Abstract
Nephrectomized rats have widely been used to study chronic renal failure. Interestingly, renal cell carcinoma occurred in the remnant kidney after uninephrectomy (UNX). In this study, we probed insulin-like growth factor (IGF)-1 signaling pathway in UNX-induced renal cancer. Adult male Sprague-Dawley rats were randomized into two groups: UNX rats (n = 22) and sham-operated rats (n = 12). Rats were killed at 3, 7, and 10 months. After 7 months after nephrectomy, the UNX rats developed renal cell carcinoma with increased expression of proliferating cell nuclear antigen, and 68.2% (15/22) of the animals exhibited invasive carcinoma. Western blot demonstrated significant down-regulation of IGF binding protein 3 contrasting with the up-regulation of protein kinase Cζ and Akt/protein kinase B in the renal cancer tissues. These findings indicate a unique rat model of UNX-induced renal cancer associated with enhanced IGF-1 signaling pathway.
Introduction
Nephrectomized animal models have widely been used in studies of high blood pressure [1,2] and renal dysfunction [3]. Interestingly, we found renal cell carcinoma in uninephrectomized (UNX) rats unexpectedly. However, the molecular mechanism underlying the renal cancer is unknown. Previous studies suggested that carcinogenesis linked to insulin-like growth factor-1 (IGF-1) signaling pathway. Key IGF-1 signaling molecules such as IGF binding protein 3 (IGFBP-3), Akt/protein kinase B (PKB) and protein kinase C (PKC) ζ contribute to malignant transformation of proliferating cells and promote cancer cell growth and invasion.
IGF-1 has long been recognized to play an important role in cell growth and carcinogenesis [4]. Elevated blood IGF-1 level was reported in previous studies during compensatory renal growth in unilateral or subtotal nephrectomized human subjects and animals [5,6]. Furthermore, administration of IGF-1 was found to stimulate early growth of renal cell carcinoma in vivo [7]. Conversely, cancer growth stimulated by IGF-1 is associated with a low level of IGF-1 binding proteins (IGFBPs) [8]. Among the IGFBPs, IGFBP-3 is identified for its inhibitory effects on cancer growth. Reduction in IGFBP-3 levels contributes to uncontrolled cell growth by increasing IGF bioavailability and diminishing its inhibitory effect on the IGF pathway. Indeed, lower IGFBP-3 level is linked to increased risk of relapse and unfavorable prognosis in patients with different types of cancers [9,10]. The cancer-promoting effects of IGF are mediated by the phosphoinositide 3-kinase (PI3K) signaling pathway [4], in which Akt/PKB has been well recognized for its tumor-stimulating effects by enhancing protein synthesis and suppressing apoptosis [11]. Similarly, PKCζ of the PI3K signaling pathway is another key molecule in cancer biology [12]. Activation of PKCζ has been found to stimulate protein synthesis [12] and mediate cell proliferation, cell survival, cell migration, and angiogenesis during cancer progression [13].
Cancer growth is characterized by uncontrolled cell proliferation. Proliferating cell nuclear antigen (PCNA) is a key protein expressed in the nuclei of actively dividing cells during the DNA synthesis phase of the cell cycle [14]. PCNA is involved in a wide range of cellular functions including DNA replication, repair, and epigenetic maintenance [15]. PCNA level in cancer cells is usually several-fold higher than those in normal cells [16]. Clinically, PCNA serves as a general proliferative marker, especially in predicting prognosis of cancer progression and metastasis [17]. In this study, we probed the protein expression levels of PCNA, IGFBP-3, PKCζ, and Akt/PKB in UNX-induced renal cancer to reveal the role of the IGF-1 signaling pathway in renal carcinogenesis.
Materials and Methods
Experiments were performed under the Animals (Control of Experiments) Ordinance and received the approval of Animal Research Ethics Committee of Hong Kong.
Animals
Three-month-old male Sprague-Dawley rats between 250 and 300 g were obtained from the Laboratory Animal Services Centre at the Chinese University of Hong Kong. The animals were caged in pairs, housed at 23 ± 1°C with artificial lights on from 6:00 am to 6:00 pm, and had free access to water and standard laboratory rat diet (5001 Rodent Diet; LabDiet America, St Louis, MO). Rats were randomized into either left nephrectomy (UNX, n = 22) or sham operation (sham, n = 12). At 3 months after operation, 2 rats from each group were killed for histologic assessment of renal cancer, whereas 10 UNX and 5 sham rats were killed at 7 and 10 months after operation. The total duration of observation was 10 months.
