femur and tibia, but not ilium and fibula, of Atf4−/− embryos at this stage (data not shown). Extensive mineralization was detected in all long bones by E15 in WT embryos. However, at this stage, and even at E16 and E17, mineralization in Atf4−/− embryos remained absent in some vertebrae (Fig. 2G) and digits (Fig. 2G and see Fig. S1B in the supplementary material). In the post-natal growth plate, 3D-computed microtomographic analysis revealed the presence of a larger gap formed by non-mineralized chondrocyte matrix in Atf4−/− tibiae, as compared with WT littermates, at 2 weeks and 2 months of age (Fig. 2H). Together, these results demonstrate that deletion of Atf4 causes a general delay in growth plate development and subsequent ossification. Therefore, Atf4 is indispensable for chondrocyte hypertrophic mineralization during endochondral ossification.
Proliferative chondrocyte disorganization and delayed hypertrophy in Atf4−/− growth plates
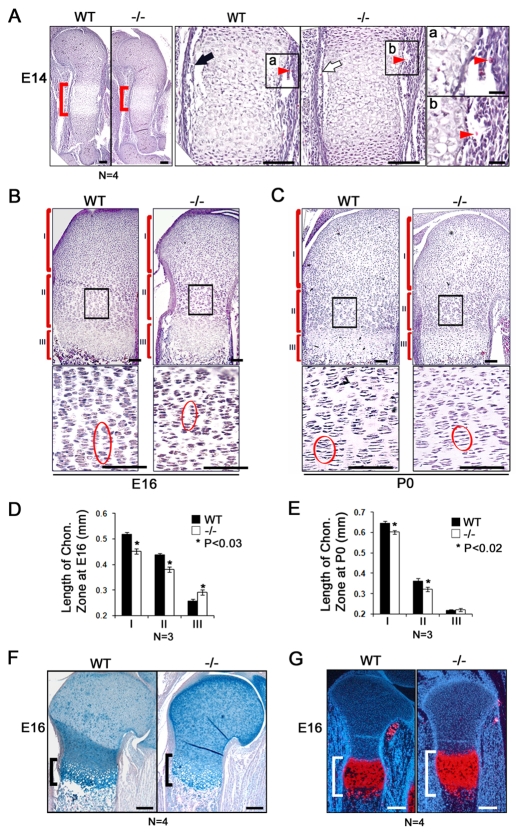
To further understand the cause of the Atf4−/− mutant phenotypes, we performed histological analyses on developing long bones of the forelimb. At E14, the hypertrophic zone in the center of the WT humerus shaft contained enlarged cells, which were surrounded by invading vasculature and a newly formed cortical bone collar in the perichondrium. The hypertrophic zone in Atf4−/− humeri was shorter (Fig. 3A, brackets), suggesting a delay in hypertrophy. In the Atf4−/− perichondrium, the cortical bone collar started to form (Fig. 3A, black arrows) and vascular invasion occurred (Fig. 3Aa,b, arrowheads), despite the reduction in the number of enlarged hypertrophic chondrocytes. These data demonstrate that Atf4 is not required for the onset of angiogenesis that initiates osteogenesis and bone collar formation, which is consistent with our previous observations (Yang et al., 2004). Furthermore, at E16, compared with the columnar pattern formed by the long and organized stacks of actively proliferating chondrocytes in the WT, the stacks were short and disorganized in Atf4−/− growth plates (Fig. 3B, bottom panels). At birth, columnar chondrocyte stacks were visible but remained disorganized in Atf4−/− growth plates (Fig. 3C, bottom panels). The length of the reserve zones was reduced by 13.5% at E16 and 8.3% at birth in Atf4−/− growth plates, and the length of the proliferative zones was decreased by 12% at E16 and 11% at birth compared with WT controls (Fig. 3D,E). Unexpectedly, despite the overall shortening and the delay in appearance of hypertrophic mineralization in the growth plate of developing Atf4−/− long bones, the length of the hypertrophic zone was increased by 12% at E16 and remained slightly extended at birth (Fig. 3B-F), which was confirmed by expanded zones of Col10a1-expressing chondrocytes (Fig. 3G). Collectively, these results demonstrate that Atf4 is required to induce timely hypertrophy at an early stage (i.e. E14), but to inhibit premature hypertrophy at later stages (i.e. E16 and P0) during skeletal development.
Fig. 3.
