Abstract
In recent years, a small number of cells that have stem cell properties were identified in human gliomas called brain tumor stem cells (BTSCs), which were thought to mainly contribute to the initiation and development of gliomas and could be identified by the surface marker CD133. However, recent studies indicated that the expression of CD133 might be regulated by environmental conditions such as hypoxia and that there might be CD133- BTSCs. Genetic mouse models demonstrated that some gliomas originated from transformed neural stem cells (NSCs). Therefore, we investigated the expression of CD15, a surface marker for NSCs, in tumor spheres derived from astrocytoma and ependymoma. CD15+ cells isolated from these tumor spheres had properties of BTSCs including self-renewal, multidifferentiation, and the ability to recapitulate the phenocopy of primary tumors. CD15 exhibited stable expression in long-term cultured tumor spheres, which sustained BTSCs properties, whereas CD133 expression decreased significantly in late passages. Furthermore, CD15+CD133- cells isolated from early or late passages of tumor spheres showed similar characteristics of BTSCs. Examination of glioma samples by immunohistochemistry showed that CD15 was expressed in a subset of human brain tumors. Therefore, CD15 can be used as a marker of stem-like cells derived from brain tumors that might contain CD133- BTSCs.
Introduction
The identification of brain tumor stem cells (BTSCs) marks a step toward finding new and effective ways to treat malignant brain tumors, one of the most lethal cancers afflicting both children and adults [1]. The concept of BTSCs has constructive significance for clinical practices because it has been elucidated that BTSCs contribute to relapse and chemoresistance or radioresistance of brain tumors [2–4]. To date,many studies exploring the property of BTSCs built on the assumption that BTSCs express a cell surface marker, CD133 [5,6]. However, it has been indicated that expression of CD133 could be regulated by environmental conditions such as hypoxia [7], and contrary results have been reported that there are CD133- BTSCs [8,9]. The existence of both CD133-positive and -negative BTSCs implies that further characterization of BTSCs is of tremendous interest. This also implies that one persistent challenge is our inability to recognize BTSCs, and many issues about the BTSCs are to be answered. For example, what is the significance of CD133 expression in BTSCs? Are there other markers that can specifically identify BTSCs? What is the relationship between BTSCs and neural stem cells (NSCs)? Is glioma derived from ancestor BTSCs or are BTSCs emerged after the forming of tumors?
The identification of NSCs provided new possible targets of tumorigenic transformation of gliomas [10]. In fact, many evidences support the idea that gliomas are derived from transformed NSCs [11]. Recent studies using genetic mouse models suggested that at least a portion of gliomas were originated from transformed NSCs, which have the characteristics of reported BTSCs and are responsible for the formation of tumors [12,13]. In addition, many functional and molecular similarities have been elucidated between BTSCs and normal NSCs. Both BTSCs and NSCs have immortal proliferative potential in the presence of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) and can differentiate into neuronal and glia lineages when withdrawing these growth factors and adding serum [6,14,15]. Both of them have an environmental niche that is important for their maintenance of stemness [16,17]. Many signaling pathways critical for NSCs are also important for BTSCs, but some of them were aberrantly regulated [4,18]. These facts imply that it would be necessary to study the relationship between NSCs and BTSCs, and importantly, BTSCs might retain some properties of NSCs.
NSCs in adult brains can be recognized by the surface marker CD15 [19]. CD15 (leukocyte cluster of differentiation 15), which is the trisaccharide 3-fucosyl-N-acetyllactosamine or FAL, also known as stage-specific embryonic antigen 1 or leX antigen, is highly expressed in many types of pluripotent stem cells [19]. Recent studies suggested that, besides the traditionally recognized NSCs that reside in the subventricular zone, CD133+/CD24- cells, but not CD24+ cells in the ependyma represent another population of NSCs [20]. Therefore, it is more likely that there are at least two types of NSCs in normal brains identified by CD133 and CD15. However, to our knowledge, although CD133 has been widely studied, the expression of CD15 has not been fully explored in BTSCs. Previous studies focused on the expression of CD15 in gliomas got contradictory results [21–24], and studies on BTSCs showed that glioma-derived neural spheres expressed CD15 [15]. Given the similarity between NSCs and BTSCs and recent disputes about CD133 as a BTSCs marker, we performed this study to further explore the expression of CD15 in BTSCs and to investigate if CD15 can be used to identify a group of BTSCs different from CD133+ cells.
Materials and Methods
Glioma Samples
Seven glioma samples used for cell culture were provided by the Department of Neurosurgery, Xijing Hospital, the Fourth Military Medical University, including one grade 2 oligoastrocytoma, two anaplastic astrocytoma (World Health Organization [WHO] grade 3), three glioblastoma (WHO grade 4), and one ependymoma (WHO grade 2; Table 1). Additional 54 samples were used to investigate CD15 expression in gliomas by Immunohistochemical analysis. Tumors were histopathologically classified according to the WHO classification. Informed consent was obtained from each patient, and experiments were approved by the local ethics committee.
Table 1.
Summary of Patients Information.
| Patients No. | Sex | Age (Years) | Tumor Location | Pathologic Subtype | Tumor Sphere Formation |
| 1 | Female | 32 | Right lateral fissure | Grade 3 astrocytoma | 5 passages |
| 2 | Male | 72 | Right frontoparietal | Grade 3 astrocytoma | >20 passages |
| 3 | Male | 44 | Left parietal | Grade 4/glioblastoma | No |
| 4 | Female | 8 | Fourth ventricle | Grade 2 ependymoma | >10 passages* |
| 5 | Female | 57 | Right frontal | Grade 4/glioblastoma | >20 passages |
| 6 | Male | 38 | Right frontal | Grade 2 oligoastrocytoma | No |
| 7 | Female | 31 | Left parietal | Grade 4/glioblastoma | >10 passages |
Grew as adherent tumor spheres.
