Abstract
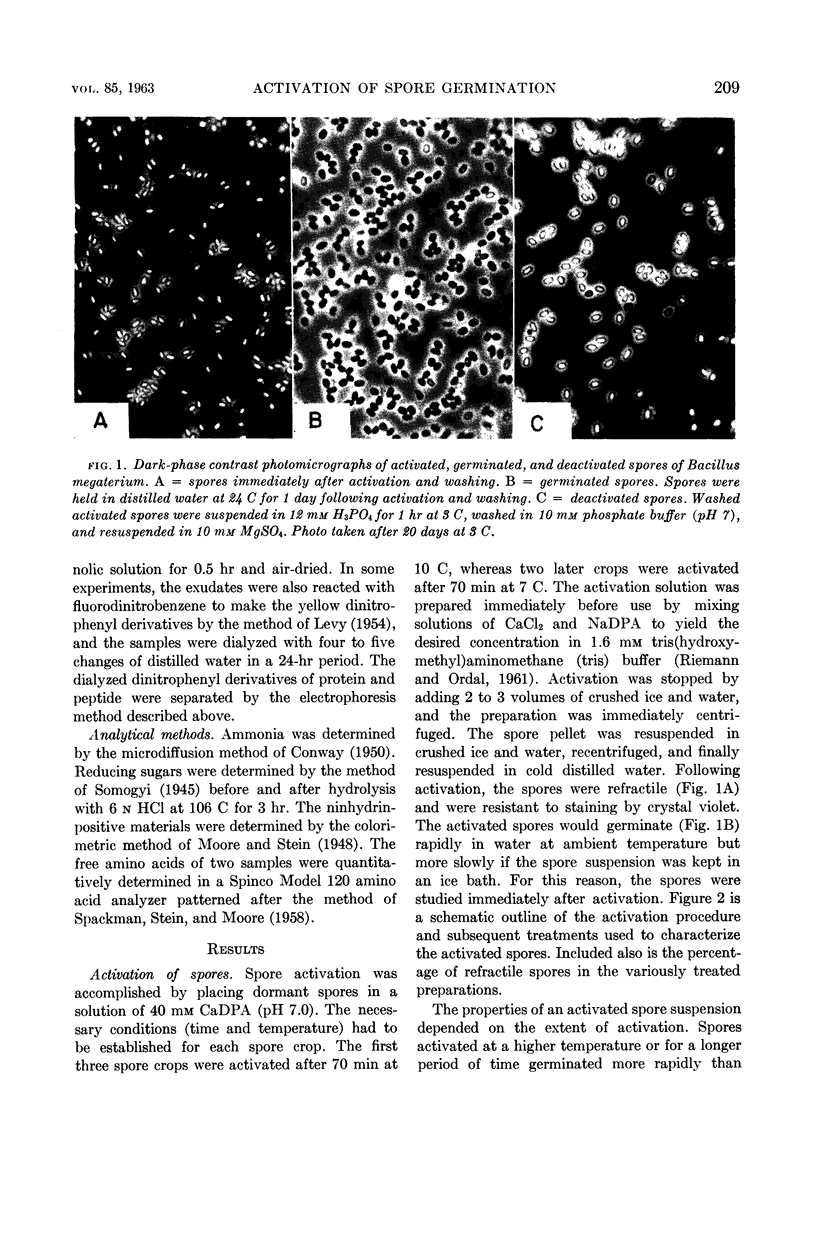
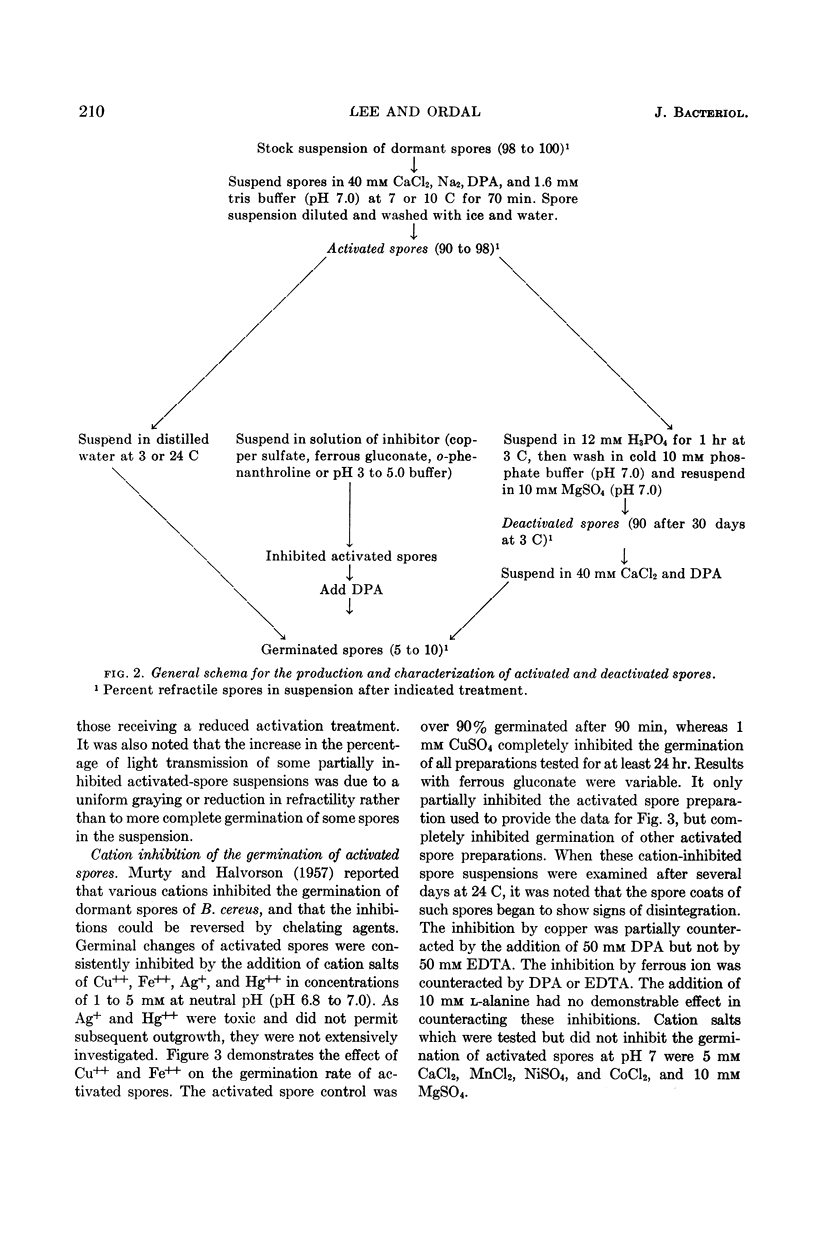
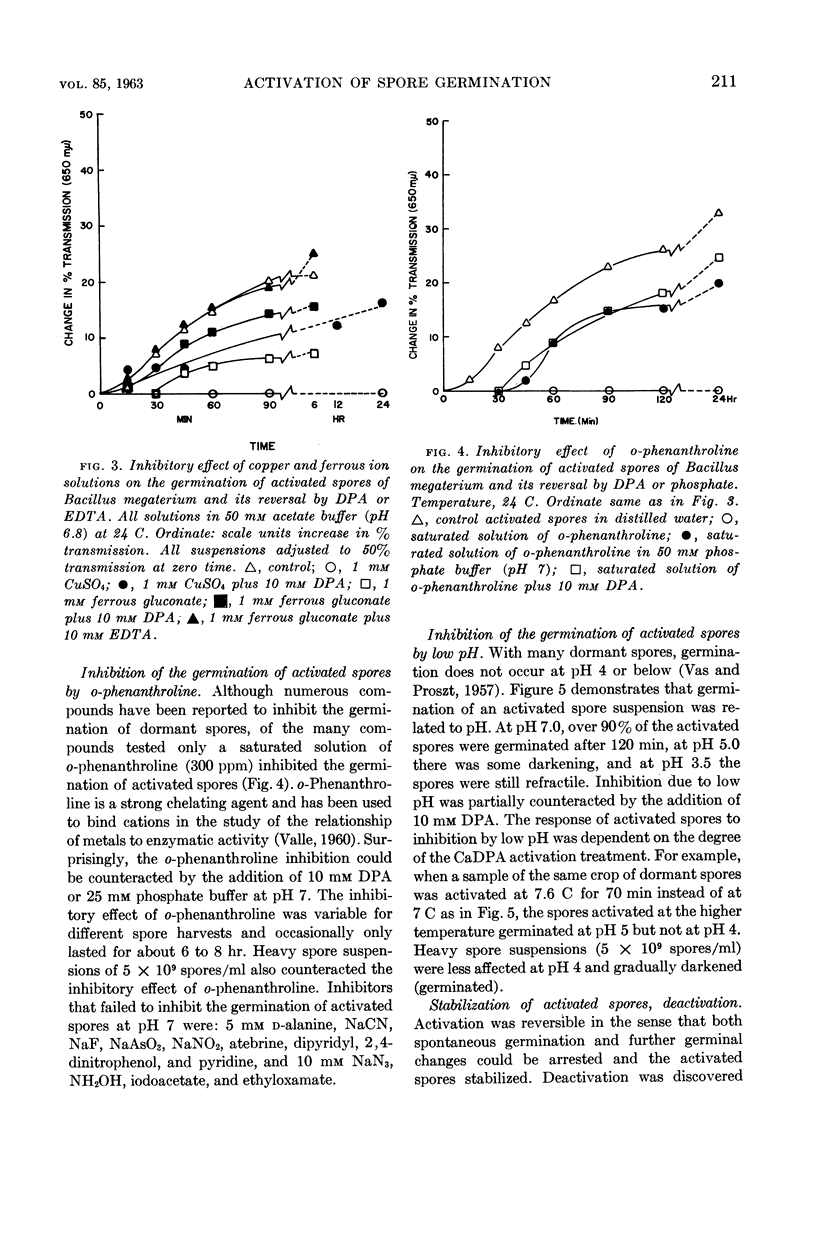
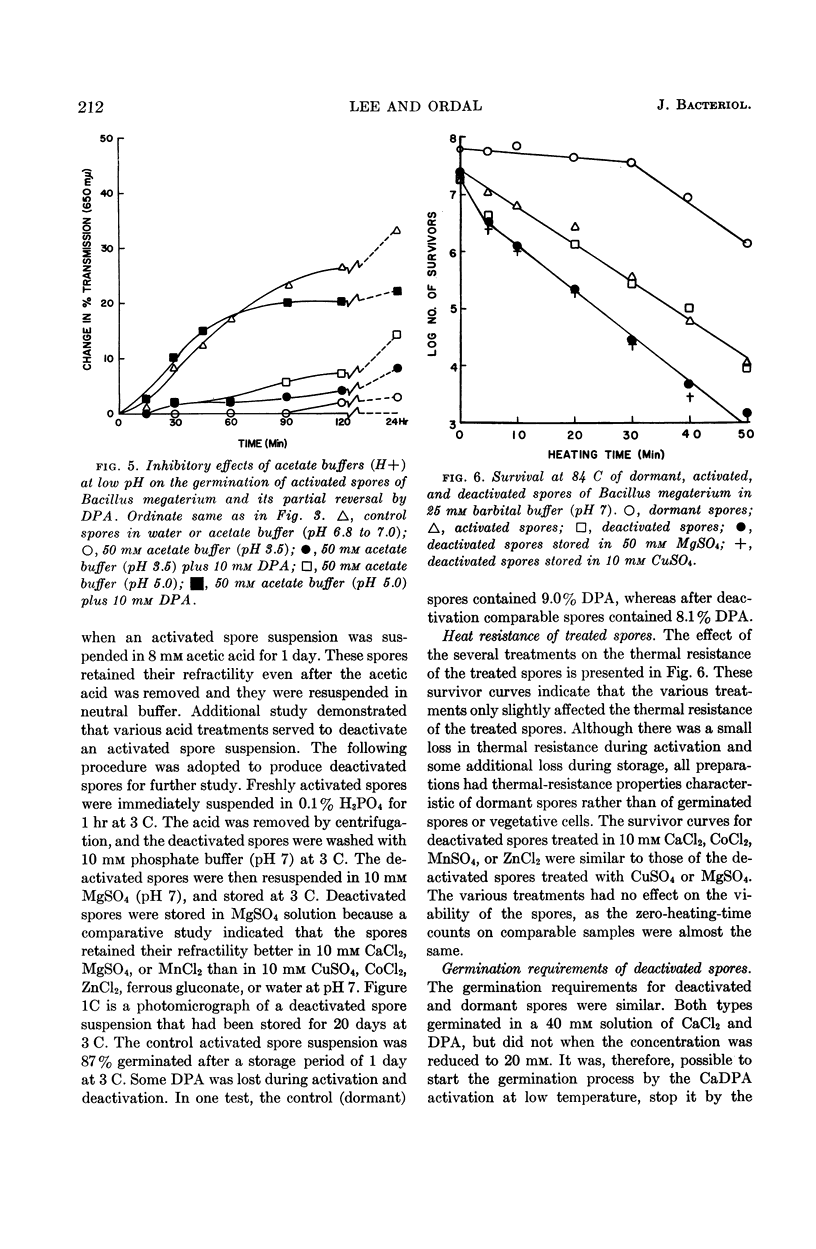
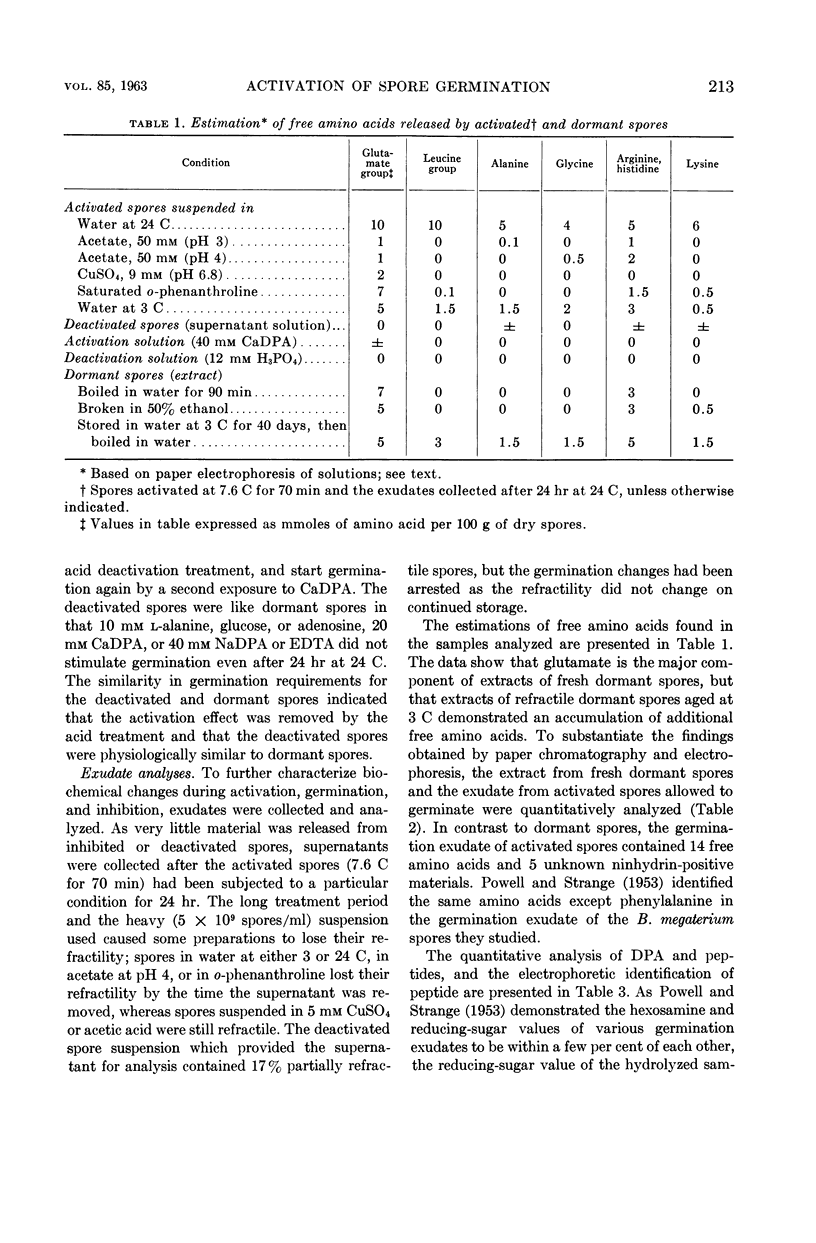
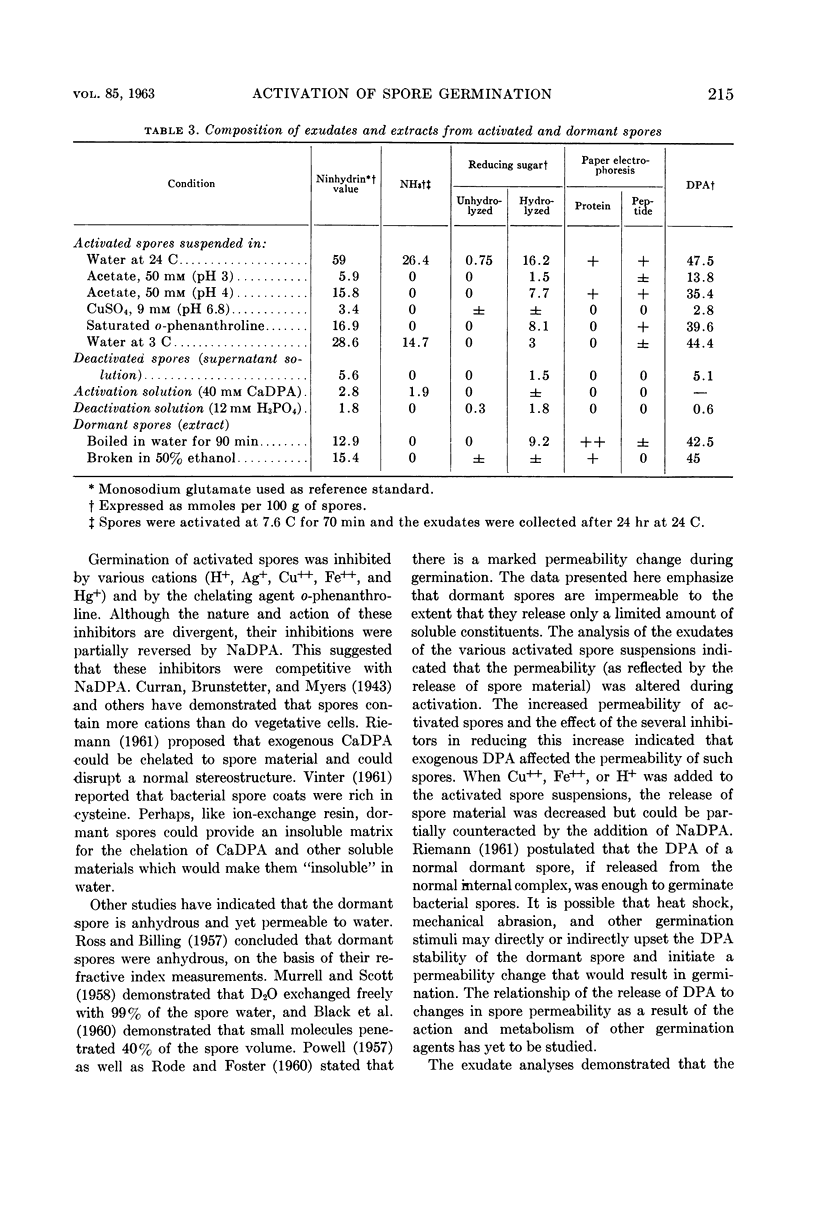
Lee, W. H. (University of Illinois, Urbana) and Z. John Ordal. Reversible activation for germination and subsequent changes in bacterial spores. J. Bacteriol. 85:207–217. 1963.—It was possible to isolate refractile spores of Bacillus megaterium, from a calcium dipicolinate germination solution, that were activated and would germinate spontaneously in distilled water. Some of the characteristics of the initial phases of bacterial spore germination were determined by studying these unstable activated spores. Activated spores of B. megaterium were resistant to stains and possessed a heat resistance intermediate between that of dormant and of germinated spores. The