Abstract
BACKGROUND
Shift-work and a sedentary lifestyle are risk factors for raised blood pressure (BP). Exercise can reduce BP in diurnally-active individuals, but it is unknown whether postexercise hypotension persists when people are active and eating at night. We present the first investigation into the acute effects of exercise on BP monitored during simulated night-work.
METHODS
Nine normotensive participants, aged 20–42 years, completed at least two crossover trials beginning at 1800 hours. Between 1900 and 2000 hours, participants either rested or exercised at 50% peak oxygen uptake (VO2peak) and then remained awake throughout the night, completing various tasks until 0515 hours. Six participants completed a total of four trials in which they exercised or rested, whereas either one standardized (60 kJ/kg) meal at 2200 hours or two smaller (30 kJ/kg) meals at 2200 and 0200 hours were eaten. Systolic and diastolic BP, mean arterial pressure (MAP), heart rate (HR), and wrist activity were recorded every 30 min.
RESULTS
Following exercise, MAP was significantly (P < 0.0005) lower throughout the night-shift compared with no prior exercise (95% confidence limits for reduction: 4–7 mm Hg). The postexercise reductions in systolic BP and MAP were not moderated by diet, but the reduction in diastolic BP was slightly greater when only one meal was eaten (P < 0.0005). BP was lower even though wrist activity and HR were significantly higher following exercise (P < 0.0005).
CONCLUSIONS
These data indicate that prior exercise lowers BP throughout a subsequent 8-h night-shift in healthy individuals within the normotensive range. Therefore, regular low-intensity exercise might moderate the well-known association between shift-work participation and raised BP.
In developed countries, shift-workers comprise 15–20% of the working population and have an increased risk of cardiovascular morbidity and mortality compared with day-workers.1,2 The exact mechanisms for the increased risk of cardiovascular disease in shift-workers are unknown, although disrupted circadian rhythms in cardiovascular function, disturbed sleep, and other lifestyle-related problems have all been implicated.1,2 It is known that shift-workers have an elevated concentration of serum triglycerides and a lower concentration of high-density lipoproteins compared with day-workers.3 Obesity, diabetes mellitus, and metabolic syndrome have also been reported to be more common in shift-workers and all these diseases contribute to increased cardiovascular risk.1 Therefore, it seems plausible that the risk of hypertension is also greater among shift-workers compared with day-workers.
Data from cross-sectional studies have indicated that blood pressure (BP) is indeed elevated in shift-workers.4 It has also been reported that there is a greater proportion of “nondippers” among shift-workers compared with day-workers.5 In view of the difficulties in interpreting differences between samples of day- and shift-workers, Suwazono et al.6 completed a 14-year longitudinal study on the BP of a large number of shift-workers. Shift-work was found to be a more significant risk factor for increased BP than age or body mass index. Recently, Lo et al.7 found that the BP of normotensive shift-workers was found to increase during sleep following a night-shift and was also slower to return to baseline. In an accompanying editorial, the relations between these acute changes within the normotensive range and longer-term-raised BP status were highlighted.8
Eating behavior influences BP9 and can be different among shift-workers compared with day-workers.10 The timing and type of food eaten by shift-workers have been found to be determined more by the opportunity afforded by the work schedule than by hunger.10 During night-work, there might be additional problems due to the unavailability of palatable food and the influence of the “body clock,” which is not synchronized to the individual’s wake-sleep routine.10 Night-workers tend to “graze” on snacks during the night-shift rather than eat a substantial meal in the middle of it.11 Although the relationships between ingestion of food and BP have been investigated previously in diurnally-active individuals,12 we are unaware of any study in which such relationships have been explored specifically during night-work.
During shift-work, the amount, type, and timing of leisure-time physical activity can be altered.1 Shift-workers are generally less active than day-workers, and those shift-workers who do exercise tend to schedule it before, rather than after, the work period. Such differences make it difficult to extrapolate the general health benefits of physical activity to shift-workers. Moreover, there has been no previous study on the effects of exercise on BP during night-work, which is surprising given the evidence that exercise can help manage hypertension.13 An exercise program has been reported to reduce BP by a similar degree as dietary salt restriction.14 BP can be reduced for up to 22 h after a single bout of exercise in diurnally-active normotensive and hypertensive people.15-18 This “postexercise hypotension” has been found to be less marked when exercise is taken in the early morning,17,18 but these studies involved diurnally-active participants. No researcher has explored whether exercise can reduce BP when participants are awake, active, and eating at night, as many shift-workers are. Therefore, our aims were to examine the acute effects of evening exercise on BP monitored throughout a subsequent 8-h night-shift, and to explore whether such effects are moderated by meal frequency.
