Abstract
AIMS: Nodal spread is the single most important prognostic factor of survival in gastric cancer patients. In this study, genes that were upregulated in the lymph node metastases of gastric cancer were identified and may serve as putative novel therapeutic target. METHODS: Complementary DNA (cDNA) microarray analysis and quantitative real-time polymerase chain reaction of primary gastric carcinomas and matched lymph node metastasis were carried out. Immunohistochemistry with anti-SPARC antibodies was performed on large tissue sections of 40 cases with primary gastric carcinoma (20 diffuse, 20 intestinal) and the corresponding lymph node metastases, as well as on tissue microarrays of 152 gastric cancer cases. RESULTS: A cDNA microarray identified SPARC as being upregulated in primary gastric carcinoma tissue and the corresponding lymph node metastasis compared with the nonneoplastic mucosa. SPARC was expressed in fibroblasts and, occasionally, in tumor cells. However, the level of immunoreactivity was particularly strong in stromal cells surrounding the tumor. The level of expression of SPARC, determined by immunohistochemistry, correlated in intestinal-type gastric cancer with the local tumor growth, nodal spread, and tumor stage according to the International Union Against Cancer. CONCLUSIONS: Our study provides transcriptional and translational evidence for the differential expression of SPARC in gastric cancer tissue. On the basis of our observations and those made by others, we hypothesize that SPARC is a promising novel target for the treatment of gastric cancer.
Introduction
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related deaths worldwide surpassed only by lung cancer [1,2]. Among various prognostically relevant variables of gastric cancer, the lymph node status and the ratio of metastasis-positive/metastasis-negative lymph nodes are the strongest markers of gastric cancer prognosis [3,4]. The N-ratio (metastatic/examined lymph nodes) has been validated as an independent prognostic factor in a large multicenter series, even where less than the recommended 15 lymph nodes have been examined [5,6]. The 5-year survival rate for patients with metastases in 1 to 6 lymph nodes is 44% and drops to 30% for 7 to 15 lymph node metastases, ending with 11% for more than 15 lymph node metastases. Unfortunately, most patients presenting with advanced gastric cancer already have lymph node metastases [7]. Gastrectomy with or without accompanying adjuvant radiotherapy and/or chemotherapy is the treatment of choice, promising complete cure in early stages. However, more than half of the patients receiving potential curative surgery will finally experience relapse. For them and for most patients presenting with advanced disease stages, the therapeutic options are systemic chemotherapy, radiotherapy, or both [1]. Because the currently used chemotherapeutic regimens and radiotherapy have limited efficacy in the metastatic stage, in this patient group, therapy-resistant disease progression usually leads to tumor-related death within a year. This underscores the urgent need for novel therapeutic targets in the treatment of gastric cancer, and identifying factors contributing to nodal spread may help to improve gastric cancer prognosis. Using a gene array-based approach, genes that were upregulated in the lymph node metastases of gastric cancer were identified, and the differential expression was confirmed by quantitative reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry. Using tissue microarrays (TMAs), we demonstrate on a larger patient series that SPARC is differentially expressed in gastric cancers and that its expression correlates with tumor progression and nodal spread. Hence, targeting SPARC may be a novel treatment target for metastatic gastric cancer.
Materials and Methods
Patient Characteristics and Tissue Samples
For histologic and immunohistochemical studies, formalin-fixed (10% neutralized formalin) and paraffin-embedded tissue samples from the archive of the Department of Pathology of the University of Magdeburg were obtained from 174 gastric cancer patients (105 men and 68 women), who had undergone either complete or partial gastrectomies between 1995 and 2005. The age of the patients ranged from 26 to 84 years (mean = 64.6 ± 11.9 years). For molecular biologic studies, unfixed tissue samples from the nonneoplastic mucosa, primary tumor, and the corresponding lymph node metastases were collected immediately after surgery from six patients with gastric cancer, shock-frozen in liquid nitrogen, and stored at -80°C until further use. Gastric cancer was classified according to Laurén [8]. The tumor (T category), node (N category), and metastasis (M category) stage was determined according to the International Union Against Cancer (UICC) guidelines and was based on histologic confirmation using hematoxylin and eosin-stained sections [9]. All cases were reviewed before study inclusion. This study is in accordance with the guidelines of the local ethics committee. Data were encoded to ensure patient protection.
Cell Culture
The human gastric cancer cell lines AGS, KATOIII, MKN28, MKN45, and NCI-N87 were obtained from the Riken Cell Bank (Tsukuba, Japan) and American Type Culture Collection (Rockville, MD). Cell lines were maintained in RPMI-1640 medium (PAA Laboratories, Cölbe, Germany), supplemented with 10% fetal calf serum (PAA Laboratories). The cells were grown in a tissue culture hood at 37°C with 5% carbon dioxide atmosphere. Cells were washed with phosphate-buffered saline (Sigma, Deisenhofen, Germany) and harvested with Trypsin-EDTA (PAA Laboratories).
Gene Array Analyses
For the gene array, large tissue samples from a single site were used each from the primary gastric cancer (poorly differentiated intestinal-type gastric cancer; pT3 pN3 pMX) and a large corresponding lymph node metastasis (>1.5 cm diameter) of a 69-year-old male white patient. After extraction of RNA using the RNeasy Kit (Qiagen, Hilden, Germany), the preparation and labeling of the complementary DNA (cDNA) (LabelStar; Qiagen) was carried out according to the LabelStar Array Handbook (Qiagen). Briefly, 20 µg of total RNA in 20 µl of RNase-free H2O was incubated at 65°C and added to 5 µl of 10x reverse transcriptase buffer, 5 µl of dNTP mix C, 1 µl of cyanine 3- or cyanine 5-labeled dCTP, 5 µl of oligo-dT primer, 0.5 µl of RNase inhibitor, 2.5 µl of LabelStar reverse transcriptase in a total volume of 50 µl. After incubating at 37°C for 120 minutes, the reaction was stopped, and the labeled cDNA was purified according to the manufacturer's instructions. Hybridization of the labeled cDNA with the cDNA gene array (Human 10k Array A; MWG-Biotech, Ebersberg, Germany) was performed in cyanine 3 and cyanine 5 hybridization buffer. The samples were incubated overnight and washed and dried the next day. Arrays were then evaluated using the GenePix 4000B microarray scanner by means of the GenePix Pro 4.0 Software (Axon Instruments Europe GmbH, Hamburg, Germany).
