Abstract
Objective
We investigated whether the covert preparation of saccadic eye movements results in spatially specific modulations of somatosensory processing.
Methods
ERPs were recorded in a spatial cueing experiment where auditory cues preceded tactile stimuli delivered to the left or right hand. In the Saccade task, cues signalled that an eye movement towards the left or right hand had to be prepared. In the Covert Attention task, cues signalled the direction of a covert shift of tactile attention.
Results
A lateralised component previously observed during cued shifts of spatial attention (ADAN) was elicited in the cue-target interval in both tasks. The somatosensory N140 component was enhanced for tactile stimuli presented to the hand on the cued side. This modulation was present not just in the Covert Attention task, but also in the Saccade task. Longer-latency effects of spatial cueing were only present in the Covert Attention task.
Conclusions
Covert shifts of attention and saccade preparation have similar effects on early stages of tactile processing, suggesting that both are mediated by overlapping control processes.
Significance
These findings support the premotor theory of attention by demonstrating that the programming of eye movements has spatially selective effects on somatosensory processing.
Keywords: attention, spatial, eye movement, event-related brain potentials, somatosensory processing
Introduction
To select relevant information from the environment, we typically orient our attention by moving the eyes toward a specific location. However, visual attention can also be oriented in a covert fashion in the absence of overt eye movements (Eriksen and Colegate, 1971; Eriksen and Hoffman, 1972; Posner et al., 1980). The question whether and how eye movement preparation and spatial attention are linked is currently a focal topic in cognitive neuroscience. Neuroimaging studies have uncovered an overlapping network of cortical regions including the frontal eye fields (FEFs) and several parietal regions that are activated during endogenous covert shifts of attention as well as during the preparation and execution of overt eye movements (Astafiev et al., 2003; Beauchamp et al., 2001; Corbetta et al. 1998; Perry and Zeky, 2000). Neurophysiological studies on non-human primates have shown that cortical areas such as the FEFs (e.g., Bizzi, 1968; Bruce et al., 1985) and posterior parietal cortex (e.g., Mountcastle et al., 1975; Robinson et al., 1978; Andersen et al., 1987) are activated during visually guided saccadic eye movements as well as during purely attentional tasks in the absence of any eye movements (Schall et al., 1995; Bushnell et al., 1981; Steinmetz et al., 1994; Colby et al., 1996). Microstimulation of the sites from which saccadic eye movements can be evoked can enhance attentional performance (Moore and Fallah, 2001). In humans, transcranical magnetic stimulation (TMS) over the FEF can modulate attentionally guided performance in visual search tasks (Muggleton et al., 2003), or disrupt shifts of attention (Grosbras and Paus, 2002).
The anatomical overlap between attentional and oculomotor systems suggests that these two systems might also be functionally linked. According to the premotor theory of attention, the activation of any of the pragmatic motor maps for eye, arm, or hand movements during spatially directed response preparation will result in concomitant shifts of spatial attention (Rizzolatti, 1983; Rizzolatti and Camarda, 1987; Rizzolatti et al., 1987, 1994). This theory was originally formulated to account links between attention and eye movements. The main difference between overt movements of the eyes and covert shifts of attention is that in the latter case the oculomotor program is activated but not overtly executed (Rizzolatti et al., 1987). If attention shifts are triggered toward the saccade target whenever an oculomotor program is activated, as predicted by the premotor theory, the processing of stimuli presented close to the saccade goal location should be improved in a way that is comparable to the improvements found as a result of covert attention shifts in the absence of eye movements. Single-cell studies on monkeys have indeed found enhancements of neural responses to visual stimuli presented close to the saccade goal location which can arise well before the eyes begin to move (Goldberg and Bushnell, 1981; Robinson et al., 1978; Wurtz and Goldberg, 1972; Wurtz et al., 1982; Wurtz and Mohler, 1976). In addition, behavioural studies in humans have demonstrated superior performance for visual events presented at intended saccade target locations even before the eyes begin to move (e.g., Deubel and Schneider, 1996; Hoffman and Subramaniam, 1995; Irwin and Gordon, 1998).
The hypothesis that a common network of frontoparietal cortical areas is involved in both spatial attention and oculomotor programming, and the related assumption that both spatial attention and eye movement preparation result in spatially specific modulations of visual processing have recently also found support from ERP studies (Eimer et al., 2006; Van der Lubbe et al., 2006; Van der Stigchel et al., 2006; Wauschkuhn et al., 1998). These studies have compared ERP correlates of the control mechanisms activated during saccade preparation and covert shifts of visual attention under conditions where spatial cues signalled the direction of attentional orienting or of an upcoming saccadic eye movement, respectively. Very similar lateralized ERP components were observed during covert attentional orienting and during saccade preparation. A negative deflection contralateral to the cued side triggered at anterior electrodes between 300 and 600 ms after cue onset (‘anterior directing attention negativity’, ADAN) was followed by a relative positivity over posterior scalp sites contralateral to the cued side (‘late directing attention positivity’, LDAP). These components are supposed to reflect brain activity within anterior and posterior regions of the attentional control network. The ADAN has been localised in dorsal premotor cortex (PMd; Praamstra et al. 2005) and the FEF (Van der Lubbe et al., 2000, 2006), while the LDAP is assumed to be generated in the occipitotemporal cortex (Mathews et al., 2006; Praamstra et al., 2005), although it has also been tentatively localized in the ventral intraparietal sulcus (VIP, Van der Lubbe et al., 2006). The fact that similar lateralised ADAN and LDAP components were found both during covert shifts of visual attention and during saccade preparation suggests not only that analogous brain areas are activated during these processes, but also that their temporal dynamics are very similar (Eimer et al., 2007; Van der Lubbe et al., 2006; but see also Wauschkuhn et al., 1998 for different results).
In addition to comparing lateralised ERP components triggered during attentional shifts and eye movement preparation, respectively, we have recently also tested whether saccade preparation can result in spatially specific modulations of early visual processing, as has previously been demonstrated for covert spatial attention (Eimer et al., 2006, 2007). In these studies, where participants had to covertly prepare a left or right saccade, task-irrelevant peripheral visual probe stimuli were presented during the saccade preparation interval at the saccade target location or on the opposite side. Visual N1 components to these probe stimuli were enhanced when they were presented at the saccade goal, in line with the prediction that covert saccade preparation induces a spatial bias for the processing of visual information.
Given that links between eye movement preparation and visual attention have now been reliably demonstrated with various neuroscientific methods, one important additional question is whether such links might extend beyond the visual domain. One might argue that spatially selective processing benefits for visual stimuli at saccade target locations are not surprising, given that the shared function of saccadic eye movements and covert visual attention is to improve the efficiency of visual processing. If it could be shown that saccade preparation has similar effects for other sensory modalities such as audition or touch, this would provide compelling evidence for the generality of the effects of oculomotor programming on sensory processing. There are indeed a number of studies that have suggested links between eye movement preparation and auditory processing. Receptive fields of multimodal audio-visual neurons in the lateral intra-parietal area (LIP) of the parietal cortex shift with gaze direction of gaze (Mazzoni et al., 1996; Stricanne et al., 1996), suggesting that the same neurons may be involved in the preparation of eye movements towards auditory as well as visual targets. Similarly, a group of neurons in the frontal eye field becomes active before a saccade is executed either toward an auditory or a visual target (Russo and Bruce, 1994). In addition, behavioural studies have demonstrated improved performance for sounds at fixation (Gopher, 1973; Hublet et al., 1976, 1977; Jones and Kabanoff, 1975; Morais et al., 1980; Reisberg et al., 1981), as well as for sounds presented at the destination of an upcoming saccade (Rorden and Driver, 1999; Lie and Coslett, 2006), suggesting that eye gaze may affect auditory processing in a spatially specific fashion.
