Abstract
Background. Sevelamer carbonate is an improved, buffered form of sevelamer hydrochloride developed for the treatment of hyperphosphataemia in CKD patients. Sevelamer carbonate formulated as a powder for oral suspension presents a novel, patient-friendly alternative to tablet phosphate binders. This study compared the safety and efficacy of sevelamer carbonate powder with sevelamer hydrochloride tablets in CKD patients on haemodialysis.
Methods. This was a multi-centre, open-label, randomized, crossover design study. Thirty-one haemodialysis patients were randomly assigned to either sevelamer carbonate powder or sevelamer hydrochloride tablets for 4 weeks followed by a crossover to the other regimen for an additional 4 weeks.
Results. The mean serum phosphorus was 1.6 ± 0.5 mmol/L (5.0 ± 1.5 mg/dL) during sevelamer carbonate powder treatment and 1.7 ± 0.4 mmol/L (5.2 ± 1.1 mg/dL) during sevelamer hydrochloride tablet treatment. Sevelamer carbonate powder and sevelamer hydrochloride tablets are equivalent in controlling serum phosphorus; the geometric least square mean ratio was 0.95 (90% CI 0.87–1.03). No statistically significant or clinically meaningful differences were observed in calcium × phosphorus product and lipid levels between sevelamer carbonate powder and sevelamer hydrochloride tablets. Serum bicarbonate levels increased 2.7 ± 3.7 mmol/L (2.7 ± 3.7 mEq/L) during sevelamer carbonate treatment. No statistically significant change in bicarbonate was observed during sevelamer hydrochloride treatment. Sevelamer carbonate powder and sevelamer hydrochloride were well tolerated during this study.
Conclusions. Sevelamer carbonate powder and sevelamer hydrochloride tablets are equivalent in controlling serum phosphorus and well tolerated in CKD patients on haemodialysis. Bicarbonate levels improved only during sevelamer carbonate treatment. Sevelamer carbonate powder should provide a welcomed new option for the treatment of hyperphosphataemia for CKD patients on dialysis.
Keywords: clinical trial, haemodialysis patients, phosphate binder, powder formulation, sevelamer carbonate
Introduction
Chronic kidney disease (CKD) patients as a whole and dialysis patients in particular have a high intake of medications. Dialysis patients typically take >13 different types of medication and >40 tablets a day. Studies suggest that patients fail to take 18–20% or more of their prescribed phosphate binders [1]. The overall pill burden for dialysis patients may be a substantial element contributing to poor compliance with medications. Large pill burden has specifically been cited as one cause for patients’ poor adherence to phosphate binder [1,2]. Powder form of phosphate binder would provide choice to patients in how their binder is delivered and be useful for patients seeking to decrease the number of tablets they take.
It is not just the number of tablets that creates problems for dialysis patients, but difficulty in swallowing tablets or capsules may also contribute to poor compliance in some patients. In a survey of 792 patients conducted by community pharmacists, ∼60% of the patients reported experiencing difficulties swallowing solid dosage forms and 69% admitted skipping a dose of medication due to swallowing difficulties [3]. In another survey, approximately one-quarter (26%) of >6000 patients seen by general practitioners reported problems in swallowing tablets [4]. The size, texture and taste were the most frequent complaints described in this survey. Age-related physiological changes, including age-related declines in salivary gland function and swallowing reflexes, may contribute to swallowing difficulties. Medical conditions such as Parkinson's disease, stroke and cancer can also lead to swallowing difficulties. Many phosphate binders are only available in a solid dosage form, and the nature of phosphate binding requires that several tablets a day will have to be taken. An alternative powder dosage form that can be suspended in water may benefit patients who dislike or have difficulty using solid dosage forms of medications.