Decreased proliferation zone and expanded hypertrophic zone of Atf4−/− growth plate chondrocytes. (A) Hematoxylin and Eosin (H&E) staining of sections through E14 WT and Atf4−/− mouse humerus. The center of the humeri (red brackets) are magnified in the middle pair of panels. The black arrow indicates newly formed cortical bone in the WT humerus, which is much thinner in the Atf4−/− humerus (white arrow). (a,b) Higher magnification of the boxed regions of the humerus center showing vascular invasion (arrowheads) in WT and Atf4−/− humeri. (B,C) H&E staining of E16 and P0 WT and Atf4−/− humerus sections. Reserve (I), proliferative (II) and hypertrophic (III) chondrocyte zones are indicated. Boxed regions showing proliferating chondrocytes are magnified beneath. The pattern of well-aligned columnar chondrocytes in the E16 WT proliferating zone is completely disorganized in the Atf4−/− growth plate (circled). (D,E) Quantification of the length of the different chondrocyte zones. Error bars indicate s.e.m. In E, P=0.1 for III. Statistical analysis was performed by paired Student's t-test. (F) Alizarin Red and Alcian Blue staining of growth plates of the radius showing that the hypertrophic zone (bracket) in Atf4−/− growth plates is expanded compared with its WT littermates at E16. (G) In situ hybridization showing that the zone of Col10a1-expressing chondrocytes (red) is increased compared with WT control. Scale bars: 0.1 mm in A; 0.02 mm in Aa,b; 0.2 mm in F,G.
Reduced chondrocyte proliferation in Atf4−/− long bones
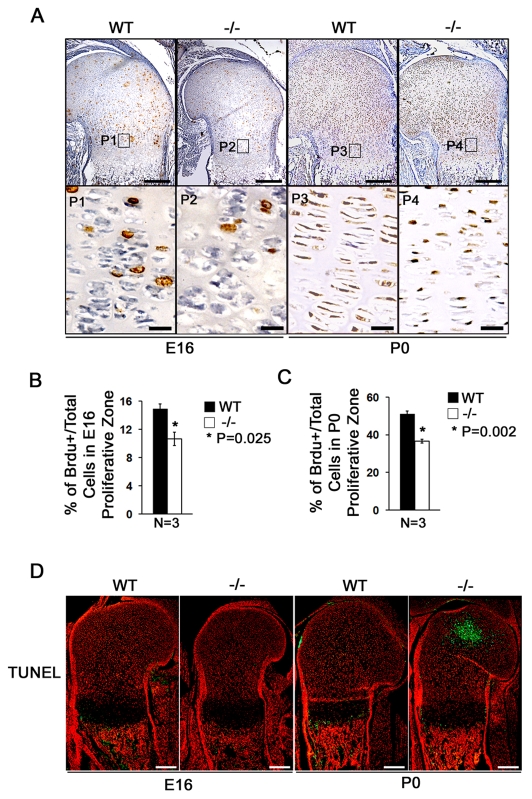
Before hypertrophy, longitudinal cartilage growth relies on fast division of proliferative chondrocytes and slow division of reserve chondrocytes. After hypertrophy and subsequent ossification, longitudinal skeletal growth is dependent on proliferation of both chondrocytes within the growth plate and osteoblasts within the bone (St-Jacques et al., 1999). The decrease in the reserve and proliferative chondrocyte zone length in Atf4−/− long bones led us to hypothesize that Atf4 might be a regulator of chondrocyte proliferation. To address this, we performed in vivo BrdU incorporation assays and found that the chondrocyte proliferative index was significantly decreased in proliferating chondrocytes in Atf4−/− E16 embryos and P0 pups (Fig. 4A-C and see Fig. S1C in the supplementary material), in agreement with the observed reduction in proliferative zone size (Figs 2,3). Therefore, Atf4 is required to maintain the high rate of chondrocyte proliferation in the rapidly growing long bones. In addition, in situ TUNEL assay revealed that there was no difference in the number of apoptotic cells between WT and mutant samples from E16 humeri, although there was an increased number of apoptotic hypertrophic chondrocytes in the center of the reserve zone in P0 Atf4−/− cartilage (Fig. 4D, green). This result rules out any contribution by delayed apoptosis to the hypertrophic expansion in E16 and P0 Atf4−/− growth plates.
Fig. 4.
Atf4 is required for chondrocyte proliferation. (A) BrdU immunohistochemistry of E16 and P0 WT and Atf4−/− mouse humerus sections. Boxed regions (P1-P4) are magnified beneath, showing that there are fewer BrdU-positive (brown) proliferative chondrocytes in Atf4−/− growth plates in E16 embryos and P0 pups. (B,C) Quantification of proliferation rate in proliferating chondrocytes, represented by the ratio of BrdU-positive cells normalized to total cells in WT and Atf4−/− E16 (B) and P0 (C) humeri. Error bars indicate s.e.m. (D) TUNEL assay. In E16 WT and Atf4−/− humerus, no apoptotic cells are present in the cartilage, but some TUNEL-positive cells (green) appear in the primary spongiosa. At P0, there are abundant apoptotic cells in the secondary ossification center of the Atf4−/− humerus, but not in the WT. There are also some TUNEL-positive cells in primary spongiosa and hypertrophic chondrocyte zones in both WT and Atf4−/− growth plate. n=5. Scale bars: 0.2 mm in A,D; 0.02 mm in P1-P4 of A.