Unilateral Nephrectomy
Rats were anesthetized with ketamine (75 mg/kg; Alfasan, Woerden, the Netherlands) and xylazine (10 mg/kg; Alfasan). The left kidney was exposed through left flank incision and was removed, leaving the adrenal gland intact in the abdominal cavity. Sham-operated rats underwent anesthesia and ventral laparotomy without removal of the left kidney. After surgery, animals were recovered from anesthesia under a heating lamp with food and water freely available.
Metabolic and Biochemical Studies
Body weight and daily intake of water and food were monitored monthly. Eight-hour fasting blood samples for sera preparation were collected by cardiac puncture when the rats were killed. Fasting blood samples were sent to the Department of Chemical Pathology in the Prince of Wales Hospital for the measurement of renal function. Serum urea was measured by enzymatic method using a Modular Analytics analyzer (Roche Diagnostics GmbH, Mannheim, Germany).
Histologic Studies of Kidneys
Rats were longitudinally killed at 3, 7, or 10 months after operation. Kidneys from all the rats were removed, weighted, and processed for light microscopy. Tissue samples were fixed in 10% neutral formaldehyde and embedded in paraffin. Serial longitudinal sections (4 µm) were spliced parallel to the longest axis of the kidney and stained with periodic acid-Schiff. Stained slides were examined with a Zeiss Axioplan 2 imaging microscope (Carl Zeiss, Inc, Hamburg, Germany), and representative images were captured using a SPOT digital camera (Diagnostic Instruments, Inc, Sterling Heights, MI).
Western Blot Assays
Tissue total proteins from renal cortex were extracted. Briefly, tissue was homogenized in a buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1% sodium deoxycholate, 1% Triton X-100, 1% sodium dodecyl sulfate, and 5% protease inhibitor cocktail (catalog no. P2714; Sigma, St Louis, MO). The homogenate was centrifuged at 13,000 rpm for 10 minutes at 4°C. The resulting supernatant was removed, and protein concentrations in the supernatant were determined by the BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA). Tissue lysates (100 µg) and prestained molecular weight markers (Bio-Rad, Hercules, CA) were loaded onto sodium dodecyl sulfate-polyacrylamide electrophoresis gels with 4% acrylamide stacking gel and 10% running gel. The resolved proteins were then transferred onto nitrocellulose membranes. The membranes were blocked for 1 hour at room temperature with 5% skimmed milk, incubated with primary antibodies of IGFBP-3 (dilution 1:1000; Santa Cruz Biotechnology Santa Cruz, CA), Akt1/2/3 (dilution 1:1000; Cell Signaling Technology, Danvers, MA), PKCζ (dilution 1:1000; Santa Cruz Biotechnology), and mouse anti-PCNA (dilution 1:1000; Dako, Carpinteria, CA) in TBS containing 0.05% Tween 20 (TBS-T) with 5% skimmed milk overnight at 4°C. After washing with TBS-T, membranes were incubated with antigoat, antirabbit, or antimouse secondary antibody conjugated to horseradish peroxidase (Upstate, Temecula, MA) with dilution of 1:2000. Proteins were detected by enhanced chemiluminescence (Amersham, Piscataway, NJ) on Hyperfilm. The major protein bands with approximately 42 kDa for IGFBP-3, 60 kDa for Akt1/2/3, 80 kDa for PKCζ, and 30 kDa for PCNA were detected. To ensure equal loading of proteins, membranes were incubated and probed with a rabbit anti-β-actin antibody (Abcam, Cambridge, MA) with dilution of 1:10,000, which recognizes β-actin at approximately 43 kDa. Signals were quantitated by densitometry and corrected for the β-actin signal, using the Kodak Digital Image station 440CF and the ID Image Analysis software program (Kodak, Rochester, NY).
Statistical Analysis
Data are mean ± SD unless specified. The statistical significance of difference noted in the biochemical parameters was evaluated using 2-tailed t-test or Mann-Whitney U test. P < .05 was taken as criterion for a statistically significant difference.
