Abstract
Cryopreservation would potentially very much facilitate the inventory control and distribution of laboratory-produced organs and tissues. Although simple freezing methods are effective for many simple tissues, bioartificial organs and complex tissue constructs may be unacceptably altered by ice formation and dissolution. Vitrification, in which the liquids in a living system are converted into the glassy state at low temperatures, provides a potential alternative to freezing that can in principle avoid ice formation altogether. The present report provides a brief overview of the problem of renal vitrification. We report here the detailed case history of a rabbit kidney that survived vitrification and subsequent transplantation, a case that demonstrates both the fundamental feasibility of complex system vitrification and the obstacles that must still be overcome, of which the chief one in the case of the kidney is adequate distribution of cryoprotectant to the renal medulla. Medullary equilibration can be monitored by monitoring urine concentrations of cryoprotectant, and urine flow rate correlates with vitrification solution viscosity and the speed of equilibration. By taking these factors into account and by using higher perfusion pressures as per the case of the kidney that survived vitrification, it is becoming possible to design protocols for equilibrating kidneys that protect against both devitrification and excessive cryoprotectant toxicity.
Keywords: biobanking, cryopreserved, cryoprotective agents, ice-free cryopreservation, organ preservation, organ banking, long-term organ preservation, vitreous, vitrification solutions
Introduction
The long-term banking of human organs or their engineered substitutes1 for subsequent transplantation is a long-sought2–4 and important1,2,5–11 goal. Given that the full demand for vital and non-vital organ replacements may be over one million per year in the United States alone, supply chain management issues may become more and more critical as the success of laboratory construct creation increases.1 Contemplating the possible development of emergency organ replacements with generic allografts without the availability of organ biobanking is a bit like trying to envision attempting to distribute human blood with a 24-hour shelf life limitation.
Biobanking of organ and tissue replacements has not been widely discussed perhaps in part because the technology for doing this without damage to the graft is not in hand. Although freezing can achieve limited success for some organs,7,9,12–15 freezing of the heart, liver or kidney has not been accomplished with subsequent life support function following cooling to temperatures low enough for long-term preservation, despite work on this problem dating back to the 1950s.3,6 Kidneys and hearts have been the most widely studied organs, but neither has been reproducibly recovered after freezing to temperatures lower than about −20°C,16–20 evidently due at least in part to mechanical damage from ice itself,21–24 although in the case of kidneys at least, sporadic survival has sometimes been claimed after freezing to about −40 to −80°C.25–28
Some time ago, one of us (GMF), after witnessing transplanted dog kidneys turning deep blue and passing urine that resembled whole blood after freezing to only −30°C with 3 M glycerol (unpublished observations using the same methodology29 used for rabbit kidney freezing), proposed a way of cooling organs to cryogenic temperatures without incurring the consequences of ice formation.30–33 This is possible because high concentrations of cryoprotective agents reduce the likelihood and the speed of ice crystal formation, and sufficiently high concentrations can prevent ice formation completely, even at the low cooling and warming rates that are applicable to organ-sized objects.1,34–36 Cooling an ice-free biological system to a low enough temperature eventually results in a transition from a mobile fluid state to a molecularly arrested glassy state (this transition being referred to as vitrification, or the glass transition). A glass is essentially a liquid that cannot flow over most time scales of interest to the observer,36 and a vitrified biological system can theoretically be stored for virtually any desired length of time due to the extreme slowing of all diffusion-driven change below the glass transition temperature37 (TG). “Vitrification solutions”38 are solutions of cryoprotective agents that are sufficiently concentrated to enable vitrification or virtual vitrification of a living system at the cooling rates employed for that purpose.
Major advances in vitrification technology have recently been reported,6,39 and it is now possible to vitrify entire organs,6 but to do so with full recovery of viability after transplantation is still difficult due in large part to devitrification. Devitrification is ice formation during rewarming, and it arises because ice nuclei, which form initially only at temperatures too low for appreciable crystal growth,36,40 encounter temperatures during warming that maximize ice growth.40,41 To date, small ovaries,42–45 blood vessels,11 heart valves,46 corneas47 and similar structures48 that can all be cooled and rewarmed rather rapidly so as to avoid devitrification, are the only macroscopic structures that have been reported to recover at least in part after vitrification.
Research on vitrification of organs that require immediate vascular anastomosis upon transplantation has been carried out primarily on the rabbit kidney.5,6,39,49–51 The rabbit kidney provides a useful illustration of the general problems of preserving both natural and laboratory-generated organ replacements. In this article, we describe the special problems of vitrifying the kidney and progress made toward their solution, including the first case of life support after vitrification and rewarming.
Results
Survival of the first large solid organ after vitrification and transplantation: a case history.
In late 2002 and early 2003, several rabbit kidneys were perfused with the M22 vitrification solution,6 vitrified and transplanted52 back to their original donors (autografts) with immediate contralateral nephrectomy either to evaluate survival or to evaluate short-term blood reflow only for the first several minutes in vivo. No rabbit survived when perfused with M22 at 40–60 mmHg, but one of two survived after perfusion with M22 at 80 mmHg for 25 min, and the second rabbit in this small group lived for 9 days after transplantation, which was longer than any other non-surviving rabbit studied. Although anecdotal, the sole survivor proves that organ cryopreservation by vitrification can result in life-supporting function after transplantation, and a detailed examination of this case reveals many interesting aspects of the problem of successfully preserving an organ by vitrification.
The events during perfusion of the surviving kidney are shown in Figure 1A and, with the exception of the elevated perfusion pressure during M22 perfusion, are typical of protocols we have described for several years.5,6,49 The venous concentration just before cooling the kidney to below TG was 96.4% of the arterial concentration, and the absolute arteriovenous concentration difference was 330–340 mM. Under the conditions of this perfusion, this venous concentration predicts6 an inner medullary tissue concentration that is 92.1% of the arterial concentration, which is sufficient to permit vitrification on cooling although insufficient to preclude devitrification.6
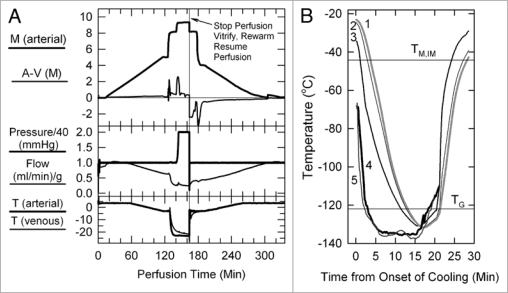
Figure 1.