Atf4 is required for Ihh expression in chondrocytes
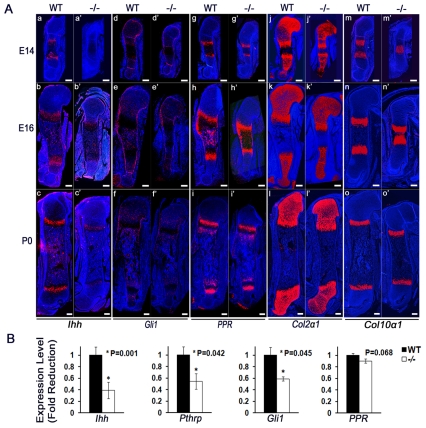
To understand the molecular mechanisms that mediate Atf4 function, and particularly to identify potential Atf4 targets, we performed in situ hybridization using chondrocyte markers. At E14, Ihh was expressed in prehypertrophic chondrocytes and its expression level increased at E16 and P0 in WT humeri (Fig. 5Aa-c). At E16, a low level of Ihh expression was found in osteoblasts in the center of WT long bones. By contrast, Ihh expression was not detectable at E14 and barely detectable at E16 in Atf4−/− humeri. Although detectable in Atf4−/− humeri at P0, the Ihh expression level was substantially lower than that in WT counterparts (Fig. 5Aa′-c′). These results indicate that Atf4 deletion leads to a delay in the onset of, and a decrease in, Ihh expression.
Fig. 5.
Ihh expression is decreased in Atf4−/− cartilage. (Aa-o′) In situ hybridization of sections of E14, E16 and P0 WT and Atf4−/− mouse humeri. Note the decrease in Ihh and Gli1, but normal PPR (Pth1r), expression in Atf4−/− growth plates. Although the Col2a1-positive zones are shorter and the Col10a1-positive zones are slightly longer than their WT counterparts at every stage examined, the expression of Col2a1 and Col10a1 was unchanged in Atf4−/− growth plates. Scale bars: 0.2 mm. (B) qRT-PCR analysis showing decreased levels of Ihh, PTHrP (Pthlh) and Gli1 and normal levels of PPR mRNA in E14 Atf4−/− cartilage. Data are normalized to expression levels in WT cartilage and 18S rRNA (n=3).
Consistently, the expression of transcription factor Gli1, a target and effector of Hh signaling, was decreased in perichondrium and proliferative chondrocytes in Atf4−/− humeri at all stages examined (Fig. 5Ad-f′). In E14 WT and Atf4−/− humeri, similar expression levels of PTH/PTHrP receptor (PPR; Pth1r — Mouse Genome Informatics) were detected in prehypertrophic chondrocytes, the same domain in which Ihh was expressed (Fig. 5Ag,g′). Interestingly, PPR expression transiently expanded to cells of the periosteum and to osteoblasts at E16, yet diminished in osteoblasts at birth in both WT and Atf4−/− humeri (Fig. 5Ah-i′).
There was no difference in Col2a1 expression between WT and Atf4−/− humeri, although the zone of Col2a1-expressing chondrocytes was shorter in Atf4−/− bones (Fig. 5Aj-l′). At E14 in WT humeri, Col10a1-expressing hypertrophic chondrocytes had already separated into two distinct zones, whereas they appeared to be one single mass at the center in Atf4−/− humeri (Fig. 5Am,m′). There were no differences in the Col10a1 expression level between WT and mutant humeri at any of the stages examined (Fig. 5Am-o′). However, at E16, mutant Col10a1-expressing hypertrophic chondrocyte zones were slightly expanded compared with their WT counterparts (Fig. 5An,n′). At birth, Col10a1-expressing hypertrophic chondrocyte zones remained unchanged, or were expanded, if one takes into account the overall length of the humeri (Fig. 5Al,l′). The pattern of Col10a1 expression was consistent with the delay in long-bone mineralization and with the transient expansion of the hypertrophic zone at E16 (Figs 2,3).
Since the level of Ihh transcripts, as well as that of its downstream target gene Gli1, appeared to be reduced in the absence of Atf4, as judged by in situ hybridization, we further quantified the expression of these genes. qRT-PCR results confirmed a 60% decrease in Ihh expression in E14 Atf4−/− cartilage. Consistently, we observed a decrease in the expression of PTHrP (45%) and Gli1 (41%), two downstream targets of the Hh signaling (Fig. 5B). Furthermore, and consistent with the in situ hybridization results, the expression of PPR was normal in E14 Atf4−/− cartilage. Taken together, these data strongly suggest that Atf4 is specifically required for Ihh expression in chondrocytes in vivo and that the decrease in Ihh expression is not a generalized consequence of Atf4 deficiency.