Results
Carcinogenesis of Remnant Kidneys
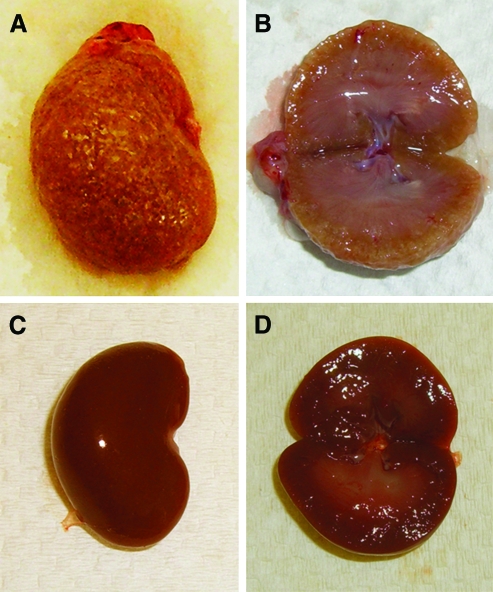
Grossly, UNX rats showed a double-sized remnant kidney (4.5 ± 0.9 vs 2.1 ± 0.2 g in sham rats, P < .001) with pallor, grainy surface Figure 1). The remnant kidneys with cancer showed diffuse thickening of renal cortex in longitudinal sections (Figure 1, A and B). In sham rats, the remnant kidneys were reddish with lighter outer cortex and darker medulla (Figure 1, C and D).
Figure 1.
Gross examination of the remnant kidney in UNX rats. (A) UNX rats exhibited a enlarged granular, grayish remnant kidney. (B) Cross-sectional view of the remnant kidney in UNX rats demonstrates diffuse thickening of cortex. (C) Sham rats showed a smooth, reddish right kidney. (D) Cross-sectional view of the right kidney in sham rats demonstrates lighter cortex and darker medulla.
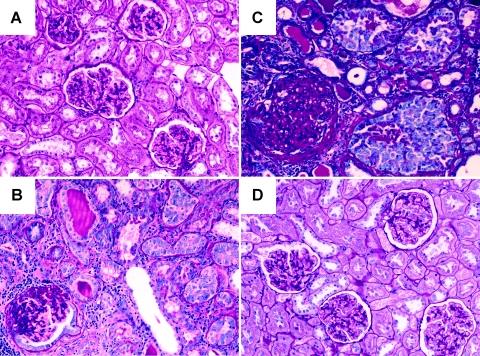
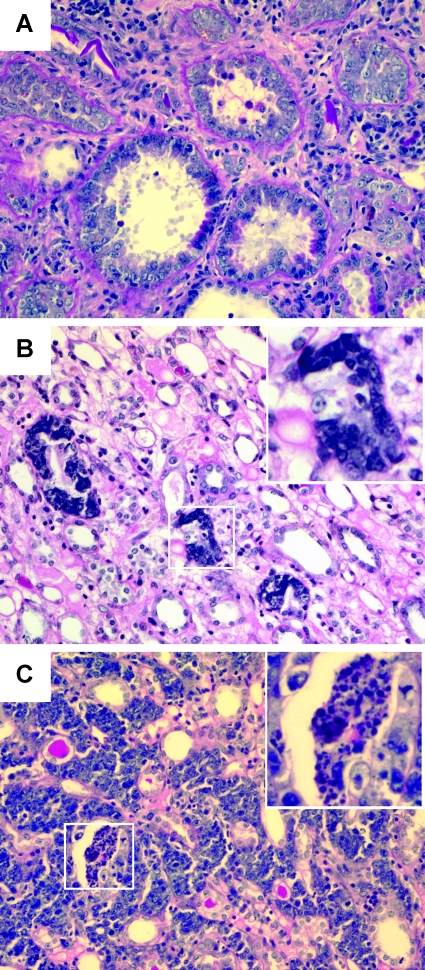
A spectrum of renal carcinogenesis from neoplastic proliferation of tubular epithelial cells to invasive carcinoma was observed by longitudinally microscopic examination of the remnant kidneys (Figure 2). At 3 months after operation, microscopic examination revealed glomerular hypertrophy and tubular cell neogenesis without malignant transformation in the remnant kidney (Figure 2A). At 7 months after operation, renal cell carcinoma was found in 7 of 10 UNX rats killed at this time point (Figure 2B). At 10 months after operation, renal cell carcinoma was found in 8 of 10 UNX rats (Figure 2C). In contrast, sham rats showed normal renal structure during the observation period (Figure 2D). The cancer lesions in the remnant kidneys included neoplastic proliferation of tubular epithelial cells (Figure 3A), in situ carcinoma (Figure 3B, inset), invasive carcinoma with central necrosis, and invasion of nonneoplastic tubules (Figure 3C, inset).