(A) Perfusion protocol for renal survival after vitrification and rewarming. M, molarity; A-V (M), arteriovenous difference in molarity; T, temperature in degrees Celsius. The protocol, as usual,6,39 employs an initial 5 M plateau, a second plateau at 8.4 M to allow cooling to −22°C without freezing, and a final plateau during M22 perfusion. In the experiment shown, the perfusion was interrupted at the point shown to enable the kidney to be vitrified, rewarmed and reperfused with 8.4 M cryoprotectant at −3°C. (B) Thermal history of the transplanted kidney based on invasive temperature measurements in a model rabbit kidney cooled and rewarmed by a procedure identical to that used for the vitrified-transplanted rabbit kidney. Line 1: inner medullary temperature, as documented by a thermocouple located 1.2 cm below the renal surface; line 2: outer medullary temperature, measured 7 mm below the renal surface; line 3: cortical temperature 2 mm below the renal surface; line 4: environmental temperature of the test kidney; line 5: environmental temperature of the kidney that was transplanted after previous vitrification. TM,IM, estimated melting point of inner medullary tissue (upper horizontal line); TG, estimated glass transition temperature of inner medullary tissue (lower horizontal line).
Figure 1B shows the thermal history of the kidney during cooling and warming and indicates that all parts of the transplanted kidney were below TG for about 8 min, the thermal nadir being about −130°C for the cortex, outer medulla and inner medulla (approximately 7–8°C below the estimated TG of the inner medulla6). The warming rate of the inner medulla from TG to −60°C was about 15°C/min, and declined to 6°C/min from −60°C to the predicted inner medullary melting point (TM,IM) of ∼−44.2°C. During the removal of M22, the kidney perfused normally, and during transplantation, the urine was not bloody and the kidney appearance was reasonable and seemed to be recovering at closure.
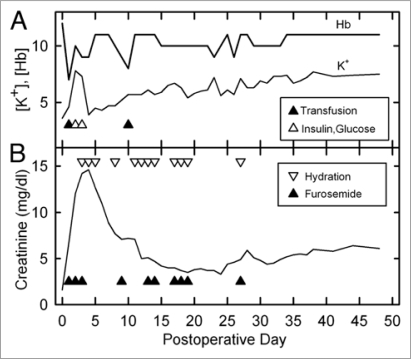
The animal became anemic on the first postoperative day and again on day 10 (Fig. 2A). This symptom was not previously seen after cooling to −45°C.6 Fortunately, the anemia spontaneously resolved after being successfully treated, suggesting recovery of adequate renal production of erythropoietin. Acute hyperkalemia developed on days 2 and 3 but was successfully controlled. Thereafter, K+ levels slowly rose until reaching a stable value by about day 32. Serum creatinine peaked at 14.6 mg/dl (Fig. 2B) on day 4 and then fell to a nadir of 3.3 mg/dl on day 24 independent of diuresis and hydration. It then slowly rose again until reaching an apparently stable value of 6.0–6.4 by day 38.
Figure 2.
(A) Changes in blood levels of hemoglobin and potassium after transplantation of a previously vitrified rabbit kidney and interventions to correct both (triangles). Hyperkalemia was corrected by intravenous glucose (20 ml of 5% dextrose in 0.45% NaCl) and insulin (0.4 ml of 1 U/ml, IV). Anemia was corrected with 20 ml of whole rabbit blood (∼6–8 ml/kg) on each occurrence. Blood levels were measured before corrective interventions given on the same day. [Hb], hemoglobin concentration in g/dl); [K+], potassium concentration in meq/l. (B) Postoperative creatinine levels and diuretic support history. Lower triangles indicate furosemide administration (generally 5–10 mg, IV or IM); upper triangles indicate hydration (generally 100–200 ml, consisting of equal volumes of 0.9% NaCl and 0.45% NaCl plus 5% glucose, subcutaneously). Blood levels were measured before corrective interventions given on the same day.
Clinically, the animal regained normal drinking behavior, a normal fecal output score, and a normal urine volume output score by about 1–2 weeks postoperatively, but food consumption and to a lesser extent water consumption and urine output declined on balance after day 24. The rabbit lost about 18% of its body weight by the fifth postoperative day and thereafter maintained this weight while also maintaining normal posture and behavior other than some sluggishness.
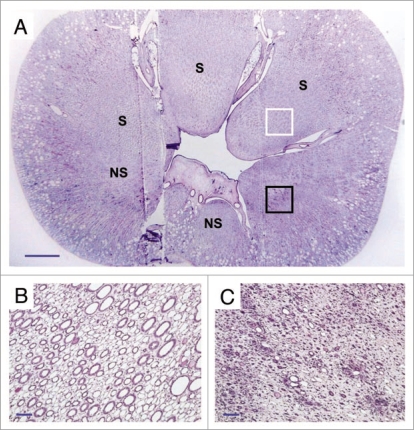
After ensuring that the animal appeared capable of living indefinitely using the vitrified kidney as the sole renal support, it was euthanized for histological follow-up on day 48. Ice formation during warming was not expected in the cortex but was expected to be equivalent to 1–2% of the total inner medullary mass,6 so the fate of the renal medulla was of special interest. To our surprise, examination of an entire renal cross section showed that medullary damage was essentially confined to one side of the kidney, the medullary portion of the peripelvic columns on the opposite side displaying remarkably good survival (Fig. 3). This raises fascinating but still unresolved mechanistic questions about the origin of the observed damage, but indicates that under the experimental conditions achieved, the delivery of M22 to the medulla was sufficient to allow survival of considerable medullary mass, inspiring hope that relatively small improvements in medullary cryoprotectant delivery might enable full survival of the renal medulla.