Atf4 directly regulates Ihh transcription
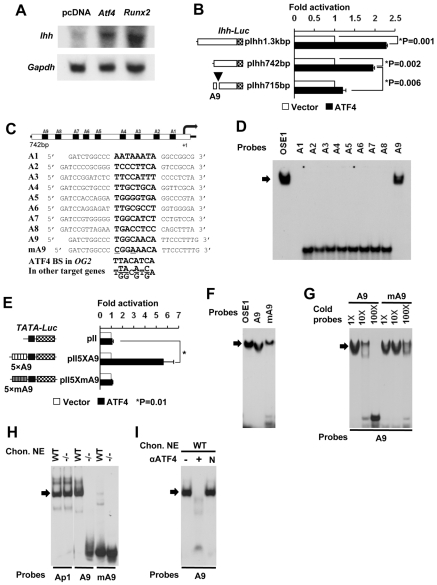
To test whether Ihh is a direct transcriptional target of Atf4, we examined whether overexpression of Atf4 could affect endogenous Ihh mRNA levels in TMC23 chondrocytes (Xu et al., 1998). Atf4 overexpression induced endogenous Ihh expression to an extent similar to that induced by Runx2 overexpression (Fig. 6A). To address whether this function of Atf4 was direct, we co-transfected Ihh reporter constructs (pIhh-1.3-Luc and pIhh-742-Luc) individually with an Atf4 expression plasmid into COS1 cells. Both reporter constructs were transactivated by Atf4 to a similar extent (Fig. 6B), suggesting that the 742 bp promoter fragment of Ihh might contain an Atf4 binding site(s).
Fig. 6.
Atf4 binds to the Ihh promoter to activate transcription. (A) Northern blot analysis showing that Atf4 and Runx2 stimulate endogenous Ihh mRNA expression in TMC23 cells. Gapdh serves as a loading control. (B) Atf4 activates Ihh promoter reporters in COS1 cells. Co-transfection assay showing that Atf4 activates Ihh promoter reporters (pIhh-1.3kbp and pIhh-742bp, as shown to left) but not the construct containing a mutated A9 (arrowhead), the Atf4 binding site. (C) Sequences of nine putative Atf4 binding sites (A1-A9) in the proximate 742 bp promoter region of the mouse Ihh gene. Core sequences are indicated in bold, being identical to those within the Atf4 consensus sequence [i.e. TTACATCA, OSE1 in osteocalcin (OG2) and T(T/G)(A/G)C(A/G)T(C/G)A in other Atf4 target genes]. (D) Atf4 binds to A9 in the Ihh promoter. EMSA using the nine 32P-labeled putative Atf4 binding sites, A1-A9, as probes with purified His-tagged Atf4 recombinant protein. OSE1 serves as a positive control. Arrow, Aft4-probe complex. (E) A9 mediates Atf4 transactivation of Ihh. p5XA9-Luc and p5XmA9-Luc contain five copies of WT and mutant A9 [which binds Atf4 only weakly (see F,G)], respectively, linked to a TATA-box vector. (F) EMSA using 32P-labeled probes at equal counts per minute of OSE1, A9 and A9 mutant (A9mut). Note the large amount of unbound A9mut probe at the bottom of the gel. (G) Competition EMSAs. Fold molar excess of unlabeled double-stranded DNA competitor over labeled probe is indicated. (H) Endogenous Atf4 binds to A9. The Ap1 probe, the cJun/cFos binding site, was used as a control for nuclear extract quality. (I) Supershift EMSAs showing that an antibody against Atf4 inhibits the binding of endogenous Atf4 to A9 (lane +). N, unrelated antibody.
We examined the 742 bp promoter fragment of Ihh and located nine putative Atf4 binding sites, named A1 to A9 (Fig. 6C). EMSA revealed that Atf4 bound strongly to A9, but not to the other eight probes (Fig. 6D). Consistently, Atf4 failed to transactivate luciferase activity on pIhh-715-Luc, a construct in which A9 is deleted (Fig. 6B). Furthermore, Atf4 transactivated a reporter containing five repeats of A9 (pII5xA9-Luc) more than 5-fold (Fig. 6E), yet did not activate a reporter containing five copies of a mutant A9 sequence (PII5xmA9-Luc) that binds Atf4 with ten times lower affinity (Fig. 6F,G).
EMSA revealed that endogenous Atf4 from WT, but not Atf4−/−, primary chondrocytes bound to A9 (Fig. 6H). In addition, Atf4 antibody inhibited protein-A9 complex formation, whereas an unrelated control antibody had no effect (Fig. 6I). Taken together, these results confirm that the A9 sequence of the Ihh promoter serves as an Atf4 binding site, mediating Atf4 transactivation of Ihh transcription.
An Ihh agonist partially restores the length of Atf4−/− limbs
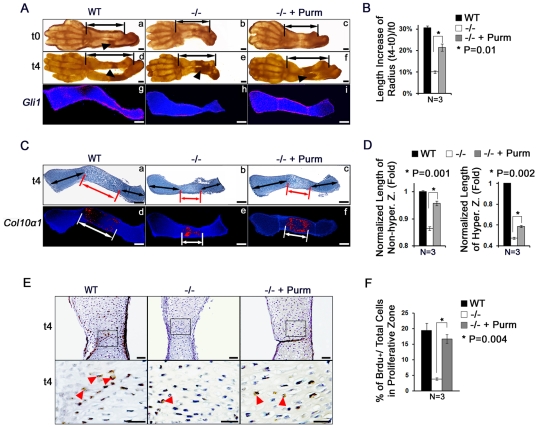
Since Col2a1-Ihh transgenic mice were not viable, we designed a rescue experiment based on an organ culture system. Limb explants from WT and Atf4−/− E14 embryos were cultured in the presence or absence of purmorphamine, a synthetic compound that directly targets smoothened to activate Hh signaling (Sinha and Chen, 2006). At the beginning of the culture (t0), the primary ossification center was formed in WT ulna, but not in WT radii. As expected, no visible ossification center was present in mutant long bones at this stage (Fig. 7A, arrowheads). After 4 days of growth (t4), ossification centers were observed in both the ulna and radii in both genotypes (Fig. 7A, arrowheads), indicating a normal sequence of limb development in this organ culture system.