In a recent ERP study (Gherri et al., in press), we investigated the potential impact of covert saccade preparation on auditory processing in a task where an auditory cue signalled the direction of an eye movement that was to be executed or withheld in response to a subsequent auditory stimulus. An enhanced negativity with an onset latency of about 180 ms was observed for lateral auditory non-target stimuli presented at the cued saccade target location, as compared to auditory stimuli presented on the opposite uncued side. These effects very similar, albeit reduced in amplitude, to the ERP modulations observed for another task where the cue indicated the direction of a covert endogenous shift of auditory attention. These results suggest that saccade preparation and covert attentional orienting may have similar spatially selective effects on auditory processing.
The aim of the present experiment was to use ERP measures to further investigate effects of eye movement preparation on sensory processing, but this time for the somatosensory modality. Only very few experiments to date have studied links between saccade preparation and the processing of tactile events. In a behavioural study, Rorden et al. (2002) demonstrated faster responses to tactile stimuli that were presented near the target location of a currently prepared saccade. This effect disappeared when tactile stimuli were likely to be presented contralateral to an upcoming eye movement, suggesting that links between eye movement preparation and tactile processing are not fully automatic, but can be modulated by top-down control processes. In the present experiment, we directly compared ERP correlates of covert shifts of tactile attention to the left or right side with the effects obtained under conditions where leftward or rightward saccades had to be prepared. In the Covert Attention task, participants were instructed to direct their attention to the side, as indicated by an auditory cue (S1) presented at the start of each trial, and to respond whenever an infrequent tactile target stimulus (S2) was presented at the cued side. They had to ignore tactile non-target S2 stimuli on the cued side, and all tactile stimuli on the uncued side (both target and non-target). Tactile non-targets were continuous stimulations of the left or right index finger (100 ms duration), while tactile targets had a gap where this stimulation was interrupted for 10 ms. In the Saccade task, participants had to prepare a saccade towards the side indicated by the auditory cue (S1), and to execute this saccade whenever an infrequent auditory S2 stimulus was presented from the central loudspeaker (go stimulus). No saccade was to be executed whenever a tactile S2 stimulus was presented to the left or right hand (nogo stimuli). Given these task instructions, participants were expected to endogenously shift tactile attention toward the cued side in the Covert Attention task, while no such voluntary shifts of tactile attention should occur in the Saccade task, where auditory go stimuli were always delivered from the central loudspeaker. In both tasks, the stimulus onset asynchrony (SOA) separating the auditory cue (S1) and the subsequent S2 was 900 ms. Hands and forearms were covered to prevent their visibility, and the positions of the left and right index finger were spatially aligned with the left and right saccade target location (see Figure 1).
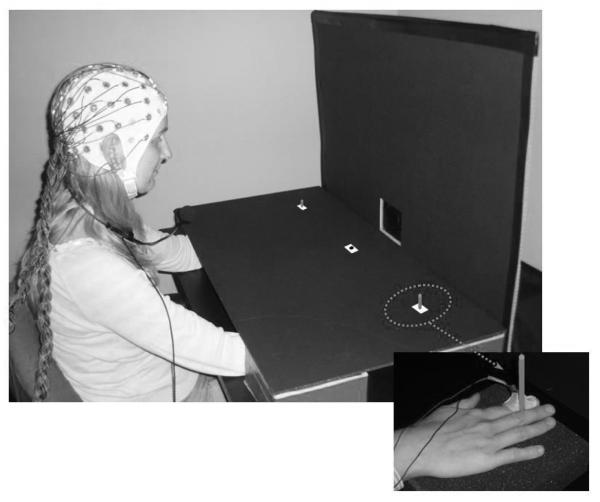
Figure 1.
Stimulus setup used in this study. Sounds were presented from a central loudspeaker that was mounted behind the vertical panel. Red markers were used to indicate the central fixation point and the two lateral saccade target positions. These were located on top of the horizontal panel that was used to cover participants’ hands and forearms. Note that these markers are colored white in this photograph to enhance their visibility. Tactile stimulation was applied to the top phalanx of the left or right index finger. Hands were located underneath the two lateral position markers, and were kept in place by two small vertical sticks, whose top parts were visible on the horizontal panel. The small inset (bottom right) shows the view of the right hand underneath the horizontal panel with the solenoid attached with adhesive medical tape to the index finger and the stick held between index and middle fingers.
Separate analyses were conducted for ERPs elicited in the S1-S2 interval in response to cues directing tactile attention to the left or right side (in the Covert Attention task), or signalling an upcoming leftward or rightward eye movement (in the Saccade task), and for ERPs elicited in response to tactile S2 stimuli delivered to the left or right hand. In the first analysis, we identified and compared lateralised ERP components (ADAN, LDAP) expected to be triggered during covert shifts of tactile attention and covert saccade preparation. Because these components are known to be elicited during cued shifts of spatial attention towards task-relevant visual, auditory, or tactile events (e.g., Eimer et al., 2002, 2003), they should also be observed during tactile-spatial attention shifts in the Covert Attention task. These components were also expected to be present during the S1-S2 interval in the Saccade task, as previous ERP studies have already demonstrated that ADAN and LDAP components are reliably also triggered during covert saccade preparation (Eimer et al., 2006, 2007; Van der Lubbe et al., 2006). The presence of similar ADAN and LDAP components in the Covert Attention and Saccade tasks would provide additional support for the claim of the premotor theory of attention that covert attention shifts are triggered during leftward or rightward saccade preparation.
The second analysis compared somatosensory ERPs in response to tactile non-target S2 stimuli on the cued versus uncued side, as obtained in the Covert Attention and Saccade tasks. In previous ERP studies that investigated the impact of endogenous shifts of tactile attention on somatosensory processing, directing tactile attention to one hand versus the other has been found to result in an enhancement of the somatosensory N140 component, although modulatory effects on earlier somatosensory components (N80, P100) have also been observed (Eimer and Forster, 2003; Michie, 1984; Josiassen et al., 1982). The N140 is elicited bilaterally and is thought to be generated in secondary somatosensory cortex (SII; Frot et al. 1999). Attentional modulations of the N140 are usually followed by a sustained attentional negativity (Eimer and Forster, 2003; Forster and Eimer, 2004; García-Larrea et al., 1995; Michie et al., 1987). These attentional modulations of somatosensory ERPs were expected to be observed in the current Covert Attention task. If covert eye movement preparation affected the processing of tactile stimuli in a spatially specific fashion, as suggested by the behavioural findings of Rorden et al. (2002), modulations of somatosensory ERPs that result from the spatial cueing of eye movement direction in the Saccade task should be similar to the attentional cueing effects obtained in the Covert Attention task.
Methods
Participants
Fifteen paid volunteers participated in the experiment. Four were excluded due to poor eye gaze control in the cue-target interval (see below), and one was excluded due to an insufficient number of trials after artefact rejection. Thus ten paid volunteers (4 females), aged 23-40 years (mean age: 29.7 years), remained in the sample. Nine participants were right-handed, one was left-handed, and all had normal or corrected to normal vision. The experiment was performed in compliance with relevant institutional guidelines, and was approved by the Birkbeck School of Psychology ethics committee.