Culture of Primary Glioma Spheres
Samples were stored in Dulbecco's modified Eagle's medium (DMEM)-F12 culture medium (Invitrogen, Carlsbad, CA) and processed within 1 hour after resection. Tumor samples were washed by DMEM-F12 medium and incubated for 20 minutes at 37°C in TrypLE (Invitrogen). Then the tumor samples were dissociated to a single-cell suspension using a fire-polished Pasteur pipette. The dissociated tumor cells were centrifuged for 5 minutes at 800g and resuspended in either serum-free medium (SFM) consisting of DMEM-F12 medium, EGF (20 ng/ml; Invitrogen), bFGF (20 ng/ml; Invitrogen), and B27 (1:50; Invitrogen) or serum-containing medium (SCM) consisting of DMEM-F12 medium with 10% fetal bovine serum (Gibco BRL Life Technologies, Rockville, MD).
Limiting Dilution Assay and Primary Sphere Formation Assay
Limiting dilution assay was performed as described previously [25]. Tumor spheres were washed and dissociated to a single-cell suspension as described above. Then dissociated tumor cells were resuspended in DMEM-F12 medium to assess viable cell numbers by Trypan Blue (Sigma, St Louis, MO) exclusion. Acutely dissociated tumor cells were plated in 96-well microwell plates in 0.2 ml of SFM. Final cell dilutions ranged from 200 cells per well to 1 cell per well. Cultures were fed 0.025 ml of SFM every 2 days until day 14. The percentage of wells not containing spheres for each cell plating density were calculated and plotted against the number of cells per well. The number of cells required to form one tumor sphere, which reflected the proportion of tumor stem cells in the cell population, was then determined from the point at which the line crossed the 0.37 level. For primary sphere formation assays, this analysis was performed on the entire acutely dissociated tumor cell population to quantify stem cell frequency within the tumor.
Cell Proliferation Assays
Tumor spheres were dissociated and plated in 96-well microwell plates in 0.2-ml volumes of SFM supplemented with growth factors at a density of 2000 cells per well. Tumor spheres were collected from six wells every 2 days after plating and dissociated to get single cells as mentioned previously to assess the total number of cells.
Differentiation Assay of Tumor Spheres
Tumor spheres were plated onto glass coverslips coated with poly-l-lysine (Sigma) in SCM in individual wells of a 24-well culture plate. Cells were fed with SCM every 2 days, and coverslips were processed at different days after plating.
Immunofluorescence
Immunofluorescence was performed on primary brain tumor stem-like cells, serum-cultured cells, and glioma cell lines as described previously [5]. Briefly, for immunostaining of undifferentiated tumor spheres, cells were plated onto poly-l-lysine-coated glass coverslips in SCM for 4 hours. Cells were then fixed with 4% paraformaldehyde and incubated with the following antibodies: CD133/1 (1:50, mouse monoclonal immunoglobulin G1 [IgG1]; Miltenyi Biotec, Bergisch Gladbach, Germany), CD15 (1:150, MMA, mouse monoclonal; BD Biosciences, San Jose, CA), Nestin (1:100, 10C2, mouse monoclonal; Abcam, Cambridge, MA), β-III-tubulin (1:500, mouse monoclonal IgG1; Abcam), NeuN (1:1000, mouse monoclonal IgG1; Chemicon, Temecula, CA), glial fibrillary acidic protein (GFAP, 1:5000, rabbit polyclonal; Abcam), Galc (1:200, rabbit polyclonal; Chemicon), and Ki67 (1:100, rabbit monoclonal; Abcam). Appropriate secondary antibodies (Texas Red goat antirabbit and Alexa 488 goat antimouse; Molecular Probes, Invitrogen) were used. For immunostaining of differentiated tumor cells, immunofluorescence was performed on differentiated tumor spheres cultured in SCM. For immunostaining of serum-cultured cells and glioma cell lines, serum-cultured cells and U87, U251, and SHG44 glioma cells were plated onto glass coverslips for 24 hours, and immunocytochemistry was performed as described previously. Cells were additionally counterstained with Hoechst 33342 (Sigma) to permit counting of cell nuclei. Quantification of cells stained with each antibody was averaged and estimated as a percentage of total nuclei counted.
Isolation of CD15+ and CD15+CD133- Cells
CD15+ and CD15+CD133- cells were isolated by magnetic bead sorting to investigate their capacity to form tumor spheres. Cultured tumor spheres were dissociated to get single-cell suspensions as described previously. Then, the dissociated cells were centrifuged at 800g for 5 minutes. Cells were incubated with anti-CD15 antibody and biotin-conjugated secondary antibody. Then biotin-labeled cells were rinsed and labeled by streptavidin-coupled Dynabeads (Dynal; Invitrogen) for 30 minutes. Cells were rinsed with phosphate-buffered saline during each step. Magnetic separation was carried out on the Dynal magnet (Dynal; Invitrogen) to separate CD15+ and CD15- cells. For CD15+CD133- cells, CD133- cells were negatively selected. Then CD15+CD133- cells were isolated from these CD133- cells as described previously. After separation, tumor sphere formation and differentiation assays were performed on CD15+, CD15-, and CD15+CD133- cells.
Chromosome Analysis
Cells were treated with 100 ml of Colcemid (10 g/ml; Invitrogen) for 2 hours. Then cells were resuspended in hypotonic solution (75 mM KCl) for 30 minutes at 37°C, centrifuged, and fixed in methanol and acetic acid. Metaphase spreads were prepared on slides, dried, and Giemsa-stained after trypsin pretreatment.