Figure 3.
(A) Cross-section of the vitrified/rewarmed kidney (PAS staining) showing surviving (S) and non-surviving (NS) medullary areas; white box designates the region depicted in (B), and black box identifies the location of (C). Non-surviving areas are confined to one side of the kidney. Scale bars: in (A), 3 mm; in (B and C), 100 microns.
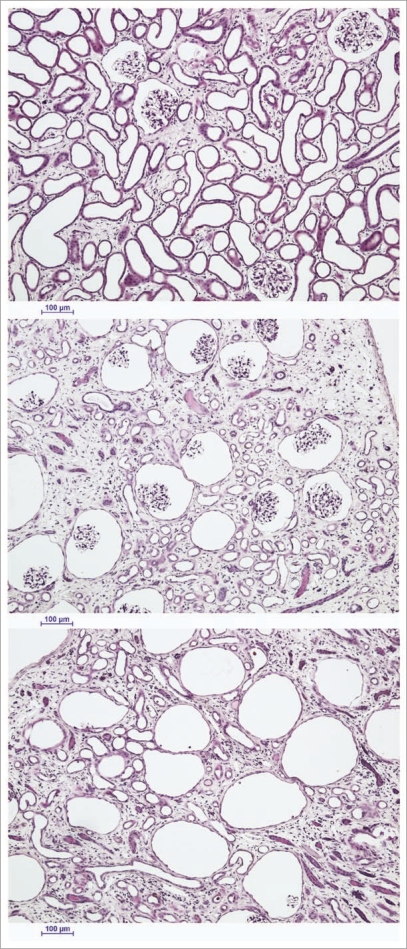
Despite lack of expected freezing in the renal cortex, considerable cortical injury was observed as well. This damage ranged from reasonably mild loss of superficial cortical tubules (Fig. 4, top) to predominant loss of tubules in the cortex corticis with persistence of glomeruli (Fig. 4, middle) to loss or atrophy of both superficial tubules and associated glomeruli (Fig. 4, bottom). We speculate that this injury is the result of previously undiscovered stress-strain phenomena in the outer cortex caused by the establishment of large thermal gradients in relatively stiff and brittle tissue near the glass transition temperature. Lowering cooling or warming rates to avoid this form of injury is feasible in principle but will require still better distribution of M22 into the renal medulla because medullary devitrification will otherwise be exacerbated by lower cooling and warming rates, as verified by direct observation (unpublished results).
Figure 4.
The spectrum of renal cortical responses to vitrification and rewarming. Top: area showing predominant survival of both tubules and glomeruli. Scale bars all represent 100 microns. Middle: transitional zone between predominantly surviving superficial renal cortex and non-surviving cortex, showing loss of tubules but survival of glomeruli. Bottom: non-surviving superficial cortex, showing loss of both tubules and glomeruli, with ballooning of Bowman's capsule. PAS stain.
The problem of renal medullary water replacement.
These results identify the renal medulla as a tissue that seems to be poised at the dividing line between the success and the failure of vitrification. The survival of the medulla presumably depends on the relationship between medullary cryoprotectant delivery and medullary ice formation, and deeper insight into this relationship will be fundamental for understanding the requirements for successful vitrification and recovery of the kidney and, by extension, for the recovery of vitrified organized tissues in general.
The anatomy of the renal vasculature is organized so as to constrain medullary blood flow to a small fraction of total renal blood flow,53 an arrangement that allows the kidney to concentrate urine but makes the task of delivering cryoprotectant to the medulla a difficult one. Anatomically, the medullary circulation is provided by the vasa recta, which originate either directly from widely-spaced points along the arcuate arteries or indirectly from efferent arterioles of juxtamedullary glomeruli, which comprise about 9% of the total number of glomeruli;54 in either case, the originating blood vessels subdivide into many parallel vascular channels, each of which carries a small fraction of the flow that enters the originating vessel.
These anatomical limitations are a given, but medullary delivery of cryoprotectant can be influenced by factors such as perfusate viscosity, cryoprotectant delivery protocol, and the permeability and diffusivity of the cryoprotectants in the vitrification solution. In addition, the vascular system is not the only route of delivery for cryoprotectants. The medulla consists also of tubules and collecting ducts that can convey permeable cryoprotectants along their lengths, diffusing as they go. At the temperatures of our experiments6 (−22°C to 3°C, and particularly ∼−22°C or −3°C for delivery of the highest concentrations of cryoprotectants), and in the presence of more than 8 molar cryoprotectant (<∼48% v/v water), no appreciable renal metabolism can be expected, and therefore tubular delivery of cryoprotectants to the medulla is presumably entirely passive and driven only by filtration at the glomerulus followed by local diffusion (no secretion, no active reabsorption, just diffusion) until delivery into the pelvis. Given that medullary blood flow amounts to only about 10% of total renal blood flow under ordinary conditions,53 a filtration fraction in the vicinity of just 10%, which we have observed for rabbit kidneys,49 would be sufficient to deliver enough ultrafiltrate to the medulla to match the total volume flowing through the medullary blood vessels.
The best vitrification solution known for the kidney to date is M22,6 whose critical cooling rate (the cooling rate above which ice formation is not observed) is 0.1°C/min, and whose critical warming rate (the warming rate above which ice formation is not observed) is 0.4°C/min.1,34 As determined from the cooling and warming curves of Figure 1B, the rabbit kidney can, conservatively, be cooled and warmed by conduction at about 8°C/min or more, which implies that 100% equilibration of the medulla with M22 is not required. Key questions are, what is the level of equilibration that is required, what is required to achieve it, and how can we know when we have achieved it? These questions are taken up in the next section.
Measuring and achieving adequate equilibration.