METHODS
Participants
Following an estimation of the sample size required for the primary comparisons (see “Statistical Analysis”), nine healthy normotensive participants (8 men and 1 woman; aged 20–42 years) gave their written informed consent to take part in this crossover experiment, which was approved by the local ethics committee. All participants were nonsmokers, had no history of cardiovascular disease, were not taking any medication and engaged in regular physical activity (defined as greater than 2 h/week). The female participant was always tested during the first week of the follicular phase of her menstrual cycle. In terms of “chronotype,” two of the participants were “ morning-types,” five were “intermediates,” and two were “evening-types.” Other characteristics of the sample, including their BP status, are shown in Table 1.
Table 1. Participant characteristics.
| Mean (s.d.) age (years) | 30.5 (8.0) |
| Mean (s.d.) weight (kg) | 75.3 (6.8) |
| Mean (s.d.) height (m) | 1.8 (0.1) |
| Mean (s.d.) BMI (kg/m2) | 22.9 (1.7) |
| Mean (s.d.) VO2peak (ml/kg/min) | 49.2 (6.7) |
| Mean (s.d.) systolic BP (mm Hg) | 117 (3) |
| Mean (s.d.) diastolic BP (mm Hg) | 69 (5) |
| Mean (s.d.) MAP (mm Hg) | 87 (3) |
| Mean (s.d.) HR (bpm) | 58 (5) |
BMI, body mass index; BP, blood pressure; bpm, beat/min; HR, heart rate; MAP, mean arterial pressure; VO2peak, peak oxygen uptake.
Experimental design
After visits to the laboratory for preliminary measurements, familiarization, and a test of peak oxygen uptake (VO2peak), all nine participants completed at least two main trials (exercise and no-exercise control) in a randomized order. Forty-eight hours prior to all trials, participants refrained from exercise, alcohol, and caffeine and recorded their eating habits in a food diary. The night before each trial, participants slept between 2300 and 0700 hours and were then instructed to abstain from food after 1000 hours. Water was consumed ad libitum and time and amount was recorded by the participant. During all trials, participants were given 100 ml of water to consume every 60 min throughout the night. The light level within the laboratory was maintained at 200 lux, with room temperature being maintained at 21 °C throughout all trials.
All trials began at 1800 hours with the consumption of a standard meal. The energy content of this meal was equivalent to 60 kJ/kg body mass, with 52% of the energy derived from fat, 38% from carbohydrate, and 10% from protein. The participants were instructed to consume the meal within 15 min. The participants then performed either cycling at an exercise intensity corresponding to 50% VO2peak or rested in the seated upright position between 1900 and 2000 hours. Subsequently, participants then worked on laptop computers, read books, watched television and listened to music until 0515 hours. Systolic BP, diastolic BP, mean arterial pressure (MAP), and heart rate (HR) were recorded at 30-min intervals throughout the testing period. General physical activity was measured from 2000 to 0515 hours. At 2100, 0100, and 0500 hours, participants completed standard tests of mental and physical performance, lasting ~20 min.
Six of the nine participants completed both the exercise and resting control trials under two conditions of meal frequency during the night-shift (a total of four trials completed). The remaining three participants completed two trials (exercise and control) in only one of the meal conditions. Two of the three participants completed two trials (exercise and control) under the one-meal condition. One participant completed two trials (exercise and control) under the two-meal condition. During the one-meal trial, participants consumed, at 2200 hours, a meal with the same energy content as the standard meal consumed at 1800 hours. In the two-meal trial, meals were consumed at 2200 and 0200 hours, with the energy content of each of these meals equivalent to 30 kJ/kg body mass. All these meals had the same relative energy contributions as the standard meal eaten at 1800 hours. All meals were required to be ingested within a 15-min period.
Preliminary visit
During this visit, height, body mass (and therefore body mass index) as well as resting BP were recorded. Resting BP was determined with the DINAMAP Pro100 blood pressure monitor (Critikon, Tampa, FL) from the average of three serial measurements. Chronotype was assessed using a questionnaire.19 Participants were also familiarized with the cycle ergometer and other equipments at this time.
Measurement of VO2peak
On the second visit, all participants completed a test of VO2peak using an incremental and continuous protocol on the cycle ergometer.20 Ten minutes of submaximal exercise was completed as a standard warm-up prior to the test, which began at a power output of 100 W and comprised increments of 25 W every 2 min until volitional exhaustion was reached. The criteria for volitional exhaustion included the point at which the participant could no longer maintain the required pedal cadence (>60 r.p.m.), the respiratory exchange ratio being >1.15, a plateau in VO2 and HR being close to the age-predicted maximum.