Implantation into Nude Mice
Single cells dissociated from primary tumor spheres, isolated CD15+, or CD15- cells were resuspended in 10 µl of phosphate-buffered saline, in aliquots of 5000 or 10,000 cells. These aliquots were injected stereotactically into the right striatum of 6- to 8-week-old nude mice brains (n = 6 each; Center of Experimental Animals, Fourth Military Medical University, Xian, China), after administration of general anesthesia. The injection coordinates were 2 mm to the right of the midline, 0.5 mm anterior to the coronal suture, and 3 mm deep. The implanted mice were killed at 10 weeks after implantation by intracardiac perfusion-fixation with 4% paraformaldehyde and examined for tumors on the brain sections. All of the animals used were experimented in strict accordance with the animal experiments guidelines enforced at the Fourth Military Medical University.
Immunohistochemistry
For staining of human and mouse paraffin sections, tissue was fixed overnight in 4% paraformaldehyde, transferred to 70% ethanol, paraffin-embedded, and sectioned at a thickness of 1 µm. Sections were mounted on microscope slides and then dried overnight at 60°C, dewaxed in xylene, and rehydrated with distilled water. Hematoxylin and eosin staining and immunohistochemical staining of the following antibodies were performed on the sections: human-specific Nestin (hNestin; 10C2, mouse monoclonal; Abcam), CD15, GFAP, and β-III-tubulin. With the exception of sections stained for GFAP, all sections were treated with heat-induced epitope retrieval technique using a citrate buffer at pH 6.0. Then the sections were blocked for endogenous peroxidase and biotin before the application of the primary antibody. Incubation of primary antibodies was performed for 3 hours at room temperature. The Elite Vector Stain ABC System (Vector Laboratories, Burlingame, CA) was used as detection system and diaminobenzidine was used as a chromogen. The counterstain of preference was hematoxylin for nuclear detail.
Statistical Analysis
Statistical analyses were performed using Student's 2-tailed t test. P < .05 was considered statistically significant. Statistical analyses were done using SPSS v.13.0.0 (SPSS, Inc, Chicago, IL).
Results
Formation of Tumor Spheres
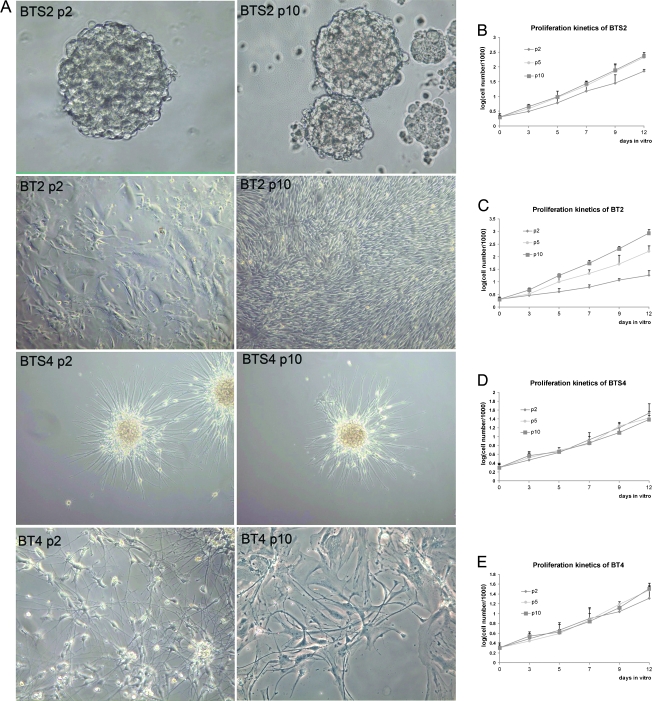
SFM containing human recombinant EGF and bFGF were used to favor the growth of glioma spheres. Glioma spheres from the seven patients were presented as BTS1, BTS2, BTS3, BTS4, BTS5, BTS6, and BTS7, and the cells cultured in SCM were marked correspondingly as BT1, BT2, BT3, BT4, BT5, BT6, and BT7. Of the seven brain tumor samples, numbers 1, 2, 4, 5, and 7 formed spheres within 1 month in SFM (Table 1). BTS1 sustained five passages and ceased growth. BTS2, BTS4, BTS5, and BTS7 have been cultured more than 10 passages (BTS2 and BTS5 for more than 20 passages) and were sustained now. However, tumor cells from samples 3 and 6 did not form tumor spheres within 1 month. Of the formed tumor spheres, BTS1, BTS2, BTS5, and BTS7 grew as floating spheres, whereas BTS4 grew as adherent tumor spheres (Figure 1A), consistent with previous report that some tumor spheres grew as adherent spheres [8]. The morphologies of the seven SCM cultured cells were also different from each other (Figure 1A). When SCM-cultured tumor cells of late passages (passage 10) were transformed to SFM, no tumor spheres were investigated within 1 month.
Figure 1.
Morphologies and proliferation kinetics of SFM- and SCM-cultured cells. (A) Morphologies of SFM-cultured stem-like tumor spheres (BTS2 and BTS4) and serum-cultured cells (BT2 and BT4) from different passages (p2 indicates passage 2; p10, passage 10). Tumor spheres BTS2 cultured in SFM showed constant morphology, whereas SCM-cultured cells changed apparently after long-term culturing. BTS2 got constant growth rate after first two passages (B). BT2 got extraordinary proliferation potential after a plateau at about passage 2 (C). BTS4 (D) and BT4 (E) showed slow and constant growth rate. Data represent mean values ± SD of three independent experiments.
