Abstract
Vaccines using dendritic cells (DCs) harboring leukemic antigens to stimulate T cells is a possible treatment of acute myeloid leukemia (AML). Limitations of breaking tolerance to leukemic cells and lack of specific activation of T cell-mediated cytotoxicity may explain the discouraging clinical results with this approach. To break self-tolerance against AML cells, we loaded DCs with AML antigens and a bifunctional small interference (si) RNA targeting interleukin (IL) 10 and simultaneously activating toll-like receptors (TLRs). In vitro, this active siRNA inhibited (P < .05) IL-10 production by silencing the IL-10 gene in DCs. The active siRNA stimulated production of tumor necrosis factor α, implying activation of TLRs. Vaccination in a nonimmunogenic rat model mimicking human AML with the loaded DCs induced a substantial and specific T-cell cytotoxicity. Leukemic rats treated with the active siRNA lived longer and had markedly less leukemic cell mass infiltrating their bone marrow compared with rats given inactive siRNA (P < .05). Furthermore, compared with inactive siRNA treatment, the active siRNA led to significantly less extramedullar leukemic dissemination, evidenced by reduced matrix metalloproteinase activity and smaller spleens. Our data demonstrate that this bifunctional siRNA may work as an immunomodulatory drug with antileukemic properties.
Introduction
The recognition that the graft-versus-leukemia effect is important points to immunomodulation as possible treatment of acute myeloid leukemia (AML). In addition to induction of graft-versus-leukemia, vaccines have been developed, but often with dismal results [1]. This may be due to limitations of breaking tolerance to leukemic cells and/or insufficient activation of high-affinity cytotoxic T cells [2].
Toll-like receptor (TLR) ligands can break tolerance to self-antigens and promote immune response to tumor antigens, as well as induce dendritic cell (DC) differentiation, and TLR ligands provide signals necessary for activation of innate and adaptive immune responses [3]. Although TLR ligands elicit immune responses, they also induce expression of DC-derived immunosuppressive cytokines, like interleukin (IL) 10, which can influence T cell-mediated immunity [4]. In addition, IL-10 has been associated with the development of tolerogenic DCs [4]. This inhibitory feedback mechanism may hamper effective immunity against tumor cells. Moreover, whereas IL-10 reduces the production of proleukemic cytokines in cultured AML blast cells, such an antileukemic effect in patients has been challenged [5]. Development of agents that stimulate DC maturation through TLRs and simultaneously inhibit immunosuppression may thus facilitate design of effective cancer vaccines in AML.
Among the gene-silencing approaches, RNA interference offers a novel opportunity for posttranscriptional inhibition of immunosuppressive factor expression in vitro and in vivo. Initially, the RNA interference pathway was thought to be nonfunctional in mammalian cells, where double-stranded RNA longer than 30 nucleotides induces a nonspecific antiviral response known as the interferon (IFN) pathway. It has been reported that small interference (si) RNA, when introduced into mammalian cells, did not trigger the IFN pathway but effectively silenced gene expression in a sequence-dependent manner [6]. However, we have shown that certain siRNA sequences can bind to TLRs and trigger the activation of innate immunity [7,8]. We recently designed a bifunctional siRNA that cleaves target messenger RNA and increases the production of cytokines through TLRs [9]. This siRNA efficiently inhibited IL-10 gene expression in cultured DCs and primed T cells, thus suggesting its possible usefulness as an immunomodulatory drug.
Here we examined whether self-tolerance to leukemic antigens could be broken by this siRNA when used as adjuvant in a nonimmunogenic rat model of human AML [10]. We show that this novel DC-based vaccine could inhibit the development and progression of neoplastic blood cells.