Figure 2.
Development of renal cell carcinoma in the remnant kidney of UNX rats. (A) At 3 months after nephrectomy, UNX rats demonstrated glomerular hypertrophy but not malignancy. (B) At 7 months after nephrectomy, UNX rats showed malignant transformation of tubular epithelial cells in parallel to diffuse glomerulosclerosis. (C) At 10 months after nephrectomy, UNX rats showed nests of renal cell carcinoma in parallel to global glomerulosclerosis. (D) Sham rats at 10 months after operation revealed normal renal structure.
Figure 3.
Morphology of carcinogenesis in the remnant kidneys of UNX rats at 10 months after nephrectomy. (A) Neoplastic proliferation of tubular epithelial cells. (B) In situ carcinoma (inset). (C) Invasive carcinoma with central necrosis (inset) and invasion of nonneoplastic tubules.
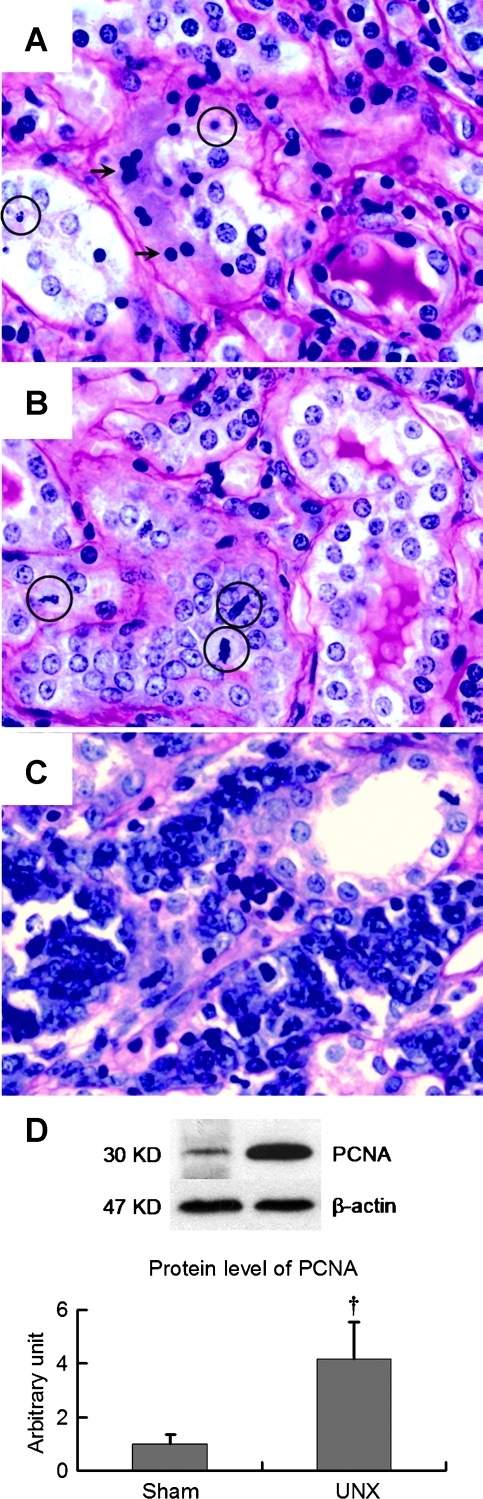
Malignant transformation of renal cell nuclei was found in parallel to the neogenesis and carcinogenesis in the remnant kidney of UNX rats (Figure 4). At 10 months after nephrectomy, tubular cell apoptosis as evident by extensive apoptotic bodies and nuclear condensation were seen in renal tubules (Figure 4A). Simultaneously, cell proliferation as evident by increased mitotic figures (mitosis) and tubular cell neogenesis (Figure 4B) was frequently observed. Histopathologic characteristics of renal cell carcinoma in the UNX rats included bizarre nuclei, frequent mitosis, and tissue invasion (Figure 4C). In agreement with the histopathologic observation,Western blot revealed a four-fold increase of PCNA protein expression in the remnant kidneys with carcinoma (Figure 4D).