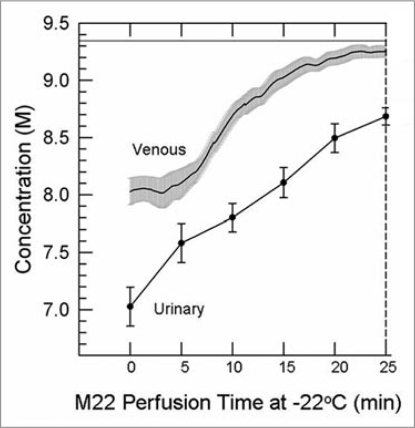
Comparisons between cryoprotectant concentrations in the urinary space and in the venous effluent revealed that the “urine” (perfusate ultrafiltrate) tends to lag far behind the venous effluent in concentration (Fig. 5). This is logical since the urine flow rate is a fraction of the arterial flow rate, and since the venous effluent disproportionately samples the overperfused renal cortex, which accounts for ∼90% of total renal perfusate flow, and therefore under-represents poorly-equilibrated areas. In addition, the urine makes three passes through the renal medulla (descending and ascending limbs of the loop of Henle followed by passage through the collecting ducts) and therefore is in intimate osmotic/diffusive communication with the renal medulla before it is collected. For these reasons, the urine is expected to reflect medullary tissue concentrations of cryoprotectant better than is the venous effluent concentration, and experimental results described below bear out this expectation.
Figure 5.
Difference between the venous concentration and the urinary space concentration during M22 perfusion at −22°C and 40 mmHg (n = 4 perfusions). Urine concentrations (discreet data points ±1 SeM) determined manually; venous concentrations (line with gray “halo” consisting of ±1 SeM) determined by computer. the time base gives time from the nominal onset of M22 perfusion, with includes a lag time as M22 makes its way through the perfusion circuit. the horizontal line near the top of the graph shows the concentration of M22, which is not fully reached even by the venous effluent by the end of M22 delivery.
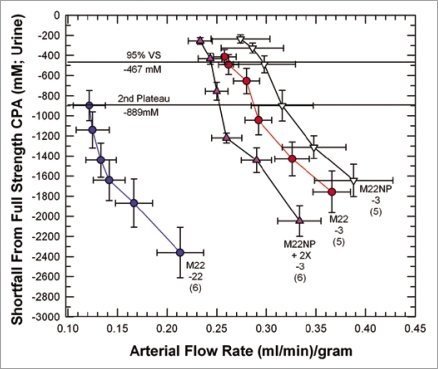
Figure 6 provides a basis for illustrating many features of medullary cryoprotectant introduction. The figure shows the effects of perfusion temperature and the polymer content of M22 on arterial flow and urine concentration equilibration with the arterial perfusate. As in Figure 1A, perfusion with the VMP transitional solution39 (8.4 M total concentration, the second concentration plateau of Fig. 1A) begins at −3°C and in the standard protocol6 continues during continuous perfusion-cooling to −22°C to allow M22 perfusion to begin at −22°C, but we see in Figure 6 that when this is done (M22-22), the urine concentration lags so far behind the arterial concentration that at the end of M22 perfusion, urine concentration is just reaching the concentration of VMP, or only about 90% of the full concentration of M22. Perfusion of VMP and M22 only at higher temperatures (−3°C) reduces viscosity and greatly improves both arterial flow and equilibration, as expected, allowing about 95% equilibration to be attained (M22-3). Removing all polymers from M22 at −3°C (M22NP) further reduces viscosity, improves flow, and improves equilibration, as expected. However, an anomaly is introduced when M22NP is supplemented with just one of the polymers of M22, namely, the commercial Supercool X-1000™ ice blocker55 (X-1000). Perfusing this solution (M22NP + 2X, containing 2% w/v X-1000, or twice the usual concentration of X-1000 in M22,6) slows the arterial perfusion rate yet ultimately allows a degree of equilibration similar to that achieved after M22NP perfusion. Therefore, urinary space equilibration is not proportional to the arterial flow rate.
Figure 6.
Equilibration shortfalls (urine concentration minus nominal arterial concentration) in rabbit kidneys perfused with M22 at −22°C (M22-22) or at −3°C (M22-3) plotted as a function of arterial flow rates (which decline as higher concentrations are reached and viscosity increases). M22NP-3 refers to M22 minus all polymers, perfused at −3°C; M22NP + 2X-3 refers to M22NP containing 2% X1000 ice blocker, perfused at −3°C. Values in parentheses indicate the number of perfusions of each type. Each data point represents urine equilibration measured at 5-min intervals, beginning at VS perfusion time zero to the right and ending at VS perfusion time = 25 min to the left. The horizontal lines are “landmark” concentrations and refer to the concentrations of VMP (2nd Plateau, which falls at a shortfall of −889 mM) and 95% of full-strength vitrification solution (VS) (which, because of the negligible molarity of the polymers of M22, is essentially the same for M22, M22NP and M22NP + 2X). error bars designate ±1 SEM. For discussion, see text.
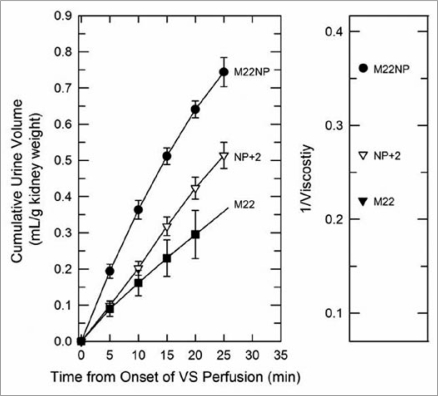
Equilibration was, however, mirrored by differences in the urine flow rates in these groups, and the latter were in turn closely accounted for by the viscosities of the M22 variant solutions (Fig. 7). Thus, it seems that urinary equilibration is more closely correlated with urine flow rate than with arterial flow rate.
Figure 7.
Left: urine accumulation during perfusion with M22, M22NP + 2% X1000 (NP + 2), and M22NP at −3°C; right: reciprocal viscosities of these three vitrification solutions (cP−1). the total accumulated urine volumes are inversely proportional to the total viscosity of each VS (M22 = 4.54 cP; M22NP + 2X = 3.71 cP; M22NP = 2.77 cP). the urine volume for M22 at 25 min was not consistently recorded and so is indicated by extrapolation. Data points represent means ±1 SEM.