RNA Extraction and cDNA Synthesis
Total RNA was extracted from tumor and nonneoplastic tissue samples using the RNeasy Kit from Qiagen following the recommended protocol. The resulting RNA was quantified spectrophotometrically using a GeneQuant (Pharmacia LKB, Freiburg, Germany) and stored at -80°C until further required. One microgram of total RNA was transcribed into cDNA using Omniscript Reverse Transcriptase (Qiagen) according to the recommended protocol. SPARC and β-actin PCR products were amplified using the Taq PCR Core Kit (Qiagen) and specific primer (SPARC: forward: 5′-AAG ATC CAT GAG AAT GAG AAG-3′, reverse: 5′-AAA AGC GGG TGG TGC AAT G-3′; β-actin: forward: 5′-CAT GTA CGT TGC TAT CCA GGC-3′, reverse: 5′-CTC CTT AAT GTC ACG CAC GAT-3′): 2 µl of cDNA was amplified with 10 µl of 10x PCR buffer, 0.4 µl of dNTPs, 1 µl of each primer, 4 µl of Q-solution, and 0.1 µl of Taq polymerase and the following temperature profile: at 95°C for 5 minutes; 40x (at 94°C for 30 seconds, at 53°C for 60 seconds, and at 72°C for 30 seconds); and at 72°C for 10 minutes.
Fluorescence-Mediated Quantitative Real-time RT-PCR
Fluorescence-mediated quantitative real-time RT-PCR was performed using the Lightcycler (Roche Diagnostics, Mannheim, Germany). External standards were established by purifying the appropriate PCR product using the Nucleospin Extract II kit (Macherey & Nagel, Düren, Germany) as instructed. The PCR product DNAwas quantified spectrophotometrically, and the copy number of the PCR products was calculated. Serial dilutions of the purified DNA samples were used as external standards in every run to create a standard curve for the calculation of mRNA levels. Quantitative RT-PCR was performed using the SYBR Green Two-Step RT-PCR Kit (Qiagen). All reactions contained 2 µl of the cDNA template, primer (see above), and 10 µl of the 2 x PCR buffer in a final volume of 20 µl. A hot start for 15 minutes at 95°C to activate the Taq polymerase was followed by 35 cycles, each with a denaturation at 94°C for 15 seconds, annealing at 54°C for 20 seconds, and extension at 72°C for 15 seconds. Melting curve analysis of the amplified products was performed between 65°C and 95°C and verified the absence of substantial side products. The fluorescence intensity of the double-strand-specific SYBR Green I, reflecting the amount of actually formed PCR product, was measured at the end of each cycle during the 72°Celongation step (previous melting curve analyses had determined the melting points of the PCR products). The time point at which the linear increase of PCR product started (threshold cycle) was determined for each sample. Using the threshold cycle values, the mRNA copy number was calculated from the standard curve (serial dilutions of the corresponding PCR product). The expression levels of β-actin were calculated in the same manner and used to normalize cDNA contents for any variability in RNA amounts or integrity.
Tissue Microarray
For the evaluation of SPARC expression, TMAs were generated using a precision instrument (Beecher Instruments, Silver Spring, MD) as described previously [10]. In brief, a minimum of 6 tissue cylinders of 0.6 mm in diameter were punched randomly from each tumor-bearing donor block and 12 tissue cylinders (six from antrum and six from corpus mucosa) from corresponding nonneoplastic mucosa, constructing 20 blocks of TMAs. Overall, 6.1 ± 2.7 spots per carcinoma from different tumor areas were eligible for analysis, resulting in a total of 2617 spots scored for SPARC expression.
Immunohistochemistry
For immunostaining of paraffin-embedded sections, the slides were deparaffinized and rehydrated in a graded alcohol series. Immunostaining was performed with an antibody directed against SPARC (monoclonal mouse, dilution 1:900; Takara Bio, Inc, Otsu, Japan). After antigen retrieval (1 mM EDTA, 12 minutes, 8 minutes, 450 W), incubation with the primary antibody was carried out in a moist chamber at 37°C for 1 hour. Biotinylated polyvalent antimouse/antirabbit immunoglobulin G (Immunotech, Marseilles, France) served as a secondary antibody (30 minutes at room temperature). Slides were washed between steps with Tris-buffered saline. Immunoreactions were visualized through an avidin-biotin complex, using the Vectastain ABC Alkaline Phosphatase Kit (distributed by CAMON, Wiesbaden, Germany), with Fast Red/NaphtholMx (Immunotech) as chromogen. The specimens were counterstained with hematoxylin. Omission of primary antibodies served as negative controls. Sections were evaluated by an experienced pathologist, and a score was applied to quantify the extent of expression: 0 = no expression, 1 = low expression, 2 = moderate expression, 3 = strong expression. For statistical analyses of the TMA results, an immunoreactivity score (IRS) was calculated by dividing the sum of the individual staining intensities observed in the tissue cylinders of a single case by the number of cylinders available from each case. The IRS ranged from 0 to 3.
Statistics
Tables 2 and 3 present a summary of the analyzed medical parameters in form of mean ± SD or frequency distributions for discrete parameters, respectively. The mean comparisons were carried out using the two-sample t-test (the Satterthwaite approximation to compute the degree of freedom). The correlations between SPARC expression and tumor classification were evaluated using Fisher exact test. Survival times were evaluated by the log-rank test, and the results are presented with Kaplan-Meier curves. All statistical decisions were made 2-tailed with a critical probability of α = 5% without α adjustment. For that reason, the results should be interpreted in an exploratory manner. To support the interpretation, P values of the statistical tests were added in Tables 2 and 3. Statistical analyses were carried out with SPSS 15.0 (SPSS, Inc, Chicago, IL) or SAS 9.1 (SAS Institute, Inc, Cary, NC).
Table 2.
Correlation of the SPARC Expression in TMAs with Various Clinicopathologic Characteristics.