Materials and Methods
siRNA and Leukemic Cell Protein Lysate
To activate TLRs, the anti-IL-10 siRNA was designed to contain a significant number of uridines (Eurogentec, Seraing, Belgium) [11]. A 2′-O-methyl uridine-modified siRNA targeting mouse basigin was used as an inactive control because this modification abolishes siRNA sensing by TLRs [12]. The target site sequences of the siRNA were as follows:
active siRNA, 5′-ACGCUGUCAUCGAUUUCUC-3′
inactive (control) siRNA, 5′-GAGGCAAUCACCAAUAGCA-3′
Leukemic cells from the Brown Norwegian (BN) rat model of human AML were suspended at a density of 1 x 107 cells/ml, frozen at -80°C, and disrupted by four freeze-thaw cycles. Then the cell suspensions were sonicated, and after that, the supernatants were passed through a 0.2-µm filter.
Generation of DCs
Blood cells from healthy BN rats were separated according to density before the monocytes were purified using plastic adherence and cultured in complete RPMI medium supplemented with granulocyte-macrophage colony-stimulating factor (50 ng/ml) and IL-4 (100 ng/ml) to generate immature DCs. On day 5, these DCs were transfected with either the active or the inactive siRNA (20 µg/2 x 106 cells) using cationic liposomes (DOTAP; 20 µg/ml; Boehringer Mannheim, Mannheim, Germany). In both cases, 50 µg of leukemic cell protein extracts was added to the transfection mixture. Subsequent to overnight incubation, cells were harvested, washed, and then immediately used for immunization.
Assays of siRNA Effects in DCs
To test for siRNA-mediated anti-IL-10 activity, immature DCs (1 x 106/ml) were transfected with siRNA (2 µg/transfection) using electroporation before stimulating with lipopolysaccharide (25 ng/ml) for 18 hours. We used ELISA kits (PromoKine, Heidelberg, Germany) to measure IL-10 and tumor necrosis factor a (TNFα) in the supernatants. IL-10 messenger RNA (mRNA) from DC was analyzed by reverse transcription-polymerase chain reaction using SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA) and the ABI PRISM 7700 equipment (Applied Biosystems, Foster City, CA). Glyceraldehyde phosphate dehydrogenase was used as an internal control gene. The following primers were used:
IL-10 forward 5′-AAAAGCAAGGCAGTGGAGCA-3′ and reverse 5′-AAGGAGTTGCTCCCGTTAGC-3′ (product 200 bp) GADH forward 5-CATGCCATCACTGCCACTCA-3′ and reverse 5′-GTTTCTCCAGGCGGCATGTC-3′ (product 200 bp).
The mRNA expressions was quantified by the comparative cycle threshold method [13].
Transplantation Protocol
The protocol conformed to the national legislation and guidelines (permission no. S-2006/30555). The animals were tested negative for microbes [14]. We used the BN model of AML because it reliably mimics several important features of human AML including development and progression of the disease as well as response to chemotherapy [10]. Syngeneic BN rats (n = 10 per group) were randomly assigned to be injected subcutaneously with 2 million DCs transfected with either active or inactive siRNA, and loaded with the leukemic lysate. One week later (day 0), this procedure was repeated before we transplanted the rats with leukemic cells [15]. We also included 10 untreated leukemic rats as controls. The rats were killed with an intraperitoneal overdose of barbiturate when they developed obvious signs of disease.
Assays of Leukemia Development and Serum Cytokines
Bone marrow leukemic cellularity was assessed with antibody staining of a leukemic cell-specific antigen and flow cytometry [16]. As a measure of leukemic metastasis, we quantified serum matrix metalloproteinase (MMP) with zymography [15]. Activation of T cell-mediated cytotoxicity was assessed by determination of 51Cr release [17].
Serum cytokines were measured using a Lincoplex Kit (Linco Research, St Charles, MO) according to the instructions of the manufacturer and as described elsewhere [18].
Statistics
Unless otherwise stated, values are medians (interquartile range). We used 2-tailed Mann-Whitney or Kruskal-Wallis tests followed by the Bonferroni correction, as appropriate. P < .05 was taken to indicate statistical significance.