Sevelamer hydrochloride (Renagel®) is a non-absorbed, calcium- and metal-free phosphate-binding polymer that has been available since 1998. Sevelamer hydrochloride effectively controls serum phosphorous and calcium × phosphorus product while maintaining normal serum calcium levels in haemodialysis patients when dosed three times per day [5–10]. However, treatment with sevelamer hydrochloride may be associated with an increase in serum chloride and/or reduction in serum bicarbonate and the potential to aggravate the metabolic acidosis frequently experienced by CKD patients [11,12]. The chloride anion of sevelamer hydrochloride may contribute to these effects. Therefore, sevelamer carbonate (Renvela®) was developed as a buffered form of sevelamer. Sevelamer carbonate is an anion-exchange resin with the same polymeric structure as sevelamer hydrochloride where carbonate is an alternative counterion to chloride. While the counterions differ for the two salts, the polymer itself, the active moiety responsible for the binding of phosphate, is the same. Sevelamer carbonate contains bicarbonate and carbonate equivalent to 0.14–0.21 g of bicarbonate per gram of sevelamer carbonate active pharmaceutical ingredient.
Sevelamer carbonate tablets have been found to be well-tolerated and equivalent to sevelamer hydrochloride tablets in controlling serum phosphorus in haemodialysis patients [13]. In addition to the tablet formulation, sevelamer carbonate has also been designed specifically as a powder formulation for aqueous suspension to provide more options to patients. Sevelamer carbonate powder has a light citrus flavour. Dosing instructions recommend mixing at least 60 mL of water with each 2.4 g of powder. Even when taken three times per day, this amount of water should not negatively impact patients whose fluid intake is restricted.
The current study was designed to compare the safety and efficacy of sevelamer carbonate powder with sevelamer hydrochloride tablets in CKD patients on haemodialysis.
Methods
Patient selection
Patients aged ≥18 years receiving maintenance haemodialysis for 3 months or longer at seven nephrology centres in England who were maintained on sevelamer hydrochloride alone or in combination with other binders formed the candidate population. Laboratory criteria for inclusion included a serum phosphorus level ≥1.76 mmol/L (5.5 mg/dL) after phosphate binder washout, an intact parathyroid hormone (iPTH) measurement of ≤800 ng/L (800 pg/mL) and a serum calcium level within the normal range [2.13–2.58 mmol/L (8.5–10.3 mg/dL)]. Patients were excluded from the study if they had a severe gastrointestinal motility disorder, poorly controlled diabetes mellitus, hypertension or any other clinically significant unstable medical condition.
The protocol and informed consent were reviewed and approved by an Independent Ethics Committee. All patients signed written, informed consent prior to the initiation of any study-related activities. This research was carried out in accordance with Good Clinical Practice guidelines, applicable regulations as well as the ethical principles that have their origin in the Declaration of Helsinki.
Study design
This was a multi-centre, open-label, randomized, crossover design study. Patients were screened, and enrolled patients discontinued all phosphate binder(s) for a 2-week washout period. At the end of the washout period, the patients with a serum phosphorus ≥1.76 mmol/L (5.5 mg/dL) continued into a 4-week sevelamer hydrochloride run-in period. The binder dose that each patient was taking prior to the washout period was replaced with an equivalent number of 800 mg sevelamer hydrochloride tablets. There were two opportunities during the run-in period to adjust the dose of sevelamer hydrochloride, if necessary, to keep serum phosphorus levels within a target level between 1.12 and 1.76 mmol/L (3.5 and 5.5 mg/dL), inclusive. Following the run-in period, the patients were randomized in a 1:1 fashion to one of two treatment sequences: sevelamer carbonate powder dosed TID with meals for 4 weeks followed by sevelamer hydrochloride tablets dosed TID with meals for 4 weeks or sevelamer hydrochloride tablets dosed TID with meals for four weeks followed by sevelamer carbonate powder dosed TID with meals for 4 weeks. The dose was individualized based on the patients’ most recent sevelamer hydrochloride dose during the run-in period. The patients were maintained at a fixed dose throughout both treatment periods. The patients’ haemodialysis prescription regarding dialysate bicarbonate, calcium concentrations and treatment time was to be maintained throughout both treatment regimens. Adherence was assessed by pill or sachet count twice during each treatment period. At the end of the second treatment period, study medication was discontinued and the patients were instructed to return to their pre-study phosphate-binder medication. The patients returned for a follow-up visit after 7 days.