| Gastric Cancer Patients | Total | Tumor | P SPARC 0 vs >0 | Stroma | P SPARC 0 vs >0 | Stroma IRS ≥ 1 | P IRS ≥1 vs <1 |
| Patients [n (%)] | 152 (100) | 11 (7.0) | 146 (96.1) | 104 (68.4) | |||
| Age [mean (SD)] | 65.03 (12.54) | 65.94 (13.03) | |||||
| Mean age SPARC-positive [years (n)] | 66.79 (2) | .748 | 65.03 (57) | 65.94 (46) | .198 | ||
| Mean age SPARC-negative [years (n)] | 64.96 (55) | - | 61.21 (11) | ||||
| Mean survival of SPARC-positive patients [days (n)] | 379.5 (2) | .932* | 548.5 (57) | - | 553.7 (48) | .363* | |
| Mean survival of SPARC-negative patients [days (n)] | 554.4 (57) | - | 525.73 (11) | ||||
| Sex | |||||||
| Men [n (%)] | 92 (60.5) | 7 (7.6) | 1.000 | 89 (96.7) | .681 | 62 (67.4) | .856 |
| Women [n (%)] | 60 (39.5) | 4 (6.7) | 57 (95.0) | 42 (32.6) | |||
| Tumor type | |||||||
| Intestinal [n (%)] | 102 (67.1) | 10 (9.8) | .102 | 99 (97.1) | .395 | 75 (73.5) | .064 |
| Diffuse [n (%)] | 50 (32.9) | 1 (2.0) | 47 (94.0) | 29 (58.0) | |||
| T category | |||||||
| pT1 [n (%)] | 15 (9.9) | 3 (20.0) | .266 | 14 (93.3) | .737 | 8 (53.3) | .121 |
| pT2A [n (%)] | 19 (12.5) | 2 (10.5) | 19 (100) | 16 (84.2) | |||
| pT2B [n (%)] | 48 (31.6) | 3 (6.3) | 45 (93.8) | 28 (58.3) | |||
| pT3 [n (%)] | 60 (39.5) | 3 (5.0) | 58 (96.7) | 45 (75.0) | |||
| pT4 [n (%)] | 10 (6.6) | 0 (0.0) | 10 (100) | 7 (70.0) | |||
| N category | |||||||
| pN0 [n (%)] | 42 (27.6) | 6 (14.3) | .198 | 41 (97.6) | .248 | 23 (54.8) | .016 |
| pN1 [n (%)] | 52 (34.2) | 1 (1.9) | 51 (98.1) | 43 (82.7) | |||
| pN2 [n (%)] | 36 (23.8) | 3 (8.3) | 32 (88.9) | 21 (58.3) | |||
| pN3 [n (%)] | 19 (12.5) | 1 (5.3) | 19 (100) | 15 (78.9) | |||
| pNX [n (%)] | 3 (2.0) | 0 (0.0) | 3 (100) | 2 (66.7) | |||
| M category | |||||||
| pM0 [n (%)] | 1 (0.7) | 0 (0.0) | .713 | 1 (100) | 1.000 | 1 (100) | .267 |
| pM1 [n (%)] | 26 (17.1) | 1 (3.9) | 25 (96.2) | 21 (80.8) | |||
| pMX [n (%)] | 125 (82.2) | 10 (8.0) | 120 (96.1) | 82 (65.69) | |||
| UICC tumor stage | |||||||
| Stage I [n (%)] | 36 (23.7) | 6 (16.7) | .022† | 35 (97.2) | .140 | 20 (55.6) | .324 |
| Stage II [n (%)] | 35 (23.0) | 0 (0.0) | 35 (100) | 25 (71.4) | |||
| Stage III [n (%)] | 39 (25.8) | 3 (7.7) | 35 (89.7) | 28 (71.8) | |||
| Stage IV [n (%)] | 42 (27.6) | 2 (4.8) | 41 (97.6) | 31 (73.8) | |||
| Grading | |||||||
| G 1 [n (%)] | 3 (2.0) | 0 (0.0) | .747† | 3 (100) | 1.000† | 0 (0.0) | .854† |
| G 2 [n (%)] | 46 (30.3) | 4 (8.7) | 44 (95.7) | 33 (71.7) | |||
| G 3 [n (%)] | 94 (61.8) | 7 (7.4) | 90 (95.7) | 68 (72.3) | |||
| G 4 [n (%)] | 9 (5.9) | 0 (0.0) | 9 (100) | 3 (13.3) |
P value of the log-rank test.
P value of a 2 x 2 contingency table.
Boldface font indicates statistically significant results.
Table 3.
Evaluation of the Expression of SPARC (Given as Mean IRS) in TMAs.
| Cell Type | No. of Cases (n)* | Mean IRS ± SD (Range) | P, t-Test |
| Nonneoplastic foveolar epithelium | 122 | 0.004 ± 0.032 (0–0.300) | |
| Stromal fibroblasts of nonneoplastic stomach (total) | 148 | 0.100 ± 0.1900 (0–1.00) | |
| Cancer cells | |||
| All gastric cancers | 152 | 0.029 ± 0.129 (0–1.20) | |
| Diffuse-type gastric cancer | 50 | 0.010 ± 0.071 | (0–0.50) .138 |
| Intestinal-type gastric cancer | 102 | 0.037 ± 0.149 (0–1.20) | |
| Cells of the desmoplastic stroma | |||
| All gastric cancers | 152 | 1.251 ± 0.685 (0–3.00) | |
| Diffuse-type gastric cancers | 50 | 1.012 ± 0.608 (0–2.30) | .002 |
| Intestinal-type gastric cancers | 102 | 1.369 ± 0.693 (0–3.00) | |
| Intestinal-type gastric cancers (pT1) | 10 | 0.860 ± 0.427 (0.2–1.5) | .002 |
| Intestinal-type gastric cancers (pT2–pT4) | 92 | 1.424 ± 0.695 (0–3.00) | |
| Intestinal-type gastric cancers (pN0) | 32 | 1.122 ± 0.562 (0.2–2.20) | .008 |
| Intestinal-type gastric cancers (pN1–pN3) | 70 | 1.481 ± 0.726 (0–3.00) | |
| Intestinal-type gastric cancers (UICC stage I) | 28 | 1.079 ± 0.531 (0.2–2.20) | .003 |
| Intestinal-type gastric cancers (UICC stage II–IV) | 74 | 1.478 ± 0.718 (0–3.00) |
The TMAs did not enclose all histoanatomic structures equally.
Results
Gene Array and RT-PCR
Homogenized tissue samples were obtained from the primary tumor and the corresponding lymph node metastasis from a 69-year-old patient with a poorly differentiated, intestinal-type gastric cancer. Among the 62 genes studied, 32 were upregulated and 30 were downregulated (Table 1). The mean fold change factors were 3.2 (range = 1.8–5.1) for the upregulated genes and 3.4 (range = 1.8–6.2) for the downregulated genes. From the upregulated genes, we selected SPARC for further validation studies because SPARC had been shown to be involved in tumor progression [11–17], although only limited data were available for gastric cancer. Furthermore, monoclonal antibodies were available commercially to further test the putative significance of SPARC on the translational level.
Table 1.
Differential Expression of mRNA in a Patient with Metastatic Gastric Cancer.
