Abstract
Nowadays, It is easy to define optimal conditions (cryoprotective agent, speed and steps of freezing, speed of warming) for the cryopreservation of a homogeneous cell population or a one cell-layer tissue. Meanwhile, It is still hard to obtain cryopreservation of composite organs. Each tissue has its own requirements and its own reactivity to the cryopreservation process. The challenge consists of, on the one hand, to select the ideal combination of cryoprotective agents that can fit the needs of the different tissues, and the definition of adequate technical parameters, on the other hand. All the experimental trials have studied the survival rate of non-vascularized cryopreserved tissues. The aim of our experimental work is to demonstrate the feasibility of cryopreserving a composite organ with its nutrient vessels “artery and veins” in order after thawing to revitalize it by reestablishing the blood irrigation by microsurgical vascular anastomosis. We report our experimental results on the cryopreservation of composite organs—amputated digits—xenotransplanted in the rabbit. Digital segments were cryopreserved, then revitalized after warming using vascular microsurgical techniques. Preliminary results are encouraging and may pave the way in the future to the microvascular allotransplantation of cryopreserved composite organs.
Keywords: xenotransplantation, composite tissues, organs, cryopreservation, skin, animals, digits, vessels, microsurgery, vascular anastomoses
Introduction
Until now, research on cell and tissue cryopreservation has been limited to the study of homogenous tissues and focused on the allotransplantation of non-vascularized tissue segments. The allotransplantation program we propose to develop differs markedly from the latter experimental conditions. It addresses composite organs composed of different tissues (bone, vessels, tendons, cartilage, nerves, ligaments, etc.,) which will be cryopreserved, thawed and revitalized by microsurgical revascularization. To validate the feasibility of this allotransplantation project of cryopreserved composite organs, we developed an experimental protocol of xenotransplantation of composite organs, subjected to cryopreservation, to warming after elimination of the cryoprotective agents and finally to revitalization by microvascular anastomosis in the rabbit. This protocol was registered with the Ministry of Research and Technology.
The experimental protocol involves three phases:
Sampling of digital xenotransplants
Cryopreservation
Thawing and microsurgical revascularization.
Recovery Phase of the Digital Xenotransplants
As a Hand and Plastic Surgeon involved in a Hand Surgery Unit, viable digital segments were possible to be recovered after hand surgery in a very particular and predefined situtations. The first situation was that of an emergency when the severed finger did not fulfill conditions for microsurgical revascularization, like a zone II amputation of the index from a hand laborer (Fig. 1A). The second situation was that of planned amputation of a stiff finger that hindered functioning of the hand (Fig. 1B). If recovery of the finger was possible, detailed information was given to the patient, and oral and written consent were obtained.
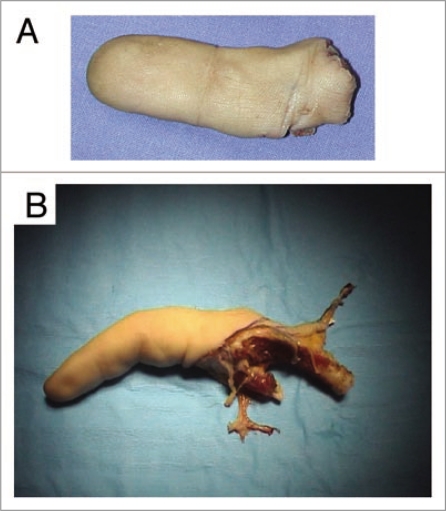
Figure 1.
Recovered xenografts. (A) An index severed in zone II from a hand laborer, which did not fulfill conditions allowing microsurgical revascularization. (B) A stiff finger that hindered functioning of the hand was removed during a scheduled operation.