Measurements during the main trials
BP and HR were measured at 30-min intervals throughout all trials using an automated BP monitor (DINAMAP Pro100). The nondominant arm was used for measurement using an appropriate-sized cuff. If the arm circumference was ≥31 cm, a large cuff was used. Each participant was required to rest in the seated position for 5 min prior to each BP reading, which was obtained by the same researcher. Prior to the experiment, the BP monitor was cross-validated with three resting BP readings obtained by a research assistant using a mercury sphygmomanometer according to British Hypertension Society guidelines.21 Reinders et al.22 found that the DINAMAP Pro100 blood pressure monitor achieved all the required criteria of the International Protocol of the European Society of Hypertension. The mean (s.d.) differences between the DINAMAP Pro100 blood pressure monitor and the nine sequential same-arm measurements, alternating between two trained observers for systolic and diastolic BPs, respectively, were 2.5 (5.4) and 0.5 (4.5) mm Hg. This degree of bias and random error falls within the pass criteria for the Association for the Advancement of Medical Instrumentation Standard (ANSI/AAMI SP10).
The Actiwatch AW4 (CamNtech, Cambridge, UK) resembles a wrist-watch and is a light-weight (16 g) device which contains a miniature uniaxial accelerometer. Accelerometry data equivalent to over 0.05 g of mass is measured 32 times per second and processed to provide the digital integration of the amount and duration of movement within a given period, or epoch. The Actiwatch has a variable epoch length of between 5 s and 15 min. Actigraph data (activity counts) were recorded using 10-s epochs, and average counts were calculated across 30-min data bins for the purpose of statistical analysis.
Statistical analysis
The primary outcomes in this study were systolic BP and diastolic BP. The primary comparison was the difference between exercise and no-exercise trials in terms of the average postexercise (2030–0500 hours) values of these outcomes subtracted from their respective baseline values. Baselines were calculated from the average of measurements made at 1800, 1830, and 1900 hours. For estimation of sample size, it was deemed from past research work15-18 that exercise would mediate a clinically significant reduction of 5 mm Hg in systolic and/or diastolic BP compared with the control trial. This reduction is typically found in normotensive individuals and is likely to be clinically important, given that exercise-related reductions in BP are generally more pronounced in people with hypertension.23 For our crossover-type experiment, which generally holds more statistical power than a cross-sectional study (because data are paired in nature), it was estimated that seven participants would allow this difference to be deemed statistically significant (statistical power = 80%, s.d. of differences ≤4 mm Hg using a one-tailed paired t-test).
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software (version 14.0; SPSS, Chicago, IL). Systolic BP, diastolic BP, MAP, HR, and wrist activity were analyzed using a linear mixed model with the factors being exercise (exercise vs. no exercise), meal frequency (one meal vs. two meals), and measurement time (every 30 min). Linear mixed modeling is the most appropriate and powerful approach to analyzing unbalanced repeated measures experimental designs.24 Exploration of cross-correlations between repeated measures levels indicated that a covariance type of compound symmetry was appropriate. Data are presented throughout the text and figures as mean (s.e.) and 95% confidence intervals (CIs). Exact P values are cited (values of P of “0.000” provided by the statistics package are reported as “<0.0005”). Statistical significance was delimited at P < 0.05.
RESULTS
Systolic BP
Compared to baseline values (Table 2), exercise mediated a significant reduction in systolic BP of 7 (2) mm Hg throughout the subsequent night-shift compared to a slight increase of 1 (2) mm Hg during the night-shift without prior exercise (Figure 1). The resulting difference between exercise and no-exercise trials in systolic BP during the night-shift was 8 (1) mm Hg (CI = 6 to 9, P < 0.0005). This difference between trials was consistent over time, evidenced by the nonsignificant interaction between the exercise and time factors (P = 0.74). No significant effects of meal frequency were found on systolic BP (P > 0.10). Meal frequency was also found not to moderate the effects of prior exercise on systolic BP (exercise × meal interaction: P = 0.17).
Table 2. Calculated mean baseline values and ±s.d. of systolic BP, diastolic BP, MAP, heart rate, and activity for all 9 participants.
| Baseline measures | ||||
|---|---|---|---|---|
| 1-Meal group with 8 participants | 2-Meal group with 7 participants | |||
| Variable | No exercise | Exercise | No exercise | Exercise |
| Systolic BP (mm Hg) | 110.1 (3.9) | 108.6 (4.1) | 118.9 (3.9) | 116.1 (4.1) |
| Diastolic BP (mm Hg) | 69.1 (2.3) | 55.5 (2.4) | 68.6 (2.3) | 68.8 (2.4) |
| MAP (mm Hg) | 91.0 (2.5) | 80.9 (3.6) | 88.1 (2.5) | 88.7 (3.6) |
| Heart rate (bpm) | 59.2 (3.9) | 50.9 (4.1) | 50.4 (3.9) | 58.3 (4.1) |
| Activity (counts/min) | 78.3 (20) | 129.8 (22) | 78.3 (20) | 189.2 (22) |
BP, blood pressure; bpm, beat/min; MAP, mean arterial pressure.
Figure 1.