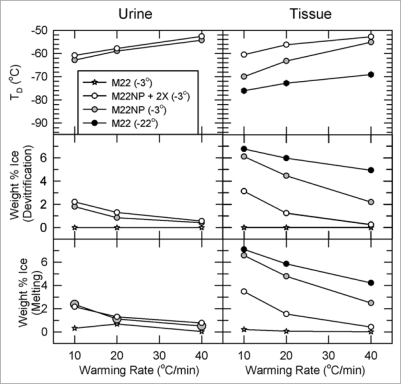
Figure 8 answers the question of “how much equilibration is enough” and brings out a number of other important points. The left panels describe the devitrification temperatures, percent ice formed at the point of devitrification, and percent of ice melted at the tissue melting point, for urine samples collected at the end of the perfusion, and the right panels report the same information for inner medullary tissue samples (all data obtained by differential scanning calorimetry).
Figure 8.
Temperature, extent and warming rate dependence of ice formation in urine (left) and tissue samples (right) obtained from kidneys subjected to the four protocols of Figure 6 and relationship between the amount of ice formed during devitrification and the amount of ice that thawed upon complete rewarming. Urine was not collected from the M22 kidneys perfused at −22°C. Upper: devitrification temperatures (TD); middle: the percentage of sample mass that crystallizes during devitrification; lower: the percentage of sample mass that melts upon continued warming. each point represents the mean of generally 5–6 independent measurements; devitrification temperatures are averaged only for those samples that devitrified. No devitrification event was observed for any specimen in the M22 −3°C group. Error bars omitted for clarity. Groups are represented as indicated in the inset. For discussion, see text.
The first thing to note is that perfusion of M22 at −22°C causes about 7% of inner medullary mass to crystallize as ice during rewarming, and this result is little affected by the warming rate. This amount of ice is substantially greater than was predicted for our surviving vitrified kidney, presumably because in the experiments of Figure 8 we perfused at 40 mmHg rather than at 80 mmHg, which is known to make a significant difference.6 In complete contrast, perfusion of M22 at −3°C (stars) results in no tissue ice formation at any warming rate. Therefore, the required degree of urinary space equilibration lies between 90% and 95%, and is probably close to the latter limit.
Second, perfusing M22NP at −3°C results in less ice formation than perfusing M22 at −22°C even though M22NP is a more dilute and intrinsically less stable solution; this is undoubtedly because the higher equilibration level of M22NP delivers more net cryoprotectant despite its lower total concentration. Finally, adding 2% X1000 to M22NP greatly suppresses tissue ice crystal formation, which demonstrates the ability of X1000 to usefully penetrate into and protect inner medullary tissue.
Comparing tissue results to urine results shows that tissue generally devitrifies at a lower temperature than does urine from the same kidney, and that the amount of ice formed in tissue is accordingly higher than it is in urine from the same kidney, indicating that tissue concentrations lag behind urine concentrations. Interestingly, for both urine and tissue, in most cases the percentage of sample mass that melts upon thawing is the same as the percentage that freezes during devitrification, meaning that vitrification is generally complete on cooling with the regimen used for tissue analysis.
Visual assessment of ice formation.
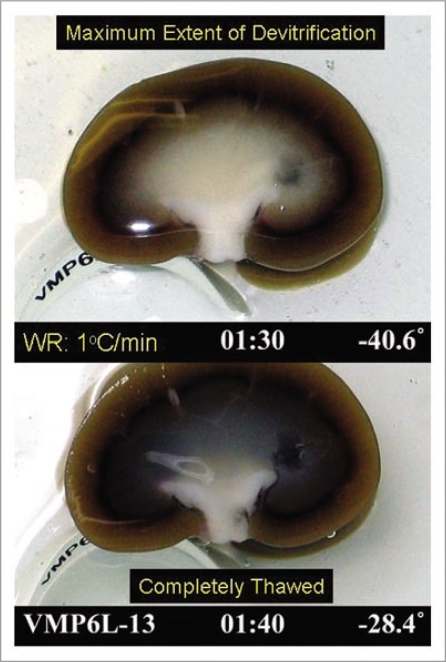
Although tissue biopsies allow quantitative results to be obtained as presented in Figure 8, we have been interested in developing methods for visualizing ice formation across entire renal cross-sections in order to be able to judge the two and three-dimensional extent of ice formation. Although these methods are still in development, we present an example of the type of information that can be obtained in Figure 9. In this example, the warming rate was about 1°C/min, and therefore more ice is expected than with the more rapid warming used in Figure 8. Nevertheless, the maximum extent of ice formation, judged by whitening of the tissue during rewarming, did not include the cortex in this example, and the ice that formed appeared to be uniformly distributed.
Figure 9.
Visual appearance of ice in an exemplary rabbit kidney cross-section during rewarming. the kidney was perfused with M22 at −22°C, cut in half, immersed in M22, vitrified in a CryoStar freezer at −135°C, and eventually rewarmed at about 1°C/min while being photographed from time to time. Rewarming was accomplished by transfer of the kidneys to an insulated box through which liquid nitrogen vapor was circulated slowly so as to allow steady warming of the contained atmosphere from just below Tg to well above the renal melting points. Times (1:30 and 1:40) represent times in hours and minutes since the onset of slow warming, and temperatures refer to ambient atmospheric temperatures near the kidney but not within the kidney itself. The upper panel shows the kidney at the point of maximum ice cross-sectional area, and the lower panel shows the kidney after complete ice melting. Both panels show the site of an inner medullary biopsy taken for differential scanning calorimetry.
