Abstract
Osteoporosis is a common complication of chronic kidney disease (CKD), and the latter is a major risk factor for cardiovascular mortality. Recent studies have elucidated some of the mechanisms by which CKD is a cardiovascular risk, and they relate to osteoporosis. Thus, the mechanisms of CKD induced cardiovascular risk provide valuable insight into the relationship between cardiovascular disease and osteoporosis, and they are reviewed here. Observational studies have determined hyperphosphatemia to be a cardiovascular risk factor in chronic kidney disease. Mechanistic studies have elucidated that hyperphosphatemia is a direct stimulus to vascular calcification, which is one cause of morbid cardiovascular events contributing to the excess mortality of chronic kidney disease. Hyperphosphatemia in chronic kidney is due to failure of excretion by the kidneys and excess bone resorption. It stimulates vascular cells to mineralize atherosclerotic plaques through osteoblastic processes. Hyperphosphatemia in chronic kidney disease is a distinct syndrome characterized by disordered skeletal remodeling, heterotopic mineralization and cardiovascular morbidity. The heterotopic mineralization stimulated by CKD is relevant to osteoporosis.
Keywords: phosphorus, cardiovascular disease, osteoporosis, kidney disease, osteoblasts
Introduction
In chronic kidney disease (CKD), hyperphosphatemia is associated with significant pathophysiology. This pathophysiology contributes to the high rates of mortality observed in CKD (1). Approximately 11-15% of Americans have CKD (2-4), and their risk of death due to a cardiovascular event related cause is higher than their risk of surviving and needing renal replacement therapy for end stage kidney disease (ESKD) (1, 2, 4). The mortality rates of patients surviving CKD and receiving hemodialysis are extremely high such that a 30 year old patient with ESKD has a life expectancy similar to a ninety year old with normal renal function (2). The mechanisms of this excess risk of cardiovascular disease are not completely understood. The well characterized risks of cardiovascular disease in the general population do not explain the increased risk in CKD (1, 3). Observational studies suggest that the well known propensity of ESKD patients to develop heterotopic mineralization of soft tissues including the vasculature is an important component of the cardiovascular risks of ESKD (5, 6). Furthermore, several observational studies demonstrate that hyperphosphatemia is an independent cardiovascular risk factor in CKD (7-9). Hyperphosphatemia has been linked to vascular calcification (10-12).
Pathogenesis of hyperphosphatemia in CKD
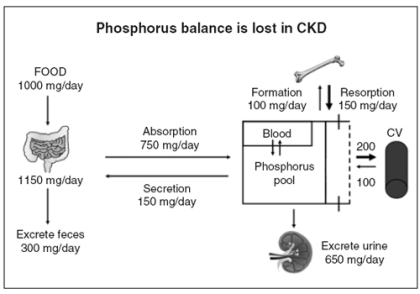
Kidney injury impairs the ability of mammals to maintain phosphorus balance, and in human chronic kidney disease, phosphorus homeostasis is lost and positive phosphate balance occurs in the later stages (4and5) of kidney diseases (13, 14). Loss of phosphorus homeostasis due to excretion failure in chronic kidney disease results in hyperphosphatemia (15, 16) due to positive balance increasing the concentration in the exchangeable phosphorus pool, often when the pool size is reduced as in the adynamic bone disorder (Fig.1). Surprisingly and not generally adequately considered, the skeleton contributes to hyperphosphatemia in CKD and ESKD through the effects of disordered bone remodeling. There are multiple skeletal remodeling disorders discussed below in CKD, but all of them are associated with excess bone resorption compared to bone formation. Thereby, they contribute to hyperphosphatemia and effectively block the skeleton from exerting its normal reservoir function when positive phosphate balance occurs.
Figure 1.