Figure 4.
Apoptosis, neogenesis, carcinogenesis, and protein expression of PCNA in the remnant kidneys of UNX rats at 10 months. (A) Tubular cell apoptosis as evident by apoptotic bodies (circles) and nuclear condensation (arrows). (B) Tubular cell neogenesis as evident by mitotic figures (circles). (C) Renal cell carcinoma is characterized by tissue invasion and bizarre nucleation. Protein abundance of PCNA was four-fold high in the remnant kidney with cancer in UNX rats. Data are mean ± SD; †P < .05 versus sham rats.
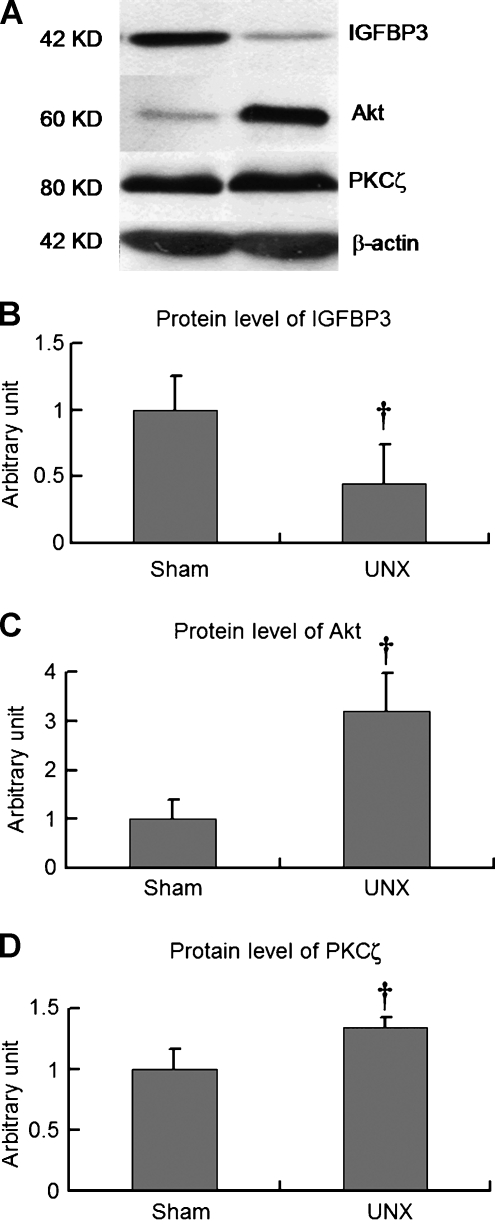
Protein Expression Levels of IGF Signaling Pathway
Protein expression levels of IGF signaling molecules such as IGFBP-3, Akt/PKB, and PKCζ were measured by Western blot. The detected protein levels were normalized to the expression level of β-actin and then adjusted to the corresponding quantities in sham rats (Figure 5). In the remnant kidneys of UNX rats, protein expression of the growth inhibitory factor IGFBP-3 was significantly reduced approximately to 50% (Figure 5, A and B). In contrast, protein expression levels of growth-promoting factors Akt/PKB and PKCζ in the remnant kidneys of the UNX rats were significantly increased (Figure 5, A, C, and D). These findings indicated down-regulation of IGFBP-3 protein expression coupled with up-regulation of Akt and PKCζ in the remnant kidneys with cancer.
Figure 5.
Expression of IGFBP-3, Akt/PKB, and PKCζ by Western blot. Renal tissue specimens were obtained from sham and UNX rats after 10 months after operation. Protein expression of IGFBP-3 reduced approximately 50% (panels A and B), whereas protein abundance of Akt/PKB (panels A and C) and PKCζ was increased (panels A and D). Data are mean ± SD; †P < .05 versus sham rats.
Uninephrectomy-Induced Renal Impairment
In this study, fasting serum urea and creatinine levels at 10 months after operation were measured to reflect renal function. Compared with sham-operated rats, UNX rats had significantly higher serum urea (19.6 ± 7.3 vs 7.0 ± 1.9 mM, P < .001) and creatinine (98.5 ± 25.5 vs 42.9 ± 9.8 µM, P < .001). Body weight (564 ± 49 vs 593 ± 42 g, P = .225) and daily food consumption (34 ± 6 vs 33 ± 5 g, P = .723) were similar between the UNX rats and sham rats, whereas UNX rats had more water consumption (73 ± 9 vs 41 ± 7 ml, P < .001) than sham rats.