A series of serological tests were performed on the donor to evaluate eventual contamination of the xenograft (anti-HCV, HBs antigens, anti HBc, anti-HIV 1&2, HIV antigen, anti-HTLV 1&2 and syphilis). After recovery of the finger, the arteriovenous vessels were localized and all manipulations carried out at low temperature (4°C) to limit ischemic tissue damage. The dominant nurrishing collateral palmar artery was catheterized with a gray catheter “16 gauge” and the finger was rinsed abundantly with Custodiol® solution until the venous flow-through was clear (Fig. 2). The washing solution was sampled for bacteriological examination before perfusion of the finger with a mixture of antibiotics “gentamycin and vancomycin” to limit microbial contamination.
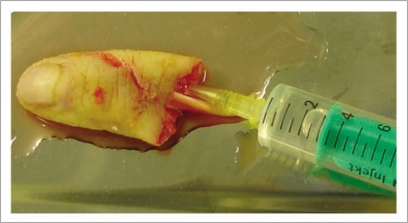
Figure 2.
After recovery of the finger, the dominant palmar collateral artery was catheterized and perfused abundantly with Custodiol® solution until flow through from the vein was clear.
A code bar was assigned to each digital xenograft to insure its traceability. The file's code was affixed to all complementary analyses and manipulations of the finger. The xenotransplant was then placed in a double-walled, Cryokit®-type container (Fig. 3), maintained at 4°C and delivered as rapidly as possible to the Cryopreservation Unit where the phase of cryopreservation itself was begun.
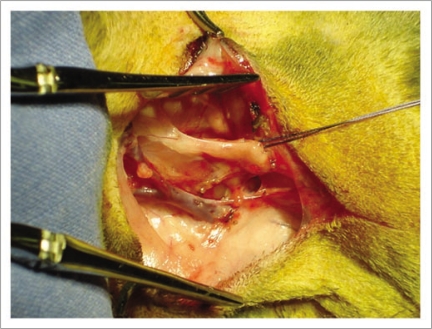
Figure 3.
Double walled container “Cryokit®” used for packaging the xenotransplant allowing a safe and constant hibernation during transport.
Cryopreservation of the Xenotransplant
All manipulation were carried out under sterile conditions under a laminar flow hood at 4°C to reduce ischemic tissue damage. The finger was taken from the Cryokit® container and washed abundantly with a solution of 4% albumin diluted to 1/3 in physiological serum.
A sample of this solution was again taken for bacteriological examination and a fragment from the extensor tendon and the skin was harvested and kept in a tissue bank to insure traceability.
Cryoprotector was then administered intra-arterially to impregnate the whole digit vascular network. We chose dimethyl sulfoxide (DMSO) prepared as follows: 27% albumin A, 58% physiological serum, and 15% DMSO. After sufficient irrigation of the finger with an evident venous flow-through, the catheter was withdrawn from the collateral artery and the xenotransplant placed in a special bag with three vents, through which the solution containing the cryoprotector was administered until the xenotransplant was completely submerged with no bubbles (Fig. 4). A sample of the cryoprotector solution was recovered and served as a control of the freezing phase. The phase of cryoprotector administration was performed rapidly to minimize the time of contact of the cryoprotector agent with the tissues, which is toxic at room temperature.
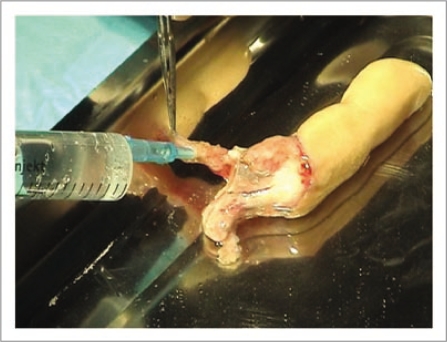
Figure 4.
After intra-arterial administration of the cryoprotector, the finger was placed in a specially adapted, three-vent bag and immersed in the cryoprotective solution with no air bubbles.
The bag and the control sample were then placed vertically in a programmable Nicol® freezer and the temperature progressively lowered by predefined levels to attain that of liquid nitrogen, −196°C. The frozen finger was then stored horizontally in a liquid nitrogen container, where, theoretically, it can be kept indefinitely.
Thawing and Revitalization of the Xenotransplant
The rabbit was chosen as a recipient model and the left carotid artery and jugular vein as the site of revascularization (Fig. 5). These vessels are easily accessible and have diameters close to those of digital vascular structures. The finger can be secured around the neck region and thus be protected from inopportune animal movements.
