Abstract
The ovarian cancer microenvironment recruits an array of immune cells to the site of tumor growth. Within the peritoneal ascites of both humans and mice, the predominant population of tumor-infiltrating leukocytes is a CD11c+CD11b+ population variably referred to as vascular leukocytes (VLCs), tumor-associated macrophages, and immature dendritic cells. We have previously shown that these cells are critical for tumor growth because their selective elimination from the peritoneal tumor microenvironment inhibited tumor progression. However, the underlying mechanism by which this therapy was efficacious is poorly understood. Here, we use the murine ID8 ovarian tumor model to demonstrate that the tumor microenvironment induces in vivo immunosuppression of T cells and that the SR-A+ VLCs mediate this suppression. Importantly, the elimination of SR-A+ VLCs from the peritoneum of tumor-bearing mice relieves the T cell suppression. Moreover, the profound changes that VLC elimination has on the immune system are T cell-dependent because the protective antitumor effect of VLC elimination does not occur when CD8 T cells are concomitantly depleted. These results were confirmed and extended with the use of a genetic model for VLC depletion, which demonstrated that short-term therapeutic depletion of VLCs alleviates immunosuppression and allows for efficacious vaccination against model tumor antigens in tumor-bearing mice. These studies provide a mechanistic explanation for how leukocytes contribute to ovarian tumor progression and, correspondingly, how leukocyte depletion inhibits tumor growth.
Introduction
Tumor growth in a variety of tissues is associated with an influx of immune cells including T regulatory cells, tumor-associated macrophages, inhibitory dendritic cells, and myeloid-derived suppressor cells (MDSCs). These cells are co-opted by the tumor to sculpt the local tumor environment into an area permissive for tumor growth and metastasis through immunosuppression, angiogenesis, and tissue remodeling [1–6]. Indeed, leukocyte accumulation in tumor tissue is correlated with poor clinical outcome [7,8].
Specifically, both human and murine ovarian carcinomas and their associated ascites are infiltrated with a variety of immune cells [9–12]. The ID8 mouse model of ovarian cancer [13] faithfully reproduces the clinical progression in this regard in that it produces a robust peritoneal ascites into which it recruits an influx of CD11c+CD11b+SR-A+ leukocytes, which have been referred to as tumor-associated macrophages, immature DCs, and vascular leukocytes (VLCs) [14–16]. Importantly, these peritoneal VLCs are critical to tumor progression: by exploiting the expression of scavenger receptor-A (SR-A) on VLCs, we previously demonstrated that the targeted elimination of VLCs from the peritoneum of ID8-challenged mice led to a reduction in ascites volume and tumor burden [14]. Subsequent in vitro studies demonstrated that VLCs isolated from the tumor ascites function as MDSCs, inhibiting CD8 and CD4 T cell responses through an arginase-1 (ARG1)-dependent mechanism [17].
The induction of MDSCs at the site of primary tumor growth is associated with the presence of suppressive CD11b+Gr-1+ MDSCs in secondary lymphoid tissues and suppression of global immune responses [18]. Because ID8-induced VLCs constitute an immunosuppressive cell population, we hypothesized that ID8 tumor growth will lead to dysregulation of immune responses distal to tumor growth and that this suppression potentiates tumor growth. However, it is currently unknown whether VLCs abet tumor progression through their function as MDSCs nor is it known whether in vivo removal of these immunosuppressive cells from the ovarian tumor microenvironment can relieve the immunosuppression.
In this study, we demonstrate that the ID8 ovarian tumor induces both local and systemic in vivo T cell immunosuppression. Importantly, we show that VLCs are required to mediate this effect, and moreover, VLC depletion reverses the immunosuppressive tumor microenvironment and alleviates the T cell immunosuppression. Indeed, even short-term therapeutic VLC depletion relieves immunosuppression and allows for the induction of a peptide-specific immune response. Consistent with previous studies [14,15], we demonstrate that VLC depletion is sufficient to impair peritoneal ID8 tumor progression. Here, we tie together the role of VLCs as immunosuppressive cells and their function as obligate tumor-promoting cells by defining their in vivo effect on the immune system. We demonstrate that the suppressive effect of tumor growth on the host immune system is reversed through VLC depletion. Relevant to their role as immunosuppressive cells, we show that the reduced tumor growth mediated by VLC depletion is partially dependent on CD8+ T cells. These results will be discussed in the context of tumor-induced immune dysfunction and the implications on current immunotherapies against cancer.
Materials and Methods
Mice and Antibodies
Female C57Bl/6 and CB6/F1 mice (4–6 weeks old) were purchased from the National Cancer Institute (Fredericksburg, MD). C57Bl/6-RAG-/- and Balb/c-RAG-/- were purchased from Jackson Laboratories (Bar Harbor, ME) and CB6/F1 RAG-/- mice were used in indicated experiments. MAFIA mice [19] were purchased from Jackson Laboratories under agreement with Ariad Pharmaceuticals (Cambridge, MA). Animal experiments were approved by the Dartmouth Medical School Institutional Animal Care and Use Committee. Antimouse Fc-Block and antimouse CD11c (HC3) were purchased from BD Biosciences (San Jose, CA). Antimouse CD3 antibody (145-2C11), CD11b (M1/70), andGr-1 (RB6-8C5)were purchased from eBiosciences (SanDiego, CA). Antimouse CD8 antibody (53–6.7) was purchased from Biolegend (San Diego, CA).