Discussion
In this report, we longitudinally described the development and progression of renal cell carcinoma in the remnant kidneys of UNX rats. Molecular mechanisms underlying the renal carcinogenesis focus on the IGF signaling pathway involving key molecules such as IGFBP-3, Akt/PKB, and PKCζ.
Elevation of renal tissue IGF-1 is associated with the enlargement of remnant kidney in UNX rats [18]. Renal mass loss, as induced by UNX in this study, may stimulate IGF-1 production and diminish IGF-1 degradation and excretion from the remnant kidney, thus, consequently, augments local and systemic IGF-1 levels. In this regard, IGF-1 can be both a cause and a consequence of compensatory renal growth. Although renal IGF-1 levels were not measured in this study, a two-fold enlargement of remnant kidneys in UNX rats compared with the corresponding right kidneys in sham-operated rats was observed in previous study [19]. Hence, it is possible that the increased Akt/PKB and PKCζ expression may result from the activated receptors of growth factors including IGF-1 and insulin that are required by the compensatory renal cell hyperplasia and proliferation. Therefore, normal IGF-1 signaling pathway such as the regulation of IGF-1 by IGFBP-3 in normal renal tissue might be disturbed in the remnant kidneys of UNX rats.
The PI3K/Akt pathway is believed to be an important cellular adaptation toward cell growth and malignant transformation. It has been shown that activation of Akt/PKB is tumor-specific [20] and that the PI3K/Akt pathway is constitutively activated in human cancers of several organs including ovary, prostate, and lung [21]. Elevation of Akt/PKB has been reported in several human renal cell carcinoma cell lines, indicating a constitutive activation of Akt/PKB in the pathogenesis of human renal cell carcinoma [20]. Our findings showed an elevated level of Akt/PKB in the remnant kidneys with cancer, suggesting that the PI3K/Akt signaling pathway is common to renal cell carcinogenesis in vivo. However, growth inhibition of cancer cells by the inhibitors of PI3K and Akt/PKB is not complete even at high concentrations [20], indicating that other growth/survival pathways also play a role in the development of renal cell carcinoma.
High incidence of cancer was noticed in human kidney transplant recipients. In a retrospective study, renal transplant recipients were found to have a 4.3 times higher risk of developing any type of cancers including renal cancer, compared with an age- and sex-matched population of the same geographical area [22]. Increased risk of cancer was also reported in Australian [23] and French [24] people who underwent renal transplantation. In contrast, only a two-fold increased incidence for some of the most common tumors in the general population, such as lung or colorectal cancers, was found in the kidney transplant recipients [22]. In addition to immunosuppression that predisposes to viral carcinogenesis, compensatory renal allograft growth associated with abnormal IGF-1 signaling pathway might be crucial. Interestingly, several clinical trials on renal transplant recipients revealed that therapies based on inhibition of mammalian target of rapamycin, a crucial factor activated by Akt/PKB in PI3K pathway, significantly reduced the risk of developing any malignancy compared with all other immunosuppressive regimens [25,26]. This finding suggests a pivotal role of PI3K pathway in the process of renal carcinogenesis.
One should be cautious in extrapolating from animal observations to human disease because profound differences exist between humans and rats. In this rodent study, most rats progressively developed chronic renal impairment after UNX. In human kidney donors, renal function test results are normal or only mildly impaired after uninephrectomy [27–29]. Nevertheless, physiopathologic changes observed in this animal model can shed light on the area of cancer biology especially in patients with chronic renal failure. In fact, a significant decrease in glomerular filtration rate was found in human kidney donors and other single-kidney survivors [30]. Long-term effects of kidney donation and renal transplantation on cancer risk warrant decades of clinical follow-up.