spontaneous germination of activated spores was inhibited by copper, iron, silver, or mercury salts, saturated o-phenanthroline, or solutions having a low pH value, but not by many common inhibitors. These inhibitions could be partially or completely reversed by the addition of sodium dipicolinate. The activated spores could be deactivated and made similar to dormant spores by treatment with acid. Analyses of the exudates from the variously treated spore suspensions revealed that whatever inhibited the germination of activated spores also inhibited the release of spore material. The composition of the germination exudates was different than that of extracts of dormant spores. Although heavy suspensions of activated spores gradually became swollen and dark when suspended in solutions of o-phenanthroline or at pH 4, the materials released resembled those found in extracts of dormant spores rather than those of normal germination exudates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. H., HASHIMOTO T., GERHARDT P. Calcium reversal of the heat susceptibility and dipicolinate deficiency of spores formed "endotrophically" in water. Can J Microbiol. 1960 Apr;6:213–224. doi: 10.1139/m60-023. [DOI] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H. Dependence of the heat resistance of bacterial endospores on their dipicolinic acid content. Nature. 1959 Jan 10;183(4654):124–125. doi: 10.1038/183124a0. [DOI] [PubMed] [Google Scholar]
- Curran H. R., Brunstetter B. C., Myers A. T. Spectrochemical Analysis of Vegetative Cells and Spores of Bacteria. J Bacteriol. 1943 May;45(5):485–494. doi: 10.1128/jb.45.5.485-494.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R. H., HALVORSON H. Comparison of electron transport systems in vegetative cells and spores of Bacillus cereus. J Bacteriol. 1961 Jan;81:51–58. doi: 10.1128/jb.81.1.51-58.