| Upregulated in the Lymph Node Metastasis | Fold Change Factor | Downregulated in the Lymph Node Metastasis | Fold Change Factor |
| HLA-DR antigens associated invariant chain (p33) | 5.1 | Desmin | 6.2 |
| hcerN3 gene mRNA for N snRNP associated protein | 4.9 | 20-kDa myosin light chain (MLC-2) | 5.7 |
| (23k/2) ubiquitin-conjugating enzyme UbcH2 | 4.9 | 22-kDa smooth muscle protein (SM22) | 5.3 |
| Lymph node homing receptor | 4.8 | Gastricsin | 5.1 |
| Immunoglobulin light-chain variable region (lambda IIIb subgroup) from IgM rheumatoid factor | 4.5 | 2′,3′-cyclic nucleotide 3′-phosphodiesterase | 4.6 |
| Ribosomal protein L32 | 4.2 | Urokinase plasminogen activator receptor | 4.3 |
| Ribosomal protein S25 | 4.1 | NRD1 convertase | 4.2 |
| Ribosomal protein L29 (humrpl29) | 4.0 | Neurogranin (RC3) | 4.1 |
| Ig rearranged light-chain mRNA V region | 3.9 | MOP4 | 3.8 |
| Neuropeptide Y-like receptor | 3.7 | Antizyme inhibitor | 3.8 |
| H5, and platelet glycoprotein Ib beta chain | 3.7 | Carbonic anhydrase IV | 3.7 |
| Translationally controlled tumor protein | 3.6 | (Clone pGHSCBS) cystathionine beta-synthase subunit (CBS) | 3.6 |
| Prothymosin alpha | 3.3 | Transducin (beta) like 1 protein | 3.4 |
| Prothymosin alpha mRNA (ProT-alpha) | 3.2 | “HMG-Y protein isoform mRNA (HMGI gene); clone 11D” | 3.1 |
| Thymosin beta-4 | 3.0 | Endothelial-monocyte activating polypeptide II | 3.1 |
| LERK5 (LERK5) | 3.0 | KIAA0411 | 3.0 |
| Elongation factor 1-alpha 1 (PTI-1) | 2.9 | Treacher Collins syndrome (TCOF1) | 2.9 |
| Wilm tumor-related protein (QM) | 2.8 | Type 1 inositol 1,4,5-trisphosphate receptor | 2.8 |
| Ribosomal protein (homologous to yeast S24) | 2.6 | Vascular smooth muscle alpha-actin | 2.6 |
| KIAA0130 gene | 2.6 | Reelin (RELN) | 2.6 |
| GDP dissociation inhibitor beta | 2.5 | Bleomycin hydrolase | 2.3 |
| Ribosomal protein | 2.5 | K12 protein precursor | 2.2 |
| alpha 2 delta Calcium channel subunit isoform II | 2.4 | Ninjurin-1 | 2.1 |
| Visinin-like peptide 1 homolog | 2.4 | KIAA 0207 gene | 2.1 |
| SPARC/osteonectin | 2.3 | Hepatoma-derived growth factor | 2.1 |
| Signal-transducing guanine nucleotide-binding regulatory (G) protein beta subunit | 2.2 | Redox factor | 2.0 |
| PLSTIRE for serine/threonine protein kinase | 2.1 | Prolyl 4-hydroxylase beta-subunit | 2.0 |
| 5′-fragment for vimentin N-terminal fragment | 2.1 | alpha-1-Antichymotrypsin | 1.9 |
| beta 2-Microglobulin | 2.0 | Procarboxypeptidase B | 1.8 |
| beta 2-mu, beta 2-Microglobulin | 1.9 | ||
| Translationally controlled tumor protein | 1.8 |
SPARC mRNA in Gastric Tissue and Gastric Cancer Cell Lines
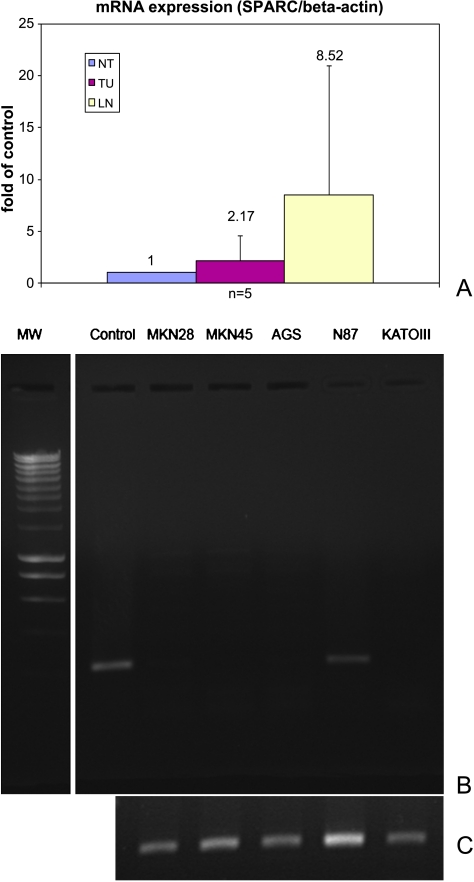
As shown in Figure 1, SPARC mRNA was found in the primary intestinal-type gastric cancers of an independent set of five random patients. The quantitative real-time RT-PCR data were used to compare the expression level of SPARC mRNA in nonmalignant specimens with the corresponding gastric cancers as well as the lymph node metastases. The SPARC mRNA values were normalized against the results for β-actin mRNA from the same samples. The average amount of SPARC mRNA from the cancerous tissue was 2.17-fold higher than that in noncancerous tissue, and at the afflicted lymph nodes, it was increased approximately 8.52-fold (Figure 1A). However, because of the small sample number, this did not reach statistical significance.
Figure 1.
Quantitative RT-PCR analysis of SPARCmRNA expression. Total RNA was extracted from the human gastric cancer cell lines (L-R: MKN28, MKN45, AGS, NCI-N87, KATO III) or from tissue samples of gastric cancer (TU), lymph node metastases (LN), and corresponding nonneoplastic tissue (NT) from five separate patients. (A) Using fluorescence-mediated quantitative real-time RT-PCR and product-specific primer, themRNA copy number for SPARC was calculated and normalized against the expression levels of β-actin to account for any variability in RNA amounts or integrity (upper panel). (B) SPARC mRNA was only found in the NCI-N87 gastric cancer cell line (N87). ASPARC-positive gastric tumor served as a control (control). (C) β-Actin mRNA served as loading control.
SPARC mRNA expression was examined in five different gastric cancer cell lines. SPARC mRNA was found only in the NCI-N87 gastric cancer cell line. AGS, KATOIII, MKN28, and MKN45 did not express SPARC mRNA. The differences in the expression of SPARC in homogenized ex vivo gastric cancer tissue enclosing the epithelial and nonepithelial components of tumor tissue and gastric cancer cell lines lead to the conjecture that SPARC in tumor tissue may arise from a cell type other than malignant epithelial cells (Figure 1B).
Immunohistochemistry on Large Tissue Sections
To evaluate where and which cells express SPARC in gastric cancer, we performed immunohistochemistry on 40 gastric tissue specimens and their corresponding lymph node metastases. The samples were obtained from both diffuse-type (20 cases) and intestinal-type (20 cases) gastric cancers, with both cohorts comparable in age (67.7 years vs 62.7 years; not statistically significant) and sex. Each block contained both noncancerous and cancerous tissues. We examined the expression of SPARC in the nonneoplastic mucosa and surface epithelium, the primary carcinomas, and the corresponding lymph node metastases.