Results and Discussion
The Active siRNA Inhibits IL-10 and Stimulates TNFα in Cultured DCs
To determine the IL-10 silencing potency of the active siRNA, we analyzed the production of IL-10 sampled from cultured DCs. The concentration of IL-10 in supernatants from DCs transfected with active siRNA was approximately five-fold reduced compared with those given inactive siRNA or untransfected DCs (median 199 vs 1075 vs 1035 pg/ml, respectively, P < .01, n = 6 per group). In support of these findings, DCs transfected with the active siRNA showed very low IL-10 mRNA levels compared with DCs transfected with the inactive siRNA (Figure 1). We then investigated the immunostimulatory potential of the active siRNA. Transfection of DCs with the active siRNA increased the supernatant concentration of TNFα, a marker of TLRs stimulation, approximately nine-fold compared with DCs transfected with the inactive siRNA or untransfected DCs (median 1353 vs 150 vs 194 pg/ml, P < .01, n = 6 per group). Addition of neutralizing anti- IL-10 antibodies to transfected or untransfected DCs did not alter the supernatant concentrations of either IL-10 or TNFα (data not shown). Notably, DCs might have an additional level of self-/non-self-discrimination that functions at the level of phagosome maturations [19]. Therefore, conjugation of self-antigen and TLRs ligands through liposomes is expected to improve immune response. We recently identified several genes involved in different pathways such as antigen presentation, cell motility, and endosome maturation induced by immunostimulatory siRNA [12]. Moreover, these genetic analyses indicated that the active siRNA most likely stimulated TLRs 7 and 8.
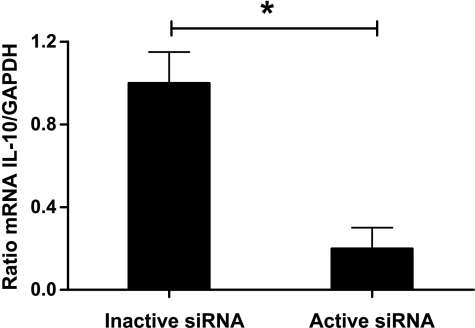
Figure 1.
The mRNA expression of IL-10 relative to that GAPDH was markedly reduced in DCs transfected with active compared with inactive siRNA. Values are mean + SEM, n = 4, *P < .05.
The Active siRNA Induces Specific T-Cell Cytotoxicity
To examine if DCs transfected with the active siRNA could induce a T cell-specific response, rats were immunized with DCs loaded with leukemic protein lysates and siRNA. T-cell cytotoxicity against leukemic cells was determined 4 weeks later. Figure 2 shows that T cells from rats given DCs with active siRNA had a profound increase in cytolytic capacity compared with T cells from rats given DCs with inactive siRNA, demonstrating induction of T cell-specific antileukemic activity. Cytotoxicity from T cells sampled from healthy rats was similar to those from rats given DCs transfected with inactive siRNA (data not shown). Whereas anti-major histocompatibility complex (MHC) class II antibodies had no effect (data not shown), anti-MHC class I antibodies abrogated this response (Figure 2). Moreover, we could not detect any antibody specifically directed against the leukemic protein lysates (data not shown). Collectively, these findings further indicate the specificity of the T cell-mediated cytotoxicity.
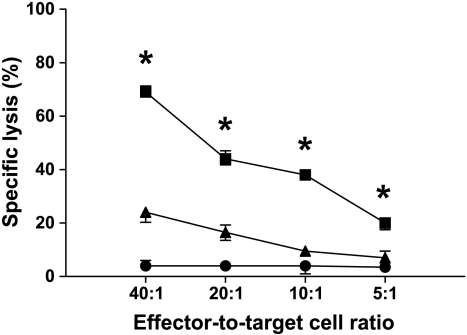
Figure 2.
The active siRNA induced specific T-cell cytotoxicity. Lysis of autologous leukemic cells were increased at all effector-to-target cell ratios tested when T cells from leukemic rats treated with DCs transfected with active siRNA (squares) were compared with those given inactive siRNA (triangles). The specific lysis of autologous leukemic cells given anti-MCH class I antibodies (circles) was negligible. MHC class I expression did not differ between untransfected DCs or DCs transfected with active or inactive siRNA (data not shown). T cells and target cells were harvested from blood and spleen before further purification with monoclonal antibodies and flow cytometric sorting. *P < .05. Values are mean and SEM, n = 10 per group.