Efficacy and safety analyses
The effects of sevelamer carbonate powder and sevelamer hydrochloride tablets, each dosed TID with meals, on the control of serum phosphorus was determined using equivalence testing. The natural log-transformed time-weighted average of the serum phosphorus assessments from the last four visits during the last 2 weeks of each treatment regimen were used in the analysis. The time-weighted average of the serum phosphorus assessments during the last 2 weeks of each treatment regimen was used to give a more accurate assessment of phosphorus control than would be attained by individual measurements. The analysis of variance (ANOVA) model included a random subject effect and fixed sequence, period and treatment effects. The two one-sided hypotheses were tested at the 5% level of significance for log-transformed serum phosphorus by constructing a 90% confidence interval for the ratio of sevelamer carbonate to sevelamer hydrochloride geometric means. This test required that the 90% confidence interval for the ratio was within the interval (0.80, 1.25) to establish equivalence. Similar ANOVA models were used to compare calcium × phosphorus product and lipids between treatment regimens at the 5% level.
Percent compliance and average actual daily dose were summarized by treatment regimen. Safety was evaluated on the basis of adverse experiences and changes in laboratory values.
Allowing for withdrawals, a sample size of 12 patients per sequence (powder/tablet versus tablet/powder) for a total of 24 patients was required to be randomized to achieve 90% power to detect equivalence.
Results
Patients
A total of 75 individual patients were enrolled in this study to randomize 31 patients (17 to the sevelamer carbonate powder/sevelamer hydrochloride tablet sequence and 14 to the sevelamer hydrochloride tablet/sevelamer carbonate powder sequence). Seven patients discontinued during sevelamer carbonate treatment (two due to adverse events and five withdrew consent). A total of 24 (77.4%) completed the study (14 in the sevelamer carbonate powder/sevelamer hydrochloride tablet sequence and 10 in the sevelamer hydrochloride tablet/sevelamer carbonate powder sequence).
The patient demographics and renal history are shown in Table 1. Overall, the baseline characteristics of the patients were similar between treatment sequences.
Table 1.
Patient demographics and renal history
| Characteristic | (N = 31) |
|---|---|
| Age (years) | |
| Mean ± SD | 52.9 ± 13.2 |
| Median (range) | 51 (27–80) |
| Gender, n (%) | |
| Male | 21 (68) |
| Female | 10 (32) |
| Ethnicity, n (%) | |
| Caucasian | 22 (71) |
| Black | 3 (10) |
| Asian | 6 (19) |
| Primary cause of end-stage renal disease, n (%) | |
| Othera | 18 (58) |
| Glomerulonephritis | 8 (26) |
| Diabetes | 4 (13) |
| Hypertension | 1 (3) |
| Time on dialysis (years) | |
| Mean ± SD | 7.2 ± 8.0 |
| Median (range) | 4.4 (0.2–30.3) |
| Pre-study phosphate binder, n (%) | |
| Sevelamer hydrochloride | 18 (58) |
| Sevelamer hydrochloride and calcium | 11 (36) |
| Other | 2 (7) |
| Using IV or oral vitamin D, n (%) | 25 (81) |
| Kidney transplant prior to study, n (%) | 8 (26) |
| Parathyroidectomy prior to study, n (%) | 4 (13) |
aOf the 18 patients with ‘other’ cited as the primary cause, the aetiology of CKD was recorded as unknown in five patients, IgA nephropathy in two patients, polycystic kidneys in two patients and interstitial nephritis, pyelonephritis, congenital, renovascular disease, reflux nephropathy, road traffic accident, hereditary nephritis, Goodpasture syndrome and Alport's syndrome in one patient each.