SPARC was more commonly expressed in gastric cancer cells (30 patients, 75%) than in nonneoplastic surface epithelium (0%). Interestingly, SPARC was expressed more commonly (20 intestinal type, 100%; 18 diffuse type, 90%) in the cells of the desmoplastic stroma surrounding the tumor cells than in the tumor cells themselves (Figure 2). The immunoreactivity in the intestinal cancer was mild in 7 (35%), moderate in 11 (55%), and strong in 2 (10%) cases. Diffuse gastric cancer cells showed a mild reaction in 8 (40%), moderate in 9 cases (45%), and strong in 1 (5%) case. A gradient of SPARC expression was found in 30 cases (17 intestinal type and 13 diffuse type) with a prominent immunoreaction of stromal cells at the invasion front of the primary gastric cancers and decreased staining intensity in the tumor center. The stromal cells of the nonneoplastic mucosa also frequently expressed SPARC (36, 90%). However, the immunoreaction was weaker than in the cells of the desmoplastic stroma. SPARC was not observed in the smooth muscle cells of either the muscularis mucosae or muscularis propria.
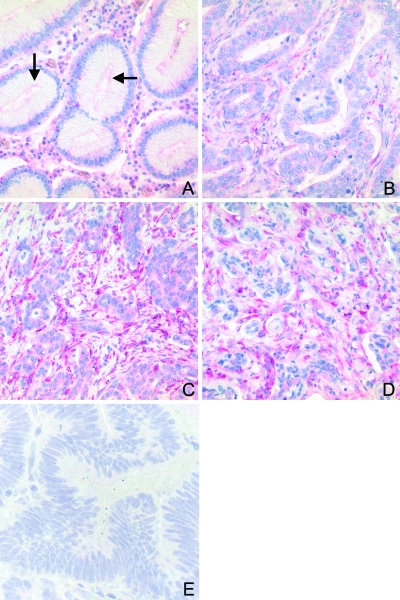
Figure 2.
Immunohistochemical analysis of SPARC expression in whole tissue sections. Formalin-fixed and paraffin-embedded tissue sections were immunostained with anti-SPARC antibodies and counterstained with hematoxylin. A faint SPARC immunostaining was found in the apical region of foveolar epithelium (A; arrows). Although intestinal type tumor cells were frequently immunonegative, the stromal cells within and surrounding the tumor (B, C) showed staining of variable intensity: mild to moderate staining was found in the tumor center (B) and strong staining was observed in the invasion front (C) and lymph node metastases (D). All pictures from panels A to D were taken from the same section. Omission of the primary antibody served as a negative control (E).
The immunohistochemical analysis of SPARC expression in the corresponding lymph node metastases showed a similar staining pattern as the primary tumor. SPARC was expressed in carcinoma cells as well as in the cells of the desmoplastic stroma (Figure 2). Again, staining of cells of the desmoplastic stroma was more intense compared with staining of the epithelial cancer cells.
Immunohistochemistry on TMA Sections
To determine whether the expression of SPARC in primary gastric cancers correlates with clinicopathologic parameters, sections from TMAs with samples from 152 gastric cancers were stained with anti-SPARC antibody. Staining intensities were given as an IRS ranging from 0 to 3 (Table 2). Similar to the large tissue sections, SPARC was found in gastric cancer cells (11 cases, 7.0%; mean IRS = 0.029 ± 0.129, range = 0–1.2), the stromal cells of the desmoplastic stroma (146, 96%;mean IRS = 1.251 ± 0.685, range = 0–3.0), and occasionally in the nonneoplastic foveolar epithelium (2, 2%; mean IRS = 0.004 ± 0.032, range = 0–0.300], and stromal cells of the nonneoplastic mucosa (46, 31%; mean IRS = 0.1 ± 0.19, range = 0–1.0; Figure 3). The frequencies and IRS of the SPARC expression are summarized in Tables 2 and 3.
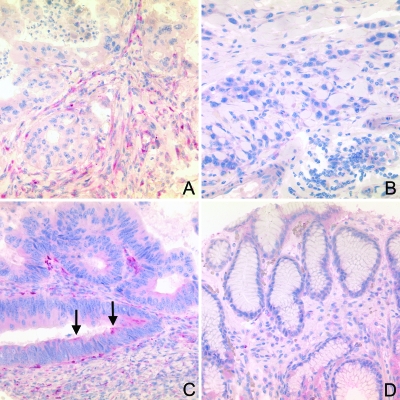
Figure 3.
Immunohistochemical analysis of SPARC expression in TMAs. Formalin-fixed and paraffin-embedded tissue sections from TMAs were immunostained with anti-SPARC antibodies and counterstained with hematoxylin. SPARC was expressed most strongly in stromal cells of the desmoplastic stroma of intestinal-type gastric cancer (A), less common in the stromal cells of diffuse type cancer (B), and occasionally, a cytoplasmic staining was found in intestinal-type tumor cells (C; arrows). Connective tissue and gastric foveolar epithelium were almost always negative (D).
Correlation of SPARC Expression with Clinicopathologic Parameters
Statistical analyses were carried out to evaluate correlations between SPARC expression assessed in TMAs (given either as mean IRS, IRS 0 vs >0, and IRS <1 vs ≥ 1) and various clinicopathologic parameters (Tables 2 and 3).
The differences in the mean IRS were highly significant (P < .01) between the following: epithelial cancer cells versus nonneoplastic foveolar epithelium; desmoplastic stroma versus stromal cells of the nonneoplastic mucosa; desmoplastic stroma of intestinal-type versus desmoplastic stromal of diffuse-type gastric cancer. In addition, the IRS of SPARCin cells of the desmoplastic stroma highly significantly correlated with tumor type (P = .002).
The IRS of SPARC was categorized and divided into two groups: 0 versus >0 and <1 versus ≥1, respectively (Table 2). Only the N category correlated significantly with the IRS <1 versus ≥1 of cells of the desmoplastic stroma, whereas a nonsignificant difference (P = .064) was noted for the tumor type. No other variable, for example, age, sex, T or M category, UICC tumor stage, or tumor grade of the entire study population, that is, including intestinal- and diffuse-type gastric cancers, correlated with the IRS of SPARC in either tumor cells or stromal cells.
The correlation between SPARC expression of stromal cells and clinicopathologic parameters was then analyzed separately in intestinal- and diffuse-type gastric cancers. Interestingly, in intestinal-type gastric cancers, the mean IRS of SPARC in cells of the desmoplastic stroma correlated highly significantly with the T category (P = .002), N category (P = .008), and UICC tumor stage (P = .003; Table 3). No correlations were found for diffuse-type gastric cancer.
SPARC Survival Analysis
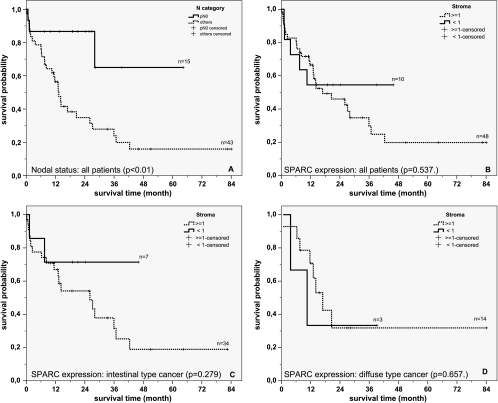
Univariate analysis showed that SPARC expression in stromal cells of intestinal-type gastric cancers was associated with a decreased patient survival. However, because of low patient numbers (n = 41), this did not reach statistical significance (P = .279; Figure 4). As a control for our study population, we also analyzed the influence of nodal spread on patient survival. A significant negative correlation was found between patient survival and the presence of lymph node metastases, with patients with a negative nodal status (N = 0) living longer than those with N > 0 (P < .01). The difference remained significant for intestinal-type gastric cancers (P = .046) and, again, because of low case numbers, was not significant for diffuse-type gastric cancers (P = .125; not shown).