Fig. 7.
Reactivation of Hh signaling by purmorphamine rescues limb defects in Atf4−/− embryos. (A) Purmorphamine, an agonist of the Hh signaling pathway, restores the length of the Atf4−/− forelimb in organ cultures. (a-f) Limb explants at the beginning (t0, a-c) and the end (4 days, t4, d-f) of culture. n=3. Purmorphamine partially rescued the longitudinal growth of the Atf4−/− radius (double-headed arrow, f) as compared with the control (e). Arrowheads indicate BrdU-positive cells. (g-i) In situ hybridization of Gli1 expression in WT (g), vehicle-treated Atf4−/− (h), and purmorphamine-treated Atf4−/− (i) radii. n=3. Purmorphamine restored Gli1 expression in Atf4−/− radii (i) to a level similar to that in WT controls (h), indicating activation of Hh signaling in Atf4−/− explants. (B) Quantification of the increase in radius length upon purmorphamine treatment of WT and Atf4−/− limbs. The percentage increase in radius growth after 4 days in culture is shown. P=0.01 by paired Student's t-test. (C) Purmorphamine partially corrects the delayed chondrocyte hypertrophy in Atf4−/− growth plates in organ culture. (a-c) Alcian Blue, Alizarin Red and Hematoxylin staining of radius sections of 4-day cultures. (d-f) In situ hybridization for Col10a1 in purmorphamine-treated and vehicle-treated limb explants. n=3. (D) Quantification of the relative length of the non-hypertrophic and hypertrophic zones upon purmorphamine treatment of WT and Atf4−/− radii. Error bars indicate s.e.m. of the length of the non-hypertrophic (left) and hypertrophic (right) zones normalized to the respective WT controls at 4 days in culture. (E) Purmorphamine increases chondrocyte proliferation in Atf4−/− growth plates. Immunohistochemistry of BrdU-labeled chondrocytes in limbs cultured for 4 days in the absence and presence of purmorphamine. The sections were counterstained with Hematoxylin. Boxed regions are magnified beneath to show BrdU-positive cells (arrowheads). n=3. (F) Quantification of proliferation rate represented by the ratio of BrdU-positive cells (brown) to total cells upon purmorphamine treatment of WT and Atf4−/− radii. Error bars indicate s.e.m. Scale bars: 0.2 mm in A,C; 0.5 mm in E.
Hh signaling reactivation upon purmorphamine treatment was confirmed by increased Gli1 expression in purmorphamine-treated Atf4−/− limbs as compared with vehicle-treated samples (Fig. 7Ah,i). Within 4 days of growth, the length of the radii increased by 31% in WT and by 21% in purmorphamine-treated Atf4−/−limbs, whereas it increased only 10% in vehicle-treated mutant limbs (Fig. 7B). These results indicate that reactivation of Hh signaling improves the longitudinal growth of Atf4−/− limbs in culture. The increase in size in purmorphamine-treated Atf4−/− limbs was associated with elongated zones of proliferative and hypertrophic chondrocytes (Fig. 7Ca-c,D). In addition, purmorphamine reduced the delayed hypertrophy in Atf4−/− limbs, as shown by the increase in the Col10a1-expressing chondrocyte area in purmorphamine-treated versus vehicle-treated Atf4−/− radii (Fig. 7Ce,f,D). The BrdU proliferative index in proliferative chondrocytes (Fig. 7E, arrowheads) was significantly increased upon purmorphamine treatment of Atf4−/− limbs (Fig. 7F). These results indicate that activation of the Hh pathway in Atf4−/− limbs can partially rescue the defects in chondrocyte proliferation and hypertrophy characteristic of this mutant, further reinforcing the notion that Atf4 and Ihh lie in the same pathway for the regulation of limb development and long-bone growth.
DISCUSSION
This study reveals the transcription factor Atf4 as a crucial regulator of chondrogenesis and identifies Ihh as a transcriptional target of Atf4 in chondrocytes. Mice lacking Atf4 exhibit dwarfism and are characterized by markedly reduced growth plates, decreased chondrocyte proliferation and an abnormally expanded hypertrophic zone. These phenotypic abnormalities are similar to those of Ihh−/− mice (St-Jacques et al., 1999), which, together with the dramatic decrease in Ihh expression observed in Atf4−/− growth plates, indicate that Atf4 and Ihh lie in the same genetic pathway regulating chondrogenesis during skeletal development.