Primary sphere formation assay showed that the stem cell frequencies in the investigated samples (samples 1, 2, 4, 5, and 7) were between 3.7% and 20.1% (12.48% ± 6.53%). Tumor spheres cultured in SFM grew at a relatively slow rate at the first two passages, whereas they got a constant and greater growth rate after two passages (Figure 1B). Most SCM-cultured tumor cells showed a highly heterogeneous morphology and proliferated at constant rates. BT2 showed initial growth followed by a plateau phase, and then some cells got a much greater growth rate and proliferated dominantly at later passages (Figure 1C). For ependymoma-derived tumor spheres that showed adherent growth pattern, BTS4 exhibited constant and a relatively slow growth rate (Figure 1, D and E).
Primary Tumor Spheres Cultured in SFM Showed BTSCs Properties
Besides BTS1, tumor spheres (BTS2, BTS4, BTS5, and BTS7) cultured in SFM could be serially passaged more than 10 times (Table 1), indicating that these stem-like cells have the potential of self-renewal, one critical feature of BTSCs. Immunofluorescence staining showed that both astrocytoma- and ependymoma-derived spheres expressed stem cell markers CD133, Nestin, CD15 (Figure 2, A–F), and proliferative marker Ki67 (Figure 2, G and H). One property of BTSCs was their potential to differentiate in serum medium without growth factors. Therefore, differentiation potential was examined in all cultured tumor spheres. Tumor spheres of BTS1, BTS2, BTS4, BTS5, and BTS7 were plated onto poly-l-lysine-coated coverslips and cultured in SCM. After culturing in this differentiation medium, all tumor spheres adhered and cells migrated from the spheres (Figure 3A). Immunofluorescence staining showed that neuronal and glial lineage markers NeuN, β-III-tubulin, GFAP, and Galc were expressed in the differentiated cells. However, a considerable portion of differentiated cells expressed both neuronal and glial markers, implying the aberrant differentiation of tumor stem-like cells (Figure 3B).
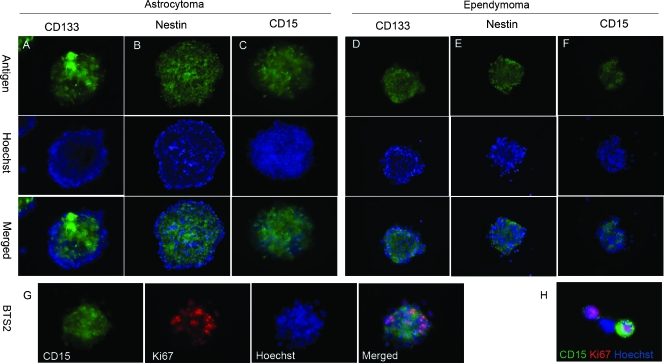
Figure 2.
Expression of stem cell markers and proliferate marker Ki67 in stem-like tumor spheres. (A–F) Tumor spheres BTS2 and BTS4 were shown as representative to be labeled with antibodies to CD133, Nestin, and CD15 (green). Nuclei were counterstained with Hoechst 33342. The merged images were also shown. Some CD15+ cells were Ki67-positive investigated in both tumor spheres (G) and dissociated single cells (H).
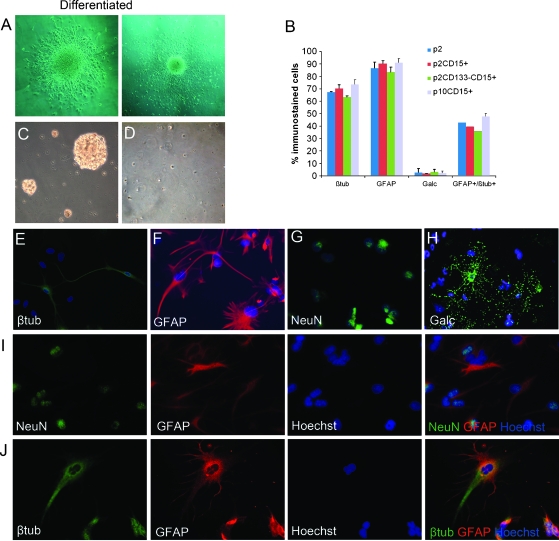
Figure 3.
Differentiation of tumor spheres (BTS2 was shown as representative) in SCM. (A) Cells in tumor spheres cultured in SCM-migrated from the spheres and differentiated into glial and neuronal lineages. (B) Differentiated cells positive for each antigen and double-positive for glial and neuronal antigen were counted (p2 indicates BTS2 at passage 2; p2CD15+, CD15-TS from passage 2; p2CD15+CD133-, tumor spheres formed by CD15+CD133- cells of passage 2; p10CD15+, CD15-TS from passage 10. Columns represent mean values ± SD of three independent experiments). CD15+ cells formed tumor spheres in SFM (C), whereas CD15- cells did not (D). (E–H) Differentiated cells from the tumor spheres showed expression of neuronal marker β-III-tubulin and NeuN, astrocyte marker GFAP, and oligodendrocyte marker Galc. (I and J) Some differentiated cells expressed both neuronal and glial markers. βtub indicates β-III-tubulin.
CD15+ Cells Isolated from Tumor Spheres Showed Potential of BTSCs
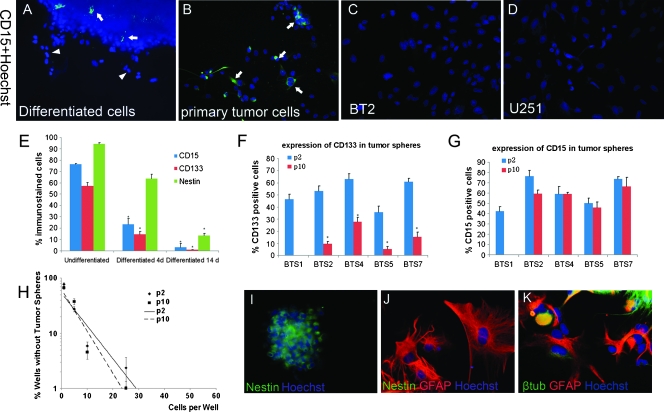
It has been suggested that CD15 was a marker of NSCs. We detected the expression of CD15 in early passages (passage 2) of SFM-cultured tumor spheres. As a result, CD15 was detected in all the tumor spheres derived from the five samples that formed stem cell-like spheres (Figure 2, C and F), including BTS1 that sustained only five passages. Interestingly, although it was elucidated that CD15 was not expressed in normal ependyma cells [19], CD15 was detected in ependymoma-derived tumor spheres (Figure 2F). Immunostaining for proliferative marker Ki67 showed that some CD15+ cells were positive for Ki67 (Figure 2, G and H), indicating that brain tumor stem-like cells showed increased proliferative capacity [6].