Using this method and differential scanning calorimetry will eventually allow us to determine the extent to which medullary ice formation can be tolerated by the kidney. We have been able to show that medullary damage can be assessed in the acute postoperative period by removing kidneys 30 min after transplantation, flushing them to remove blood, and examining the extent of medullary blood trapping by inspection of renal cross-sections (unpublished observations). Although preliminary, such observations have identified conditions that allow blood trapping to be avoided, and as our methods improve, we should be able to use such methods to determine how much medullary ice formation, if any, is acceptable, and to select perfusion methods for evaluation by permanent transplantation.
Materials and Methods
Procedure for obtaining survival after rabbit kidney vitrification.
A 12.7 gram rabbit kidney was perfused with M22, a 9.3 M vitrification solution with very low critical cooling and warming rates,1,6,34 in an LM5 carrier solution under computer control56 using a variation of our standard protocol6 on December 10th, 2002 (Fig. 1A). Perfusion began with Renasol-14 containing 2% w/v B. Braun hydroxyethyl starch (HES) and no cryoprotectant and continued, after a pause at 5 M cryoprotectant to allow the arteriovenous (AV) concentration gradient to level, to VMP39 in LM5 containing no HES. To distribute M22 more thoroughly than usual while minimizing damage from perfusion pressures over 40 mmHg, perfusion pressure was raised to 80 mmHg only during the 25-min period of exposure to M22 itself. The kidney was removed from the perfusion apparatus at the end of M22 perfusion and cooled in rapidly-moving nitrogen vapor6 (Fig. 1B) The intra-renal thermal history was determined by inserting a three-point needle thermocouple (beads at 2, 7 and 12 mm depths; PhysiTemp, Huron, PA) into an identically-treated but non-transplanted kidney.
Rewarming was accomplished by slowly raising the environmental temperature to about −115°C in order to bring the cortical temperature to just above TG, at which point the kidney was returned to the perfusion machine and further warmed by pouring M22 at −22°C over the renal surface for 8 min. Rewarming was completed by perfusing the kidney with VMP at −3°C, after which cryoprotectant washout was completed as usual6 (Fig. 1A), transitioning from VMP in LM5 to Renasol-14 + 2% HES, and the kidney was transplanted according to our published method52 with immediate contralateral nephrectomy.
Perfusion of kidneys with M22 and alternative vitrification solutions at 40 mmHg.
All perfusions were carried out under computer control in the general manner represented in Figure 1A. However, because B. Braun discontinued the manufacturing of HES and because the use of all alternative forms of HES was associated with higher post-transplantation peak creatinine levels (unpublished results), we replaced HES with 2% w/v decaglycerol (dG) in the carrier solution at the beginning of the perfusion. We also used TransSend-4 at the beginning and end of each experiment, but retained LM5 as the carrier for VMP, M22, and their variants. Remaining protocol details other than the perfusion pressure were as reported in Figure 1A and elsewhere.6
End point measurements.
Determination of tissue freezing points (devitrification temperatures), percent ice formation and percent ice melted were all carried out by differential scanning calorimetry. The cooling and warming protocol for inner medullary samples was to cool to −120°C at 10°C/min and to rewarm at 10, 20 or 40°C/min when the endpoint was devitrification. Heats of devitrification and of melting were obtained by integrating peak areas and were converted from units of joules/gram into percent ice formation by dividing by 3.34.6 The temperatures of devitrification were taken to be the temperatures at the tops of the observed peaks.
Cryoprotectant concentrations were determined from refractive index readings on the basis of appropriate calibration curves. Baseline data were freshly derived for each experiment during priming of the perfusion system. All refractive indices were recorded continuously at ∼0°C using ice-immersed in-line process refractometers (AFAB Enterprises, Eutis, FL, Model PR-111) at the beginning (priming) and experimental phases of each perfusion except that urine refractive index was determined using a bench-top Bellingham Stanley RFM 330 refractometer at room temperature and converted to concentration using a separate room temperature calibration obtained using the same refractometer. Viscosities were measured using a Gilmont falling-ball viscometer (Cole Parmer) at room temperature.
Conclusions
Clearly, the problem of eliminating or sufficiently limiting ice formation throughout the kidney without inducing unacceptable toxicity is a complex and many-faceted one. So far, the most promising single approach seems to be the one described in Figure 1, which resulted in survival after transplantation. However, the many lessons that have been learned since that experiment will undoubtedly result in methods for protecting the kidney that are more effective than those used in Figure 1, and that will allow better and more consistent survival to be obtained after vitrification and rewarming. Certainly, the availability of new methodologies to evaluate renal tissue resistance to ice formation will be helpful, and the use of microwave rewarming to reduce the likelihood of damage from devitrification could also be highly beneficial for our efforts to solve the very complex problem of fully successful renal vitrification.
Because of its unique vascularization, the kidney may be the most challenging organ of them all to vitrify and rewarm successfully. If so, continued progress with the kidney should be encouraging for the future vitrification and recovery of other complex living systems, including laboratory-produced organ and tissue replacements, whose accessibility to cryoprotectant may be significantly greater than that of the renal medulla.