Distribution of Atf4 in chondrocytes
Atf4 was originally termed osteoblast-specific factor 1 (Osf1) because it was first identified as a specific binding activity of osteoblast nuclear extracts to OSE1, the osteoblast-specific element 1 found in the osteocalcin (Bglap) promoter (Ducy and Karsenty, 1995). Subsequent studies revealed that the cell specificity of Atf4 is regulated at the post-translational level (Yang and Karsenty, 2004). However, nuclear extracts of primary chondrocytes were not tested in previous studies, and the evidence for involvement of Atf4 in chondrogenesis was lacking. In this study, a systematic analysis of the expression pattern of Atf4 demonstrated that it is expressed at high levels in embryos, limbs and primary chondrocytes, supporting its role in chondrogenesis.
Atf4 as a novel transcriptional regulator of Ihh
A major finding of this study is that Atf4 directly regulates chondrocyte proliferation by affecting the transcription of Ihh, a molecule that plays crucial roles in both chondrogenesis and osteogenesis. We demonstrated, for the first time genetically and molecularly, that Atf4 acts as a direct transcriptional activator of Ihh, the expression of which is dramatically decreased in Atf4 mutant mice. Atf4 binds and transactivates Ihh in chondrocytes and forced expression of Atf4 enhances endogenous Ihh mRNA synthesis. In organ cultures, reactivation of Hh signaling by a synthetic compound that bypasses the need for Ihh ligand almost completely rescues the proliferation defects and partially rescues the delay in hypertrophy in Atf4−/− limbs. As a result, the short limb phenotype in Atf4−/− animals was partially corrected. Together, these results strongly suggest that Atf4 and Ihh act in the same pathway to regulate chondrocyte proliferation and hypertrophy. The fact that the hypertrophic defect in Atf4−/− limbs was not fully rescued by purmorphamine might suggest that Atf4 regulates chondrocyte hypertrophy by an additional and Ihh-independent mechanism(s), or, more likely, it might reflect a limitation of the organ culture system.
The functions of Atf4 and Runx2, another transcriptional activator of Ihh, are not redundant as removal of Atf4 or Runx2 gives rise to severe chondrocyte proliferation or differentiation defects, respectively. Although our current study cannot dissect the relative contributions of Runx2 and Atf4 to the control of chondrogenesis, the fact that Runx2−/− animals completely lack hypertrophic chondrocytes in their skeletons (Komori et al., 1997; Otto et al., 1997), and that the role of Runx2 is in the induction, rather than inhibition, of chondrocyte hypertrophy (Takeda et al., 2001; Ueta et al., 2001), suggest that the function of Runx2 in the control of chondrocyte biology could be Ihh-independent and antiproliferative. In fact, a recent study showed that Runx2 inhibits chondrocyte proliferation and hypertrophy through regulation of Fgf18 transcription in the perichondrium (Hinoi et al., 2006). It is unlikely that Atf4 regulates Fgf18 directly because overexpressing Atf4 has no effect on luciferase activity driven by Fgf18 promoter constructs in DNA co-transfection assays (Dr M. Naski, personal communication). Consistently, we observed no differences in the expression of Fgf18 in WT and Atf4−/− cartilage (see Fig. S2A in the supplementary material). Other studies indicate that Runx2 mRNA synthesis can be downregulated by PTH/PTHrP signaling (Guo et al., 2006), yet Atf4 mRNA and protein are upregulated by parathyroid hormone (Yu et al., 2008), supporting the notion that Atf4 and Runx2 play different, but related, roles in the regulation of chondrogenesis.
Atf4 regulates chondrocyte differentiation
The fact that the hypertrophic chondrocyte zone in Atf4−/− long bones is transiently increased at E16 and then remains the same as in WT, and that PTHrP expression is downregulated in Atf4 mutant growth plates, indicate that Atf4 plays a role in the control of hypertrophic chondrocyte differentiation. This provides indirect confirmation that Ihh expression is downregulated in Atf4−/− growth plates. PTHrP and its receptor, which lie downstream of Ihh signaling, play multiple roles in regulating chondrocyte proliferation and differentiation (Kronenberg, 2006). PTHrP acts as an inhibitor to prevent premature chondrocyte hypertrophy (Karaplis et al., 1994), which ensures the maintenance of a pool of proliferative chondrocytes. PTHrP also directly stimulates chondrocyte proliferation in an organ culture system (Mau et al., 2007). However, it is unclear whether Atf4 is a direct transcriptional regulator of PTHrP or not. Our DNA transfection data suggest that the downregulation of PTHrP expression in Atf4−/− chondrocytes might be due to an indirect mechanism, most likely involving impaired Hh signaling, as: (1) Atf4 did not transactivate luciferase constructs driven by 4.5 or 1.1 kb PTHrP promoter fragments, whereas these two reporter constructs responded to Gli2, a known transcriptional activator of PTHrP (Sterling et al., 2006; Zhao, 2005) (see Fig. S2B,C in the supplementary material); and (2) we could not locate any Atf4 binding consensus site within this promoter region.