Generation of Tumors and Harvest of Tumor-Associated Leukocytes
Ovarian tumors were generated and harvested as previously described [14]. Briefly, mice were injected intraperitoneally (i.p.) with 5 x 106 ID8 cells. Approximately 6 weeks later, peritoneal ascites were harvested. The cellular fraction was treated with ACK lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA) to remove red blood cells, and the remaining cells were resuspended in 0.5% BSA in PBS or medium for analysis. In indicated experiments, single-cell suspensions of spleens were generated from naive or tumor-challenged mice by digestion with collagenase/DNAse and passage through a 70-µM cell strainer (BD Biosciences).
Flow Cytometry
Cells from murine ascites were resuspended at 1 x 106 cells/ml in 0.5% BSA in PBS with Fc-blocking antibody and subsequently stained with the indicated antibodies. Flow cytometry was performed at the NCCC Englert Cell Analysis Laboratory using a FACS Calibur or FACS Aria (BD Biosciences) and analyzed with FloJo 8.8.4 Software (FloJo, Ashland, OR).
Splenocyte Activation Assays
Splenocytes were derived from mice as described previously. A total of 106 splenocytes were resuspended in medium and, as indicated, stimulated with 1 µg of plate-bound αCD3 antibody or a combination of 50 nM phorbol 12-myristate acetate (PMA) and 0.5 µM ionomycin (Sigma, St Louis, MO) and incubated at 37°C. After 72 hours, the cells were spun down, and supernatants were removed for ELISA. Supernatants were assayed for interferon-γ (IFN-γ) content using the murine DuoSet ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. In indicated experiments, CD11b+ cells from tumor-bearing or naive mice were isolated from splenocyte preparations through positive selection using Miltenyi CD11b magnetic beads (Miltenyi Biotec, Auburn, CA). Where indicated, 105 CD11b+ cells were added to naive splenocytes as previously mentioned.
Anti-SR-A Depletion of VLCs and CD8 Depletion
Preparation of anti-SR-A immunotoxin: 4.8 µg of Rat-ZAP in the absence (ZAP) or presence of 4 µg of clone 2F8 rat antimouse SR-A antibody (SRA-ZAP) was incubated on ice for 30 minutes. CB6/F1 mice were injected i.p. with 5 x 106 ID8-C3 cells, in conjunction with SRA-ZAP or ZAP, on day 0. Additional administrations of SRA-ZAP or ZAP were then given every 7 days. Indicatedmice were injected with 250 µl of anti-CD8 antibody, 2.43 mAb, a generous gift of Dr. Mary Jo Turk (Dartmouth Medical School, Lebanon, NH). Mice were weighed weekly to monitor tumor progression and were killed when an increase in body weight of 75% was observed. In indicated experiments, all mice were killed after 7 weeks, ascites volume was measured, and ascites cellularity was assessed after ACKlysis of RBC; in some cases, peritoneal lavage was necessary for mice with ascites less than 5 ml. Cells were stained with anti-CD11c antibody, and numbers of CD11c+ (CD11c+GFP-) and tumor cells (CD11c-GFP+) in the peritoneum of the mice were calculated by flow cytometry. Data are derived from three separate experiments, with a total of at least six mice in each treatment group.
Serum ELISAs
Ascites was collected from the peritoneum of tumor-bearing mice under the indicated treatment regimen, and samples were centrifuged for 10 minutes at 15,000g. Serum was collected and centrifuged once more for 10 minutes at 15,000g to remove any additional debris. Interleukin 10 (IL-10) and IFN-γ content was assessed by ELISA using the murine DuoSet ELISA (R&D Systems) according to the manufacturer's protocol.
AP20187 Treatment
AP20187 was obtained under agreement with Ariad Pharmaceuticals. MAFIA mice were treated with AP20187 as previously described [19,20]. Briefly, mice were treated with 10 µg of AP20187 per mouse in 450 µl of injection solution consisting of 4% ethanol, 10% PEG-400, and 1.7% Tween-20 in water. Control mice were treated with 450 µl of injection solution alone. To test the efficacy of AP20187 depletion of VLCs, MAFIA mice were injected with 1 x 106 ID8 cells; 6 weeks later, mice were treated with AP20187 or injection solution for three straight days. Mice were killed, and the post-RBC lysis fraction of ascites was stained with anti-CD11c antibody and assessed by flow cytometry.
Immunization and VLC Depletion
MAFIA mice were injected with 1 x 106 ID8 cells. At 5 to 6 weeks after tumor injection, mice were treated with AP20187 or injection solution, every other day for 5 days, for a total of three injections. On day 5, indicated mice were injected retro-orbitally with 2 µg of SIINFEKL peptide/10 µg of lipopolysaccharide/100 µg of agonistic aCD40 antibody (gift of the Noelle Laboratory, Dartmouth Medical School). After 72 hours, splenocytes were harvested as previously mentioned and were incubated with 1 µg/ml SIINFEKL peptide, or 50 nM PMA and 0.5 µM ionomycin, and 10 µg/ml brefeldin A in complete medium at 37°C for 5 hours. Cells were stained with anti-CD8, fixed, and rendered permeable before staining with anti-IFN-γ. Percentages of CD8+ and IFN-γ+ cells were determined in comparison to non-tumor-treated, vehicle-injected, MAFIA mice.