Phosphorus homeostasis is lost in chronic kidney disease due to failure of excretion. Despite reductions in the fraction of filtered phosphorus that is reabsorbed, eventually the filtered load becomes insufficient to maintain homeostasis, and positive phosphorus balance ensues. Kidney disease decreases the exchangeable phosphorus pool size by inhibiting bone formation. The skeletal mineralization fronts at the sites of new bone formation are significant components of the exchangeable phosphorus pool. Positive phosphate balance is associated with establishment of heterotopic mineralization sites in soft tissue organs and the vasculature. Exit from the exchangeable phosphorus pool into the vasculature is portrayed as a bidirectional process because we have been able to demonstrate that stopping the exit into the vasculature results in diminishment of established vascular calcification levels.
The normal function of the skeleton as a reservoir when phosphate balance is positive is seen in several syndromes of hyperphosphatemia in mammalian pathophysiology. All of them except immobilization and chronic kidney disease are associated with increased skeletal mass and mineralization due to phosphorus deposition into the skeletal storage reservoir. In chronic kidney disease, there is a complex set of losses and adaptations in skeletal function that produce bone disorders that complicate the state (see section on “Renal osteodystrophy”). Recent discoveries characterize all forms of skeletal function disorder in chronic kidney disease as having excess bone resorption rates compared to bone formation rates (see section on “Osteoporosis in chronic kidney disease”). Therefore, the skeleton is contributing to hyperphosphatemia in chronic kidney disease, and the reservoir function of the skeleton that is supposed to act in the presence of positive phosphorus balance is blocked. The outcome of this change in physiology to a new pathophysiology requires that a new phosphate reservoir for the positive balance is established. This new reservoir is soft tissue organs including the vasculature (Fig.1). The problem with establishing the new reservoirs of phosphate storage is that they produce disease. One of the new reservoirs for phosphate deposition established when excretion is no longer sufficient to maintain balance, the vasculature (Fig.1), is especially disease causing. Vascular calcification in CKD is not well tolerated as it produces blood vessel stiffness. There are two forms of vascular calcification prominent in CKD, calcification of atherosclerotic neointimal plaques and arterial medial calcification. CKD markedly stimulates both forms. The atherosclerotic calcification is especially appreciated in the coronary arteries as it is measured by the increasingly popular imaging techniques for determining coronary artery calcification (17, 18). However, arterial medial calcification is as clinically important, as it is the likely the most important factor in vascular stiffness and increased pulse pressure in CKD. The vascular calcification so prominent in CKD is not specific to CKD as it is seen in type 2 osteoporosis of elderly subjects and in patients with diabetes. Thus, the discovery of pathogenetic mechanisms of vascular calcification in CKD most likely has relevance to osteoporosis and diabetes as well.
Methods, Results, and Discussion
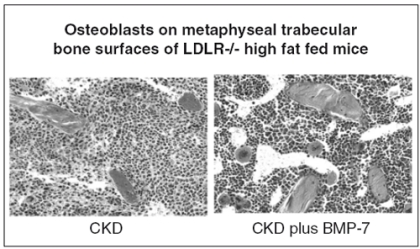
In order to study the mechanisms of CKD stimulated vascular calcification, we developed a translational animal model. We started with a model of atherosclerosis that develops cardiac valvular and atherosclerotic neointimal plaque calcification, the low density lipoprotein receptor deficient mouse (LDLR-/-) fed a high fat diet. To the model we added ablation induced kidney failure, and demonstrated marked stimulation of aortic atherosclerotic calcification (19). We discovered that bone morphogenetic protein-7 (BMP-7) prevented the development of CKD stimulated vascular calcification (19). Furthermore, the high fat fed CKD mice exhibited hyperphosphatemia that was corrected by BMP-7 (20). In investigating the mechanism of hyperphosphatemia correction by BMP-7 we found that bone formation was stimulated correcting the adynamic bone disorder that complicates the kidney failure in these mice (20) (Fig.2). Renal phosphate excretion was not increased, and we questioned how much of the BMP-7 effect on vascular calcification was due to bone formation induced correction of hyperphosphatemia. To examine this, we added phosphate binders to the high fat diet in an attempt to isolate hyperphosphatemia correction as a single entity separate from the other actions of BMP-7. To our surprise, phosphate binders were very effective in preventing vascular calcification. We first used CaCO3(20), but subsequently have studied sevelamer carbonate (21) and LaCO3with similar results.