Figure 5.
The rabbit was used as an animal model. Xenograft implantation was performed in the cervical region with vascular attachment to the carotid artery and the jugular vein.
The rabbit was anesthetized by intramuscular injection of ketamine. An intravenous catheter was placed in the anterior auricular vein and anesthesia maintained by administration of ketamine at a rate of 0.1 cc when necessary. The rabbit was laid on its right side and the left carotid-jugular pedicle was reached through a longitudinal lateral cervical skin approach on the left side.
The xenotransplant in its bag was plunged into a water bath at 37°C in the operating room until all ice was completely thawed. The bag was then opened and the digital xenotransplant recovered. It was abundantly rinsed with physiological serum. The dominant collateral artery was again catheterized and the finger abundantly washed with 4% albumin to eliminate all traces of cryoprotector agent from the vascular network. A heparin wash was performed to prepare the capillary network and to avoid the constitution of thrombi and particularly the no-Reflow Phenomenon when blood circulation was re-established (Fig. 6). Following thawing and washing, tissues recovered their normal texture and consistency.
Figure 6.
The dominant collateral artery was again catheterized and the finger abundantly rinsed with physiological serum until tissues recovered a normal consistency. Heparin was administered intravascularly to avoid non-reflow phenomenon.
The digital xenotransplant was then fastened with three sutures to the recipient skin and microsurgical revascularization begun under a microscope by establishing an end-to-end arterial anastomosis between the left carotid artery of the rabbit and the dominant palmar collateral artery of the finger. Before releasing the arterial clamp, a perfusion of 3,000 IU of Streptase® (1 cc), 100 IU Heparin (2 cc) and 0.1 cc of Xylocain 1% diluted in 2 cc of physiological serum was administred intravenously to the rabbit. This bolus was followed by a solution of 12,000 IU of Streptase® (4 cc), 400 IU of Heparin (8 cc) and 4 cc of Xylocain 1% diluted in 8 cc of physiological serum. It was delivered by an electronic syringe at a rate of 1 cc/24 hours. The latter treatment was maintained throughout the duration of xenotransplantation.
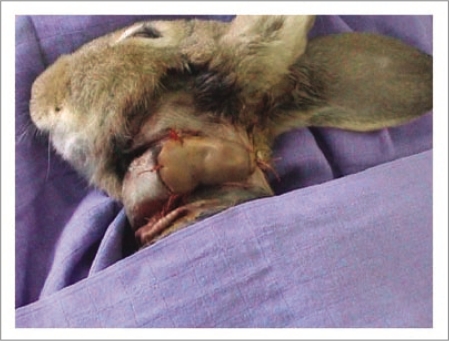
Following irrigation and a satisfactory venous flow-through, an end-to-end anastomosis was done between a dorsal draining digital vein and the jugular vein. The finger was then placed around the rabbit's cervical region and fixed with proximal sutures to the skin and distally with a suture between the finger's pulpo-ungueal zone and the rabbit's dorsal skin (Fig. 7). A circular cervical bandage was placed to protect the xenotransplant. The rabbit was placed in a cage under monitoring, with the temperature and humidity kept constant. The xenotransplant was inspected twice daily for temperature, color, capillary pulse and bleeding through cutaneous scarification (Fig. 8). Lastly, the rabbit received an intramuscular injection of antibiotics every three days.
Figure 7.
Following microsurgical vascular attachment, the xenograft was positioned around the neck and secured with sutures at both extremities.
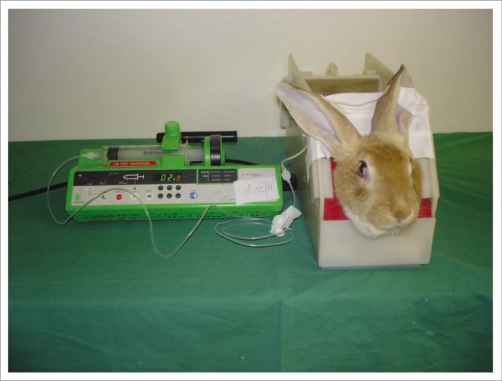
Figure 8.