Figure 4.
Overall survival. Kaplan-Meier curves depicting overall patient survival according to nodal status (A; P = .010), expression of SPARC (<1 vs ≥1) in cells of the desmoplastic stroma in all patients (B; P = .537), patients with intestinal-type gastric cancer (C; P = .279) and patients with diffuse-type gastric cancer (D; P = .657).
Because the expression of SPARC in cells of the desmoplastic stroma correlated with the N category (Table 2) and because patient survival is influenced by nodal status, we performed a Cox regression with a variable reduction procedure (Wald forward) using nodal status (N = 0 vs N > 0) and SPARC expression as covariables. This procedure showed that only the nodal status is of importance for this model for all patients (P = .027) as well as patients with intestinal-type gastric cancer (P = .064).
Discussion
In this study, we show that secreted protein acidic and rich in cysteine (SPARC; syn. osteonectin, BM-40) is differentially expressed in metastastic gastric cancer, localized primarily in the stromal cells of the desmoplastic stroma surrounding the epithelial tumor cells, and correlates significantly in intestinal-type gastric cancer with the local tumor growth, nodal spread, and tumor stage. On the basis of our results, we hypothesize that SPARC is involved in the pathology of gastric cancers.
SPARC is a calcium-binding glycoprotein with three domains: the N-terminal acidic domain with a low affinity for calcium, a copper-binding domain, and the C-terminal, calcium-binding region [18]. The single-copy gene is located on chromosome 5, and the secreted gene product has a molecular weight of 43 kDa. It was first described as being a constituent of human and bovine bones [19], but in the embryo, SPARC is expressed in all germ lines and is also found in the skin, heart, kidney, lung, testicle, thyroid, and intestine.
Matricellular proteins, such as SPARC, play important roles in the remodeling of the extracellular matrix without being a structural component of it. Several other matricellular proteins, including SPARC-like 1, testican 1 to 3, SMOC 1 to 2, CCN 1 to 6, thrombospondin 1 to 5, osteopontin, tenascin-C, tenascin-X, and galectin, have been recently identified [20]. All these proteins exhibit a high level of expression during embryogenesis and minimal or no expression in the adult organism, with reexpression occurring after tissue injury, inflammation, and tumorigenesis [21,22]. The up-regulation of SPARC observed in this study in gastric cancer tissues, its expression in epithelial and stromal cells, and the increased expression at the invasion front, where tissue remodeling is most prominent, are in line with previous observations and its known biologic functions as matricellular protein.
SPARC in Gastric Cancer
Our study also supports previous investigations regarding differential expression of SPARC in gastric cancer, including three groups using a gene array-based approach [23–25]. In keeping with our own observations, all previous studies collectively reported an up-regulation of SPARC on transcriptional, translational, or both levels in gastric cancer compared with nonneoplastic mucosa [23–27]. However, there are discrepancies regarding the histoanatomic distribution of SPARC in the stomach. Wewer et al. [27] described a differential expression of SPARC in the epithelial and stromal compartments of six gastric cancer specimens. Maeng [26] found SPARC only in stromal cells of their 31 gastric cancer patients and not in any epithelial cancer cell. Wang et al. [25] also found a differentially expressed SPARC in gastric cancer patients as assessed by gene array analysis, quantitative RT-PCR, and immunostaining. However, Wang et al. [25] found SPARC in normal gastric epithelial cells, marked in gastric cancer cells, and in low levels in the surrounding stromal cells of the tumor tissue. These discrepancies cannot be fully explained. The size of the study populations, the choice of primary antibodies, antibody dilutions, staining protocols, and tissue specimens may all contribute to variable staining patterns among nonneoplastic epithelium, cancer, and stromal cells. Even in our study, which until now presents the largest series of gastric cancer patients investigated for SPARC expression, we observed a discrepancy between nonneoplastic epithelium in large tissue sections and sections from TMA. The latter may be explained by the fact that nonneoplastic mucosa in large tissue sections was usually adjacent to the tumor area, which may have influenced SPARC expression. Our sections from TMA included nonneoplastic mucosa more distant from the primary tumor site and may explain the reduced expression of SPARC. Indeed, we noticed in the large tissue sections that mucosa adjacent to the tumor expressed SPARC more commonly than mucosa at a distance. These details were not considered in previous studies and may have influenced the results.
SPARC in Tumor Biology
Our immunohistochemical studies showed that SPARC expression in cells of the desmoplastic stroma highly significantly correlated with tumor type, favoring the intestinal type of gastric cancer. Similar observations were previously made in breast cancer. Watkins et al. [28] showed that ductal adenocarcinomas of the breast with a cohesive tumor growth pattern, similar to the intestinal-type gastric cancer, had higher SPARC levels than lobular breast cancers, with a decohesive growth pattern resembling that of diffuse-type gastric cancer. In addition, many studies have provided evidence that the desmoplastic tumor stroma is not a passive mold for the epithelial tumor cells, but influences tumor cell differentiation, proliferation, apoptosis, and mobility, thereby playing a prominent role in tumor biology [29–31]. It is to be expected that the expression of SPARC in cells of the desmoplastic stroma of gastric cancer correlates with the histologic tumor type. However, on the basis of our data, the nature of the interaction remains obscure. SPARC expression in stromal cells may depend on the histologic type of the tumor and vice versa. Indeed, transfection of melanocytes with SPARC was associated with a change of their phenotype from dendritic to fibroblast-like [32]. Mechanisms of SPARC action were also examined in SPARC wild-type and SPARC knockout mice injected with ovarian cancer cells. Wild-type mice had higher levels of tissue inhibitor of matrix metalloproteinases 1 and 2 and reduced levels of matrix metalloproteinases, leading to decreased vascular endothelial growth factor levels, a reduced activation of integrins on the cell surface, and an increased deadhesion of the tumor cells [33,34]. A similar mechanism may be active in the diffuse type of gastric cancer, where a reduced expression of SPARC in stromal cells may support a decohesive phenotype.