The equivalence analysis was based on a comparison of serum phosphorus control using the per protocol set (PPS; n = 21) as this is the appropriate population for equivalence testing. Confirmatory analyses of serum phosphorus and the analyses of the secondary endpoints, calcium × phosphorus product and lipids were performed using the intent to treat (ITT) population (n = 30). Safety was assessed in all patients who received at least one dose of randomized study medication (n = 31).
Study treatment exposure
In the PPS, the mean actual daily dose was 6.0 ± 3.1 g/ day of sevelamer carbonate powder and 6.4 ± 3.3 g/day of sevelamer hydrochloride tablets. No patients changed their sevelamer daily dose during randomized treatment. Treatment compliance was similar with both sevelamer carbonate powder (86%) and sevelamer hydrochloride tablets (84%).
Efficacy
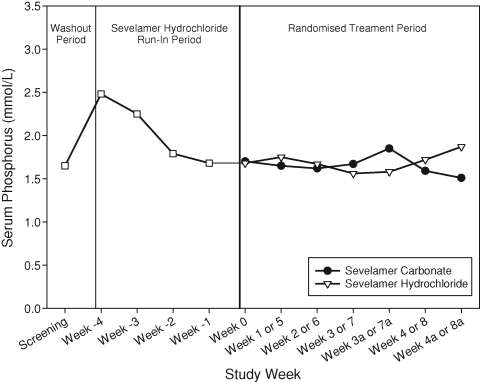
The mean time-weighted average serum phosphorus was 1.6 ± 0.5 mmol/L (5.0 ± 1.5 mg/dL) during sevelamer carbonate powder treatment and 1.7 ± 0.4 mmol/L (5.2 ± 1.1 mg/dL) during sevelamer hydrochloride tablet treatment. For assessing phosphorus equivalence, the treatment response across sequences was pooled since there was no sequence effect (P = 0.932). The geometric least square mean ratio (sevelamer carbonate powder/sevelamer hydrochloride tablets) was 0.95 with a corresponding 90% confidence interval of 0.87–1.03. The confidence interval is within the interval of 0.80–1.25, indicating that sevelamer carbonate powder and sevelamer hydrochloride tablets are equivalent in controlling serum phosphorus. The results of a confirmatory analysis conducted with the ITT population corroborate the PPS analysis. The mean serum phosphorus at each study visit is presented in Figure 1. This figure demonstrates that similar serum phosphorus control was attained during treatment with sevelamer carbonate and sevelamer hydrochloride throughout the study.
Fig. 1.
Serum phosphorus over time.
Table 2 presents the calcium × phosphorus product and lipid results. No statistically significant or clinically meaningful differences were observed in calcium × phosphorus product and lipid levels between sevelamer carbonate powder and sevelamer hydrochloride tablets dosed TID with meals.
Table 2.