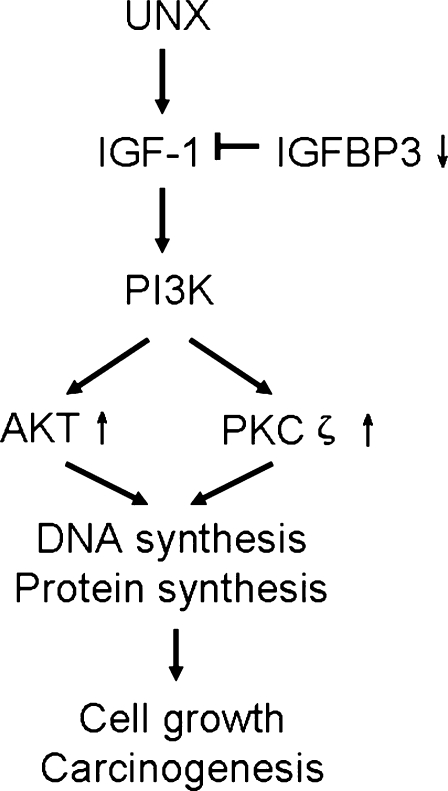
On the basis of the findings from this study, we propose a hypothesis of renal carcinogenesis induced by uninephrectomy (Figure 6). Evidence in this study suggests that IGF signaling pathway characterized by decreased IGFBP-3 contrasting with increased Akt/PKB and PKCζ expression may stimulate cell malignant transformation from limited cell proliferation, which may, in turn, predispose proliferating cells to cancer cells. Currently unknown are the detailed expressions of each factor mediating the process of malignant transformation. Clarification of the molecular mechanisms in this process awaits additional investigation and may be of value in cancer prevention and control.
Figure 6.
Proposed IGF-1 signaling pathway in renal carcinogenesis induced by uninephrectomy. Down-regulation of IGFBP-3 in IGF-1 signaling pathway contrasting with up-regulation of Akt/PKB and PKCζ expression may enhance cell proliferation, malignant transformation, cancer growth, and invasion.
Acknowledgments
The authors thank Liu Li-zhong and Stanley Cheung for the antibodies they provided.
Footnotes
The work is supported by a grant from the Research Grants Council of Hong Kong (CUHK4462/06M) and by the Hong Kong Jockey Club Charities Trust (JCICM-P2-05, CUHK).
Declarations of interest
All the authors have no conflicts of interest.
References
- 1.Lopez-Hernandez FJ, Flores O, Lopez-Novoa JM, Montero MJ, Carron R. Antihypertensive action of trandolapril and verapamil in spontaneously hypertensive rats after unilateral nephrectomy. J Cardiovasc Pharmacol. 1998;32:284–290. doi: 10.1097/00005344-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Seymour AA, Davis JO, Freeman RH, DeForrest JM, Rowe BP, Stephens GA, Williams GM. Hypertension produced by sodium depletion and unilateral nephrectomy: a new experimental model. Hypertension. 1980;2:125–129. doi: 10.1161/01.hyp.2.2.125. [DOI] [PubMed] [Google Scholar]
- 3.Lopes GS, Lemos CC, Mandarim-De-Lacerda CA, Bregman R. Effect of unilateral nephrectomy on renal function of diabetic rats. Histol Histopathol. 2004;19:1085–1088. doi: 10.14670/HH-19.1085. [DOI] [PubMed] [Google Scholar]
- 4.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 5.Yildiz B, Kural N, Colak O, Ak I, Akcar N. IGF-1, IGFBP-3, VEGF and MMP-9 levels and their potential relationship with renal functions in patients with compensatory renal growth. Clin Physiol Funct Imaging. 2008;28:107–112. doi: 10.1111/j.1475-097X.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 6.Flyvbjerg A, Schrijvers BF, De Vriese AS, Tilton RG, Rasch R. Compensatory glomerular growth after unilateral nephrectomy is VEGF dependent. Am J Physiol Endocrinol Metab. 2002;283:E362–E366. doi: 10.1152/ajpendo.00007.2002. [DOI] [PubMed] [Google Scholar]
- 7.Rosendahl AH, Holly JM, Celander M, Forsberg G. Systemic IGF-I administration stimulates the in vivo growth of early, but not advanced, renal cell carcinoma. Int J Cancer. 2008;123:1286–1291. doi: 10.1002/ijc.23642. [DOI] [PubMed] [Google Scholar]
- 8.Toniolo P, Bruning PF, Akhmedkhanov A, Bonfrer JM, Koenig KL, Lukanova A, Shore RE, Zeleniuch-Jacquotte A. Serum insulin-like growth factor-I and breast cancer. Int J Cancer. 2000;88:828–832. doi: 10.1002/1097-0215(20001201)88:5<828::aid-ijc22>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 11.Petley T, Graff K, Jiang W, Yang H, Florini J. Variation among cell types in the signaling pathways by which IGF-I stimulates specific cellular responses. Horm Metab Res. 1999;31:70–76. doi: 10.1055/s-2007-978701. [DOI] [PubMed] [Google Scholar]