Dendritic Cells Harboring the Active siRNA Inhibit Leukemogenesis in Rat AML
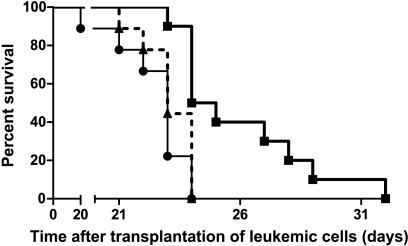
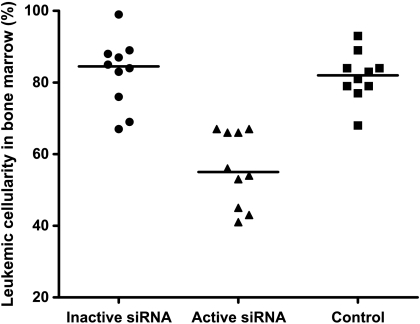
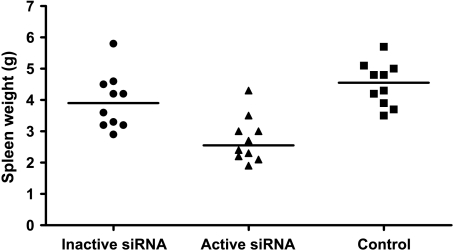
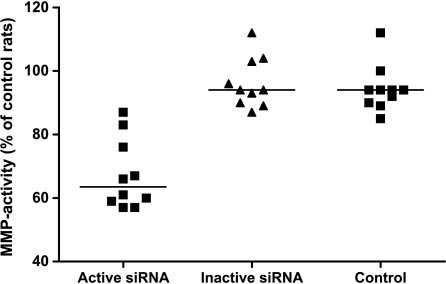
Given that self-tolerance can be broken by delivering a TLR signal and blocking IL-10 expression in DC, we next examined whether IL-10-silenced DCs would induce antileukemic immunity in vivo. Kaplan-Meier analysis revealed a survival advantage among rats given DCs transfected with active siRNA compared with those given DCs transfected with inactive siRNA (Figure 3; P = .035, log-rank test). Moreover, in rats treated with DCs transfected with active siRNA, median leukemic cell load constituted 55% of bone marrow cellular content, a marked reduction compared with the 85% and 82% in median leukemic cellularity in rats treated with DC transfected with the inactive siRNA or in untreated leukemic rats, respectively (Figure 4). The spleen mass is a valid measure of leukemic dissemination in this model [20]. Whereas median splenic weight among rats given DCs transfected with inactive siRNA or untreated rats were 3.9 and 4.6 g, respectively, it was substantially lower (P < .05) in rats treated with DCs transfected with active siRNA: 2.6 g (Figure 5). Activation of MMP is required for extravasation of leukemic cells [21]. Further supporting the inhibitory effect of the active siRNA on leukemic dissemination, the median MMP activity in rats given DCs transfected with active siRNA was only 63.3% (P < .05) of that measured among untreated rats, whereas the corresponding percentage was 94 among rats given DCs transfected with the inactive siRNA (Figure 6).
Figure 3.
Increased survival on treatment with active siRNA. Leukemic rats treated with DCs transfected with active siRNA (thick filled line) lived longer compared with those given either inactive siRNA (thin solid line) or with untreated rats (dashed line). n = 10 per group.
Figure 4.
The bone marrow leukemic cellularity was significantly lower among rats treated with DCs transfected with the active siRNA (triangles) compared with those given inactive siRNA (circles), or with untreated rats (squares). Individual values are shown and are given as percentages of the total number of nucleated cells. The horizontal lines represent median values. n = 10 per group.
Figure 5.
Spleen weight was significantly reduced in rats treated with DCs transfected with the active siRNA (triangles) compared with those given inactive siRNA (circles) or with untreated rats (squares). Values are individual weights. n = 10 per group.
Figure 6.
MMP-related activity decreased significantly in rats given DCs transfected with the active siRNA (triangles) compared with those given inactive siRNA (circles) or control rats (squares). Individual values are given and expressed as a percentage of the mean value obtained among untreated leukemic rats. n = 10 per group.