End of treatment serum calcium × phosphorus product and lipids
| Laboratory parameter | Sevelamer carbonate powder TID | Sevelamer hydrochloride tablets TID | P-value |
|---|---|---|---|
| Calcium × phosphorus product | 0.749 | ||
| (mmol2/L2) (mg2/dL2) | |||
| n | 25 | 28 | |
| Mean ± SD | 3.7 ± 1.1 (45.9 ± 13.8) | 3.7 ± 0.8 (45.8 ± 10.0) | |
| Median | 3.8 (46.6) | 3.8 (46.9) | |
| Total cholesterol (mmol/L) (mg/dL) | 0.218 | ||
| n | 22 | 27 | |
| Mean ± SD | 3.5 ± 0.7 (135.4 ± 26.9) | 3.3 ± 0.8 (129.1 ± 31.6) | |
| Median | 132 (3.4) | 127 (3.3) | |
| LDL cholesterol (mmol/L) (mg/dL) | 0.109 | ||
| n | 22 | 27 | |
| Mean ± SD | 1.8 ± 0.5 (70.4 ± 18.3) | 1.8 ± 0.7 (67.7 ± 25.4) | |
| Median | 67 (1.7) | 65 (1.7) | |
| HDL cholesterol (mmol/L) (mg/dL) | 0.537 | ||
| n | 22 | 27 | |
| Mean ± SD | 1.2 ± 0.5 (44.5 ± 17.7) | 1.1 ± 0.4 (43.7 ± 13.9) | |
| Median | 43 (1.1) | 42 (1.1) | |
| Triglycerides (mmol/L) (mg/dL) | 0.992 | ||
| n | 22 | 27 | |
| Mean ± SD | 2.2 ± 1.6 (192.7 ± 139.6) | 2.1 ± 1.5 (188.9 ± 131.9) | |
| Median | 144 (1.6) | 142 (1.6) |
The serum bicarbonate, calcium and iPTH over time are presented in Table 3. During sevelamer carbonate powder treatment, there was a statistically significant increase from the end of the run-in period (baseline) to the end of the treatment period (final) in serum bicarbonate [mean change 2.7 mmol/L (2.7 mEq/L) from 18.0 mmol/L (18.0 mEq/L); P = 0.001]. No statistically significant change in bicarbonate was observed during sevelamer hydrochloride treatment. The observed change in serum bicarbonate was statistically significantly different between treatment regimens, with greater increases in the sevelamer carbonate regimen (P = 0.001). There was no statistically significant change in serum calcium (albumin-adjusted) during either treatment, and no statistically significant difference in calcium (albumin-adjusted) change between the treatment regimens. There was no statistically significant difference in the iPTH change between the treatment regimens.
Table 3.
Laboratory measurements over time
| Laboratory parameter | Sevelamer carbonate powder TID (N = 31) | Sevelamer hydrochloride tablets TID (N = 28) | P-value* |
|---|---|---|---|
| Bicarbonate (mmol/L) (mEq/L) | 0.001 | ||
| Baseline | 18.0 ± 3.1 (18.0 ± 3.1) | 17.8 ± 3.2 (17.8 ± 3.2) | |
| Final | 20.2 ± 2.8 (20.2 ± 2.8) | 18.0 ± 3.1 (18.0 ± 3.1) | |
| Change | 2.7 ± 3.7 (2.7 ± 3.7) | 0.1 ± 3.3 (0.1 ± 3.3) | |
| P-value* | 0.001 | 0.791 | |
| Calcium (mmol/L) (mg/dL) | 0.665 | ||
| Baseline | 2.3 ± 0.2 (9.1 ± 0.9) | 2.3 ± 0.2 (9.1 ± 1.0) | |
| Final | 2.3 ± 0.2 (9.1 ± 0.8) | 2.3 ± 0.2 (9.1 ± 0.9) | |
| Change | −0.0 ± 0.1 (−0.1 ± 0.5) | −0.0 ± 0.2 (−0.0 ± 0.9) | |
| P-value* | 0.173 | 0.734 | |
| iPTH (pmol/L) (pg/mL)a | 0.404 | ||
| Baseline | 31 (291) | 33 (310) | |
| Final | 41 (390) | 43 (408) | |
| Change | 3 (30) | 4 (42) | |
| P-value* | 0.272 | 0.019 |
aSerum iPTH presented as median.
*Wilcoxon signed-rank test used to compare change from baseline between treatment regimens and to assess change from baseline within each treatment.
The number of observations varies in the statistics shown.