Type II collagen secretion is normal in Atf4−/− chondrocytes
Given the indispensable role of Atf4 in the regulation of stress responses (Harding et al., 2000; Harding et al., 2003; Rutkowski and Kaufman, 2003) and in type I collagen synthesis in osteoblasts (Yang et al., 2004), Atf4 may be required for secretion of type II collagen in chondrocytes. Our immunohistochemistry results using a monoclonal antibody developed by Dr Thomas F. Linsenmayer (Tufts Medical School, Boston, MA, USA) revealed that type II collagen secretion is normal in Atf4−/− cartilage (see Fig. S2C in the supplementary material). Furthermore, we also observed a normal expression level of hypoxia-inducible factor 1 (see Fig. S2E in the supplementary material), a master regulator of oxygen homeostasis and a key element to cellular survival and adaptation and a regulator of chondrogenesis (Amarilio et al., 2007). These results cannot rule out the possibility that Atf4 plays a role in the response to oxygen level in chondrocytes, given the hypoxic environment in which chondrocytes exist.
This study reveals a novel mechanism by which Atf4 regulates chondrocyte proliferation and differentiation via upregulating Ihh expression. It remains unclear, however, what upstream signals regulate Atf4 expression and activity and what downstream effector molecules of Hh signaling are controlling chondrocyte proliferation and differentiation. Many extracellular regulators, including members of the FGF, BMP, Igf1, Wnt and PTHrP families, are reported to regulate chondrocyte proliferation and differentiation. Whether they act on chondrocytes by modifying Atf4 activity remains to be determined. Lastly, and importantly, it is of interest to establish whether the same mechanisms are responsible for the bone defects seen in the Atf4−/− mice.
Supplementary Material
Acknowledgements
We thank Drs C. Chiang, M. Patel and T. Schinke for critical comments on the manuscript and Drs G. Mundy, M. Naski and J. Sterling for sharing unpublished data. The monoclonal anti-Col II antibody developed by Dr Thomas F. Linsenmayer was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Science, Iowa, IA 52242, USA. This work was funded by grants from the March of Dimes Foundation (to X.Y.) and National Institute of Arthritis and Musculoskeletal and Skin Diseases (to X.Y. and E.F.). Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing commercial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/24/4143/DC1
References
- Albrecht J. H., Poon R. Y., Ahonen C. L., Rieland B. M., Deng C., Crary G. S. (1998). Involvement of p21 and p27 in the regulation of CDK activity and cell cycle progression in the regenerating liver. Oncogene 16, 2141-2150 [DOI] [PubMed] [Google Scholar]
- Alcedo J., Noll M. (1997). Hedgehog and its patched-smoothened receptor complex: a novel signalling mechanism at the cell surface. Biol. Chem. 378, 583-590 [DOI] [PubMed] [Google Scholar]
- Amano K., Ichida F., Sugita A., Hata K., Wada M., Takigawa Y., Nakanishi M., Kogo M., Nishimura R., Yoneda T. (2008). MSX2 stimulates chondrocyte maturation by controlling Ihh expression. J. Biol. Chem. 283, 29513-29521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilio R., Viukov S. V., Sharir A., Eshkar-Oren I., Johnson R. S., Zelzer E. (2007). HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 134, 3917-3928 [DOI] [PubMed] [Google Scholar]
- Chung U. I., Schipani E., McMahon A. P., Kronenberg H. M. (2001). Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J. Clin. Invest. 107, 295-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. F., Yang Y. (2008). Wnt and hedgehog signaling pathways in bone development. J. Bone Joint Surg. Am. 90Suppl. 1, 19-24 [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Vuorio T., Karsenty G., Maity S., Rutheshouser E. C., Goldberg H. (1991). Transcriptional control mechanisms for the expression of type I collagen genes. Ann. Rheum. Dis. 50Suppl. 4, 872-876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P., Karsenty G. (1995). Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 15, 1858-1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P., Zhang R., Geoffroy V., Ridall A. L., Karsenty G. (1997). Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747-754 [DOI] [PubMed] [Google Scholar]
- Guo J., Chung U. I., Yang D., Karsenty G., Bringhurst F. R., Kronenberg H. M. (2006). PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev. Biol. 292, 116-128 [DOI] [PubMed] [Google Scholar]
- Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. (2000). Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099-1108 [DOI] [PubMed] [Google Scholar]
- Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., et al. (2003). An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619-633 [DOI] [PubMed] [Google Scholar]
- Hinoi E., Bialek P., Chen Y. T., Rached M. T., Groner Y., Behringer R. R., Ornitz D. M., Karsenty G. (2006). Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 20, 2937-2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaplis A. C., Luz A., Glowacki J., Bronson R. T., Tybulewicz V. L., Kronenberg H. M., Mulligan R. C. (1994). Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8, 277-289 [DOI] [PubMed] [Google Scholar]
- Karp S. J., Schipani E., St-Jacques B., Hunzelman J., Kronenberg H., McMahon A. P. (2000). Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 127, 543-548 [DOI] [PubMed] [Google Scholar]