Stimuli and Apparatus
Participants sat in a dimly lit experimental chamber wearing a head mounted microphone and facing a vertical black cardboard panel (85 × 60 cm) at a viewing distance of approximately 67 cm (Figure 1). One loudspeaker was mounted at the centre of the panel at an angle of 30° below eye level.
Tactile stimuli were presented using 4.5V solenoids that were driving a metal rod with a blunt conical tip to the fingers, and made contact with the skin whenever a current was passed trough the solenoid. Two tactile stimulators were used, each attached with adhesive medical tape to the left and right index finger, placed so that the metal rod made contact with the inner side of the top phalanx. Participants were instructed to place their hands on the table over two pieces of foam. A black horizontal cardboard panel (85 × 45 cm) was occluding the hands and lower parts of the arms to prevent their visibility (see Figure 1). This panel was placed above the hands and attached to the vertical panel at an angle of 90°. A red square (1 × 1 cm) located at the centre of this horizontal panel, aligned with the centre of the loudspeaker mounted on the vertical panel, was used as fixation point. The fixation point was located at a viewing distance of approximately 57 cm (10 cm in front of the intersection of the two panels). Saccade target locations were marked by two additional red squares (1 × 1 cm) that were located 28 cm to the right or left of the fixation cross (corresponding to an angular distance of 26°), horizontally aligned with central fixation. Left and right hands were placed under the horizontal panel, with index fingers and left and right saccade target locations spatially aligned. To ensure the accurate alignment of unseen index fingers and saccade target locations throughout all experimental blocks, participants had to hold a small stick between their middle and the index finger with each hand (see small inset in Figure 1). The top of these sticks was visible through small holes that were inserted in the centre of the lateral saccade target markers.
Auditory cues (S1) were high or low tones (500 or 2000 Hz at 72 dB SPL), presented for 100 ms from the central loudspeaker. On each trial, a second stimulus (S2), either tactile or auditory, was presented. Auditory S2 stimuli consisted of a 100 ms burst of white noise (20 ms rise and fall times, 70 db SPL) presented from the central loudspeaker. Tactile S2 stimuli were either continuous (non gap stimuli), consisting of one rod contacting one finger for 100 ms, or contained a 10 ms gap where this contact was interrupted after a duration of 45 ms (gap stimuli). Throughout the experimental blocks, white noise (62 dB SPL) was continuously delivered from an additional loudspeaker centrally located behind the horizontal panel, to mask any sounds made by tactile stimulators.
Procedure
The experiment consisted of 16 experimental blocks with 80 trials per block. Each trial started with a 100 ms presentation of an auditory cue (S1) from the central loudspeaker which was followed after an interval of 800 ms by the presentation of the S2 stimulus (auditory or tactile) for 100 ms. Intertrial interval was 1800 ms. Participants performed two tasks (Covert Attention task and Saccade task), each consisting of eight successive blocks. The order in which these tasks were delivered was balanced across participants.
In the Covert Attention task, the auditory cue (S1) was always followed by a tactile stimulus (S2) presented to left or right hand. Participants were instructed to direct their attention to the side indicated by the auditory cue (with the mapping of high and low frequency cues to the left or right hand, or vice versa, counterbalanced across participants) and to vocally respond by saying ‘yes’ whenever a tactile target (gap stimulus) was delivered to the cued hand. Tactile non-targets (non gap stimuli) on the cued side, as well as all tactile stimuli (target and non-target) on the uncued side were to be ignored, and central fixation had to be maintained. Each experimental block contained 48 trials where tactile non-targets (non-gap stimuli) were presented with equal probability on the left or right side and were preceded with equal probability by a left or right cue. Target stimuli (gap stimuli) were presented in the remaining 32 trials per block. Twenty-four of these targets were delivered on the cued side (equiprobably on the left or right side) and thus required a vocal response. On eight trials per block, tactile targets appeared on the uncued side, and no response was required on these trials.
In the Saccade task, the auditory cue (S1) was identical to the Covert Attention task, and was followed either by an auditory S2 stimulus (100 ms burst of white noise) presented from the central loudspeaker, or by a tactile S2 stimulus (100 ms non gap stimulus) delivered to the left or right index finger. Participants were instructed prepare a saccade towards the side indicated by the auditory cue (with mappings of high and low frequency cues to saccade direction counterbalanced across participants), and to execute the saccade on trials where the auditory S2 (go stimulus) was presented, but to maintain central fixation on trials where a tactile S2 (nogo stimulus) was delivered instead. Each experimental block contained 56 trials where a tactile S2 stimulus was delivered with equal probability to the left or right hand and no eye movement response was required. In the remaining 24 trials, an auditory S2 stimulus was presented from the central loudspeaker and the prepared eye movement (left or right with equal probability) had to be executed.
In the Covert Attention task participants were required to use the information provided by the cue (S1) to direct their attention to the cued location in order to respond as quickly and accurately as possible when the response-relevant S2 stimulus (target stimulus) was presented at the cued location. They were also required to ignore targets presented on the uncued side, as well as non targets presented on both sides. Participants were explicitly reminded to maintain central eye fixation throughout the task. In the Saccade task, participants were instructed to covertly prepare either a leftward or rightward saccadic eye movement according to the information provided by the cue (S1), and to initiate the movement as soon as the auditory go stimulus was presented. On go trials, they had to move their gaze to the cued target location, and then immediately back to the central fixation point. Participants had to maintain fixation during the S1-S2 interval. On trials where a nogo stimulus was presented, they had to maintain fixation throughout the trial.
Recording and Data Analysis
EEG was DC-recorded from 23 Ag-AgCl electrodes (Fpz, F7, F3, Fz, F4, F8, FC5, FC6, T7, C3, Cz, C4, T8, CP5, CP6, P7, P3, Pz, P4, P8, PO7 and PO8) relative to a left earlobe reference. Horizontal EOG was recorded unipolarly from the outer canthi of both eyes. Electrode impedance was kept below 5 kΩ, and the impedances of the earlobe electrodes were kept as equal as possible. Data were recorded with an upper cutoff filter of 40 Hz. EEG and EOG were sampled with a digitization rate of 250 Hz and stored on disk. No additional filters were applied after recording to the EEG or EOG data. EEG was digitally re-referenced to the average of the left and right earlobe.
EEG was epoched offline into 1500 ms periods, starting 100 ms prior to cue onset and ending 500 ms after the onset of S2. Separate averages were computed for ERPs recorded in the S1-S2 interval (relative to a 100 ms baseline preceding cue onset) and for ERPs elicited by subsequent tactile S2 stimuli (relative to a 100 ms baseline preceding the onset of these stimuli). In the Covert Attention task, ERPs were computed only for non-target tactile S2 stimuli (non gap stimuli), to avoid contamination by vocal responses. In the Saccade task, ERPs were computed in response to physically identical tactile S2 stimuli that now signalled a nogo trial where no eye movements were required. Trials with vocal responses to non-targets in the Covert Attention task and trials with eye movements to tactile nogo stimuli in the Saccade task were excluded from analysis, as were trials with eyeblinks (Fpz exceeding ±60 μV relative to baseline), horizontal eye movements (HEOG exceeding ±30 μV relative to baseline), or other artefacts (a voltage exceeding ±80 μV at any electrode location relative to baseline) in both tasks. To detect smaller systematic deviations of eye position, indicating residual tendencies to move the eyes toward the cued side, averaged waveforms in the cue-target interval in response to left versus right cues were examined for each participant. HEOG deviations exceeding ±3 μV led to the disqualification of four participants.