Safety
Sevelamer carbonate powder and sevelamer hydrochloride were well tolerated during this study. A total of nine events in 7 (22.6%) of the 31 randomized patients were considered by the investigator as treatment related during the sevelamer hydrochloride run-in period including dyspepsia, abdominal distension, abdominal pain, diarrhoea, gastritis, nausea and stomach discomfort. No treatment-related adverse events were reported during treatment with sevelamer hydrochloride tablets during the randomized treatment period. Four adverse events in three (9.7%) patients (nausea and vomiting in one patient, nausea in one patient and constipation in one patient) that occurred during sevelamer carbonate treatment were assessed as treatment related. The two patients who experienced nausea and vomiting withdrew from the study due to these events. No serious AEs were considered to be related to either study treatment by the investigator. No patients died during either treatment period.
Discussion
In this study, sevelamer carbonate powder and sevelamer hydrochloride tablets, both dosed TID with meals, were equivalent in controlling serum phosphorus in CKD patients on haemodialysis. Sevelamer carbonate powder was well tolerated by the patients in this study. The safety profile of sevelamer carbonate powder was similar to the known safety profile of sevelamer carbonate tablets and sevelamer hydrochloride tablets. These findings are particularly supportive of sevelamer carbonate as an effective phosphate binder since despite changes in both the formulation (tablet to powder) and the counterion (hydrochloride to carbonate), equivalent phosphorus control with sevelamer hydrochloride was demonstrated.
Sevelamer carbonate for oral suspension may be particularly beneficial for several patient populations. Elderly and other patients who have difficulty in taking solid dosage forms of medications may find a suspension easier to swallow. The patients who are overwhelmed by the number of pills that they must take each day may find an oral suspension preferable. A powder formulation for oral suspension may also provide a more suitable formulation than tablets for paediatric patients. Thus, the availability of an oral suspension provides the patient with a dosage option that could result in increased compliance with therapy that could improve patient outcomes. The similar compliance rates for the powder and tablet formulations seen in this study may be related to this being a well-controlled clinical trial of short duration and likely does not accurately reflect long-term compliance with each formulation.
Reduced serum bicarbonate levels (<22 mmol/L) are frequently observed in CKD patients indicating that many of the patients have an underlying acidosis [14]. Acidosis has been associated with adverse effects on bone metabolism [15] and increased malnutrition and inflammation [14] in haemodialysis patients. In addition, metabolic acidosis with a serum bicarbonate level of <17.5 mmol/L has been independently associated with an increased risk of death in dialysis patients [16,17]. In this study, bicarbonate levels increased during sevelamer carbonate treatment. The addition of the buffering capacity provided by sevelamer carbonate could potentially decrease the burden of acidosis in CKD patients. It may also decrease the need for additional sodium bicarbonate tablet supplementation, potentially reducing the pill burden for some patients.
Sevelamer carbonate powder and sevelamer hydrochloride were well tolerated during this study. The nature and severity of adverse events was consistent with the administration of phosphate binders to patients with CKD receiving dialysis. Treatment-related adverse events during sevelamer carbonate were gastrointestinal in nature, which is consistent with the occurrence of related adverse events during the sevelamer hydrochloride run-in period and the product labelling.
Although these results are very encouraging, the study was limited by a design that was open label and did not include a washout period between the active treatment periods. The study was open label due to the practical considerations involved in blinding patients to study medication assignment (powder versus tablet). The use of a double-dummy approach would have required patients to have a considerable study medication burden, which may have caused patients to be non-compliant and could thereby compromise the validity of the study. No washout period occurred between treatment periods, as carryover effects were not expected at the time of efficacy measurements. This was supported by the lack of a sequence effect.
In summary, this study demonstrated that sevelamer carbonate powder was a well-tolerated, effective phosphate binder that was equivalent to sevelamer hydrochloride tablets in controlling serum phosphorus in CKD patients on haemodialysis. Sevelamer carbonate powder should provide a welcomed new option for the treatment of hyperphosphataemia for CKD patients.
Acknowledgments
This study was supported by Genzyme Corporation.
Conflict of interest statement. Stanley Fan has received speaker's honoraria from Genzyme Corporation and the department has received educational and research grants. Jeremy Heaton, John Hunter and Melissa Plone are employees of Genzyme Corporation.