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLYNN F. V., DE MAYO P. Microelectrophoresis of protein on filter-paper. Lancet. 1951 Aug 11;2(6676):235–239. doi: 10.1016/s0140-6736(51)93239-4. [DOI] [PubMed] [Google Scholar]
- KRISHNA MURTY G. G., HALVORSON H. O. Effect of enzyme inhibitors on the germination and respiration of and growth from Bacillus cereus var. terminalis spores. J Bacteriol. 1957 Feb;73(2):230–234. doi: 10.1128/jb.73.2.230-234.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY A. L. A paper chromatographic method for the quantitative estimation of amino-acids. Nature. 1954 Jul 17;174(4420):126–127. doi: 10.1038/174126a0. [DOI] [PubMed] [Google Scholar]
- LONG S. K., WILLIAMS O. B. Method for removal of vegetative cells from Bacterial spore preparations. J Bacteriol. 1958 Sep;76(3):332–332. doi: 10.1128/jb.76.3.332-332.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson H. S., Wrigley A. S. Spore Germination and Emergence of Bacillus megaterium. Science. 1960 May 6;131(3410):1382–1382. doi: 10.1126/science.131.3410.1382. [DOI] [PubMed] [Google Scholar]
- POWELL J. F. Isolation of dipicolinic acid (pyridine-2:6-dicarboxylic acid) from spores of Bacillus megatherium. Biochem J. 1953 May;54(2):210–211. doi: 10.1042/bj0540210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Biochemical changes occurring during the germination of bacterial spores. Biochem J. 1953 May;54(2):205–209. doi: 10.1042/bj0540205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIEMANN H., ORDAL Z. J. Germination of bacterial endospores with calcium and dipicolinic acid. Science. 1961 May 26;133(3465):1703–1704. doi: 10.1126/science.133.3465.1703. [DOI] [PubMed] [Google Scholar]
- ROSS K. F., BILLING E. The water and solid content of living bacterial spores and vegetative cells as indicated by refractive index measurements. J Gen Microbiol. 1957 Apr;16(2):418–425. doi: 10.1099/00221287-16-2-418. [DOI] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. MECHANICAL GERMINATION OF BACTERIAL SPORES. Proc Natl Acad Sci U S A. 1960 Jan;46(1):118–128. doi: 10.1073/pnas.46.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. A cell-wall lytic enzyme associated with spores of Bacillus species. J Gen Microbiol. 1957 Feb;16(1):236–249. doi: 10.1099/00221287-16-1-236. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., POWELL J. F. Hexosamine-containing peptides in spores of Bacillus subtilis, B. megatherium and B. cereus. Biochem J. 1954 Sep;58(1):80–85. doi: 10.1042/bj0580080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAS K., PROSZT G. Effect of temperature and hydrogen-ion concentration on the germination of spores of Bacillus cereus. Nature. 1957 Jun 22;179(4573):1301–1302. doi: 10.1038/1791301a0. [DOI] [PubMed] [Google Scholar]