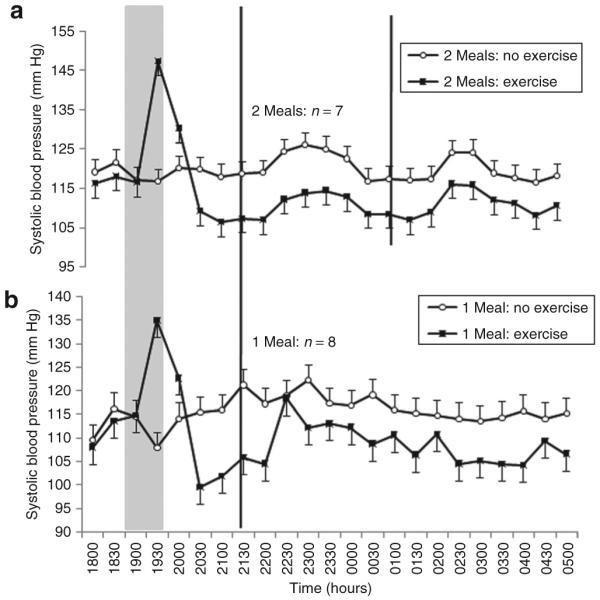
Mean ± s.e. systolic blood pressure measured every 30 min during a simulated night-shift with, and without, exercise prior to the beginning of the shift. (a) Consumption of two smaller (30 kJ/kg body mass) meals during the shift at 2200 and 0200 hours. (b) Consumption of one larger (60 kJ/kg body mass) meal during the shift at 2200 hours. The gray panel indicates time of exercise or rest. Black vertical lines indicate time of meal consumption.
Diastolic BP
Prior exercise reduced diastolic BP by 4 (2) mm Hg throughout the subsequent night-shift compared to a slight increase of 1 (2) mm Hg without exercise (Figure 2). The resulting difference between trials of 6 (1) mm Hg was statistically significant (CI = 4–7, P < 0.0005). There was a significant interaction between the exercise and time factors (P = 0.02), indicating that differences between the exercise and no-exercise trials reduced toward the end of the simulated night-shift (Figure 2). Diastolic BP was generally 2 (1) mm Hg lower when only one large meal was eaten during the shift compared to two smaller meals (P = 0.004). Meal frequency also significantly moderated the exercise-related effects on diastolic BP (P < 0.0005). Exercise reduced diastolic BP by 6 (2) mm Hg when one meal was eaten compared with a reduction of 3 (2) mm Hg in the two-meal trials.
Figure 2.
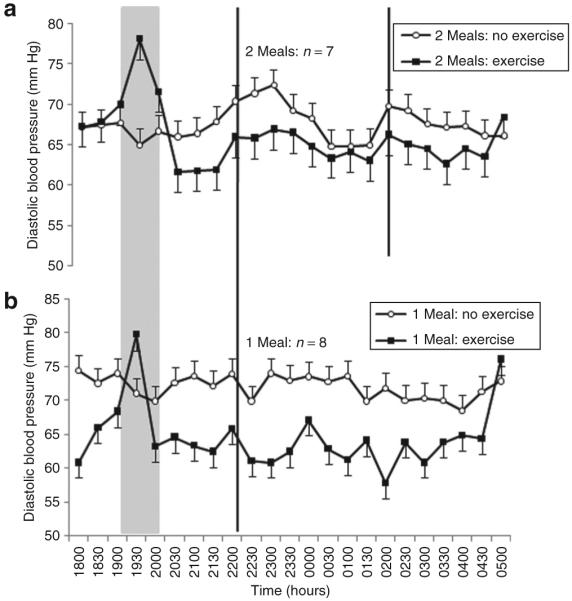
Mean ± s.e. diastolic blood pressure measured every 30 min during a simulated night-shift with, and without, exercise prior to the beginning of the shift. (a) Consumption of two smaller (30 kJ/kg body mass) meals during the shift at 2200 and 0200 hours. (b) Consumption of one larger (60 kJ/kg body mass) meal during the shift at 2200 hours. The gray panel indicates time of exercise or rest. Black vertical lines indicate time of meal consumption.
MAP
Generally, MAP reduced over the duration of the simulated night-shift (P = 0.03). Nevertheless, the reduction in MAP of 6 (2) mm Hg was significantly greater following exercise than the reduction of 1 (2) mm Hg in the no-exercise trials (CI = 4–7, P < 0.0005, Figure 3). The interaction between the meal frequency and time factors was significant (P = 0.02). In the two-meal trials, two distinct increases in MAP were observed soon after the meals, which differed from the time course of MAP in the one-meal trials (Figure 3). Nevertheless, meal frequency did not moderate the exercise-related effects on MAP (P = 0.51).
Figure 3.