Acknowledgements
Transplantation of the surviving kidney was carried out by Dr. Jun Wu, who is now in private dental practice. The authors wish to thank Ms. Perlie Tam for expert surgical assistance. Supported by 21st Century Medicine, Inc., All procedures involving animal use were done according to USDA standards and with IACUC approval.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/9974
References
- 1.Fahy GM, Wowk B, Wu J. Cryopreservation of complex systems: the missing link in the regenerative medicine supply chain. Rejuvenation Res. 2006;9:279–291. doi: 10.1089/rej.2006.9.279. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE. A look ahead at transplantation. J Surg Res. 1970;10:291–297. doi: 10.1016/0022-4804(70)90047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AU. Problems in the resuscitation of mammals from body temperatures below 0°C. Proc R Soc Lond B Biol Sci. 1957;147:533–544. doi: 10.1098/rspb.1957.0077. [DOI] [PubMed] [Google Scholar]
- 4.Karow AM., Jr . The organ bank concept. In: Karow AM Jr, Abouna GJM, Humphries AL Jr, editors. Organ Preservation for Transplantation. Boston: Little, Brown and Company; 1974. pp. 3–8. [Google Scholar]
- 5.Khirabadi B, Fahy GM. Permanent life support by kidneys perfused with a vitrifiable (7.5 molar) cryoprotectant solution. Transplantation. 2000;70:51–57. [PubMed] [Google Scholar]
- 6.Fahy GM, Wowk B, Wu J, Phan J, Rasch C, Chang A, et al. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology. 2004;48:157–178. doi: 10.1016/j.cryobiol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Chen H, Yin H, Kim S, Lin Tan S, Gosden R. Fertility after intact ovary transplantation. Nature. 2002;415:385. doi: 10.1038/415385a. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson JO, Toner M. Cryopreservation. In: Lanza RP, Langer R, Vacanti J, editors. Principles of Tissue Engineering. Second Edition. San Diego: Academic Press; 2000. pp. 293–307. [Google Scholar]
- 9.Arav A, Revel A, Nathan Y, Bor A, Gacitua H, Yavin S, et al. Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum Reprod. 2005;20:3554–3559. doi: 10.1093/humrep/dei278. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser J. New prospects for putting organs on ice. Science. 2002;295:1015. doi: 10.1126/science.295.5557.1015. [DOI] [PubMed] [Google Scholar]
- 11.Song YC, Khirabadi BS, Lightfoot F, Brockbank KG, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nat Biotechnol. 2000;18:296–299. doi: 10.1038/73737. [DOI] [PubMed] [Google Scholar]
- 12.Bedaiwy MA, Jeremias E, Gurunluoglu R, Hussein MR, Siemianow M, Biscotti C, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003;79:594–602. doi: 10.1016/s0015-0282(02)04842-2. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Madrid B, Dolmans M-M, van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82:1390–1394. doi: 10.1016/j.fertnstert.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton R, Holst HI, Lehr HB. Successful preservation of canine small intestine by freezing. J Surg Res. 1973;14:313–318. doi: 10.1016/0022-4804(73)90033-4. [DOI] [PubMed] [Google Scholar]
- 15.Fahy GM. Analysis of “solution effects” injury: rabbit renal cortex frozen in the presence of dimethyl sulfoxide. Cryobiology. 1980;17:371–388. doi: 10.1016/0011-2240(80)90044-9. [DOI] [PubMed] [Google Scholar]
- 16.Elami A, Gavish Z, Korach A, Houminer E, Schneider A, Schwalb H, et al. Successful restoration of function of frozen and thawed isolated rat hearts. J Thorac Cardiovasc Surg. 2008;135:666–672. doi: 10.1016/j.jtcvs.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 17.Toledo-Pereyra LH. Organ freezing. J Surg Res. 1982;32:75–84. doi: 10.1016/0022-4804(82)90188-3. [DOI] [PubMed] [Google Scholar]
- 18.Pegg DE, Green CJ, Walter CA. Attempted canine renal cryopreservation using dimethyl sulphoxide, helium perfusion and microwave thawing. Cryobiology. 1978;15:618–626. doi: 10.1016/0011-2240(78)90086-x. [DOI] [PubMed] [Google Scholar]
- 19.Smith AU. The effects of glycerol and of freezing on mammalian organs. In: Smith AU, editor. Biological Effects of Freezing and Supercooling. London: Edward Arnold, Ltd; 1961. pp. 247–269. [Google Scholar]
- 20.Kubota S, Lillehei RC. Some of the problems associated with kidneys frozen to −50°C or below. Low Temp Med. 1976;2:95–105. [Google Scholar]
- 21.Pegg DE, Diaper MP. The mechanism of cryoinjury in glycerol-treated rabbit kidneys. In: Pegg DE, Jacobsen IA, Halasz NA, editors. Organ Preservation, Basic and Applied Aspects. Lancaster: MTP Press, Ltd; 1982. pp. 389–393. [Google Scholar]
- 22.Karow AM, Jr, Shlafer M. Ultrastructure-function correlative studies for cardiac cryopreservation. IV. Prethaw ultrastructure of myocardium cooled slowly (<=2°C/min) or rapidly (>=70°C/sec) with or without dimethyl sulfoxide (DMSO) Cryobiology. 1975;12:130–143. doi: 10.1016/s0011-2240(75)80005-8. [DOI] [PubMed] [Google Scholar]
- 23.Hunt CJ. Studies on cellular structure and ice location in frozen organs and tissues: the use of freeze-substitution and related techniques. Cryobiology. 1984;21:385–402. doi: 10.1016/0011-2240(84)90077-4. [DOI] [PubMed] [Google Scholar]
- 24.Pollack GA, Pegg DE, Hardie IR. An isolated perfused rat mesentery model for direct observation of the vasculature during cryopreservation. Cryobiology. 1986;23:500–511. doi: 10.1016/0011-2240(86)90059-3. [DOI] [PubMed] [Google Scholar]
- 25.Halasz NA, Rosenfield HA, Orloff MJ, Seifert LN. Whole organ preservation II. Freezing studies. Surgery. 1967;61:417–421. [PubMed] [Google Scholar]
- 26.Halasz NA, Miller S. Rewarming methods for whole organ freezing. In: Norman JC, editor. Organ Perfusion and Preservation. New York: Appleton-Century-Crofts; 1968. pp. 731–737. [Google Scholar]