The EEG obtained in the S1-S2 interval was averaged for all combinations of task (Covert Attention versus Saccade) and cued side (left versus right). Mean amplitudes values were computed at lateral anterior sites (F3/4, F5/6, F7/8), lateral central sites (C3/4, CP5/6, T7/8), and lateral posterior sites (P3/4, P7/8, PO7/8), within two pre-defined post-cue time windows, 300-600 ms (where the ADAN was previously observed), and 600-900 ms (where the LDAP was previously observed). Mean amplitudes were analysed by repeated measures ANOVAs for the factors task (Covert Attentions vs. Saccade), lateralization (electrode ipsilateral versus contralateral to the cued side), cued side (left vs. right) and electrode site (F3/4 vs. F5/6 vs. F7/8 for lateral anterior electrodes, C3/4, CP5/6, T7/8 for lateral central electrodes and P3/4, P7/8, PO7/8 for lateral posterior electrodes). Additional analyses were conducted separately for the Covert Attention and Saccade tasks. In these analyses, lateralized ERP components sensitive to the side of a cued attention shift and/or to the side of a cued eye movement, such as the ADAN and LDAP, are reflected by significant main effect of the factor lateralization. Differences of ADAN and LDAP components between the two tasks will be indicated by interactions between task and lateralization.
Somatosensory ERPs elicited by tactile S2 stimuli were averaged relative to a 100 ms baseline, separately for all combinations of task, cued side, and stimulated hand (left vs. right). ERPs mean amplitudes were computed for measurement windows centered on the peak latencies of the somatosensory P100 and N140 components (90 – 115 ms and 130 – 170 ms post stimulus respectively). To investigate longer-latencies effects of spatial cueing, mean amplitudes were also computed between 190 and 290 ms after tactile stimulus onset. Analyses of somatosensory ERPs were conducted separately for lateral sites (FC5/6, C3/4, CP5/6) contralateral and ipsilateral to the stimulated hand, and for midline sites (Fz, Cz, Pz). These analyses included the factors task (Covert Attention vs. Saccade), spatial cuing (tactile stimulus presented to the hand on the cued vs. uncued side), stimulated hand (left vs. right) and electrode site (FC5/6 vs. C3/4 vs. CP5/6 for lateral electrodes and Fz vs. Cz vs. Pz for midline electrodes). Again, additional follow-up analyses were conducted separately for the Covert attention and Saccade tasks. For all analyses, Greenhouse-Geisser adjustments to the degrees of freedom were applied where appropriate.
Eye movement onset latencies in the Saccade task were measured on the basis of HEOG waveforms recorded after the onset of an auditory S2 (go stimulus). Saccade onset was defined as the latency (in ms poststimulus) of the first data point within this interval exceeding a threshold of ±100 μV (relative to a 100 ms pre-stimulus baseline), with saccade direction (left vs. right) indicated by the polarity of this value. The latency of vocal responses to targets in the Covert Attention task was measured with a voice key, and was calculated relative to the onset of the target-defining gap (45 ms after stimulus onset), as target/non-target discrimination could only start at this point. In both tasks, response times (RTs) longer than 1000 ms were excluded from the analysis. Vocal and saccade RTs obtained on trials with correct responses were further analysed with paired t-tests.
Results
Behavioural performance
Vocal RTs in the Covert Attention task were significantly slower than saccade RTs in the Saccade task (560 vs. 377 ms, respectively, t (9) = 6.2, p<.001). Separate analyses conducted for each task revealed that vocal RTs in the Covert Attention task did not differ as a function of the hand where the target tactile stimulus was delivered (552 vs. 568 ms for left and right targets, t (9) = 1.2; p=.23). Likewise, eye movement latencies in the Saccade task did not differ between leftward and rightward eye movements (377 ms for both saccade directions, t (9) = 0.17; p=.98).
In the Covert Attention task, participants missed targets on the cued side on 7.3% of these trials. Vocal RTs longer than 1000 ms were observed on 3.5% of all target trials. False Alarms to targets on the uncued side were observed on 0.3% of these trials, and False Alarms to non-targets were observed on 0.2% of all non-target trials. In the Saccade task, participants failed to execute a saccade on 0.7% of all trials where an auditory go stimulus was presented. There were no saccades to go stimuli with an onset latency beyond 1000 ms. Incorrect saccades (i.e. saccades towards the uncued side) were observed on 6.4% of all target trials. False alarms occurred on 1.1% of all trials where a lateral tactile S2 (nogo stimulus) was presented.
Lateralised ERP components in response to auditory spatial cues
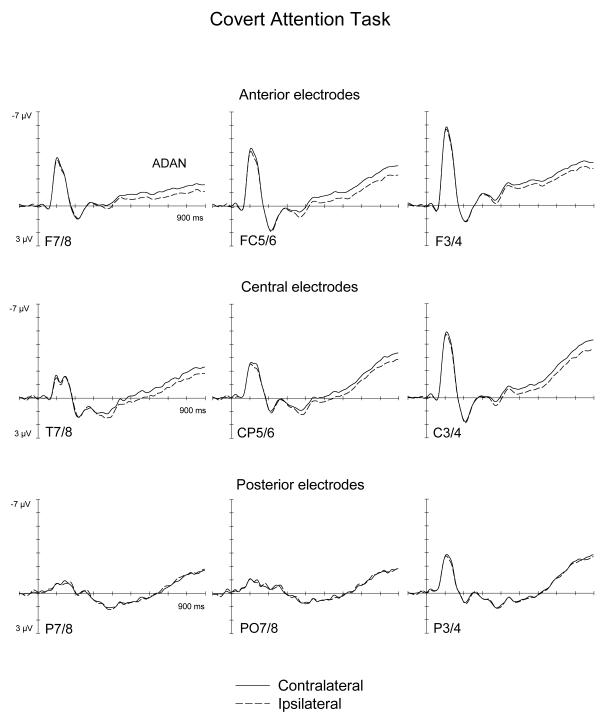
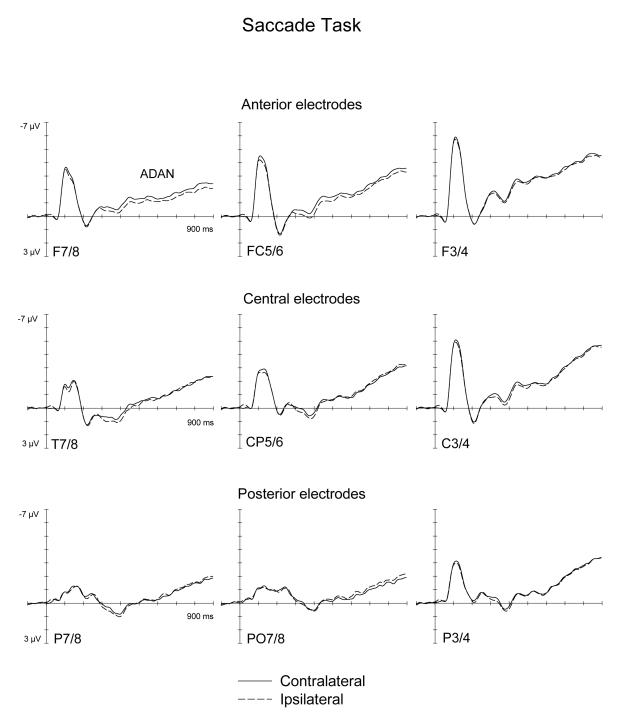
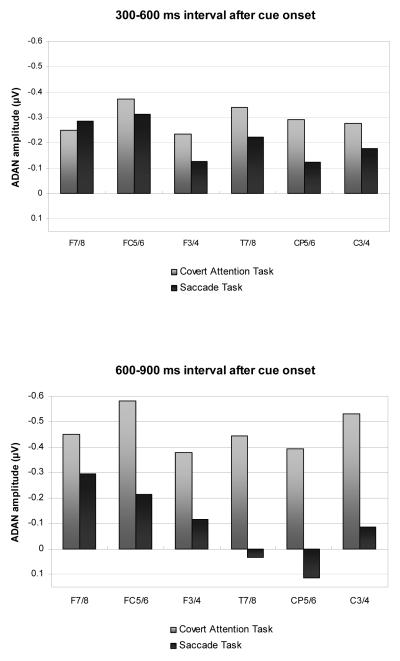