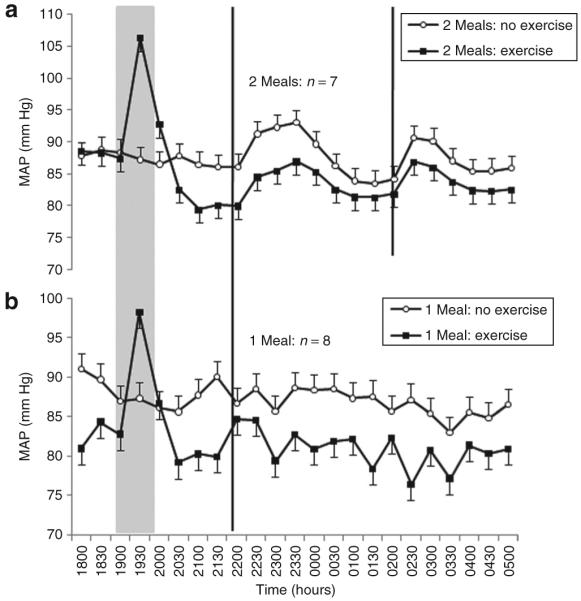
Mean ± s.e. MAP measured every 30 min during a simulated night-shift with, and without, exercise prior to the beginning of the shift. (a) Consumption of two smaller (30 kJ/kg body mass) meals during the shift at 2200 and 0200 hours. (b) Consumption of one larger (60 kJ/kg body mass) meal during the shift at 2200 hours. The gray panel indicates time of exercise or rest. Black vertical lines indicate time of meal consumption. MAP, mean arterial pressure.
HR
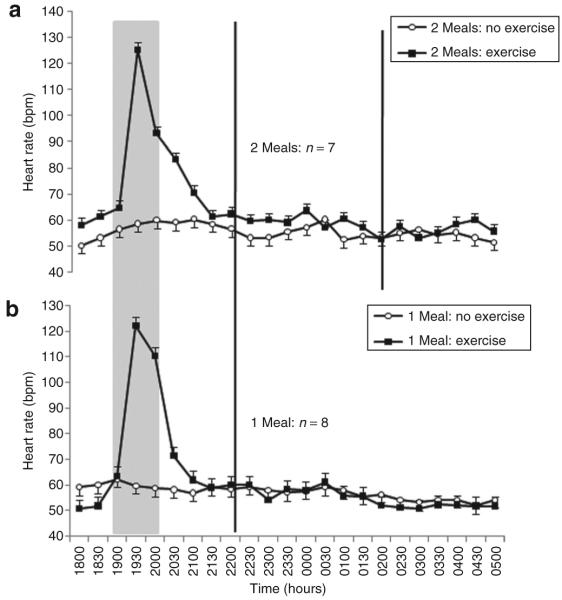
Prior exercise mediated an increase of 2 (2) beat/min throughout the subsequent night-shift compared with a slight decrease of 1 (2) beat/min without exercise (Figure 4). The difference between trials was 3 (1) beat/min (CI = 1.3–4.0, P < 0.0005). The interaction between the meal frequency and exercise factors was statistically significant (P = 0.002); in the two-meal trials, the exercise-related increase in HR (3 beat/min) was larger than that observed in the one-meal trials (1 beat/min).
Figure 4.
Mean ± s.e. heart rate measured every 30 min during a simulated night-shift with, and without, exercise prior to the beginning of the shift. (a) Consumption of two smaller (30 kJ/kg body mass) meals during the shift at 2200 and 0200 hours. (b) Consumption of one larger (60 kJ/kg body mass) meal during the shift at 2200 hours. The gray panel indicates time of exercise or rest. Black vertical lines indicate time of meal consumption.
Wrist activity
Prior exercise mediated an increase in wrist activity by 85 (11) counts/min throughout the subsequent night-shift compared with a slight reduction of 21 ± (11) counts/min without exercise. The difference between trials was 106 (8) counts/min (CI = 90–122, P < 0.0005). Meal frequency significantly moderated these exercise-related effects on activity (P < 0.0005). In the two-meal trial, the exercise-related increase in subsequent wrist activity was much larger (120 counts/min) compared with the one-meal trials (51 counts/min).
DISCUSSION
In light of the established relationship between shift-work and chronically raised BP, we have undertaken this first study into whether the aftereffects of exercise on BP persist when simulated night-work is undertaken in the postexercise period. Following a 1-h bout of moderate-intensity exercise in the evening, clinically worthwhile (CI = 4–7 mm Hg) and statistically significant reductions in MAP were observed throughout a subsequent 8-h simulated night-shift (2100–0500 hours) in healthy normotensive volunteers. The reduction in diastolic BP was slightly larger when one larger meal was ingested at the start of the shift compared with “grazing” on two smaller meals during the shift, which is a common behavior among night-workers. The exercise-mediated reductions in systolic BP and MAP were not moderated by meal frequency.