Results
Peritoneal ID8 Tumors Suppress Distal T Cell Activity through VLCs
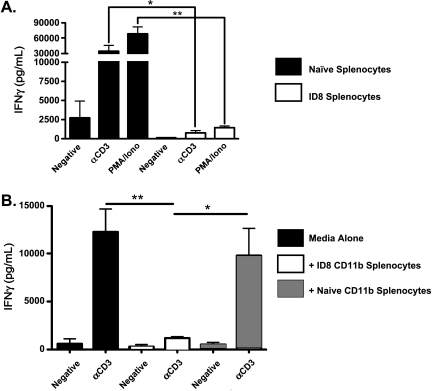
It is well established that CD11b+Gr-1+ cells harvested from tumor-bearing mice can suppress T cell responses [18,21,22]. Consistent with this, we previously reported that CD11c+CD11b+ VLCs derived from ID8 peritoneal ascites can function as MDSCs to inhibit CD8+ and CD4+ T cell responses in vitro [17]. However, during ID8 tumor progression, CD11b+Gr-1+ cells are also present in anatomy distal to the immediate tumor microenvironment, including the spleens of tumor-bearing mice. To test whether the presence of these cell populations influences T cell activity in lymphoid tissues distal to the tumor, we assayed splenic T cells from tumor-bearing and control naive mice. On activation either with an anti-CD3 antibody or with PMA/ionomycin, splenic CD8+ T cells from mice with 6-week ID8 peritoneal tumors produced substantially less IFN-γ in comparison to naive controls (Figure 1A). Because CD11b+ cells from spleens of tumor-bearing mice in a variety of tumor models are immunosuppressive, we tested whether CD11b+ cells from ID8 tumor-bearing mice are suppressive. We harvested CD11b+ cells from the spleens of naive and tumor-bearing mice and added them to stimulated naive splenocytes. CD11b+ cells from tumor-bearing mice suppress naive T cells in comparison to their naive counterparts, as assessed by IFN-γ production (Figure 1B). These results demonstrate that ID8 tumor growth causes suppression of the immune system in distal lymphoid tissues and the induction of a suppressive phenotype in the splenic CD11b+ compartment.
Figure 1.
ID8 tumor growth suppresses splenic responses. (A) A total of 1 x 106 splenocytes from naive mice (black bars) and mice bearing 6-week ID8 ovarian tumors (white bars) were stimulated with media alone (negative), αCD3 antibody (αCD3), or PMA and ionomycin (PMA) for 72 hours. Culture supernatants were harvested, and IFN-γ was assessed by ELISA. (B) A total of 1 x 106 splenocytes from naive mice were left unstimulated (negative) or stimulated with αCD3 antibody (aCD3), in the presence of media alone (black bars), 105 CD11b+ splenocytes from tumor-bearing mice (white bars), or 105 CD11b+ splenocytes from naive mice (hashed bars), and IFN-γ was assessed in the cell supernatant by ELISA 72 hours later. Statistical significance (*P < .05; **P < .01) was determined with the paired Student's t test.
VLC Depletion Inhibits Tumor-Induced Immunosuppression
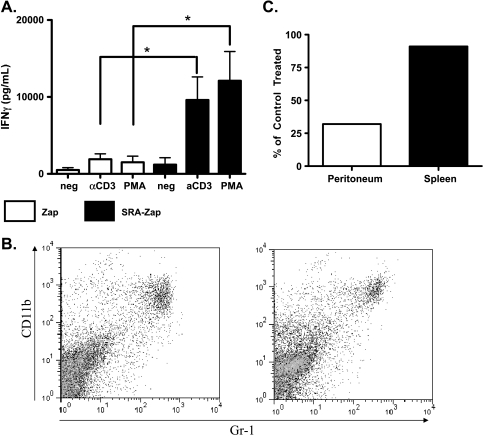
Because VLCs are immunosuppressive cells [14], we wanted to ascertain whether they are necessary for the in vivo immunosuppression seen in Figure 1. To test this, we used an SR-A immunotoxin that, as we previously demonstrated, removed tumor-promoting leukocytes within the peritoneum of ID8-challenged mice and thereby slowed ID8 tumor progression and reduced tumor burden [14]. To test whether VLCs are not only sufficient but also necessary for tumor-induced immunosuppression (Figure 1), we tested whether SR-A-mediated VLC depletion restored T cell activity in lymphoid tissues of treated mice. CD3- and PMA-stimulated T cells from mice treated with control toxin were unable to produce substantial IFN-γ after 7 weeks of tumor growth (Figure 2A). However, when mice were treated weekly with the SR-A immunotoxin, splenic T cells were then able to be activated ex vivo as assessed by IFN-γ production (Figure 2A). This occurred independently of an observable change in the splenic MDSC population (Figure 2B). Consistent with previous results, SR-A depletion effectively reduces the number of CD11c+ cells from the peritoneum of tumor-bearing mice [14] but has no effect on the splenic MDSC population (Figure 2C). These results demonstrate that removal of SR-A+ cells from the peritoneum affects distal lymphoid tissues with no demonstrable change in the number of CD11b+ cells in the spleen. Therefore, we tested the effect of SR-A+ cell depletion on the local cytokine milieu at the site of tumor growth.