Then we assessed if CD15 can identify stem cells in these tumor spheres. CD15-positive cells were isolated by magnetic bead sorting from BTS1, BTS2, BTS4, BTS5, and BTS7. Both CD15-positive and CD15-negative cells were cultured in SFM to investigate their potential to form stem-like spheres. CD15+ cells formed tumor spheres within 1 week, whereas few CD15-negative cells formed tumor spheres within 2 weeks (Figure 3, C and D). The percentage of formed tumor spheres in CD15-positive and CD15-negative cells were 41.6% ± 3.2% and 1.1% ± 0.4%, respectively (P < .001). Tumor spheres formed by CD15+ cells (CD15-TS) could be dissociated and form secondary tumor spheres, similar to their parental spheres. Besides CD15-TS from BTS1 that sustained three to four passages, CD15-TS from BTS2, BTS4, BTS5, and BTS7 were serially passaged more than five times, indicating their self-renewal capacity. When CD15-TS were induced to differentiation, both neuronal and glial cell markers β-III-tubulin, NeuN, GFAP, and Galc were expressed (Figure 3, E–H). Like their parental tumor spheres, CD15-TS showed aberrant differentiation with many cells that expressed both neuronal and glial cell markers (Figure 3, I and J). Quantification of differentiated cells showed that percentages of GFAP, β-III-tubulin, Galc-positive, and GFAP, β-III-tubulin double-positive cells in differentiated cells from CD15-TS were similar to those of their parental tumor spheres (Figure 3B).
CD15+ Cells Displayed Tumor-Specific Properties
In addition to forming stem-like neural spheres in SFM, BTSCs were expected to bear some tumor-specific features, such as unbalanced karyotypes. We conducted chromosome analysis on SFM- and SCM-cultured cells to demonstrate that cells in tumor spheres and CD15+ cells were indeed transformed and are not normal NSCs. For each SFM-cultured cell line (BTS2, BTS4, BTS5, and BTS7), identical abnormal numerical chromosomal aberrations were present in tumor sphere or CD15+ cells of both early and late passages (passages 2 and 10). As a representative, identical numerical chromosomal aberrations 47 XY, -10, +7, +21, were detected in BTS2 cells and CD15+ BTS2 cells of early and late passages (Figure 4A). However, for SCM-cultured tumor cells, more complicated numerical chromosomal aberrations were present in late passages (data not shown). These results suggested that stem-like cells cultured in SFM retained their property with little changes, whereas tumor cells cultured in SCM showed chromosome instability at late passages [15].
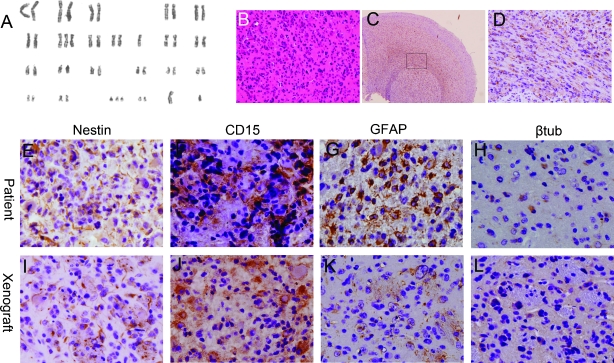
Figure 4.
CD15+ cells showed aberrant karyotypes and tumorigenesis in vivo (data from BTS2 was shown as representative). (A) Chromosome analysis demonstrated that CD15+ cells from BTS2 showed numerical chromosomal aberrations 47 XY, -10, +7, +21. Hematoxylin and eosin (B) and anti-hNestin staining (C, D) of intracranial xenograft formed by CD15+ cells transplanted into nude mice brains. Anti-hNestin staining showed that hNestin+ cells infiltrated into surrounding brain tissues (C) and migrated along the corpus callosum (D, a higher magnification of the box in C). (E–L) Immunohistologic staining for Nestin (E, I), CD15 (F, J), GFAP (G, K), and β-III-tubulin (βtub; H, L) showed that primary (E–H) and xenografts (I–L) tumors had similar immunohistologic characteristics.
CD15+ Cells Showed Tumorigenesis In Vivo
CD15+ cells were isolated from expanded BTS2, BTS4, and BTS5. Then CD15+ and CD15- cells, SCM-cultured cells BT2, BT4, and BT5 were injected into nude mice brains, in aliquots of 5000 or 10,000 cells to investigate their potential to form tumors in vivo. The mice were killed at 10 weeks after transplantation for examination of tumors. All of the nude mice that received CD15+ cells developed tumors (Figure 4B), whereas none of the mice injected with SCM-cultured cells or CD15- cells formed tumors. Anti-human Nestin-10C2 antibody (hNestin; Abcam), which does not react with mouse antigens, was used to specifically recognize human-derived cells expressing Nestin. Brains of normal nude mice were used as negative controls. hNestin was not detected in normal nude mice brain, indicating that Nestin-positive cells detected in xenografts would be derived from human tumor cells. hNestin-positive cells in the xenograft tumors demonstrated extensive infiltration into the surrounding cerebral cortex, a pathologic feature of human gliomas (Figure 4C). Furthermore, hNestin-positive cells showed a tendency for migrating along white matter tracts such as the corpus callosum (Figure 4D), as is characteristically seen with glioma cells in the brains of patients. Immunohistologic staining showed that CD15 was expressed in both primary tumor samples and tumor xenografts (Figure 4, E and I). Examination of Nestin, GFAP, and β-III-tubulin expression showed that primary and xenografts tumors had similar immunohistologic characteristics (Figure 4, E–L). These data demonstrate that CD15+ cells have the potential to form tumors in vivo, a critical property of BTSCs.