- Karsenty G. (2001). Transcriptional control of osteoblast differentiation. Endocrinology 142, 2731-2733 [DOI] [PubMed] [Google Scholar]
- Karsenty G., Wagner E. F. (2002). Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389-406 [DOI] [PubMed] [Google Scholar]
- Kaufman M. H. (1992). The Atlas of Mouse Development London: Academic Press; [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M., et al. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755-764 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332-336 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2006). PTHrP and skeletal development. Ann. N. Y. Acad. Sci. 1068, 1-13 [DOI] [PubMed] [Google Scholar]
- Mackie E. J., Ahmed Y. A., Tatarczuch L., Chen K. S., Mirams M. (2008). Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 40, 46-62 [DOI] [PubMed] [Google Scholar]
- Mak K. K., Kronenberg H. M., Chuang P. T., Mackem S., Yang Y. (2008). Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 135, 1947-1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka H. C., Townes T. M. (2002). Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood 99, 736-745 [DOI] [PubMed] [Google Scholar]
- Mau E., Whetstone H., Yu C., Hopyan S., Wunder J. S., Alman B. A. (2007). PTHrP regulates growth plate chondrocyte differentiation and proliferation in a Gli3 dependent manner utilizing hedgehog ligand dependent and independent mechanisms. Dev. Biol. 305, 28-39 [DOI] [PubMed] [Google Scholar]
- Minina E., Wenzel H. M., Kreschel C., Karp S., Gaffield W., McMahon A. P., Vortkamp A. (2001). BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128, 4523-4534 [DOI] [PubMed] [Google Scholar]
- Nilsson O., Marino R., De Luca F., Phillip M., Baron J. (2005). Endocrine regulation of the growth plate. Horm. Res. 64, 157-165 [DOI] [PubMed] [Google Scholar]
- Ornitz D. M. (2005). FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 16, 205-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto F., Thornell A. P., Crompton T., Denzel A., Gilmour K. C., Rosewell I. R., Stamp G. W., Beddington R. S., Mundlos S., Olsen B. R., et al. (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765-771 [DOI] [PubMed] [Google Scholar]
- Rutkowski D. T., Kaufman R. J. (2003). All roads lead to ATF4. Dev. Cell 4, 442-444 [DOI] [PubMed] [Google Scholar]
- Schinke T., Karsenty G. (1999). Characterization of Osf1, an osteoblast-specific transcription factor binding to a critical cis-acting element in the mouse osteocalcin promoters. J. Biol. Chem. 274, 30182-30189 [DOI] [PubMed] [Google Scholar]
- Shaywitz A. J., Greenberg M. E. (1999). CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 68, 821-861 [DOI] [PubMed] [Google Scholar]
- Sinha S., Chen J. K. (2006). Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol. 2, 29-30 [DOI] [PubMed] [Google Scholar]
- St-Jacques B., Hammerschmidt M., McMahon A. P. (1999). Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13, 2072-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling J. A., Oyajobi B. O., Grubbs B., Padalecki S. S., Munoz S. A., Gupta A., Story B., Zhao M., Mundy G. R. (2006). The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells. Cancer Res. 66, 7548-7553 [DOI] [PubMed] [Google Scholar]
- Takeda S., Bonnamy J. P., Owen M. J., Ducy P., Karsenty G. (2001). Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 15, 467-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta C., Iwamoto M., Kanatani N., Yoshida C., Liu Y., Enomoto-Iwamoto M., Ohmori T., Enomoto H., Nakata K., Takada K., et al. (2001). Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J. Cell Biol. 153, 87-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Ji X., Harris M. A., Mundy G. R., Harris S. E. (1998). A clonal chondrocytic cell line derived from BMP-2/T antigen-expressing transgenic mouse. In Vitro Cell Dev. Biol. Anim. 34, 359-363 [DOI] [PubMed] [Google Scholar]
- Yang X., Karsenty G. (2004). ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J. Biol. Chem. 279, 47109-47114 [DOI] [PubMed] [Google Scholar]
- Yang X., Matsuda K., Bialek P., Jacquot S., Masuoka H. C., Schinke T., Li L., Brancorsini S., Sassone-Corsi P., Townes T. M., et al. (2004). ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 117, 387-398 [DOI] [PubMed] [Google Scholar]
- Yoshida C. A., Yamamoto H., Fujita T., Furuichi T., Ito K., Inoue K., Yamana K., Zanma A., Takada K., Ito Y., et al. (2004). Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 18, 952-963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Franceschi R. T., Luo M., Zhang X., Jiang D., Lai Y., Jiang Y., Zhang J., Xiao G. (2008). Parathyroid hormone increases activating transcription factor 4 expression and activity in osteoblasts: requirement for osteocalcin gene expression. Endocrinology 149, 1960-1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Sterling J. A., Qiao M., Oyajobi B. O., Harris S. E., Mundy G. R. (2005). The Hedgehog signaling molecule Gli2 regulates both BMP2 and PTHrP expression in the growth plate. J. Bone Miner. Res. 20Suppl. 1, S40 [Google Scholar]
- Zuscik M. J., Hilton M. J., Zhang X., Chen D., O'Keefe R. J. (2008). Regulation of chondrogenesis and chondrocyte differentiation by stress. J. Clin. Invest. 118, 429-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.