Our data provide the first indication that the postexercise reduction in BP (commonly described as postexercise hypotension) is robust and prolonged when a simulated night-shift is undertaken after a bout of evening exercise prior to the shift. The exercise-mediated reductions in systolic and diastolic BPs we found are comparable in magnitude and duration with those reported in studies undertaken during the hours of daylight. Brandão Rondon et al.25 showed that a 45-min bout of relatively low-intensity (50% VO2max) bicycle exercise undertaken diurnally mediated prolonged (up to 22 h) reductions in the systolic and diastolic BPs of hypertensive patients. Researchers who have examined normotensive participants during the daylight hours have generally reported exercise-related reductions in BP of 5–9 mm Hg.26,27 Our findings also agree with those of Jones et al.18 who examined whether time of day moderates postexercise hypotension. Similar reductions in BP to ours were also found by Jones et al.17 following exercise at 2000 and 2200 hours, although BP was measured for only 20 min in the postexercise period by these researchers.17 It is important to note that our findings are relevant to the situation in which evening exercise is performed prior to a night-shift. The hypotensive effect of exercise prior to an early morning shift (e.g., 0600–1400 hours) may, for example, be different, given recent evidence that postexercise hypotension is generally attenuated when exercise is undertaken in the early morning.17,18
The precise mechanisms governing the acute and longer-term hypotensive response to exercise are inconclusive. It is generally thought that postexercise hypotension is the result of persistent reductions in systemic and peripheral resistance that are not offset by changes in cardiac output. Both these physiological changes are mediated via the autonomic nervous system and by vasodilatory substances.18,27 Recently, Halliwill et al.28 provided evidence that the vasodilatory effects of histamine are important in explaining postexercise hypotension. Endogenous concentrations of histamine are known to show circadian variation29 with lower values being observed at night. Endogenous nitric oxide is another important vasodilatory substance, which has also been reported to show circadian variation in normotensive but not hypertensive individuals.30 Our finding of a prolonged hypotensive effect of exercise during the night is interesting when considered alongside these studies that have attempted to identify the underlying vasodilatory substance. Of special interest is melatonin, as this secretory product of the pineal gland has vasodilatory properties and is also thought to be crucial to the circadian system.31 Nevertheless, any interactive effects of exercise and time of day on endogenous concentrations of histamine, nitric oxide, and melatonin are, at present, unclear.28,31 It would be sensible for future researchers to measure these vasodilatory substances alongside the BP measurements following exercise at different circadian times.
HR and wrist activity levels were found to be significantly higher during the simulated night-shift when prior exercise was undertaken. It has been suggested that prior moderate-intensity exercise lasting 20 min or more (but no longer than 1 h) can increase feelings of vigor and work productivity, and this could account for the increased activity and, therefore, HR following the exercise.32,33 The exercise-mediated increase in wrist activity could also be explained by the “cognitive appraisal hypothesis” in that the prior exercise could have improved mood states and mediated a more favorable perception of the simulated work that was undertaken during the night.32,33 The most important point for our study results is that sustained reductions in BP were observed in our study even though wrist activity and HR were higher following exercise. This finding agrees with those of MacDonald26 who found that postexercise hypotension is sustained during typical daytime activities in the postexercise period.
MAP was generally influenced by meal frequency, being increased by ~5 mm Hg at about the time when a meal was ingested. This increase was typically sustained for 30–60 min after a meal (Figure 3). Nevertheless, it can be seen in Figure 3 that the meal-mediated changes in MAP were clearer in the two-meal, compared with the one-meal, condition. Although these different meal frequencies did not moderate the postexercise reductions in systolic BP and MAP, postexercise diastolic BP was ~2 mm Hg lower in the one-meal trials compared with the two-meal trials. Cardiovascular responses to food are, in part, dependent upon the composition, the size, and the timing of the meal ingested as well as choice/availability and palatability.34,35 It is important to note that we controlled meal composition and total energy intake while we varied meal frequency and size of each meal ingested. The composition of the meals was also dictated by us, although checked beforehand for palatability to our participants. Although a postprandial reduction in BP is common in elderly people, Jakulj et al.34 found that the ingestion of a single high-fat (65% of total energy intake) meal amounting to 3,433 kJ led to a statistically significant increase in both systolic and diastolic BPs, as well as total peripheral resistance, in comparison with a low-fat meal of the same total energy content. Our meals also contained a relatively high-fat content in line with the known dietary habits of night-workers,10 and so our data are consistent with those of Jakulj et al.34
Large meals generally elicit a more prolonged cardiac response in comparison to small meals,35 but this was not apparent when the responses of MAP to the larger (one-meal trial) and smaller (two-meal trial) meal at 2200 hours are compared (Figure 3). The greater postprandial increment in cardiac output is the direct consequence of a greater demand in the mesenteric bed, which has been postulated to lead to greater blood flow and a decrease in BP in comparison to little or no change in cardiac reactivity following smaller meals.36,37 In the present study, even though each of the two meals was half the size of the one meal, it was apparent that two meals in the postexercise period somewhat “swamped” the exercise-related changes in BP, suggesting that meal frequency has greater effects on longitudinally studied BP than meal size. Sidery and Macdonald35 provide some support for this notion in that no differences in postprandial BP were found between three different meal sizes (1, 2, and 3 MJ with ~84% of the energy coming from carbohydrates). It is also plausible that the “grazing/snacking” approach adopted in the two-meal trials did not fulfill the immediate hunger needs of the participants, leading to a “hunger-stress” effect. Several other researchers have found that changes in eating habits associated with shift-work (a decrease in meal size but increase in snacking and smaller meal frequency) have detrimental effects on other physiological outcomes.38,39 Nevertheless, this mechanism is unlikely because meal frequency did not moderate the exercise-related reductions in systolic BP and MAP in the present study. In conclusion, these data indicate for the first time that prior exercise lowers BP throughout a subsequent 8-h night-shift. There is now good evidence that shift-work is a risk factor for chronically raised BP, and circadian-related disturbances have been postulated to be important in explaining this phenomenon. Although our findings should be limited to healthy volunteers within the normotensive range, they suggest that leisure-time physical activity may be an important lifestyle factor in managing hypertension in shift-workers in that regular low-intensity exercise has the potential to attenuate the longer-term increase in BP in shift-workers.