Figures 2 and 3 show ERPs triggered in the S1-S2 interval in response to auditory spatial cues at lateral anterior, central, and posterior electrodes ipsilateral and contralateral to the cued side, as observed in the Covert Attention task (Figure 2) and in the Saccade task (Figure 3). As can be seen from these Figures, spatial cueing resulted in an enhanced contralateral negativity (ADAN) at anterior electrodes that started about 300 ms after cue onset and remained present throughout the S1-S2 interval, and was also elicited, albeit in an attenuated fashion, at lateral central electrodes. Importantly, the ADAN appeared to be present both in the Covert Attention task as well as in the Saccade task, although its amplitude seemed smaller in the Saccade task relative to the Covert Attention task. This is further illustrated in Figure 4, which shows the absolute size of ADAN amplitudes that was obtained by subtracting mean ERP amplitudes at anterior and central electrodes ipsilateral to the cued side from contralateral ERPs, separately for the 300-600 ms time interval after cue onset (top panel), and for the 600-900 ms time window (bottom panel). In contrast, there was little evidence for the late directing positivity (LDAP) during later phases of the S1-S2 interval at lateral posterior electrodes.
Figure 2.
Grand-averaged ERPs elicited by the auditory cues during the S1-S2 interval in the 900 ms following cue onset relative to a 100 ms baseline, for anterior (top panel), central (middle panel) and posterior (bottom panel) electrodes ipsilateral (dashed lines) and contralateral (solid lines) to the cued side in the Covert Attention task.
Figure 3.
Grand-averaged ERPs elicited by the auditory cues during the S1-S2 interval in the 900 ms following cue onset relative to a 100 ms baseline, for anterior (top panel), central (middle panel) and posterior (bottom panel) electrodes ipsilateral (dashed lines) and contralateral (solid lines) to the cued side in the Saccade task.
Figure 4.
Size of ADAN component at anterior and central electrode pairs in the 300-600 ms time interval after cue onset (top panel), and in the subsequent 600-900 ms time interval (bottom panel), shown separately for the Covert Attention task (grey bars) and the Saccade task (black bars). Amplitude values were obtained by subtracting ERP mean amplitudes at electrodes ipsilateral to the cued side from mean amplitudes obtained at the corresponding contralateral electrodes.
Statistical analyses, where the presence of reliable ADAN and LDAP components are indicated by significant main effect of the factor lateralization, and differences in ADAN and LDAP amplitudes between tasks by interactions between task and lateralization (see Methods section), confirmed these informal observations. In the 300-600 ms interval, a significant main effect of lateralization at lateral anterior electrodes, F(1,9)=13.4, p<.005, as well as at lateral central electrodes, F(1,9)=14.6, p<.004, reflected the presence of the ADAN component. Importantly, there was no task x lateralization interaction at lateral anterior and lateral central recording electrodes (both F(1, 9)<1.5). This suggests that although the early phase of the ADAN was numerically larger in the Covert Attention task (see also Figure 4), ADAN amplitudes did not differ reliably between tasks during this time interval. Follow-up analyses conducted separately for both tasks confirmed significant effects of lateralisation at anterior and central electrodes for the Covert Attention task (both F(1,9)>12.4; p<.006). In the Saccade task, main effect of lateralization were statistically significant at anterior electrodes (F(1,9)=5.16; p<.05), but not at central electrodes (F(1,9)=3.34; p=.102). No significant main effect of lateralization, or task x lateralization interaction was present at lateral posterior electrode sites in the 300-600 ms interval.
In the subsequent 600-900 ms post-cue interval, significant main effects of lateralization were again found at lateral anterior and lateral central electrode sites (both F(1,9)>22; p<.001), demonstrating that the ADAN remained reliably present during this later phase of the S1-S2 interval. In contrast to the earlier time interval, a significant interaction between task and lateralization was now obtained at lateral central electrodes sites (F(1,9)=9, p<.015), and almost reached significance at lateral anterior sites (F(1,9)=4.9, p=.055). During this late time interval, ADAN amplitudes were larger for the Covert Attention task as compared to the Saccade task (see Figure 4). Follow-up analyses conducted separately for each task demonstrated that the ADAN remained reliably present at lateral anterior sites not only in the Covert Attention task (F(1,9)=25.63; p<.001), but also in the Saccade task (F(1,9)=6.3, p<.034). At lateral central sites, a significant ADAN was only found in the Covert Attention task (F(1,9)=32.38; p<.001), but not in the Saccade task (F(1,9)<1).
There was no statistical evidence for the presence of the posterior LDAP in the 600-900 ms time interval. At lateral posterior electrode sites, the main effect of lateralization was far from significant (F(1,9)<1), and there were also no task x lateralization or task x lateralization x electrode site interactions (both F(1,9)<1). In addition, follow-up analyses conducted separately on each task failed to reveal any indication of a main effect of lateralization or a lateralization x electrode site interaction for either task (all F(1,9)<1.5).