To further establish the specificity of DCs transfected with the active siRNA in reducing IL-10 concomitant with activation of TLRs, we measured selected cytokines involved in an inflammatory reaction. Table 1 shows that the serum concentrations of TNFα and IFN-γ, but not of IL-1β, were increased in rats injected with DCs transfected with active siRNA compared with those given DCs transfected with inactive siRNA. Furthermore, the serum concentrations of IL-10 were almost halved in rats treated with DCs transfected with active siRNA compared with those given DCs transfected with inactive siRNA, whereas those of IL-13, another immunosuppressive cytokine, remained similar in both groups. These data further substantiate the specific anti-IL-10 effect of the active siRNA as well as the binding of the active siRNA to TLRs 7/8.
Table 1.
Serum Concentrations of Cytokines.
| Cytokine (pg/ml) | Inactive siRNA | Active siRNA | P |
| IL-10 | 650 (525–755) | 385 (298–430) | .0023 |
| IL-13 | 305 (188–633) | 305 (225–440) | .95 |
| TNFα | 25 (5–33) | 55 (38–70) | .0022 |
| IL-1β | 55 (25–70) | 48 (30–80) | .84 |
| IFN-γ | 23 (15–53) | 400 (140–888) | .0007 |
Values are medians (interquartile range). n = 10 per group.
There are some limitations to this study. First, we adopted a preventive strategy in relation to the development of leukemia to test the efficacy of the siRNA treatment. We realize that this treatment regimen should next be tested in already established disease. Second, although some survival benefit was detected, it was small. We therefore believe that the siRNA treatment might be best suited as supplementary to other treatment options, for example, transplantation. Moreover, it could also be valuable for patients that cannot tolerate intensive treatment regimens.
To summarize, the siRNA efficiently reduced IL-10 production by silencing its gene in DCs. In addition, transfection of DCs with the siRNA enhanced production of TNFα, indicating stimulation of TLRs 7/8. Importantly, vaccination with DCs harboring this siRNA and leukemic antigens activated T cell-mediated killing of leukemic cells as well as enhanced survival and reduced the leukemic cell burden and metastasis in a rat model of AML. It has been reported that siRNA directed at IL-10 can modulate DCs to stimulate T cells in vitro [22,23]. However, the present study is the first to demonstrate a combined immunoinhibitory and immunostimulatory effect of DC siRNA in an in vivo model of human malignancy. Hence, our study demonstrates that the use of a bifunctional siRNA in a vaccine setting can break tolerance to leukemic cells and stimulate specific T-cell cytotoxicity, and thus act as an immunomodulatory drug with antileukemic properties.
Acknowledgments
The authors declare no conflict of interest.
Footnotes
We appreciate the financial support from the Throne Holst Foundation, the Western Norway Regional Health Authority, the EU 6th framework program Integrated Project “Angiotargeting,” and from the Norwegian Cancer Society.