Figure 2.
Treatment of CKD in LDLR-/- high fat fed mice corrects the adynamic bone disorder (ABD). BMP-7, 10 mcg/kg injected IP weekly increased osteoblast number and surfaces on metaphyseal trabeculae. The increase in bone formation rate restored the reduced bone volume to normal over the course of treatment.
The LDLR-/- high fat fed mouse is characterized by obesity, hypertension and insulin resistance that progresses to diabetes and severe hypercholesterolemia. Thus, the mouse model is relevant to the human metabolic syndrome, and the development of kidney disease in obese diabetics. Even the renal osteodystrophy complicating CKD, the adynamic bone disorder, is the same as observed in patients with diabetic nephropathy. The vascular calcification of the model was discovered by Towler and Semenkovich (22). They found that osteoblastic transcriptional activity was present in the aorta, and their model is the starting point to which we add CKD. At the beginning, in other words our high fat fed sham operated animals, expressed BMP-2, BMP-4, Runx2, Msx2, osteocalcin and osteopontin in the vasculature, especially the aorta. This is relevant because several investigators have demonstrated that the vasculature of patients with CKD/ESKD expresses osteoblastic markers (23-26). However, our high fat fed sham operated animals had a low level of vascular calcification that was stimulated two to four fold by the induction of CKD (19).
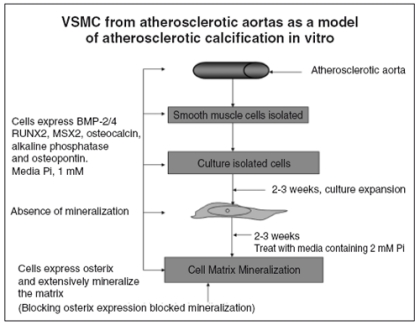
To further investigate mechanisms of atherosclerotic calcification, we developed an in vitro model to study in cell culture with the strategy of confirming discoveries made in vitro using our animal model. We began by obtaining primary cultures of human vascular smooth muscle cells (hVSMC) from areas of aortic atherosclerotic plaques, which were expanded and then exposed to an increase in media phosphorus concentrations (Fig.3). We demonstrated that the starting tissue and the primary cultures in regular media expressed BMP-2, BMP-4, Runx2, Msx2, osteocalcin and osteopontin. Thus, it appeared that BMP-2 and 4 as bone morphogens were stimulating an osteoblastic differentiation program in vascular cells directed by the osteoblast specific transcription factors. This data is in agreement with other investigators (27), including those who reported increased levels of BMP-2 and 4 in atherosclerotic lesions (28, 29). However, the primary hVSMC cultures did not mineralize the extracellular matrix as osteoblast cultures do (30). When media phosphorus was increased by 1 or 2 mM to 2 or 3 mM (equal to a serum phosphorus of 6 to 9 mg/dl), heavy matrix mineralization ensued (Fig.3). Analysis of the osteoblastic transcription program revealed that the very low levels of osterix expression in the starting cultures were increased several fold by the increase in media phosphorus. Blocking the increase in osterix expression in high phosphate media prevented matrix mineralization (31). Both our translational model in vivo and our cell culture model in vivo represent mineralization due to the atherosclerotic process. We observe mainly neointimal calcification in vivo, and the medial calcification we observe is in proximity to atherosclerotic plaques.
Figure 3.
-Schematic of the experimental plan for in vitro studies demonstrating that high phosphorus causes vascular calcification and osteoblastic gene expression. Human vascular smooth muscle cells (hVSMC) derived from atherosclerotic aortas expressed increased levels of morphogens, specific transcription factors and biomarkers of the osteoblast and decreased levels of those corresponding to contractile hVSMC. Yet the cells did not mineralize until media Pi was increased from 1 to 2 mM. High media Pi stimulated osterix expression, and when osterix expression was diminished in the presence of high media Pi there was no mineralization (31).