CD15 Expression Was Decreased in Differentiated Tumor Spheres
To further confirm if CD15 was predominantly expressed in stem-like tumor cells, we examined CD15 expression in differentiated cells and serum-cultured cells (Figure 5, A–D). Decrease of CD15 expression was detected in all SFM-cultured cell lines after they were transformed to SCM. After 4 days of differentiation, CD15 was significantly decreased in the tumor spheres and was not expressed in the migrated cells (Figure 5A). After 14 days of differentiation, CD15 was almost not detected. These results meant that CD15 expression declined with the differentiation of BTSCs and was little or not expressed in more mature cells (Figure 5E).
Figure 5.
CD15 expression and stem cell properties in differentiated and late-passage tumor spheres. (A) Expression of CD15 decreased significantly after differentiation. (B) CD15-positive cells were detected in the primary dissociated cells. Arrows in panels A and B indicate CD15+ cells, arrowhead in panel B indicates CD15- cells migrated from the tumor spheres. (C and D) CD15 was not detected in serum-cultured cells or standard glioma cell lines. (E) Stem cell markers CD15, CD133, and Nestin were downregulated in differentiated tumor spheres. (F and G) Percentage of CD133-positive cells was decreased at late passages, whereas decrease of CD15 expression was not appreciable. (H) Limiting dilution assay showed that BTS2 cells had similar potential in forming tumor spheres at early and late passages. (I–K) CD15+CD133- cells formed tumor spheres in SFM expressing Nestin (I), which differentiated to glial and neuronal lineages in SCM (J, K). Nestin expression was decreased in differentiated cells (J). Data from tumor sample 2 (BTS2 or BT2) were shown as representative in panels A–C, E, H, and I–K. p2 indicates passage 2; p10, passage 10. Columns represent mean values ± SD of three independent experiments. (E) *P < .05 versus undifferentiated. (F) *P < .05 versus p2.
Primary tumor cells were also cultured in SCM. Although investigated in dissociated single cells from primary tumors (Figure 5B), CD15 was not detected in serum-cultured cells (BT1-BT7; Figure 5C). We also tested if standard glioma cell lines cultured in serum medium contain CD15+ cells. We performed immunostaining in U87, U251, and SHG44 cell lines, but CD15 was not detected in these cell lines (Figure 5D). These results indicated that CD15 was not expressed in cells cultured in serum medium, in which stem-like cells were differentiated. These results also implied that serum-cultured cells and standard glioma cell lines shared many important properties different from SFM-cultured stem-like cells and primary tumors [15].
CD15 Identified BTSCs in Tumor Spheres of Late Passages
Because long-term culture might influence the expression of CD133, we also examined the expression of CD133 and CD15 in late passages of tumor spheres. In late passages (passage 10), the percentage of CD133+ cells were decreased significantly, whereas decreased percentage of CD15+ cells were hardly appreciable (Figure 5, F and G). Limiting dilution assay showed that stem-like cell frequencies in early and late tumor spheres were similar (Figure 5H). In addition, differentiation assay showed that CD15-TS from early and late passages had similar potential to differentiate into neuronal and glial lineages (Figure 3B). Together with the result that karyotype of BTSCs was stable in late passages, these facts suggested that SFM-cultured cells maintained their stem cell properties even in late passages and that decreased percentage of CD133+ cells in late passages might be, at least partly, due to the environmental factors as have been elucidated but not necessarily decreased stem cell potentials. Therefore, stable expression of CD15 made it more suitable to identify BTSCs from tumor spheres, especially of late passages.
CD15+ Cells Might Contain BTSCs That Are CD133 Negative
To further investigate if CD15+ cells represent one type of BTSCs different from CD133+ cells, we isolated CD15+CD133- cells and tested if they have stem cell activities. CD15+CD133- cells could be isolated from all SFM-cultured tumor spheres of both early (passage 2) and late passages (passage 10, except BTS1). Tumor sphere formation assay and differentiation assay showed that CD15+CD133- cells could also form tumor spheres in SFM, and cells in these tumor spheres differentiated into glial and neuronal lineages in SCM (Figure 5, I–K; Figure 3B). Then CD15+ cells isolated from tumor spheres formed by CD15+CD133- cells of late passages were injected into nude mice brains. Xenografts similar to those formed by early CD15+ cells were investigated. Therefore, CD15 might identify BTSCs that contain CD133- cells from both early and late passages of tumor spheres.
CD15 Was Expressed in a Subset of Human Brain Tumors
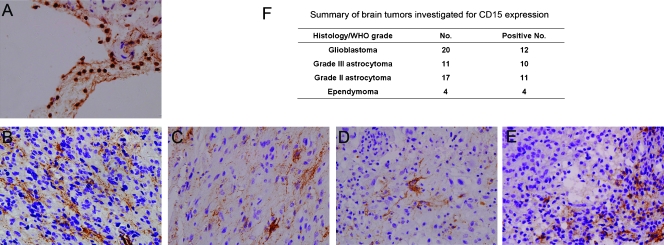
To determine whether CD15 is expressed in human brain tumors, we performed immunohistochemical analysis on 54 archival brain tumor samples, including 20 grade 4 glioblastomas, 11 grade 3 astrocytomas, 17 grade 2 astrocytomas, and 4 ependymomas. Immunoreactivity for CD15 in granulocytes was detected in all cases, serving as an internal positive control (Figure 6A). Primary antibody omission was used as negative control. CD15 was investigated in a portion of all types of astrocytomas and ependymomas (Figure 6, B–F). This means a subset of brain tumors contained CD15+ stem- or progenitor-like cells, which might have originated from transformed neural stem or progenitor cells and responsible for the development of tumors.