References
- 1.el-Shami K, Smith BD. Immunotherapy for myeloid leukemias: current status and future directions. Leukemia. 2008;22:1658–1664. doi: 10.1038/leu.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett AJ, Rezvani K. Translational mini-review series on vaccines: peptide vaccines for myeloid leukaemias. Clin Exp Immunol. 2007;148:189–198. doi: 10.1111/j.1365-2249.2007.03383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sioud M. Innate sensing of self and non-self RNAs by Toll-like receptors. Trends Mol Med. 2006;12:167–176. doi: 10.1016/j.molmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Kabelitz D, Wesch D, Oberg HH. Regulation of regulatory T cells: role of dendritic cells and toll-like receptors. Crit Rev Immunol. 2006;26:291–306. doi: 10.1615/critrevimmunol.v26.i4.10. [DOI] [PubMed] [Google Scholar]
- 5.Bruserud O. IL-4, IL-10 and IL-13 in acute myelogenous leukemia. Cytokines Cell Mol Ther. 1998;4:187–198. [PubMed] [Google Scholar]
- 6.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 7.Sioud M. Therapeutic siRNAs. Trends Pharmacol Sc. 2004;25:22–28. doi: 10.1016/j.tips.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Furset G, Sioud M. Design of bifunctional siRNAs: combining immuno-stimulation and gene-silencing in one single siRNA molecule. Biochem Biophys Res Commun. 2007;352:642–649. doi: 10.1016/j.bbrc.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 10.Martens AC, van Bekkum DW, Hagenbeek A. The BN acute myelocytic leukemia (BNML) (a rat model for studying human acute myelocytic leukemia (AML)) Leukemia. 1990;4:241–257. [PubMed] [Google Scholar]
- 11.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Sioud M, Furset G, Cekaite L. Suppression of immunostimulatory siRNA-driven innate immune activation by 2′-modified RNAs. Biochem Biophys Res Commun. 2007;361:122–126. doi: 10.1016/j.bbrc.2007.06.177. [DOI] [PubMed] [Google Scholar]
- 13.Antonov J, Goldstein DR, Oberli A, Baltzer A, Pirotta M, Fleischmann A, Altermatt HJ, Jaggi R. Reliable gene expression measurements from degraded RNA by quantitative real-time PCR depend on short amplicons and a proper normalization. Lab Invest. 2005;85:1040–1050. doi: 10.1038/labinvest.3700303. [DOI] [PubMed] [Google Scholar]
- 14.Rehbinder C, Baneux P, Forbes D, van Herck H, Nicklas W, Rugaya Z, Winkler G. FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig and rabbit experimental units. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Animal Health accepted by the FELASA Board of Management, November 1995. Lab Anim. 1996;30:193–208. doi: 10.1258/002367796780684881. [DOI] [PubMed] [Google Scholar]
- 15.Iversen PO, Sorensen DR, Tronstad KJ, Gudbrandsen OA, Rustan AC, Berge RK, Drevon CA. A bioactively modified fatty acid improves survival and impairs metastasis in preclinical models of acute leukemia. Clin Cancer Res. 2006;12:3525–3531. doi: 10.1158/1078-0432.CCR-05-2802. [DOI] [PubMed] [Google Scholar]
- 16.Martens AC, Johnson RJ, Kaizer H, Hagenbeek A. Characteristics of a monoclonal antibody (RM124) against acute myelocytic leukemia cells. Exp Hematol. 1984;2:667–671. [PubMed] [Google Scholar]
- 17.Rolstad B, Fossum S. Allogeneic lymphocyte cytotoxicity (ALC) in rats: establishment of an in vitro assay, and direct evidence that cells with natural killer (NK) activity are involved in ALC. Immunology. 1987;60:151–157. [PMC free article] [PubMed] [Google Scholar]
- 18.Semaeva E, Tenstad O, Bletsa A, Gjerde EA, Wiig H. Isolation of rat trachea interstitial fluid and demonstration of local cytokine production in lipopolysaccharide-induced systemic inflammation. J Appl Physiol. 2008;104:809–820. doi: 10.1152/japplphysiol.00846.2007. [DOI] [PubMed] [Google Scholar]
- 19.Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen BT, Jensen PO, Helledie N, Iversen PO, Ralfkiaer E, Larsen JK, Madsen MT. Changing bone marrow micro-environment during development of acute myeloid leukaemia in rats. Br J Haematol. 1998;102:458–464. doi: 10.1046/j.1365-2141.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 21.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Ng H, Akasaki Y, Yuan X, Ehtesham M, Yin D, Black KL, Yu JS. Small interference RNA modulation of IL-10 in human monocyte-derived dendritic cells enhances the TH1 response. Eur J Immunol. 2004;34:1680–1687. doi: 10.1002/eji.200425081. [DOI] [PubMed] [Google Scholar]
- 23.Chhabra A, Chakraborty NG, Mukherji B. Silencing of endogenous IL-10 in human dendritic cells leads to the generation of an improved CTL response against human melanoma associated antigenic epitope, MART-1 27-35. Clin Immunol. 2008;126:251–259. doi: 10.1016/j.clim.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]