Effects of covert spatial attention and saccade preparation on somatosensory ERPs
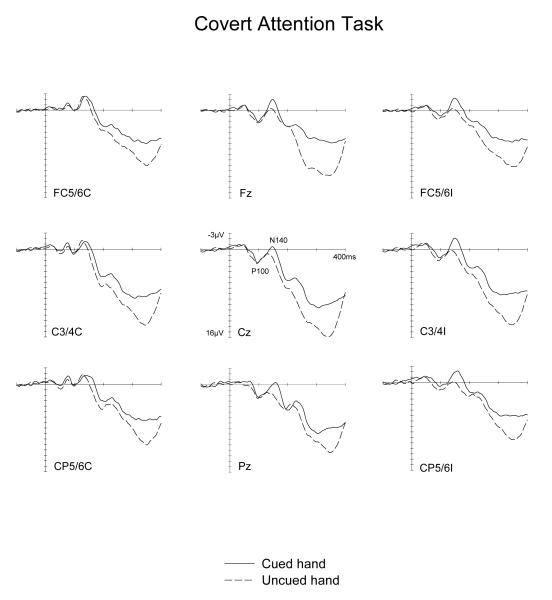
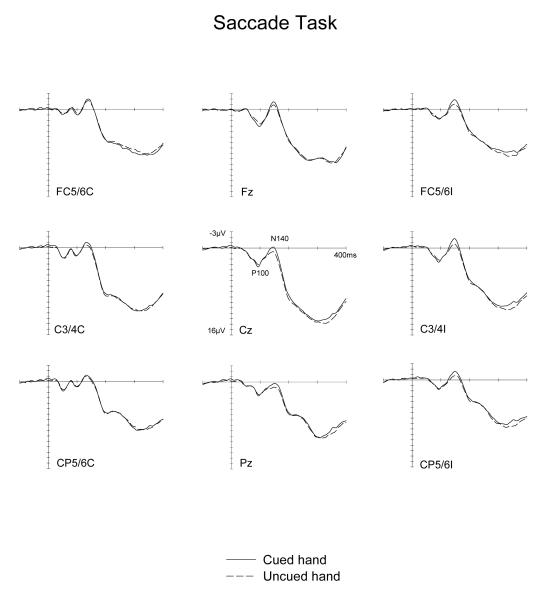
Figure 5 and 6 show ERPs elicited by tactile non-target stimuli in the Covert Attention task, and by physically identical tactile nogo stimuli in the Saccade task, displayed separately for tactile stimuli presented to the hand located on the cued side and on the uncued side. Waveforms are shown for electrode sites FC5/6, C3/4 and CP5/6 over the hemisphere contralateral (left side) and ipsilateral (right side) to the stimulated hand, as well as for midline electrodes Fz, Cz and Pz. The somatosensory N140 component appears to be enhanced for tactile stimuli presented to the cued hand, not only when tactile attention was focused on this hand (Covert Attention task, Figure 5). Importantly, a similar, albeit numerically smaller N140 amplitude modulation was also observed for tactile stimuli delivered to the hand that was located next to the current saccade target location (Saccade task, Figure 6). In contrast, a sustained attentional negativity for cued versus uncued stimuli was elicited at longer latencies in the Covert attention task, but not in the Saccade task.
Figure 5.
Grand-averaged somatosensory ERPs elicited in the Covert Attention task by tactile non-target stimuli in the 400 ms following stimulus onset (relative to a 100 ms pre-stimulus baseline) at midline electrodes and at sites contralateral (C) and ipsilateral (I) to the side of the stimulated hand. ERPs are shown in response to tactile stimuli presented to the hand on the cued side (solid line) and on the uncued side (dashed lines).
Figure 6.
Grand-averaged somatosensory ERPs elicited in the Saccade task by tactile no-go stimuli in the 400 ms following stimulus onset (relative to a 100 ms pre-stimulus baseline) at midline electrodes and at sites contralateral (C) and ipsilateral (I) to the side of stimulus presentation. ERPs are shown in response to tactile stimuli presented to the cued hand (solid line) and to the uncued hand (dashed lines).
No significant main effects of spatial cuing or task x spatial cuing interactions (all F(1,9)<1.5) were found for the P100 component (90-115 ms post-stimulus). In the N140 latency range (130-170 ms post-stimulus), main effects of spatial cuing were obtained at contralateral and ipsilateral electrodes as well as at midline sites (all F(1,9)>6.4; all p<.032), demonstrating that N140 amplitudes were reliably enhanced for tactile stimuli presented to the hand located on the cued side, relative to tactile stimulation of the opposite uncued hand. Importantly, there were no significant interactions between spatial cueing and task at contralateral, ipsilateral or midline sites (all F(1,9)<2.4; all p>.16). This suggests that although amplitude modulations of the N140 component were numerically larger in the Covert Attention task, their size did not differ reliably between the two tasks. Follow-up analyses conducted separately for each task confirmed the presence of significantly enhanced N140 amplitudes for tactile stimuli delivered to the cued versus uncued hand at ipsilateral and midline electrodes in the Covert attention as well as in the Saccade tasks (all F(1,9)>5.9, p<.038). At contralateral electrodes, these spatial cueing effects on N140 amplitudes failed to reach statistical significance in either task (F(1,9)=4.0 and 2.0; p>.07 and .17, for the Covert Attention and Saccade tasks, respectively).
In the 190-290 ms time window, main effects of spatial cuing were obtained at contralateral, ipsilateral and midline sites (all F(1,9)>7.0; all p<.03), reflecting the presence of a sustained attentional negativity for tactile stimuli delivered to the cued versus uncued hand. However, and in marked contrast to the results found for the N140 component, there were now significant interactions between task and spatial cuing at contralateral, ipsilateral and midline sites (all F(1,9)>8.2; all p<.02). As can be seen from Figures 5 and 6, these interactions were due to the fact that while this sustained negativity was clearly present in the Covert Attention task, it was absent in the Saccade task. This was confirmed by follow-up analyses conducted separately for both tasks, which revealed reliable spatial cuing effects during the 190-290 ms time interval at contralateral, ipsilateral and midline sites in the Covert attention task (all F(1,9)>9.7; all p<.012). In contrast, no such effects were elicited in the Saccade task at any of these electrodes sites (all F(1,9)<1). There was also a significant main effect of task during the 190-290 ms time window at contralateral, ipsilateral, and midline sites (all F(1,9)>9; all p<.015). ERPs were generally more positive in the Saccade task, due to the absence of a sustained attentional negativity on trials where tactile stimuli were presented to the cued hand (see Figure 6).
Discussion
To find out whether the processing of tactile events is modulated in a spatially specific fashion by covert saccade preparation, we compared somatosensory ERPs in response to tactile non-targets delivered to the left or right hand when these were preceded by central auditory cues that signalled an upcoming left or right eye movement (Saccade task), or the direction of a covert shift of tactile attention (Covert Attention task).We found that saccade preparation has systematic effects on somatosensory processing. The critical finding was that somatosensory N140 amplitudes were enhanced in response to tactile stimuli delivered to the hand located near to the goal location for a cued eye movement, as compared to the N140 elicited to tactile stimuli presented to the hand on the uncued side. Moreover, this spatial cueing effect on N140 amplitudes was statistically equivalent to the N140 effects observed in the Covert Attention task, where participants were explicitly instructed to direct their attention to the left versus right hand. These results provide new ERP evidence that covert saccade preparation affects tactile processing in a similar fashion as endogenous covert shifts of tactile attention. Together with previous studies demonstrating effects of eye movement preparation on visual and auditory processing (Eimer et al., 2006, 2007; Gherri et al., in press), they strongly support the view that saccade preparation is linked to attention shifts, and demonstrate that such links can affect the sensory processing of stimuli regardless of their modality.
While the effects of spatial cueing on N140 amplitudes were similar across both tasks, longer-latency spatial cueing effects (i.e., a sustained enhanced negativity for tactile stimuli on the cued versus uncued side) were only found in the Covert Attention task, but not in the Saccade task (see Figures 5 and 6). This difference is likely to be a consequence of differences in S2 processing demands between the two tasks. In the Saccade task, tactile S2 stimuli were always nogo stimuli, irrespective of whether they were presented at the cued or uncued side, and thus required no additional perceptual analysis. In the Covert Attention task, tactile stimuli presented to the cued hand had to be further processed in order to determine their status as targets (gap stimuli) or non-targets (non-gap stimuli). Sustained longer-latency negativities triggered in response to task-relevant attended stimuli are usually interpreted as indicators for attentional processes involved in target identification. Thus, the presence of such an attentional negativity in the Covert Attention task, and its absence in the Saccade task, is likely to be directly related to the fact that tactile stimuli delivered to the cued hand were potential targets in the former, but not in the latter task.