- 27.Guttman FM, Lizin J, Robitaille P, Blanchard H, Turgeon-Knaack C. Survival of canine kidneys after treatment with dimethylsulfoxide, freezing at −80°C, and thawing by microwave illumination. Cryobiology. 1977;14:559–567. doi: 10.1016/0011-2240(77)90166-3. [DOI] [PubMed] [Google Scholar]
- 28.Lehr H. Progress in long-term organ freezing. Transplant Proc. 1971;3:1565. [Google Scholar]
- 29.Fahy GM. Activation of alpha adrenergic vasoconstrictor response in kidneys stored at −30°C for up to 8 days. Cryo Letters. 1980;1:312–317. [Google Scholar]
- 30.Fahy GM. Prospects for vitrification of whole organs. Cryobiology. 1981;18:617. [Google Scholar]
- 31.Fahy GM, Hirsh A. Prospects for organ preservation by vitrification. In: Pegg DE, Jacobsen IA, Halasz NA, editors. Organ Preservation, Basic and Applied Aspects. Lancaster: MTP Press; 1982. pp. 399–404. [Google Scholar]
- 32.Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 33.Fahy GM, MacFarlane DR, Angell CA. Recent progress toward vitrification of kidneys. Cryobiology. 1982;19:668–669. [Google Scholar]
- 34.Wowk B, Fahy GM. Toward large organ vitrification: extremely low critical cooling and warming rates of M22 vitrification solution. Cryobiology. 2005;51:362. [Google Scholar]
- 35.Wowk B, Fahy GM. Ice nucleation and growth in concentrated vitrification solutions. Cryobiology. 2007:330. [Google Scholar]
- 36.Wowk B. Thermodynamic aspects of vitrification. Cryobiology. 2009:59. doi: 10.1016/j.cryobiol.2009.05.007. (in press) [DOI] [PubMed] [Google Scholar]
- 37.Fahy GM. Vitrification: An overview. In: Liebermann J, Tucker MJ, editors. Vitrification in Assisted Reproduction: A User's Manual and Troubleshooting Guide. London: Informa Healthcare; 2007. (in press) [Google Scholar]
- 38.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196°C by vitrification. Nature. 1985;313:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 39.Fahy GM, Wowk B, Wu J, Paynter S. Improved vitrification solutions based on predictability of vitrification solution toxicity. Cryobiology. 2004;48:22–35. doi: 10.1016/j.cryobiol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Fahy GM. The role of nucleation in cryopreservation. In: Lee REJ, Warren GJ, Gusta LV, editors. Biological ice nucleation and its applications. St. Paul: APS Press; 1995. pp. 315–336. [Google Scholar]
- 41.Fahy GM. Vitrification. In: McGrath JJ, Diller KR, editors. Low Temperature Biotechnology: Emerging Applications and Engineering Contributions. New York: American Society of Mechanical Engineers; 1988. pp. 113–146. [Google Scholar]
- 42.Courbiere B, Massardier J, Salle B, Mazoyer C, Guerin J-F, Lornage J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84:1065–1071. doi: 10.1016/j.fertnstert.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto M, Maeda S, Manabe N, Miyamoto H. Development of infantile rat ovaries autotransplanted after cryopreservation by vitrification. Theriogenology. 2000;53:1093–1103. doi: 10.1016/S0093-691X(00)00255-7. [DOI] [PubMed] [Google Scholar]
- 44.Salehnia M. Autograft of vitrified mouse ovaries using ethylene glycol as cryoprotectant. Exp Anim. 2002;5:509–512. doi: 10.1538/expanim.51.509. [DOI] [PubMed] [Google Scholar]
- 45.Migishima F, Suzuki-Migishima R, Song S-Y, Kuramochi T, Azuma S, Nishijima M, et al. Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod. 2003;68:881–887. doi: 10.1095/biolreprod.102.007948. [DOI] [PubMed] [Google Scholar]
- 46.Brockbank KG, Song YC. Morphological analyses of ice-free and frozen cryopreserved heart valve explants. J Heart Valve Dis. 2004;13:297–301. [PubMed] [Google Scholar]
- 47.Armitage WJ, Hall SC, Routledge C. Recovery of endothelial function after vitrification of cornea at −110°C. Invest Ophthalmol Vis Sci. 2002;43:2160–2164. [PubMed] [Google Scholar]
- 48.Taylor MJ, Song YC, Brockbank KG. Vitrification in tissue preservation: new developments. In: Fuller BJ, Lane N, Benson EE, editors. Life in the frozen state. Boca Raton: CRC Press; 2004. pp. 603–641. [Google Scholar]
- 49.Fahy GM, Ali SE. Cryopreservation of the mammalian kidney II. Demonstration of immediate ex vivo function after introduction and removal of 7.5 M cryoprotectant. Cryobiology. 1997;35:114–131. doi: 10.1006/cryo.1997.2026. [DOI] [PubMed] [Google Scholar]
- 50.Fahy GM, da Mouta C, Tsonev L, Khirabadi BS, Mehl P, Meryman HT. Cellular injury associated with organ cryopreservation: chemical toxicity and cooling injury. In: Lemasters JJ, Oliver C, editors. Cell Biology of Trauma. Boca Raton: CRC Press; 1995. [Google Scholar]
- 51.Khirabadi BS, Fahy GM, Ewing L, Saur J, Meryman HT. 100% survival of rabbit kidneys chilled to −32°C after perfusion with 8 M cryoprotectant at −22°C. Cryobiology. 1994;31:597. [Google Scholar]
- 52.Wu J, Ge X, Fahy GM. Ultrarapid nonsuture mated cuff technique for renal transplantation in rabbits. Microsurgery. 2003;23:1–5. doi: 10.1002/micr.10145. [DOI] [PubMed] [Google Scholar]
- 53.Ofstad J, Aukland K. Renal circulation. In: Seldin DW, Giebisch G, editors. The kidney, physiology and pathophysiology. New York: Raven Press; 1985. pp. 471–496. [Google Scholar]
- 54.Kaissling B, Kritz W. Structural analysis of the rabbit kidney. Adv Anat Embryol Cell Biol. 1979;56:1–123. doi: 10.1007/978-3-642-67147-0. [DOI] [PubMed] [Google Scholar]
- 55.Wowk B, Leitl E, Rasch CM, Mesbah-Karimi N, Harris SB, Fahy GM. Vitrification enhancement by synthetic ice blocking agents. Cryobiology. 2000;40:228–236. doi: 10.1006/cryo.2000.2243. [DOI] [PubMed] [Google Scholar]
- 56.Fahy GM. Organ perfusion equipment for the introduction and removal of cryoprotectants. Biomed Instrum Technol. 1994;28:87–100. [PubMed] [Google Scholar]