Figure 2.
VLC depletion reverses splenic immunosuppression. Mice were injected with 5 x 106 ID8-C3 cells and treated with either the ribosomal toxin saporin conjugated to the 2F8 anti-SR-A monoclonal antibody (SRA-Zap) or with untargeted toxin (Zap). Mice were given subsequent weekly injections of SRA-Zap or ZAP. After 7 weeks, mice were killed, and splenocytes were stimulated with media alone (neg), αCD3 antibody (αCD3), or PMA and ionomycin (PMA) for 72 hours. (A) Culture supernatants were harvested, and IFN-γ was assessed by ELISA. (B) Splenocytes were stained with CD11b and Gr-1 antibodies, and MDSC populations were assessed (representative FACS plot shown). (C) Total numbers of peritoneal VLCs (CD11c+GFP-) (white bar) and percentages of splenic CD11b+Gr-1+ cells (black bar) from Zap- and SRA-Zap-treated mice were compared. The population of cells in SRA-Zap-treated mice in comparison to control mice is shown as percent of control treatment. Statistical significance (*P < .05) was determined with the paired Student's t test.
VLC Depletion Modifies the Peritoneal Cytokine Milieu
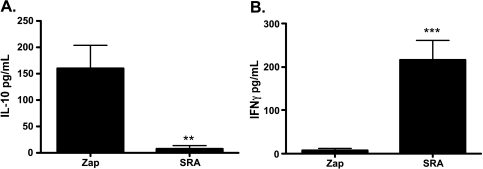
SR-A depletion relieves splenic immunosuppression in ID8-bearing mice, and T cell activity was restored despite similar splenic MDSC populations in SR-A- and control-treated mice. Therefore, we assessed whether depletion of SR-A+ cells in the peritoneum of tumor-bearing mice altered the immunosuppressive cytokine environment. We analyzed the cytokine levels of two critical cytokines known to affect antitumor immune responses: IL-10 is secreted by T regulatory cells, macrophages, and MDSCs in the tumor environment and dampens antitumor CD8+ T cell responses [23,24], whereas IFN-γ promotes antitumor responses through stimulation of the immune system [25]. Serum collected from the peritoneal ascites of tumor-bearing control mice treated with untargeted toxin contains robust IL-10 levels (Figure 3A) in conjunction with depressed levels of IFN-γ (Figure 3B). However, on VLC depletion with SR-A-targeted immunotoxin, IL-10 is diminished at the site of tumor growth (Figure 3A), whereas the amount of IFN-γ in the peritoneum of ID8 tumor-challenged mice increases on treatment with SR-A immunotoxin (Figure 3B). These results demonstrate that the depletion of SR-A+ cells in the peritoneum of tumor-bearing mice engenders a profound change in the local cytokine environment. Because our previous findings demonstrated that SR-A+ depletion slows tumor growth, we tested whether the immune system contributes to the SR-A immunotoxin effects.
Figure 3.
SR-A-mediated depletion alters the local cytokine environment. Mice were treated with tumors and SRA-Zap or Zap as in Figure 2. After 7 weeks, ascites was harvested and spun, and ascites serum was collected. Concentrations of IL-10 (A) or IFN-γ (B) were assessed by ELISA. Statistical significance (**P < .01; ***P < .005) was determined with the paired Student's t test.
Antitumor Effects of VLC Depletion Are Dependent on the Immune System
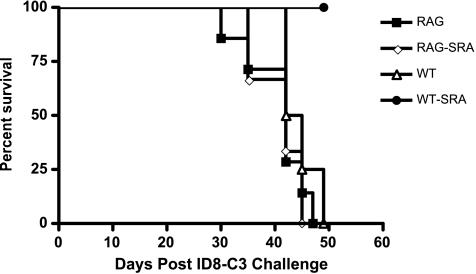
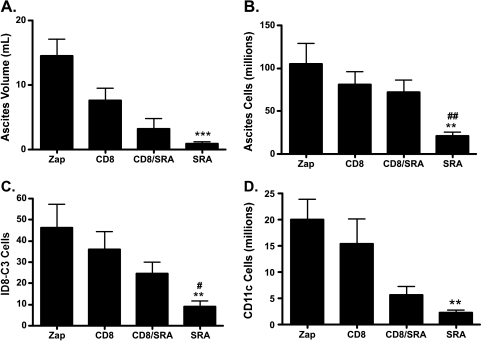
The in vitro function of VLCs as MDSCs, along with the change in cytokine milieu in ascites of VLC-depleted mice, suggested that activation of T cell activity through VLC depletion may be responsible for the observed inhibition of tumor progression. We extended these experiments to test whether SR-A immunotoxin affected the survival of tumor-challenged RAG-knockout (RAG-/-) mice, which lack an adaptive immune system. Wild type and RAG-/- were challenged with ID8 tumors expressing GFP (ID8-C3) and were given one of two treatments i.p. once weekly for 7 weeks: Zap immunotoxin (ZAP) or SR-A-targeted ZAP toxin through conjugation with an anti-SRA antibody (SRA) [14]. As previously demonstrated, the depletion of SR-A+ cells from the peritoneum of ID8 tumor-challenged mice extends their survival (Figure 4) [14]. However, when RAG-/- mice were injected with ID8 tumor cells and depleted of SR-A+ cells, the tumor growth kinetics was similar to wild-type and RAG-/- mice given Zap alone. Indeed, RAG-/- SR-A-depleted mice had a poorer survival than wild type given the SR-A immunotoxin (P < .01). Because T cell activity is proposed to contribute to tumor control in a variety of tumor models, we tested more stringently the contribution of CD8+ T cells to the efficacy of the SR-A immunotoxin. To test this hypothesis, we performed the SR-A-mediated cellular depletion experiments in conjunction with CD8-mediated depletion. Mice were challenged with ID8-C3 tumors and were given one of four treatments i.p. once weekly for 7 weeks: Zap immunotoxin (ZAP), CD8-depleting antibody (CD8), SR-A-targeted ZAP toxin through conjugation with an anti-SRA antibody (SRA) [14], and CD8-depleting antibody along with anti-SR-A antibody-conjugated ZAP toxin (CD8/SRA). After 7 weeks, all four groups were killed, and tumor growth was assessed ex vivo. Consistent with previous results [14], when SR-A+ cells are eliminated from the tumor environment, there is a marked reduction in ascites volume, ascites cellularity, ID8 tumor cells, and CD11c+ cells within the peritoneum of tumor-challenged mice (Figure 5, A–D: compare Zap with SRA). However, when a depleting CD8 antibody is administered along with the SR-A immunotoxin, the SR-A depletion is markedly less efficacious (Figure 5, A–D: compare SRA with SRA/CD8). In particular, the total numbers of ascites cells and ID8 tumor cells under conditions of CD8 and SR-A depletion are significantly increased over SR-A depletion alone (Figure 5, B and C). As a control, the number of CD11c+ cells in the peritoneum of tumor-challenged mice is unaffected when CD8+ cells are depleted along with SR-A+ cells, indicating that the anti-CD8 antibody did not affect the efficacy of the SR-A immunotoxin treatments (Figure 5D). The depletion of only CD8+ cells from the peritoneum of mice had a minimal effect on any of the parameters assessed. These data demonstrate the importance of CD8+ cells for the antitumor effects of SR-A depletion. In conjunction with our data showing that SR-A depletion changes the levels of peritoneal cytokines, this indicates that SR-A depletion relieves immunosuppression allowing for the induction of an antitumor response.
Figure 4.
SR-A depletion in RAG-/- background does not confer a survival benefit. Wild-type or RAG-/- mice were challenged with 5 x 106 ID8-C3 cells and treated with weekly peritoneal injections of either SRA-ZAP (WT-SRA or RAG-SRA) or with untargeted toxin (WT or RAG). Mice were killed after gaining 75% of weight at time of tumor injections. A Kaplan-Meier analysis was conducted on survival of each treatment group (WT vs WT-SRA, P < .01; RAG-SRA vs WT-SRA, P < .05).
Figure 5.
Depletion of SR-A+ cells requires CD8+ T cells for effect. Mice were challenged with 5 x 106 ID8-C3 cells and treated with weekly peritoneal injections of either SRA-ZAP, with untargeted toxin (Zap), anti-CD8 antibody (CD8), or with SRA-ZAP and anti-CD8 antibody (CD8/SRA). Four variables of tumor progression were assessed for each treatment group. Peritoneal exudates were measured for the ascites volume (A), total numbers of cells within the ascites (after RBC lysis; B), the quantity of GFP+ ID8-C3 tumor cells (C), and the quantity of CD11c+ VLCs (D). CD11c+ VLCs and GFP+ tumor cells were quantified by FACS analysis. Statistical significance (SRA vs ZAP, **P < .01, ***P < .005; SRA vs CD8/SRA, #P < .05, ##P < .01) was determined with the paired Student's t test.