The correlation found in this study between the expression of SPARC in stromal cells and the local tumor growth, metastasis formation, and tumor stage is reinforced by similar results in both gastric cancer and other tumor types [35]. Other groups also studied the correlation between the expression of SPARC, either on the transcriptional [25] or the translational level [23], with various clinicopathologic patient characteristics. They found a correlation between SPARC expression and local tumor growth (T category), nodal spread (N category), and UICC tumor stage, which is in line with our own findings. However, in this context, it is worth noting that Wang et al. [25] used tissue homogenates and correlated overall SPARC mRNA levels in tumor tissue without separating into the epithelial and stromal compartment. Oue et al. [23] studied gastric cancer patients with a special focus on atomic bomb survivors. Both did not find any correlation between SPARC expression and tumor type, that is, intestinal- and diffuse-type gastric cancer. This may also be related to the different study populations and different methods used and may explain this particular discrepancy to our own result. Wang et al. [25] studied only 43 patients, which may have been too few to find a difference on the transcriptional level between intestinal- and diffuse-type gastric cancer. Despite including more patients, Oue et al. [23] mixed gastric cancer with and without radiation exposure. Patients exposed to radiation were shown to have lower levels of SPARC in their tumor tissue compared with non-radiation-exposed individuals. Nevertheless, a recent article demonstrated a correlation of SPARC mRNA and protein expression with the World Heath Organization's grade in meningioma [36], indicating that there are valid grounds for a correlation between SPARC expression and tumor type.
SPARC plays a role in various biologic functions, most of which are important capabilities for local tumor growth and metastasis formation. Deregulation of any of these processes, which include cellular morphology, cell adhesion and deadhesion, migration, apoptosis, angiogenesis, and tissue remodeling, would contribute to the invasion and metastasis of tumor cells [37–45]. It has been shown that SPARC increases cell mobility and migratory behavior in glioblastoma [46], kidney [47], and prostate cancer cells, partly in a concentration-dependent manner [48].
Invasion of epithelial tumor cells is characterized and indispensably associated with the formation of the desmoplastic stroma, which provides a tumor biologic rationale for the increased expression of SPARC at the invasion front, as it was found in our series of large gastric cancer tissue sections. Tissue remodeling is most prominent at this transitional interface, where we found the highest expression of SPARC. Local tumor growth also depends on a sufficient supply of oxygen and nutrients, and neoangiogenesis is one of several hallmarks of malignancy. Depending on the local concentration and the primary structure of its proteolytic fragments generated in situ, SPARC may either promote or inhibit neovascularization [18,46,49–52]. Indeed, in our study, the expression of SPARC correlated in the intestinal-type gastric cancer with the local tumor growth and at least supports the contention that SPARC may have a proangiogenic effect in gastric cancer.
In summary, including our own study, there is now ample transcriptional and translational evidence for a differential expression of SPARC in gastric cancer tissue. The expression of SPARC correlates with the tumor phenotype, local tumor growth, nodal spread, and UICC tumor stage. On the basis of our observations and those made by others, we hypothesize that SPARC is a promising novel target for the treatment of gastric cancer.
References
- 1.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(Suppl 2):ii31–ii36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi A, Nakagohri T, Konishi M, Inoue K, Takahashi S, Itou M, Sugitou M, Ono M, Saito N, Kinoshita T. Aggressive surgical treatment for T4 gastric cancer. J Gastrointest Surg. 2004;8:464–470. doi: 10.1016/j.gassur.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Ichikura T, Tomimatsu S, Uefuji K, Kimura M, Uchida T, Morita D, Mochizuki H. Evaluation of the New American Joint Committee on Cancer/International Union against cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer. 1999;86:553–558. doi: 10.1002/(sici)1097-0142(19990815)86:4<553::aid-cncr2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H, Kikuchi S. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380–384. doi: 10.1080/00365520310008629. [DOI] [PubMed] [Google Scholar]
- 5.Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–552. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchet A, Mocellin S, Ambrosi A, de Manzoni G, Di Leo A, Marrelli D, Roviello F, Morgagni P, Saragoni L, Natalini G, et al. The prognostic value of N-ratio in patients with gastric cancer: validation in a large, multicenter series. Eur J Surg Oncol. 2008;34:159–165. doi: 10.1016/j.ejso.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 8.Laurén T. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Wittekind Ch. TNM Classification of Malignant Tumours. 6th ed. West Sussex, England: John Wiley & Sons; 2002. [Google Scholar]
- 10.Weichert W, Röske A, Gekeler V, Beckers T, Ebert MP, Pross M, Dietel M, Denkert C, Röcken C. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9:139–148. doi: 10.1016/S1470-2045(08)70004-4. [DOI] [PubMed] [Google Scholar]
- 11.Le BB, Faouzi S, Boussarie L, Guirouilh J, Blanc JF, Carles J, Bioulac-Sage P, Balabaud C, Rosenbaum J. Osteonectin/SPARC is overexpressed in human hepatocellular carcinoma. J Pathol. 1999;189:46–52. doi: 10.1002/(SICI)1096-9896(199909)189:1<46::AID-PATH392>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Massi D, Franchi A, Borgognoni L, Reali UM, Santucci M. Osteonectin expression correlates with clinical outcome in thin cutaneous malignant melanomas. Hum Pathol. 1999;30:339–344. doi: 10.1016/s0046-8177(99)90014-x. [DOI] [PubMed] [Google Scholar]
- 13.Porte H, Chastre E, Prevot S, Nordlinger B, Empereur S, Basset P, Chambon P, Gespach C. Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int J Cancer. 1995;64:70–75. doi: 10.1002/ijc.2910640114. [DOI] [PubMed] [Google Scholar]
- 14.Rempel SA, Golembieski WA, Fisher JL, Maile M, Nakeff A. SPARC modulates cell growth, attachment and migration of U87 glioma cells on brain extracellular matrix proteins. J Neurooncol. 2001;53:149–160. doi: 10.1023/a:1012201300188. [DOI] [PubMed] [Google Scholar]
- 15.Sakai N, Baba M, Nagasima Y, Kato Y, Hirai K, Kondo K, Kobayashi K, Yoshida M, Kaneko S, Kishida T, et al. SPARC expression in primary human renal cell carcinoma: upregulation of SPARC in sarcomatoid renal carcinoma. Hum Pathol. 2001;32:1064–1070. doi: 10.1053/hupa.2001.28244. [DOI] [PubMed] [Google Scholar]
- 16.Thomas R, True LD, Bassuk JA, Lange PH, Vessella RL. Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res. 2000;6:1140–1149. [PubMed] [Google Scholar]