References
- 1.Cleary DJ, Matzke GR, Alexander ACM, et al. Medication knowledge and compliance among patients receiving long term dialysis. Am J Health Syst Pharm. 1995;52:1895–1900. doi: 10.1093/ajhp/52.17.1895. [DOI] [PubMed] [Google Scholar]
- 2.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 3.Strachan I, Greener M. Medication-related swallowing difficulties may be more common than we realize. Pharm Pract. 2005:411–414. [Google Scholar]
- 4.Anderson O, Zweidorff O, Hjelde T, et al. Problems when swallowing tablets. A questionnaire study from general practice. Tidsskr Nor Laegeforen. 1995;115:947–949. [PubMed] [Google Scholar]
- 5.Chertow GM, Burke SK, Lazarus JM, et al. Poly[allylamine hydrochloride] (RenaGel): a noncalcemic phosphate binder for the treatment of hyperphosphatemia in chronic renal failure. Am J Kidney Dis. 1997;29:66–71. doi: 10.1016/s0272-6386(97)90009-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg DI, Dillon MA, Slatopolsky EA, et al. Effect of RenaGel, a non-absorbed, calcium- and aluminium-free phosphate binder, on serum phosphorus, calcium, and intact parathyroid hormone in end-stage renal disease patients. Nephrol Dial Transplant. 1998;13:2303–2310. doi: 10.1093/ndt/13.9.2303. [DOI] [PubMed] [Google Scholar]
- 7.Bleyer AJ, Burke SK, Dillon M, et al. A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis. 1999;33:694–701. doi: 10.1016/s0272-6386(99)70221-0. [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Burke SK, Dillon MA, et al. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant. 1999;14:2907–2914. doi: 10.1093/ndt/14.12.2907. [DOI] [PubMed] [Google Scholar]
- 9.Chertow GM, Dillon M, Burke SK, et al. A randomized trial of sevelamer hydrochloride (RenaGel) with and without supplemental calcium. Strategies for the control of hyperphosphatemia and hyperparathyroidism in hemodialysis patients. Clin Nephrol. 1999;51:18–26. [PubMed] [Google Scholar]
- 10.Slatopolsky EA, Burke SK, Dillon MA The RenaGel Study Group. RenaGel, a nonabsorbed calcium- and aluminum-free phosphate binder, lowers serum phosphorus and parathyroid hormone. Kidney Int. 1999;55:299–307. doi: 10.1046/j.1523-1755.1999.00240.x. [DOI] [PubMed] [Google Scholar]
- 11.Sonikian MA, Pani IT, Iliopoulos AN, et al. Metabolic acidosis aggravation and hyperkaliemia in hemodialysis patients treated by sevelamer hydrochloride. Ren Fail. 2005;27:143–147. [PubMed] [Google Scholar]
- 12.DeSanto NG, Frangiosa A, Anastasio P, et al. Sevelamer worsens metabolic acidosis in hemodialysis patients. J Nephrol. 2006;19(Suppl 9):S108–S114. [PubMed] [Google Scholar]
- 13.Delmez J, Block G, Robertson J, et al. A randomized, double-blind, crossover design study of sevelamer hydrochloride and sevelamer carbonate in patients on hemodialysis. Clin Nephrol. 2007;68:386–391. doi: 10.5414/cnp68386. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Mehrotra R, Fouque D, et al. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial. 2004;17:455–465. doi: 10.1111/j.0894-0959.2004.17606.x. [DOI] [PubMed] [Google Scholar]
- 15.Kraut JA. The role of metabolic acidosis in the pathogenesis of renal osteodystrophy. Adv Ren Replace Ther. 1995;2:40–51. doi: 10.1016/s1073-4449(12)80070-7. [DOI] [PubMed] [Google Scholar]
- 16.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 17.Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Study (DOPPS) Am J Kidney Dis. 2004;44:661–671. [PubMed] [Google Scholar]