The ERP results obtained in the S1-S2 interval in response to auditory cues confirmed the presence of the ADAN component that was previously observed during cued shifts of attention as well as during cued saccade preparation (e.g., Eimer et al., 2006; Van der Lubbe et al., 2006; Van der Stigchel et al., 2006). This component was initially elicited in a similar fashion in the Covert Attention and Saccade tasks, and remained present during the later phase of the S1-S2 interval in both tasks, although its amplitude was now reduced in the Saccade task (see Figures 2 to 4). The presence of the ADAN during saccade preparation as well as during covert shifts of tactile attention suggests that similar spatially selective control processes were activated in response to the cues in both tasks.
In marked contrast to the ADAN, no LDAP component was observed in the present experiments in either task. The complete absence of this component was unexpected, given that the LDAP has been reliably obtained in several previous ERP studies that investigated ERP correlates of covert saccade preparation and spatial orienting (Eimer et al., 2006, 2007; Van der Lubbe et al., 2006). One important difference between these studies and the present experiment was that visual and auditory stimulation devices (LEDs, loudspeakers) were in full view in all of the former studies, while vision of the hands, arms, and tactile stimulators was prevented in our experiment (see Figure 1). This procedural difference may have been responsible for the absence of the LDAP in the present experiment. We have previously shown that this component (but not the ADAN) is absent during cued shifts of tactile attention performed by congenitally blind people, and also for sighted participants who performed the same tactile attention task in absolute darkness (Van Velzen et al., 2006). These observations suggest that the LDAP is linked to attentional control processes that depend on the continuous availability of visually coded representations of external space. If this is the case, it may not be all that surprising that no LDAP was elicited in the covert tactile attention task of the present study, where the occlusion of forearms and hands had eliminated the visual cues that might otherwise have been available to guide tactile attention to the cued hand.
It has to be acknowledged that this explanation cannot readily explain the lack of an LDAP in the Saccade task. In previous experiments that investigated ERP correlates of eye movement preparation, the LDAP was present during saccade preparation tasks, but was substantially attenuated relative to the LDAP observed during covert spatial orienting tasks (see Eimer et al., 2006, 2007; Gherri et al., in press; Van der Lubbe et al., 2006). It is conceivable that a small LDAP was in fact present in the Saccade task, but has gone undetected due to the relatively small number of participants tested in the present experiment. It is also possible that the continued presence of the ADAN throughout the S1-S2 interval in the present experiment (see Figures 2 and 3) was responsible for the absence of a reliable LDAP component. Scalp-recorded ERPs can be affected by the volume conduction of currents that originate from remote brain regions. When lateralised ERP components of opposite polarity are elicited simultaneously, such as the later part of the ADAN and the LDAP, volume conduction of the underlying source currents from anterior to posterior brain regions, can result in an attenuation of component amplitudes, or even in the elimination of one of these components. Alternatively, one could assume that full visibility of the spatial layout of the experimental setting, including vision of the hands and arms, is a necessary condition for the LDAP component not just during covert shifts of attention, but also during saccade preparation. This possibility will need to be explored in future experiments.
In summary, the present study has found new electrophysiological evidence suggesting that eye movement preparation affects the perceptual processing of tactile stimuli. Results demonstrate for the first time that the N140 component of the somatosensory event-related brain potential is modulated in a spatially selective fashion as a result of saccade preparation, and that this modulation is analogous to the effects of instructed endogenous tactile attention shifts. This observation provides further evidence for the central claim of the premotor theory of attention that eye movement preparation is linked to shifts of spatial attention, and demonstrates that attention shifts elicited during saccade preparation modulate the perceptual processing of tactile events.
Acknowledgements
This research was supported by the Medical Research Council (UK). ME holds a Royal Society Wolfson Research Merit Award.
References
- Andersen RA, Essick GK, Siegel RM. Neurons of area 7 activated by both visual stimuli and oculomotor behaviour. Exp Brain Res. 1987;67:316–22. doi: 10.1007/BF00248552. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional Organization of Human Intraparietal and Frontal Cortex for Attending Looking and Pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. NeuroImage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Discharge of frontal eye field neurons during saccadic and following eye movements in unanesthetized monkeys. Exp Brain Res. 1968;6:69–80. doi: 10.1007/BF00235447. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields II Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Goldberg ME, Robinson DL. Behavioural enhancement of visual responses in monkey cerebral cortex I modulation in posterior parietal cortex related to selective attention. J Neurophysiol. 1981;46:755–772. doi: 10.1152/jn.1981.46.4.755. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual presaccadic and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical independent or overlapping neural systems? Proc Natl Acad Sci USA. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Eimer M, Forster B. Modulations of early somatosensory ERP components by transient and sustained spatial attention. Exp Brain Res. 2003;151:24–31. doi: 10.1007/s00221-003-1437-1. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Driver J. Cross-modal interactions between audition touch and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J Cogn Neurosci. 2002;14:254–271. doi: 10.1162/089892902317236885. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Forster B, Driver J. Shifts of attention in light and in darkness: an ERP study of supramodal attentional control and crossmodal links in spatial attention. Cogn Brain Res. 2003;15:308–323. doi: 10.1016/s0926-6410(02)00203-3. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Gherri E, Press C. ERP correlates of shared control mechanisms involved in saccade preparation and in covert attention. Brain Res. 2007;1135:154–166. doi: 10.1016/j.brainres.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Gherri E, Press C. Manual response preparation and saccade programming are linked to attention shifts: ERP evidence for covert attentional orienting and spatially specific modulations of visual processing. Brain Res. 2006;1105:7–19. doi: 10.1016/j.brainres.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Colegate RL. Selective attention and serial processing in briefly presented visual displays. Percept Psychophys. 1971;10:321–326. [Google Scholar]
- Eriksen CW, Hoffman JE. Temporal and spatial characteristics of selective encoding from visual displays. Percept Psychophys. 1972;11:301–204. [Google Scholar]
- Forster B, Eimer M. The attentional selection of spatial and non-spatial attributes in touch: ERP evidence for parallel and independent processes. Biol Psychol. 2004;66:1–20. doi: 10.1016/j.biopsycho.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Frot M, Rambaud L, Guénot M, Mauguière F. Intracortical recordings of early pain-related CO2-laser evoked potentials in human second somatosensory (SII) area. Clin Neurophysiol. 1999;110:133–145. doi: 10.1016/s0168-5597(98)00054-9. [DOI] [PubMed] [Google Scholar]