Acknowledgments
This research was funded by the National Prevention Research Initiative (http://www.npri.org.uk) with support from the following organizations: British Heart Foundation; Cancer Research UK; Chief Scientist Office, Scottish Government Health Directorate; Department of Health; Diabetes UK; Economic and Social Research Council; Health and Social Care Research and Development Office for Northern Ireland; Medical Research Council; Welsh Assembly Government; and World Cancer Research Fund. We also thank Laura Sutton for assistance during the data collection.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Atkinson G, Fullick S, Grindey C, Maclaren D. Exercise, energy balance and the shift worker. Sports Med. 2008;38:671–685. doi: 10.2165/00007256-200838080-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterhouse J, Folkard S, Minors D. HSE Contract Research Report. HMSO; London: 1992. Shift Work, Health and Safety. An Overview of the Scientific Literature 1978–1990. [Google Scholar]
- 3.Knutson A, Andersson H, Berglund U. Serum lipoproteins in day and shift workers: a prospective study. Br J Ind Med. 1990;47:132–134. doi: 10.1136/oem.47.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakata K, Suwazono Y, Harada H, Okubo Y, Kobayashi E, Nogawa K. The relationship between shift work and the onset of hypertension in male Japanese workers. J Occup Environ Med. 2003;45:1002–1006. doi: 10.1097/01.jom.0000085893.98441.96. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension. 1998;32:417–423. doi: 10.1161/01.hyp.32.3.417. [DOI] [PubMed] [Google Scholar]
- 6.Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Nogawa K. Shift work is a risk factor for increased blood pressure in Japanese men: a 14-year historical cohort study. Hypertension. 2008;52:581–586. doi: 10.1161/HYPERTENSIONAHA.108.114553. [DOI] [PubMed] [Google Scholar]
- 7.Lo SH, Liau CS, Hwang JS, Wang JD. Dynamic blood pressure changes and recovery under different work shifts in young women. Am J Hypertens. 2008;21:759–764. doi: 10.1038/ajh.2008.186. [DOI] [PubMed] [Google Scholar]
- 8.Ha M. Does resting for two consecutive days enable complete recovery from night work? Am J Hypertens. 2008;21:730–731. doi: 10.1038/ajh.2008.208. [DOI] [PubMed] [Google Scholar]
- 9.Lindquist TL, Beilin LJ, Knuiman MW. Influence of lifestyle, coping, and job stress on blood pressure in men and women. Hypertension. 1997;29(1 Pt 1):1–7. doi: 10.1161/01.hyp.29.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Waterhouse J, Buckley P, Edwards B, Reilly T. Measurement of, and some reasons for, differences in eating habits between night and day workers. Chronobiol Int. 2003;20:1075–1092. doi: 10.1081/cbi-120025536. [DOI] [PubMed] [Google Scholar]
- 11.Lennernas M, Lillemor A, Hambraeus L, Akerstedt T. The 24 hour intake of energy and nutrients in 3 shift workers. Ecol Food Nutr. 1994;32:157–165. [Google Scholar]
- 12.Sidery MB, Macdonald IA. The effect of meal size on the cardiovascular responses to food ingestion. Br J Nutr. 1994;71:835–848. doi: 10.1079/bjn19940190. [DOI] [PubMed] [Google Scholar]
- 13.Hamer M, Taylor A, Steptoe A. The effect of acute aerobic exercise on stress related blood pressure responses: a systematic review and meta-analysis. Biol Psychol. 2006;71:183–190. doi: 10.1016/j.biopsycho.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Hellénius ML, de Faire U, Berglund B, Hamsten A, Krakau I. Diet and exercise are equally effective in reducing risk for cardiovascular disease. Results of a randomized controlled study in men with slightly to moderately raised cardiovascular risk factors. Atherosclerosis. 1993;103:81–91. doi: 10.1016/0021-9150(93)90042-s. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald JR, Hogben CD, Tarnopolsky MA, MacDougall JD. Post exercise hypotension is sustained during subsequent bouts of mild exercise and simulated activities of daily living. J Hum Hypertens. 2001;15:567–571. doi: 10.1038/sj.jhh.1001223. [DOI] [PubMed] [Google Scholar]