Therapeutic VLC Depletion from Established Peritoneal Ascites Relieves T Cell Immunosuppression
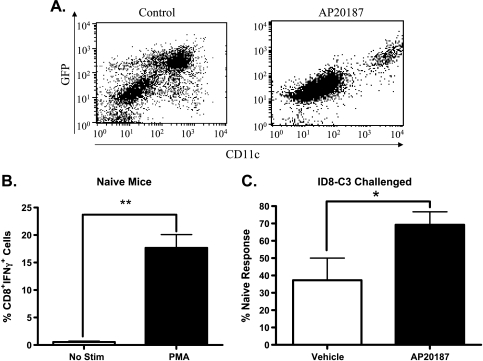
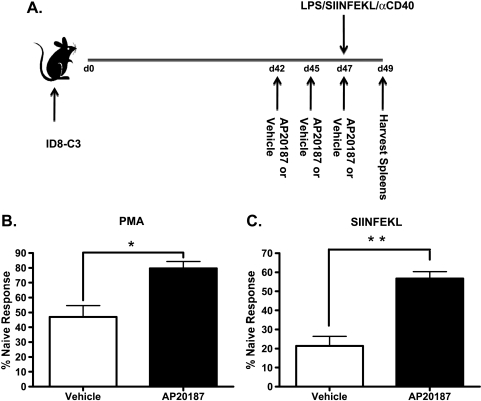
Our previous experiments that demonstrate an immunologic effect after VLC depletion used therapeutic intervention over the course of the tumor challenge (Figures 2 and 3). To extend these studies to a more therapeutically relevant intervention, we next tested whether later-stage depletion of VLCs from mice bearing established tumors affected the tumor-induced immunosuppression. To do so, we used the MAFIA strain of mice [19]. The MAFIA mice contain a knock-in GFP gene under the colony-stimulating factor-1 receptor (CSFR-1, c-fms, CD115) promoter that renders monocyte-derived cells, preferentially macrophages, GFP-positive [19]. In addition to a GFP knock-in, MAFIA mice contain a cell surface receptor that, when dimerized with the small molecule AP20187, induces cellular apoptosis [19,20]. We have previously shown that, consistent with previous reports of CD115+ as a marker MDSCs [26], the VLC population in ID8 ovarian ascites expresses GFP in the MAFIA mouse [17]. To determine whether we could deplete VLCs from mice bearing established tumors, we injected AP20187 into ID8-challenged MAFIA mice. Delivery of AP20187 depleted theCD115+CD11c+ immunosuppressive cell population when injected i.p. into 6-week tumor-bearing mice (Figure 6A). To determine whether peritoneal depletion of VLCs from mice bearing ID8 ascites affected late-stage tumor-induced immunosuppression, 6 weeks after ID8 tumor initiation, we injected AP20187 or vehicle into the peritoneum of these mice three times over the course of 5 days. The day after the last AP20187 injection, the mice were killed, splenocytes were stimulated with PMA and ionomycin, and CD8+ T cell activation was assessed by intracellular cytokine staining for IFN-γ production. In non-tumor-bearing naive mice, a significant amount of CD8+ T cells respond to PMA with the production of IFN-γ (Figure 6B). However, mice challenged with ID8 tumors and treated with vehicle had 75% less CD8+IFN-γ+ cells when stimulated with PMA compared with naive mice. After three injections of AP20187, in vitro T cell responses from tumor-bearing mice were restored to ∼70% of that of naive mice (Figure 6C). To determine whether this short-term depletion of immunosuppressive cells affected the ability to raise a peptide-specific response, we performed a variation on the previous experiment in which we immunized mice with SIINFEKL peptide, the H2-Kb-restricted epitope of chicken albumin (Figure 7A). Briefly, MAFIA mice were injected with ID8 tumors, and 6 weeks later, mice were given AP20187 injections every other day for 5 days. On day 5, mice were retro-orbitally injected with 2 µg of SIINFEKL plus adjuvant. After 72 hours, mice were killed and splenic T cells were stimulated either with PMA and ionomycin or with 1 µg/ml SIINFEKL peptide. In support of the previous results (Figure 6C), short-term administration of AP20187 partially restored the ability of CD8+ T cells to produce IFN-γ in response to PMA when compared to those treated with vehicle (Figure 7B). Interestingly, when restimulated in vitro with peptide, the CD8+IFN-γ+ cell population of AP20187-treated mice is ∼75% of immunized naive mice, in comparison to 50% for vehicle-treated mice (Figure 7C). These results provide the first demonstration that short-term depletion of VLCs improves both polyclonal and peptide-specific T cell responses in secondary lymphoid organs of ovarian tumor-bearing mice.
Figure 6.
Short-term VLC depletion relieves immunosuppression. (A) MAFIA mice were challenged with 5 x 106 ID8-C3. After 6 weeks, mice were injected with AP20187 (AP20187) or injection solution (Control) for three straight days. Mice were killed, and post-RBC lysis of ascites was stained with CD11c antibody and VLC population (GFP+CD11c+) population was assessed by FACS. (B) Splenocytes from naive MAFIA mice were harvested and left unstimulated (white bar) or stimulated with PMA/ionomycin for 5 hours (black bar). Cells were stained for CD8 and intracellular IFN-γ. CD8+IFN-γ+ cells were determined by FACS. (C) MAFIA mice were challenged with 5 x 106 ID8-C3. After 6 weeks, mice were injected with AP20187 (AP20187) or injection solution (Vehicle) every other day for 5 days. At 24 hours after the last injection, mice were killed, and splenocytes were harvested. Splenocytes from AP20187-treated (AP20187) and injection solution-treated (Vehicle) were stimulated with PMA/ionomycin. Cells were stained for CD8 and intracellular IFN-γ. CD8+IFN-γ+ cells were determined by FACS and expressed as a percentage of CD8+IFN-γ+ cells from non-tumor-bearing naive mice, as determined in panel B. Statistical significance (*P < .05) was determined with the paired Student's t test.
Figure 7.