Figure 6.
Expression of CD15 in human brain tumors. (A) CD15 was detected in granulocytes, serving as an internal positive control. (B–E) CD15 was investigated in glioblastoma (B), grade 3 astrocytoma (C), grade 2 astrocytoma (D), and ependymoma (E). (F) Summary of CD15 detection in brain tumors.
Discussion
In recent years, the concept of cancer stem cells (CSCs) gives us new insights about the tumor biology. The CSC hypothesis claimed that tumors were driven by a rear population of cells, CSCs, which have the potential of self-renewal, multidifferentiation, and the ability to recapitulate the phenotype of origin tumors. Although there are controversies [26], the research about CSCs progressed rapidly in recent years and gave researchers new direction to find more effective therapies. For brain tumor, the identification of BTSCs leads to promising progress in treating gliomas [4,6,27]. However, some critical problems are needed to be solved to get substantial understanding of BTSCs. First, the identity of BTSCs is not clear. Present isolation methods, including isolation based on surface marker CD133 or side population, have led to disputed results of identifying BTSCs [8,28,29]. Second, the origin of BTSCs and its relationship with NSCs are not clear [30]. These problems mean that we only have little knowledge about BTSCs, and further studies are needed to explore the characteristics of BTSCs.
Previous studies showed that single cells from primary gliomas formed tumor spheres in SFM containing EGF and bFGF. This method has been used for many years to study NSCs. Only stem or progenitor cells survive in this medium, whereas differentiated mature cells die, and progenitor cells can only sustain limited passages. In recent years, this medium has been used to enrich stem cells from human brain tumors [6,15]. In our study, tumor cells cultured in SFM showed a relatively slow growth rate within the first two passages and got greater and achieved a constant growth rate after two passages, possibly reflecting the select process of this SFM culture system for stem-like cells. In the early passages, differentiated, more mature progenitor and other cells gradually disappeared, and the BTSCs pool survived and proliferated. During late passages, most cells in tumor spheres were stem or progenitor cells and, therefore, showed constant growth rate. As a result, we got brain tumor spheres containing BTSCs from both glioma and ependymoma cultured in SFM. In our results, 5 (71.43%) in 7 samples formed tumor spheres within 1 month and 4 sustained more than 10 passages.
Recent studies showed that not all CD133-positive cells were BTSCs, and there were CD133-negative BTSCs [8,9,28]. For example, A2B5+ CD133- brain tumor-initiating cells were identified recently [31]. In addition, the significance of CD133 expression in BTSCs was not clear [32]. It has long been hypothesized that brain tumors might have originated from NSCs [11]. Exploration of the mechanisms of glioma tumorigenesis using genetic mouse models implied that at least a part of the gliomas might have originated from transformed NSCs [12,13]. Therefore, we examined the expression of CD15, a marker of NSCs, in tumor spheres to assess if CD15 can be used to enrich BTSCs.
CD15 is expressed in many pluripotent stem cells, including pluripotent embryonic stem cells, in germinal zones of the developing CNS, on some astrocytes in the adult CNS, and within adult neurogenic zones. CD15 has been identified as one specific marker for NSCs [19] and, recently, as a marker for tumor-propagating cells in medulloblastoma [33]. Our results demonstrated that CD15 was expressed in both glioma- and ependymoma-derived tumor spheres and that its expression decreased dramatically in differentiated tumor spheres. In addition, CD15 was not expressed in SCM-cultured cells and standard glioma cell lines, which have been suggested not containing stem cells. In fact, serum-cultured cell lines were quite different from stem-like cells in many facets. For example, SFM-cultured stem-like cells, but not serum-cultured primary cells or standard glioma cells lines (U251, U87 cells, etc.), phenocopied the critically important histopathologic features of the original human gliomas [15]. Another difference between SFM- and SCM-cultured cells was their stability of critical features. We analyzed the chromosomes of different passages of both SFM- and SCM-cultured cells and found that SFM-cultured tumor spheres largely maintained their initial genotypes, whereas, in contrast, SCM-cultured cells showed quite complex changes of genotypes. Together, these results indicated that CD15 was expressed mainly in stem-like brain tumor cells.
CD15 has been described as an NSC marker that did not contain the ependyma cells [19]. Recent studies elucidated that other types of CD133-positive NSCs residing in the ependyma were different from CD15+ NSCs because CD15 was not expressed in ependyma cells [20,34]. These facts complicated the issue of origin of BTSCs and indicated that BTSCs may also contain different types of “stem cells,” which probably originated from different types of NSCs or progenitors. It was not clear which type of NSCs, CD15+ NSCs or CD133+ NSCs, was more early, or if they represented different lineages of NSCs. Although we did not directly elucidate the existence of CD15+CD133+ cells, the percentage of CD15+ cells and CD133+ cells in early passages (e.g., 76.5% ± 0.5% and 53.3% ± 4.1%, respectively, for BTS2; Figure 5E) in tumor spheres indicated that there were CD15+CD133+ BTSCs. This is different from the situations in normal nervous systems, where CD15 and CD133 were expressed by different cells. Whatever the origin of BTSCs and the relationship between CD15+ and CD133+ NSCs, both expressions of CD15 and CD133 in BTSCs implied that the identified stemlike cells from brain tumors did have the property of stem cells but were aberrantly transformed. In addition, the expression of CD15 in ependymoma-derived tumor spheres also implied the aberrant phenotype of these stem-like tumor cells.