The rabbit was placed in a cage with its head immobilized to avoid inopportune movement. A perfusion was placed in the auricular vein to administer anticoagulants and fibrinolytics.
Results and Discussion
We performed three xenotransplantations of cryopreserved digital segments: an amputated index and ring fingers, previously revascularized following their traumatic amputation—the digits normalization were requested by the patients due to functional disability caused by the stifness of the fingers—and one ring finger amputated with a crush and stripping phenomenon with important tissue defect involving the proximal first phalanx, the skin cover and the flexor tendons representing a contra-indication for digital replantation. The three fingers were cryopreserved and thawed according to the protocol described above. Cryopreservation in liquid nitrogen averaged 8 weeks (4 to 12 weeks).
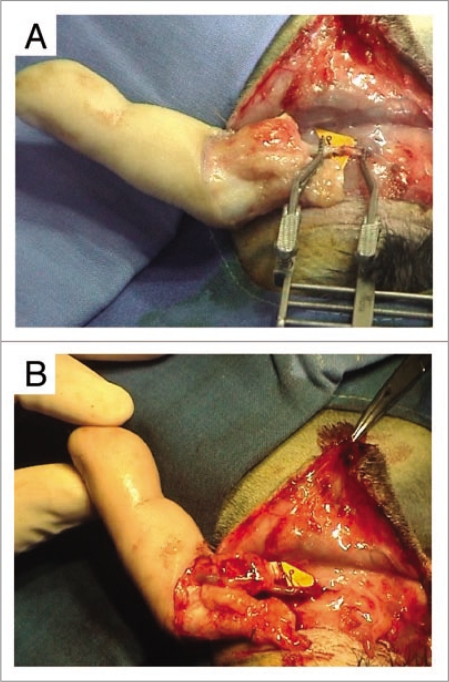
In all three cases, upon release of the arterial clamps, the digital xenograft revascularized immediately with a venous flow-through, but the finger rapidly became marbled with a hard-to-discern capillary pulse (Fig. 9A and B). In contrast, the cutaneous temperature was normal and the pulp renitent and filled out, which allowed us to evaluate the quality of digital irrigation.
Figure 9.
(A) End-to-end arterial attachment of the dominant palmar artery of the finger to the rabbit's carotid artery. (B) Upon release of the arterial clamps, xenograft revascularization was immediate, with appearance of a venous flow-through. However, the finger became rapidly marbled and the capillary pulse was hardly perceptible.
Treatment with a fibrinolytic (Streptase®), an anticoagulant (Heparin) and a vasodilator (Xylocain) was collected from protocols used to treat the absence of blood irrigation in the No-Reflow phenomenon following digital revascularization.1–4 The doses were adapted to the rabbit's weight. While the duration of perfusion with fibrinolytics in the clinical protocol was limited to 24 hours, we opted for continuous use throughout xenograft implantation. Whereas cryopreservation protects tissues and cells, it generates cellular injuries similar to those of the No-Reflow phenomenon, especially on the vascular endothelium.5 Consequently, fibrinolytic treatment must be maintained for at least 15 days, the time necessary for the reconstitution of the endothelium sheath over the inner surface of the vascular network and the development of the neo-vascular ization on the different sites of contact between the donor digit and the recipient rabbit.
We deliberately chose not to use anti-rejection therapy or immunosuppressors. Effectively, the main purpose of our experimentation as to verify restoration of circulation in the xenotransplant and revitalization of a cryopreserved organ; the immunological reaction and the inflammatory process of rejection do not appear before 10 days. We considered that a 7-day maintenance of digital vitality to be successful revitalization of a composite cryopreserved organ.