- 17.Yamanaka M, Kanda K, Li NC, Fukumori T, Oka N, Kanayama HO, Kagawa S. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol. 2001;166:2495–2499. [PubMed] [Google Scholar]
- 18.Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- 19.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 20.Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment—where there's SPARC, there's fire. J Cell Biochem. 2008;104:721–732. doi: 10.1002/jcb.21688. [DOI] [PubMed] [Google Scholar]
- 21.Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost. 2003;90:986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- 22.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oue N, Sentani K, Sakamoto N, Motoshita J, Nishisaka T, Fukuhara T, Matsuura H, Sasaki H, Nakachi K, Yasui W. Characteristic gene expression in stromal cells of gastric cancers among atomic-bomb survivors. Int J Cancer. 2009;124:1112–1121. doi: 10.1002/ijc.24060. [DOI] [PubMed] [Google Scholar]
- 24.Takeno A, Takemasa I, Doki Y, Yamasaki M, Miyata H, Takiguchi S, Fujiwara Y, Matsubara K, Monden M. Integrative approach for differentially overexpressed genes in gastric cancer by combining large-scale gene expression profiling and network analysis. Br J Cancer. 2008;99:1307–1315. doi: 10.1038/sj.bjc.6604682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CS, Lin KH, Chen SL, Chan YF, Hsueh S. Overexpression of SPARC gene in human gastric carcinoma and its clinic-pathologic significance. Br J Cancer. 2004;91:1924–1930. doi: 10.1038/sj.bjc.6602213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeng HY, Song SB, Choi DK, Kim KE, Jeong HY, Sakaki Y, Furihata C. Osteonectin-expressing cells in human stomach cancer and their possible clinical significance. Cancer Lett. 2002;184:117–121. doi: 10.1016/s0304-3835(02)00191-x. [DOI] [PubMed] [Google Scholar]
- 27.Wewer UM, Albrechtsen R, Fisher LW, Young MF, Termine JD. Osteonectin/SPARC/BM-40 in human decidua and carcinoma, tissues characterized by de novo formation of basement membrane. Am J Pathol. 1988;132:345–355. [PMC free article] [PubMed] [Google Scholar]
- 28.Watkins G, Douglas-Jones A, Bryce R, Mansel RE, Jiang WG. Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids. 2005;72:267–272. doi: 10.1016/j.plefa.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 30.Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- 31.Willhauck MJ, Mirancea N, Vosseler S, Pavesio A, Boukamp P, Mueller MM, Fusenig NE, Stark HJ. Reversion of tumor phenotype in surface transplants of skin SCC cells by scaffold-induced stroma modulation. Carcinogenesis. 2007;28:595–610. doi: 10.1093/carcin/bgl188. [DOI] [PubMed] [Google Scholar]
- 32.Robert G, Gaggioli C, Bailet O, Chavey C, Abbe P, Aberdam E, Sabatie E, Cano A, Garcia de Herreros A, Ballotti R, et al. SPARC represses E-cadherin and induces mesenchymal transition during melanoma development. Cancer Res. 2006;66:7516–7523. doi: 10.1158/0008-5472.CAN-05-3189. [DOI] [PubMed] [Google Scholar]
- 33.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 34.Said N, Najwer I, Motamed K. Secreted protein acidic and rich in cysteine (SPARC) inhibits integrin-mediated adhesion and growth factor-dependent survival signaling in ovarian cancer. Am J Pathol. 2007;170:1054–1063. doi: 10.2353/ajpath.2007.060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giatromanolaki A, Sivridis E, Koukourakis MI. The pathology of tumor stromatogenesis. Cancer Biol Ther. 2007;6:639–645. doi: 10.4161/cbt.6.5.4198. [DOI] [PubMed] [Google Scholar]
- 36.Mawrin C, Wolke C, Haase D, Krüger S, Firsching R, Keilhoff G, Paulus W, Gutmann DH, Lal A, Lendeckel U. Reduced activity of CD13/aminopeptidase N (APN) in aggressive meningiomas is associated with increased levels of SPARC. Brain Pathol. 2009 doi: 10.1111/j.1750-3639.2009.00267.x. February 18 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu A, Kligman LH, Samulewicz SJ, Howe CC. Impaired wound healing in mice deficient in a matricellular protein SPARC (osteonectin, BM-40) BMC Cell Biol. 2001;2:15. doi: 10.1186/1471-2121-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradshaw AD, Reed MJ, Sage EH. SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem. 2002;50:1–10. doi: 10.1177/002215540205000101. [DOI] [PubMed] [Google Scholar]
- 39.Chlenski A, Liu S, Guerrero LJ, Yang Q, Tian Y, Salwen HR, Zage P, Cohn SL. SPARC expression is associated with impaired tumor growth, inhibited angiogenesis and changes in the extracellular matrix. Int J Cancer. 2006;118:310–316. doi: 10.1002/ijc.21357. [DOI] [PubMed] [Google Scholar]
- 40.Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92:679–690. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- 41.Ledda MF, Adris S, Bravo AI, Kairiyama C, Bover L, Chernajovsky Y, Mordoh J, Podhajcer OL. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med. 1997;3:171–176. doi: 10.1038/nm0297-171. [DOI] [PubMed] [Google Scholar]
- 42.Puolakkainen PA, Bradshaw AD, Brekken RA, Reed MJ, Kyriakides T, Funk SE, Gooden MD, Vernon RB, Wight TN, Bornstein P, et al. SPARC-thrombospondin-2-double-null mice exhibit enhanced cutaneous wound healing and increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges. J Histochem Cytochem. 2005;53:571–581. doi: 10.1369/jhc.4A6425.2005. [DOI] [PubMed] [Google Scholar]
- 43.Reed MJ, Puolakkainen P, Lane TF, Dickerson D, Bornstein P, Sage EH. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41:1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- 44.Tremble PM, Lane TF, Sage EH, Werb Z. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol. 1993;121:1433–1444. doi: 10.1083/jcb.121.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yiu GK, Chan WY, Ng SW, Chan PS, Cheung KK, Berkowitz RS, Mok SC. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunigal S, Gondi CS, Gujrati M, Lakka SS, Dinh DH, Olivero WC, Rao JS. SPARC-induced migration of glioblastoma cell lines via uPA-uPAR signaling and activation of small GTPase RhoA. Int J Oncol. 2006;29:1349–1357. [PMC free article] [PubMed] [Google Scholar]
- 47.Kato Y, Sakai N, Baba M, Kaneko S, Kondo K, Kubota Y, Yao M, Shuin T, Saito S, Koshika S, et al. Stimulation of motility of human renal cell carcinoma by SPARC/osteonectin/BM-40 associated with type IV collagen. Invasion Metastasis. 1998;18:105–114. doi: 10.1159/000024503. [DOI] [PubMed] [Google Scholar]
- 48.Jacob K, Webber M, Benayahu D, Kleinman HK. Osteonectin promotes prostate cancer cell migration and invasion: a possible mechanism for metastasis to bone. Cancer Res. 1999;59:4453–4457. [PubMed] [Google Scholar]
- 49.Charest A, Pepin A, Shetty R, Cote C, Voisine P, Dagenais F, Pibarot P, Mathieu P. Distribution of SPARC during neovascularisation of degenerative aortic stenosis. Heart. 2006;92:1844–1849. doi: 10.1136/hrt.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC, Harris AL, Sage EH. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 51.Lane TF, Iruela-Arispe ML, Johnson RS, Sage EH. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell Biol. 1994;125:929–943. doi: 10.1083/jcb.125.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puolakkainen PA, Brekken RA, Muneer S, Sage EH. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res. 2004;2:215–224. [PubMed] [Google Scholar]