- 12.Mendez R, Kollmorgen G, White MF, Rhoads RE. Requirement of protein kinase C ζ for stimulation of protein synthesis by insulin. Mol Cell Biol. 1997;17:5184–5192. doi: 10.1128/mcb.17.9.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta K, Li J, Bhattacharya R, Gasparian L, Wang E, Mukhopadhyay D. Protein kinase C ζ transactivates hypoxia-inducible factor α by promoting its association with p300 in renal cancer. Cancer Res. 2004;64:456–462. doi: 10.1158/0008-5472.can-03-2706. [DOI] [PubMed] [Google Scholar]
- 14.Leonardi E, Girlando S, Serio G, Mauri FA, Perrone G, Scampini S, Dalla Palma P, Barbareschi M. PCNA and Ki67 expression in breast carcinoma: correlations with clinical and biological variables. J Clin Pathol. 1992;45:416–419. doi: 10.1136/jcp.45.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 16.Naryzhny SN, Lee H. Characterization of proliferating cell nuclear antigen (PCNA) isoforms in normal and cancer cells: there is no cancer-associated form of PCNA. FEBS Lett. 2007;581:4917–4920. doi: 10.1016/j.febslet.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Caputi M, Esposito V, Groger AM, Pacilio C, Murabito M, Dekan G, Baldi F, Wolner E, Giordano A. Prognostic role of proliferating cell nuclear antigen in lung cancer: an immunohistochemical analysis. In Vivo. 1998;12:85–88. [PubMed] [Google Scholar]
- 18.Flyvbjerg A, Frystyk J, Marshall SM. Additive increase in kidney insulin-like growth factor I and initial renal enlargement in uninephrectomizeddiabetic rats. Horm Metab Res. 1990;22:516–520. doi: 10.1055/s-2007-1004961. [DOI] [PubMed] [Google Scholar]
- 19.Sui Y, Zhao HL, Ma RC, Ho CS, Kong AP, Lai FM, Ng HK, Rowlands DK, Chan JC, Tong PC. Pancreatic islet β-cell deficit and glucose intolerance in rats with uninephrectomy. Cell Mol Life Sci. 2007;64:3119–3128. doi: 10.1007/s00018-007-7395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sourbier C, Lindner V, Lang H, Agouni A, Schordan E, Danilin S, Rothhut S, Jacqmin D, Helwig JJ, Massfelder T. The phosphoinositide 3-kinase/Akt pathway: a new target in human renal cell carcinoma therapy. Cancer Res. 2006;66:5130–5132. doi: 10.1158/0008-5472.CAN-05-1469. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 22.Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, Jauch KW, Guba M. The janus face of immunosuppression - de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007;71:1271–1278. doi: 10.1038/sj.ki.5002154. [DOI] [PubMed] [Google Scholar]
- 23.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 24.Kessler M, Jay N, Molle R, Guillemin F. Excess risk of cancer in renal transplant patients. Transpl Int. 2006;19:908–914. doi: 10.1111/j.1432-2277.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 25.Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, Claesson K, Stallone G, Russ G, Rostaing L, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17:581–589. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 26.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 27.Najarian JS, Chavers BM, McHugh LE, Matas AJ. 20 years or more of follow-up of living kidney donors. Lancet. 1992;340:807–810. doi: 10.1016/0140-6736(92)92683-7. [DOI] [PubMed] [Google Scholar]
- 28.Sahay M, Narayen G, Anuradha CV. Risk of live kidney donation—Indian perspective. J Assoc Physicians India. 2007;55:267–270. [PubMed] [Google Scholar]
- 29.Narkun-Burgess DM, Nolan CR, Norman JE, Page WF, Miller PL, Meyer TW. Forty-five year follow-up after uninephrectomy. Kidney Int. 1993;43:1110–1115. doi: 10.1038/ki.1993.156. [DOI] [PubMed] [Google Scholar]
- 30.Kasiske BL, Ma JZ, Louis TA, Swan SK. Long-term effects of reduced renal mass in humans. Kidney Int. 1995;48:814–819. doi: 10.1038/ki.1995.355. [DOI] [PubMed] [Google Scholar]