These experiment were not free of pitfalls. In spite of all our precautions, the first rabbit tore out the intravenous perfusion during the first night following xenotransplantation. Ceasing treatment with fibrinolytics and anti-coagulants resulted in thrombosis of the arterial anastomosis. Our second rabbit went into cardiac arrest during the post-operative period, although the xenograft was sufficiently vascularized. Our last rabbit lived 5 days, but died of cardiac arrest on the sixthest day. During those five days, the xenograft remained warm and the pulp filled out. The xenograft was recovered and fixed in formol for histological study.
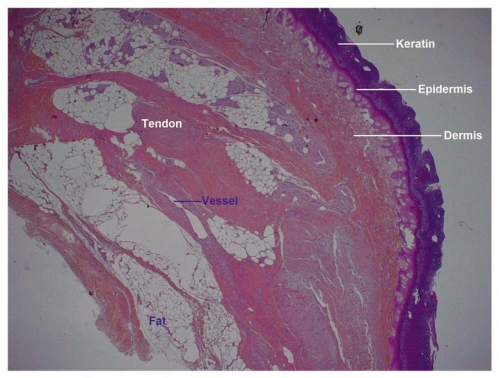
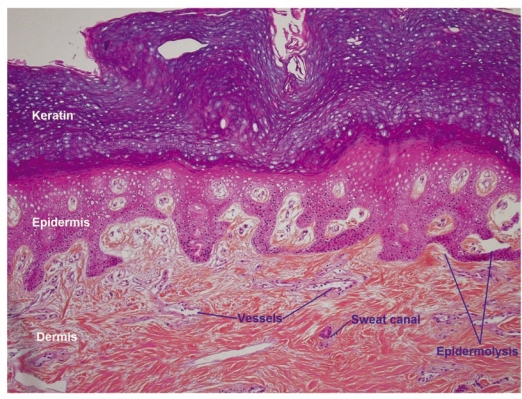
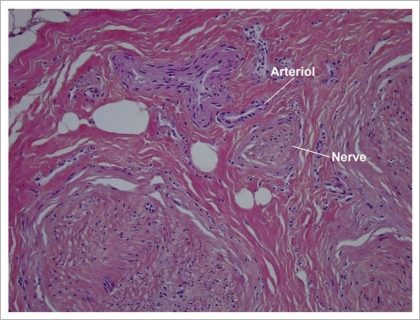
Low power examination of histological sections showed that all cutaneous, hypodermic, tendon, nervous and vascular tissues retained a normal architecture (Fig. 10). At higher magnification, detachment spaces were seen at the dermal-epidermal junction (Fig. 11), which were a prelude to epidermolysis predictably due to the temperature at which allotransplants are stored (−196°C). Such epidermolysis occurs regularly after frostbite. At very high magnification, it was interesting to note the absence of any thrombosis in both venules and arterioles (Fig. 12). This demonstrates that the xenograft remained irrigated throughout the 5 days the rabbit survived.
Figure 10.
Low power histology sections showed that cutaneous, hypodermal, tendon, neural and vascular tissues retained their normal architectures.
Figure 11.
At higher magnification one sees spaces at the dermal-epidermal junction that indicate the onset of a foreseeable epidermolysis.
Figure 12.
At very high magnification vascular thrombosis is totally absent from both venules and arterioles.
These preliminary results confirm that a cryopreserved organ can be revitalized. The use of intra-arterial fibrinolytics, anti-coagulants and vasodilators is fundamental to combat the No-Reflow phenomenon, which systematically occurs due to injuries of the endothelium and the vascular wall during cryopreservation.5–7
It is well-know that during cryopreservation of tissues, particularly vessels, part of the cells does not support the cryoprotective process and died. If we consider the endotheluim layer of the vessels, part of these cells died during cryopreservation while the other part survives. These later cells in addition to the endotheluim cells of the recipient will participate to the rehabilitation process replacing the destroyed endotheluim cells recreating a healed new endotheluim layer two to three weeks after revascularization of the allotransplant. For these reasons, it is fundamental to use fibrinolytic and anti-coagulant during this period to avoid the constitution of the No-reflow phenomenon generated at the sites of injured endotheluim cells.8–12
Further experimentation is certainly necessary, using either a model closer to man, or performing allotransplants directly on animals.