VLC depletion potentiates the induction of a peptide specific immune response. (A) MAFIA mice were challenged with 5 x 106 ID8-C3. After 6 weeks, mice were injected with AP20187 (AP20187) or injection solution (Vehicle) for every other day for 5 days. On the fifth day, mice were injected with 2 µg of SIINFEKL peptide/10 µg of lipopolysaccharide/100 µg of agonistic αCD40 antibody. As a control, naiveMAFIAmice were also immunized at this point. (B) After 72 hours, AP20187-treated (AP20187), injection solution-treated (Vehicle), and immunized naive mice were killed and spleens were stimulated with PMA/ionomycin. After 5 hours, cells were harvested and stained for CD8 and intracellular IFN-γ. Cells were analyzed by FACS, and the percentage CD8+IFN-γ+ cells compared with naive immunized mice were determined. (C) Mice were treated as in panel B, except stimulated with 1 µg/ml SIINFEKL peptide and analyzed as a percentage of the CD8+IFN-γ+ cells produced from naive mice. Statistical significance (*P < .05; **P < .01) was determined with the paired Student's t test.
Discussion
The critical role that the immune system has in modulating tumor growth is now recognized [1]. The tumor, and its resultant microenvironment, recruits a local population of dysregulated immune cells that aid in tumor growth [27]. Many murine tumor models, including lung, breast, and colon, recruit an influx of immunosuppressive leukocytes [28–30]. Specifically, both human and murine ovarian tumor growth induces an influx of CD11b+CD11c+ leukocytes to the peritoneal tumor environment that is critical to tumor progression [15,31]. These VLCs are critical to tumor growth because their selective removal slows tumor growth [14,32]. However, how VLC depletion inhibits tumor progression is not well understood. A recent clue was the elucidation that VLCs can function as MDSCs: VLCs express ARG1 and are immunosuppressive in vitro [14,33]. In numerous tumor models, the presence of MDSCs correlates with a globally depressed immune response [21,34]. Therefore, we hypothesized that ID8 ovarian tumor growth may induce immunosuppression both locally and distally to tumor growth and that depletion of the immunosuppressive VLC population may alleviate the immunosuppression.
Here we demonstrate that the growth of ID8 tumors impacts the immune status of lymphoid tissues distal to tumor growth. T cells recovered from ID8 tumor-bearing mice secrete less IFN-γ compared with naive controls (Figure 1A). Furthermore, the CD11b+ compartment within spleens of tumor-bearing mice recapitulates this phenotype, as coculture of CD11b+ cells from tumor-bearing mice causes a diminution of IFN-γ release from naive T cells when compared with CD11b+ splenocytes isolated from naive controls (Figure 1B). These data are supported by findings that splenic MDSCs from tumor-bearing hosts are consistently more suppressive than those from naive controls [18]. Relevant to human disease, our data support clinical findings because peripheral blood mononuclear cell of late-stage ovarian patients are unable to respond to stimuli as effectively as healthy controls [35,36]. Moreover, dysregulated signaling in the CD8 T cell compartment of ovarian cancer patients is well established [37]. Coupled with our previous findings that VLC depletion slows tumor growth [14], these data led us to define the effect of VLC depletion on T cell responses in ID8 tumor-bearing mice.
In tumor-challenged mice depleted of VLCs, their T cells produce more IFN-γ in response to T-cell receptor stimulation and mitogen activation than T cells from the control mice (Figure 2). One underlying reason for the increased T cell activation after VLC depletion may reside in the resultant cytokine environment. Depletion of VLCs led to the reduction in levels of IL-10 and to an increase in the concentration of IFN-γ within the tumor microenvironment (Figure 3). This switch from a tolerogenic cytokine profile to one that is associated with proinflammatory antitumor responses led us to ask whether the inhibition in tumor progression mediated by VLC depletion was dependent on T cell-dependent responses. RAG-/- mice depleted of VLCs did not exhibit the survival benefit of wild-type mice depleted of VLCs (Figure 4). Extending these results to specifically test which cellular subsets are necessary for SR-A immunotoxin effects, mice were depleted of VLCs using the SR-A immunotoxin with and without CD8 depletion. Mice depleted of CD8+ T cells along with VLCs had both higher levels of total peritoneal cells within the ascites and of ID8 tumor cells 7 weeks after tumor challenge (Figure 5, B and C). In addition, mice depleted of VLCs and CD8+ T cells did not exhibit the survival benefit of mice depleted of VLCs alone (Figure 5). These data are consistent with findings in other tumor models wherein activation of CD8+ T cell responses can limit tumor progression and lead to reduced tumor burden [34,38–40]. Interestingly, despite the substantial reduction in cellularity within the peritoneum of VLC-depleted mice relative to those that also received CD8 depletion, VLC- and VLC/CD8-depleted mice contained similar levels of ascites volume (Figure 5A). Because VLCs promote angiogenesis [2,15], the lack of substantial ascites in both instances could be due to their removal under both conditions.