Consistent with other reports [12,35], we investigated downregulation of CD133 in late passages of stem-like cells. In addition, CD133 might fail to accurately identify stem-like cells in late passages, whereas CD15 retained its property even at late passages. Recent studies discovering some mechanisms that regulate expression of CD133 in brain tumor cells indicated that expression of CD133 could be regulated by environmental conditions such as hypoxia and mitochondrial dysfunction [7]. The mechanisms of regulation of CD133 expression in brain tumors need to be further examined to elucidate their roles in stem-like cells.
The expression of CD15 in BTS1, which sustained only five passages, indicated that CD15 was also expressed in some progenitor cells. However, the existence of CD15+CD133- BTSCs demonstrated that CD15 might be one marker for BTSCs, which contained CD133- cells. Our results and other reports indicated that CD133 was not expressed in all brain tumors (data not shown) [36,37]. Therefore, CD15 would be used as one marker to identify BTSCs from brain tumors that do not express CD133. The expression of the NSC marker CD15 in brain tumor stem-like cells implied some intrinsic relationships between NSCs and BTSCs. The expression of the NSCs marker CD15 indicated that BTSCs might retain more characteristics of NSCs besides self-renewal and multidifferentiation.
These results raised other questions about NSCs and BTSCs such as the following: What is the relationship between CD15+ and CD133+ NSCs and BTSCs? How have CD15 and CD133 appeared during the formation of gliomas? Answers to these questions would lend credence to the identity of BTSCs and provide novel strategies to target these aberrantly transformed stem-like cells.
Acknowledgments
The authors thank Juan Li and Xiaoyan Chen for their technical assistance in cell culture and immunohistochemistry, Zhenzhong Zhu for his generously provision of CD15 antibody, Ganlan Bian for her technical assistance in transplantation experiments, Hui Xu for her help in chromosome analysis, and Rong Cao, Jinmei Diao for their assistance in capturing images.
References
- 1.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 2.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao XG, Zhang X, Zhen HN. Progress on potential strategies to target brain tumor stem cells. Cell Mol Neurobiol. 2009;29:141–155. doi: 10.1007/s10571-008-9310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 6.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 7.Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR, Jr, Gillespie GY. CD133 is a marker of bioenergetic stress in human glioma. PLoS ONE. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;112:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 11.Sanai N, varez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 12.Marumoto T, Tashiro A, Friedmann-Morvinski D, Scadeng M, Soda Y, Gage FH, Verma IM. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15:110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcantara LS, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, varez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 18.Clark PA, Treisman DM, Ebben J, Kuo JS. Developmental signaling pathways in brain tumor-derived stem-like cells. Dev Dyn. 2007;236:3297–3308. doi: 10.1002/dvdy.21381. [DOI] [PubMed] [Google Scholar]
- 19.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 20.Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr, Fan G, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reifenberger G, Sieth P, Niederhaus M, Wechsler W. Expression of CD15 in tumours of the nervous system. Histochem J. 1992;24:890–901. doi: 10.1007/BF01046360. [DOI] [PubMed] [Google Scholar]
- 22.Martin K, Akinwunmi J, Rooprai HK, Kennedy AJ, Linke A, Ognjenovic N, Pilkington GJ. Nonexpression of CD15 by neoplastic glia: a barrier to metastasis? Anticancer Res. 1995;15:1159–1166. [PubMed] [Google Scholar]
- 23.Gocht A, Struckhoff G, Lhler J. CD15-containing glycoconjugates in the central nervous system. Histol Histopathol. 1996;11:1007–1028. [PubMed] [Google Scholar]
- 24.Budka H, Majdic O. Shared antigenic determinants between human hemopoietic cells and nervous tissues and tumors. Acta Neuropathol. 1985;67:58–66. doi: 10.1007/BF00688124. [DOI] [PubMed] [Google Scholar]
- 25.Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 26.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 27.Yi L, Zhou ZH, Ping YF, Chen JH, Yao XH, Feng H, Lu JY, Wang JM, Bian XW. Isolation and characterization of stem cell-like precursor cells from primary human anaplastic oligoastrocytoma. Mod Pathol. 2007;20:1061–1068. doi: 10.1038/modpathol.3800942. [DOI] [PubMed] [Google Scholar]
- 28.Joo KM, Kim SY, Jin X, Song SY, Kong DS, Lee JI, Jeon JW, Kim MH, Kang BG, Jung Y, et al. Clinical and biological implications of CD133- positive and CD133-negative cells in glioblastomas. Lab Invest. 2008;88:808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 29.Zheng X, Shen G, Yang X, Liu W. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007;67:3691–3697. doi: 10.1158/0008-5472.CAN-06-3912. [DOI] [PubMed] [Google Scholar]
- 30.Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, et al. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62:505–514. doi: 10.1227/01.neu.0000316019.28421.95. [DOI] [PubMed] [Google Scholar]
- 32.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882–892. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, Febbo PG, Wechsler-Reya RJ. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15:135–147. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfenninger CV, Roschupkina T, Hertwig F, Kottwitz D, Englund E, Bengzon J, Jacobsen SE, Nuber UA. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727–5736. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- 35.Platet N, Liu SY, Atifi ME, Oliver L, Vallette FM, Berger F, Wion D. Influence of oxygen tension on CD133 phenotype in human glioma cell cultures. Cancer Lett. 2007;258:286–290. doi: 10.1016/j.canlet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 37.Christensen K, Schroder HD, Kristensen BW. CD133 identifies perivascular niches in grade II–IV astrocytomas. J Neurooncol. 2008;90:157–170. doi: 10.1007/s11060-008-9648-8. [DOI] [PubMed] [Google Scholar]