When we examined our high fat fed LDLR-/- aortas from the various groups of animals, we found that the sham operated high fat fed animals had low or undetectable levels of osterix expression that were increased several fold when CKD was induced (31). Most importantly, treatment of CKD high fat fed hyperphosphatemic animals with phosphate binders inhibited osterix expression (31). As expected without a critical osteoblastic transcription factor, matrix mineralization (neointimal calcification) was severely compromised.
The mechanism of phosphorus stimulation of matrix mineralization in vitro has been studied by Jono et al. (32) and Li et al. (33) in VSMC and by Beck et al. in osteoblastic cells (34, 35). These authors have demonstrated that the effect of media phosphorus was through activation of ERK1/2, and it was blocked by inhibition of sodium dependent phosphate transport proteins. Studies with RNAi to Pit-1 (33, 36) indicate that blocking the actions of high phosphorus culture media in vitro are similar to the effects of lowering the serum phosphorus in vivo, that of inhibiting osteoblastic stimulation of matrix mineralization.
Thus, phosphorus is more than a stimulator of vascular calcification acting through an elevated calcium-phosphorus product in CKD and ESKD. It is a signaling molecule serving to complete osteoblastic differentiation in the aorta, and an important component of the action of CKD to stimulate atherosclerotic calcification. The results of the translational animal studies and the studies in vitro just discussed are in agreement with a new clinical consensus that has lead to renaming of renal osteodystrophy by the KDIGO Foundation as the chronic kidney disease mineral bone disorder (CKD-MBD) in recognition of the roles of the skeleton in hyperphosphatemia and vascular calcification (37).
Renal osteodystrophy in the CKD-MBD
There are several disorders of bone remodeling that complicate CKD and ESKD and represent the skeletal component of the CKD-MBD. For purposes of this discussion, consideration will be limited to the most prevalent forms, a high turnover disorder, osteitis fibrosa, and a low turnover disorder, the adynamic bone disorder. Elevated remodeling rates associated with secondary hyperparathyroidism, produce an osteoblast phenotype in CKD that has reduced secretion of type 1 collagen and increased production of RANK ligand (RANKL), the critical osteoclast differentiation factor. This results in bone resorption outpacing bone formation. In addition, the high remodeling rates are characterized by insufficient replacement of newly formed atypical “woven” bone with bone formed on collagen lamellae. Thus, even with normal bone mass, skeletal frailty may be problematic in the high turnover osteodystrophy powered by secondary hyperparathyroidism in CKD/ESKD. Finally, the excess in bone resorption may lead to osteopenia and osteoporosis complicating CKD and especially ESKD.
A second skeletal remodeling disorder has become increasingly prevalent with administration of high doses of vitamin D analogs in CKD/ESKD, the adynamic bone disorder (ABD) (38-40). The ABD was originally thought to be due to suppression of osteoblast function with high doses of vitamin D analogs (38, 40). The finding that vitamin D analogs stimulate, not inhibit, bone formation and osteoblast function has put this contention to rest (41-43). What is likely the case is that the negative effects of CKD on skeletal anabolism are uncovered by suppression of PTH. This demonstrates that higher than normal PTH levels are required to maintain bone remodeling in CKD (44). In the ABD the profound suppression of osteoblast function (Fig.2) is not matched by equal suppression of osteoclast function. As a result, excess bone resorption is observed that may lead to osteopenia and osteoporosis.