Conclusion
Experimentation with different types of tissue allows defining the ideal cryopreservation protocol for each of them. The synthesis of these protocols allows selecting parameters adapted to the cryopreservation of composite organs. Our experimental results of microsurgical revascularization of cryopreserved xenografts are encouraging, provided direct intra-arterial perfusion with fibrinolytics, anti-coagulants and vasodilators is used. These preliminary results allowed us to reasonably envisage a clinical application: sampling of composite digital segments from a living donor (flexor tendons, metacarpo-phalangeal and proximal Interpalangeal joints, nerves, etc.,), cryopreservation and conservation in an organ bank and on-demand alloreconstruction of the injured hand with functional entities.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/9584
References
- 1.Oufquir A, Bakhach J, Panconi B, Guimberteau JC, Baudet J. Sauvetage des revascularisations digitales par administration intra-artérielle de fibrinolytiques. Ann Chir Plast Esthet. 2006:51471–51481. doi: 10.1016/j.anplas.2005.12.017. (Fre). [DOI] [PubMed] [Google Scholar]
- 2.Furnas H, Rosen J. Monitoring in microvascular surgery. Ann Plast Surg. 1991;26:265–272. doi: 10.1097/00000637-199103000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Yii Ni W, Evans GRD, Miller MJ, Reece GP, Langstein H, Chang D, et al. Thrombolytic therapy: what is its role in free flap salvage? Ann Plast Surg. 2001;46:601–604. doi: 10.1097/00000637-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Serletti JM, Moran SL, Orlando GS, O'Connor T, Herrera R. Urokinase protocol for free-flap salvage following prolonged venous thrombosis. Plast Reconstr Surg. 1998;102:1947–1953. doi: 10.1097/00006534-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Attkiss KJ, Walker M, Buncke HJ. Effect of cryopreservation on survival of composite tissue grafts. J Reconstr Microsurg. 1998;14:559–564. doi: 10.1055/s-2008-1040776. [DOI] [PubMed] [Google Scholar]
- 6.Fu-Chein W, Yen-Lu C, Hung-Chi C, Chwei-Ching C. Three successful digital replantations in a patient after 84, 86 and 94 hours of cold ischemia time. Plast Reconstr Surg. 1988;81:588–589. doi: 10.1097/00006534-198808000-00026. [DOI] [PubMed] [Google Scholar]
- 7.May JM. Digital replantation with full survival afer 28 hours of cold ischemia. Plast Reconstr Surg. 1981;74:565–566. doi: 10.1097/00006534-198104000-00039. [DOI] [PubMed] [Google Scholar]
- 8.Hunt CJ, Song YC, Bateson EAJ, Pegg DE. Fractures in cryopreserved arteries. Cryobiology. 1994;31:506–515. doi: 10.1006/cryo.1994.1061. [DOI] [PubMed] [Google Scholar]
- 9.Bujan J, Pascual G, Lopez R, Corrales C, Rodriguez M, Turegano F, et al. Gradual thawing improves the preservation of cryopreserved arteries. Cryobiology. 2001;42:256–265. doi: 10.1006/cryo.2001.2329. [DOI] [PubMed] [Google Scholar]
- 10.Pascual G, Rodriguez M, Corrales C, Turegano F, Garcia-Honduvilla N, Bellon JM, et al. New approach to improving endothelial preservation in cryopreserved arterial substitutes. Cryobiology. 2004;48:62–71. doi: 10.1016/j.cryobiol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Mesa F, Serra JM, Herreros J. Vascular cryopreservation in microsurgery. J Reconstr Microsurg. 1993;30:164–171. doi: 10.1055/s-2007-1000231. [DOI] [PubMed] [Google Scholar]
- 12.Castier Y, Laseche G, Palombi T, Petit MD, Cerceau O. Early experience with cryopreserved arterial allografts in below-knee revascularisation for limb salvage. Am J Surg. 1999;177:197–202. doi: 10.1016/s0002-9610(99)00010-0. [DOI] [PubMed] [Google Scholar]