To confirm the results obtained with the SR-A immunotoxin, as well as to extend our studies to test the more therapeutically relevant trial of short-term VLC depletion in mice bearing established tumors, we made use of the MAFIA strain of mice. These mice express a GFP transgene under the CD115 promoter, which previous data have shown corresponds with, and identifies, the CD11b+CD11c+ VLC population within ID8 ascites (Figure 6A and [17]). CD115+ cells in MAFIA mice also harbor a cell surface receptor which, on dimerization with the small molecule AP20187, leads to cellular apoptosis [19,20]. Intraperitoneal AP20187 injection effectively reduced the number of VLCs within late-stage peritoneal ascites (Figure 6). Importantly, three daily treatments within the sixth week of ID8-C3 tumor-challenged MAFIA mice relieved tumor-induced immunosuppression as assessed by ex vivo T cell stimulation (Figure 6C). To extend these results, we tested whether VLC depletion could improve a peptide-specific T cell response against a model tumor antigen. Depletion of VLCs for 5 days followed by immunization against the SIINFEKL peptide, improved the in vitro recall response in tumor-bearing mice (Figure 7C). These data demonstrate that depletion of immunosuppressive cells at the site of tumor growth relieves tumor-induced immune suppression and restores normal function of the immune system. Because these effects are seen in mice bearing well-established tumors, it is tempting to speculate on the therapeutic benefit of a combination of VLC depletion coupled with vaccination against known tumor-associated antigens.
These data reveal another facet in which the ID8 tumor model recapitulates the human disease in mice, making it a tractable model for studying immune dysfunction in ovarian cancer. This study outlines multiple novel interactions between ID8 tumor growth and the immune system, including the induction of distal immunosuppression in lymphoid tissues, the reversal of immune suppression through peritoneal leukocyte depletion, and the dependence of antitumor leukocyte depletion on CD8+ T cells. The ability to reverse the immunosuppression through two different modes of leukocyte depletion provides a powerful tool for further studies into tumor/immune system interactions. Because the molecular mechanisms behind tumor-induced T cell tolerance are not fully understood, models such as this will prove useful in answering these questions. As cancer therapies attempting to initiate an immune response against cancer have garnered mixed results [27], studies such as these add to the growing body of evidence that complementary depletion of immunosuppressive cells is a viable avenue for novel cancer treatments.
Acknowledgments
The authors thank the Englert Cell Analysis Laboratory for technical assistance with FACS and members of the Berwin Laboratory for helpful discussions.
Footnotes
This research was supported by National Institutes of Health grants COBRE P20RR016437 and RO1 AI067405 (B.B.) and National Institutes of Health training grants T32 AI07363 (S.P.B) and T32 GM08704 (K.H.).
References
- 1.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 2.McLean K, Buckanovich RJ. Myeloid cells functioning in tumor vascularization as a novel therapeutic target. Transl Res. 2008;151:59–67. doi: 10.1016/j.trsl.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Halin S, Rudolfsson SH, Van Rooijen N, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11:177–186. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Said NA, Elmarakby AA, Imig JD, Fulton DJ, Motamed K. SPARC ameliorates ovarian cancer-associated inflammation. Neoplasia. 2008;10:1092–1104. doi: 10.1593/neo.08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 8.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 9.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 10.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 11.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang R, Cai Z, Zhang Y, Yutzy WH, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 13.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 14.Bak SP, Walters JJ, Takeya M, Conejo-Garcia JR, Berwin BL. Scavenger receptor-A-targeted leukocyte depletion inhibits peritoneal ovarian tumor progression. Cancer Res. 2007;67:4783–4789. doi: 10.1158/0008-5472.CAN-06-4410. [DOI] [PubMed] [Google Scholar]
- 15.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, et al. Tumor-infiltrating dendritic cell precursors recruited by a β-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 16.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 17.Bak SP, Alonso A, Turk MJ, Berwin B. Murine ovarian cancer vascular leukocytes require arginase-1 activity for T cell suppression. Mol Immunol. 2008;46:258–268. doi: 10.1016/j.molimm.2008.08.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 20.Burnett SH, Beus BJ, Avdiushko R, Qualls J, Kaplan AM, Cohen DA. Development of peritoneal adhesions in macrophage depleted mice. J Surg Res. 2006;131:296–301. doi: 10.1016/j.jss.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 22.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 23.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 24.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 25.Brandacher G, Winkler C, Schroecksnadel K, Margreiter R, Fuchs D. Antitumoral activity of interferon-gamma involved in impaired immune function in cancer patients. Curr Drug Metab. 2006;7:599–612. doi: 10.2174/138920006778017768. [DOI] [PubMed] [Google Scholar]
- 26.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 27.Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117:1130–1136. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 30.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 31.Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105:679–681. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- 32.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, Benencia F, Stan RV, Keler T, Sarobe P, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart KM, Bak SP, Alonso A, Berwin B. Phenotypic and functional delineation of murine CX(3)CR1 monocyte-derived cells in ovarian cancer. Neoplasia. 2009;11:564–573. doi: 10.1593/neo.09228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130–1139. doi: 10.1158/0008-5472.can-03-1715. [DOI] [PubMed] [Google Scholar]
- 35.Berek JS, Schultes BC, Nicodemus CF. Biologic and immunologic therapies for ovarian cancer. J Clin Oncol. 2003;21:168s–174s. doi: 10.1200/JCO.2003.01.517. [DOI] [PubMed] [Google Scholar]
- 36.Gordon AN, Schultes BC, Gallion H, Edwards R, Whiteside TL, Cermak JM, Nicodemus CF. CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients. Gynecol Oncol. 2004;94:340–351. doi: 10.1016/j.ygyno.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 37.Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- 38.Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–1828. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 39.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]