- García-Larrea L, Lukaszewicz AC, Mauguière F. Somatosensory responses during selective spatial attention: The N120- to-N140 transition. Psychophysiol. 1995;32:526–537. doi: 10.1111/j.1469-8986.1995.tb01229.x. [DOI] [PubMed] [Google Scholar]
- Gherri E, Driver J, Eimer M. Eye movement preparation causes spatially-specific modulation of auditory processing: New evidence from event-related brain potentials. Brain Res. doi: 10.1016/j.brainres.2008.05.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Bushnell MC. Behavioral enhancement of visual responses in monkey cerebral cortex II Modulation in frontal eye fields specifically related to saccades. J Neurophysiol. 1981;46:773–787. doi: 10.1152/jn.1981.46.4.773. [DOI] [PubMed] [Google Scholar]
- Gopher D. Eye-movement patterns in selective listening task of focused attention. Percept Psychophys. 1973;14:259–264. [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the frontal eye-field: effects on visual perception and attention. J Cog Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Hublet C, Morais J, Bertelson P. Spatial constraints on focused attention: beyond the right-side advantage. Perception. 1976;5:3–8. doi: 10.1068/p050003. [DOI] [PubMed] [Google Scholar]
- Hublet C, Morais J, Bertelson P. Spatial effects in speech perception in the absence of spatial competition. Perception. 1977;6:461–466. doi: 10.1068/p060461. [DOI] [PubMed] [Google Scholar]
- Irwin DE, Gordon RD. Eye movements attention and transsaccadic memory. Vis Cogn. 1988;5:127–155. [Google Scholar]
- Jones B, Kabanoff B. Eye movements in auditory space perception. Percept Psychophys. 1975;17:241–245. [Google Scholar]
- Josiassen RC, Shagrass C, Roemer RA, Ercegovac DE, Straumanis JJ. Somatosensory evoked potential changes with a selective attention task. Psychophysiology. 1982;19:46–159. doi: 10.1111/j.1469-8986.1982.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Lie E, Coslett HB. The Effect of Gaze Direction on Sound Localization in Brain-Injured and Normal Adults. Exp Brain Res. 2006;168:322–336. doi: 10.1007/s00221-005-0100-4. [DOI] [PubMed] [Google Scholar]
- Mathews S, Dean PJ, Sterr A. EEG dipole analysis of motor-priming foreperiod activity reveals separate sources for motor and spatial attention components. Clin Neurophysiol. 2006;117:2675–83. doi: 10.1016/j.clinph.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Bracewell RM, Barash S, Andersen RA. Spatially tuned auditory responses in area LIP of macaques performing delayed memory saccades to acoustic targets. J Neurophysiol. 1996;75:1233–1241. doi: 10.1152/jn.1996.75.3.1233. [DOI] [PubMed] [Google Scholar]
- Michie PT, Bearpark HM, Crawford JM, Glue LCT. The effects of spatial selective attention on the somatosensory event-related potential. Psychophysiology. 1987;24:449–463. doi: 10.1111/j.1469-8986.1987.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Michie PT. Selective attention effects on somatosensory event-related potentials. Ann N Y Acad Sci. 1984;425:250–255. doi: 10.1111/j.1749-6632.1984.tb23542.x. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;9:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais J, Cary L, Vanhaelen H, Bertelson P. Postural determinants of frontal position advantage in listening to speech. Percept Psychophys. 1980;27:141–48. doi: 10.3758/bf03204302. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command function for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Juan CH, Cowey AZ, Walsh V. Human frontal eye fields and visual search. J Neurophysiol. 2003;89:3340–3343. doi: 10.1152/jn.01086.2002. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Zeki S. The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain. 2000;123:2273–2288. doi: 10.1093/brain/123.11.2273. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. J Exp Psychol Gen. 1980;109:160–174. [PubMed] [Google Scholar]
- Praamstra P, Boutsen L, Humphreys GW. Frontoparietal control of spatial attention and motor intention in human EEG. J Neurophysiol. 2005;94:764–774. doi: 10.1152/jn.01052.2004. [DOI] [PubMed] [Google Scholar]
- Reisberg D, Scheiber R, Potemken L. Eye position and the control of auditory attention. J Exp Psychol Hum Percept Perform. 1981;7:318–323. doi: 10.1037//0096-1523.7.2.318. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R. Neural circuits for spatial attention and unilateral neglect. In: Jeannerod M, editor. Neurophysiological and neuropsychological aspects of spatial neglect. North-Holland; Amserdam: 1987. pp. 289–313. [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Sheliga B. Space and selective attention. In: Umilta C, Moscovitch M, editors. Attention and Performance XV. MIT Press; Cambridge MA: 1994. pp. 231–265. [Google Scholar]
- Rizzolatti G. Mechanisms of selective attention in mammals. In: Ewert JP, editor. Advances in Vertebrate Neuroethology. Plenum; London: 1983. p. 261. [Google Scholar]
- Robinson DL, Goldberg ME, Stanton GB. Parietal association cortex in the primate: sensory mechanisms and behavioural modulation. J Neurophysiol. 1978;41:910–932. doi: 10.1152/jn.1978.41.4.910. [DOI] [PubMed] [Google Scholar]
- Rorden C, Driver J. Does auditory attention shift in the direction of an upcoming saccade? Neuropsychologia. 1999;37:357–377. doi: 10.1016/s0028-3932(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Rorden C, Greene K, Sasine G, Baylis G. Enhanced tactile performance at the destination of an upcoming saccade. Curr Biol. 2002;20:1429–1434. doi: 10.1016/s0960-9822(02)01039-4. [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Frontal eye field activity preceding aurally guided saccades. J Neurophysiol. 1994;71:1250–3. doi: 10.1152/jn.1994.71.3.1250. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15:4464–87. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz MA, Connor CE, Constantinidis C, McLaughlin JR. Covert attention suppresses neuronal responses in area 7a of the posterior parietal cortex. J Neurophysiol. 1994;72:1020–3. doi: 10.1152/jn.1994.72.2.1020. [DOI] [PubMed] [Google Scholar]
- Stricanne B, Andersen RA, Mazzoni P. Eye-centered headcentered and intermediate coding of remembered sound locations in area LIP. J Neurophysiol. 1996;76:2071–2076. doi: 10.1152/jn.1996.76.3.2071. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RHJ, Neggers SFW, Verleger R, Kenemans JL. Spatiotemporal overlap between brain activation related to saccade preparation and attentional orienting. Brain Res. 2006;1072:133–152. doi: 10.1016/j.brainres.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RHJ, Wauschkuhn B, Wascher E, Niehoff T, Kömpf D, Verleger R. Lateralized EEG components with direction information for the preparation of saccades versus finger movements. Exp Brain Res. 2000;132:163–178. doi: 10.1007/s002219900328. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Heslenfeld DJ, Theeuwes J. An ERP study of preparatory and inhibitory mechanisms in a cued Saccade task. Brain Res. 2006;1105:32–45. doi: 10.1016/j.brainres.2006.02.089. [DOI] [PubMed] [Google Scholar]
- Van Velzen J, Eardley AF, Forster B, Eimer M. Shifts of attention in the early blind: An ERP study of attentional control processes in the absence of visual spatial information. Neuropsychologia. 2006;44:2533–2546. doi: 10.1016/j.neuropsychologia.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Wauschkuhn B, Verleger R, Wascher E, Klostermann W, Burk M, Heide W, Kömpf D. Lateralised human cortical activity for shifting attention and initiating saccades. J Neurophysiol. 1998;80:2900–2910. doi: 10.1152/jn.1998.80.6.2900. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME, Robinson DL. Brain mechanisms of visual attention. Sci Am. 1982;246:100–7. doi: 10.1038/scientificamerican0682-124. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in the behaving monkey III Cells discharging before eye movements. J Neurophysiol. 1972;35:575–586. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Mohler CW. Enhancement of visual response in monkey striate cortex and frontal eye fields. J Neurophysiol. 1976;39:766–772. doi: 10.1152/jn.1976.39.4.766. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Research. 1995;13:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]