- 16.Pescatello LS, Kulikowich JM. The aftereffects of dynamic exercise on ambulatory blood pressure. Med Sci Sports Exerc. 2001;33:1855–1861. doi: 10.1097/00005768-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Jones H, George K, Edwards B, Atkinson G. Effects of time of day on post-exercise blood pressure: circadian or sleep-related influences? Chronobiol Int. 2008;25:987–998. doi: 10.1080/07420520802548044. [DOI] [PubMed] [Google Scholar]
- 18.Jones H, Pritchard C, George K, Edwards B, Atkinson G. The acute post-exercise response of blood pressure varies with time of day. Eur J Appl Physiol. 2008;104:481–489. doi: 10.1007/s00421-008-0797-4. [DOI] [PubMed] [Google Scholar]
- 19.Waterhouse J, Minors D, Waterhouse M, Reilly T, Atkinson G. Keeping in Time With Your Body Clock. Oxford University Press; Oxford, UK: 2005. [Google Scholar]
- 20.Bird S, Davison R. Physiological Testing Guidelines. British Association of Sports Sciences; Leeds: 1997. [Google Scholar]
- 21.O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 22.Reinders A, Reggiori F, Shennan AH. Validation of the DINAMAP ProCare blood pressure device according to the international protocol in an adult population. Blood Press Monit. 2006;11:293–296. doi: 10.1097/01.mbp.0000217998.96967.fb. [DOI] [PubMed] [Google Scholar]
- 23.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, American College of Sports Medicine American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 24.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Brandão Rondon MU, Alves MJ, Braga AM, Teixeira OT, Barretto AC, Krieger EM, Negrão CE. Postexercise blood pressure reduction in elderly hypertensive patients. J Am Coll Cardiol. 2002;39:676–682. doi: 10.1016/s0735-1097(01)01789-2. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald JR. Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens. 2002;16:225–236. doi: 10.1038/sj.jhh.1001377. [DOI] [PubMed] [Google Scholar]
- 27.Piepoli M, Coats AJ, Adamopoulos S, Bernardi L, Feng YH, Conway J, Sleight P. Persistent peripheral vasodilation and sympathetic activity in hypotension after maximal exercise. J Appl Physiol. 1993;75:1807–1814. doi: 10.1152/jappl.1993.75.4.1807. [DOI] [PubMed] [Google Scholar]
- 28.Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol. 1996;495(Pt 1):279–288. doi: 10.1113/jphysiol.1996.sp021592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuomisto L, Lozeva V, Valjakka A, Lecklin A. Modifying effects of histamine on circadian rhythms and neuronal excitability. Behav Brain Res. 2001;124:129–135. doi: 10.1016/s0166-4328(01)00222-4. [DOI] [PubMed] [Google Scholar]
- 30.Bode-Böger SM, Böger RH, Kielstein JT, Löffler M, Schäffer J, Frölich JC. Role of endogenous nitric oxide in circadian blood pressure regulation in healthy humans and in patients with hypertension or atherosclerosis. J Investig Med. 2000;48:125–132. [PubMed] [Google Scholar]
- 31.Atkinson G, Drust B, Reilly T, Waterhouse J. The relevance of melatonin to sports medicine and science. Sports Med. 2003;33:809–831. doi: 10.2165/00007256-200333110-00003. [DOI] [PubMed] [Google Scholar]
- 32.Turnbull M, Wolfson S. Effects of exercise and outcome feedback on mood: evidence for misattribution. J Sport Behav. 2002;25:394–406. [Google Scholar]
- 33.Gollwitzer P, Earle W, Stephan W. Affect as a determinant of egotism: residual excitation and performance attributions. J Pers Soc Psychol. 1982;43:702–709. [Google Scholar]
- 34.Jakulj F, Zernicke K, Bacon SL, van Wielingen LE, Key BL, West SG, Campbell TS. A high-fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. J Nutr. 2007;137:935–939. doi: 10.1093/jn/137.4.935. [DOI] [PubMed] [Google Scholar]
- 35.Sidery MB, Macdonald IA. The effect of meal size on the cardiovascular responses to food ingestion. Br J Nutr. 1994;71:835–848. doi: 10.1079/bjn19940190. [DOI] [PubMed] [Google Scholar]
- 36.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 37.van Baak MA. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol Behav. 2008;94:178–186. doi: 10.1016/j.physbeh.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring) 2009;17:72–77. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- 39.Di Lorenzo L, De Pergola G, Zocchetti C, L’Abbate N, Basso A, Pannacciulli N, Cignarelli M, Giorgino R, Soleo L. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord. 2003;27:1353–1358. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]