Osteoporosis in chronic kidney disease
The balance between bone formation and resorption may be either negative or positive in CKD. When positive, osteosclerosis results, but this is rare in modern medicine. In the case of negative bone balance, bone loss occurs in cortical and cancellous bone and is more rapid when bone turnover is high. In those cases, bone densitometry will detect osteopenia or osteoporosis. The prevalence of osteoporosis in the population with CKD exceeds the prevalence in the general population (45-47). Osteoporosis is observed in CKD before dialysis is required for end stage kidney failure (48). When bone turnover is high, as in secondary hyperparathyroidism with osteitis fibrosa, bone resorption rates are in excess of bone formation and osteopenia progressing to osteoporosis may result. When bone turnover is low, as in the ABD, although both bone formation rates and bone resorption are reduced, resorption is in excess and loss of bone mass occurs. Thus, osteoporosis may be observed with either high turnover (48-51) or low turnover (52) forms of osteodystrophy. When bone resorption exceeds bone formation rates in CKD, phosphorus and calcium release contribute to hyperphosphatemia and hypercalcemia. The increase in skeletal mineral deposition that should result from hyper-phosphatemia or hypercalcemia is blocked, and heterotopic mineralization is stimulated, especially in the vasculature. The failure of the skeleton to absorb positive phosphate balance in CKD is an important stimulus to heterotopic mineralization, and links the skeleton and osteoporosis in CKD to cardiovascular events and mortality. This link between osteoporosis and vascular calcification, now partly defined in CKD, is not specific to CKD. Type 2 osteoporosis is strongly associated with vascular calcification, and perhaps, the mechanisms defined in CKD related to the serum phosphorus as a signal and positive phosphate balance also apply.
The discussion of osteoporosis in CKD above is focused on osteoporosis caused by CKD itself. However, many patients with CKD have osteoporosis independent of CKD. These patients may be elderly or may have post-menopausal osteoporosis. In addition, it is clear that gonadal hormone deficiency, as in postmenopausal osteoporosis, is also caused by CKD and is another factor in the pathogenesis of osteoporosis in CKD. Thus, osteoporosis in CKD presents a difficult differential diagnosis between the type 2 osteoporosis of aging, gonadal hormone deficiency, and excess bone resorption associated with the CDK-BMD.
In conclusion, hyperphosphatemia in CKD is a distinct syndrome. It represents one component of the increased risk of cardiovascular disease in CKD that has been successfully analyzed. Hyperphosphatemia in CKD represents a signal that heterotopic sites of mineralization are being used to compensate for the failure of reservoir function of the skeleton in positive phosphate balance. In fact, hyperphosphatemia itself is one of the signals activating heterotopic deposition sites, and functions as a signaling molecule in stimulating atherosclerotic neointimal mineralization that is markedly increased in CKD. The features of hyperphosphatemia in CKD, especially the failure of the skeletal reservoir function, may not be unique to CKD and heterotopic mineralization including the vasculature, in type 2 osteoporosis and diabetes appear to be linked to a similar pathogenesis. This requires additional study but may relate to the severe cardiovascular disease leading to morbid cardiac events in these conditions similar to CKD.
Disclosure
The manuscript and the studies reported here were supported by NIH grants DK070790 and AR41677, and by grants-in-aid from Genzyme and Shire. KAH received consultation fees from Genzyme and Shire Pharmaceutical. SM received consultation fees from Genzyme, and RL received consultation fees from Abbott.
Acknowledgments
We would like to thank Helen Odle and Frank Strebeck for administrative and technical support, Celina Mount of CDRAC for artistic support, Ray Pratt and Ping Qiu (Shire) and Jose Menoyo (Genzyme) for valuable discussion. Support for this manuscript and the studies described herein were from the NIH, DK070790, AR41677, Shire, and Genzyme.
Table I -.
Hyperphosphatemic syndromes
| Increased intake |
| Transcellular shifts from intracellular to extracellular spaces |
| Excess bone resorption |
| Decreased renal excretion |
| Idiopathic hyperparathyroidism |
| Pseudohypoparathyroidism |
| FGF-23 deficiency |
| Tumoral calcinosis |
| Chronic kidney disease |
| Acromegaly |
| Artifactual |
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kid Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 3.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease: A Statement From the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, et al. Prevalence of Chronic Kidney Disease in the United States. J Am Med Assoc. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal diseases: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 6.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 7.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium X phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 8.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 9.Slinin Y, Foley RN, Collins AJ. Calcium, Phosphorus, Parathyroid Hormone, and Cardiovascular Disease in Hemodialysis Patients: The USRDS Waves 1, 3, and 4 Study. J Am Soc Nephrol. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 10.Marchais SJ, Metivier F, Guerin AP, et al. Association of hyperphosphataemia with haemodynamic disturbances in end-stage renal disease. Nephrol Dial Transplant. 1999;14:2178–2183. doi: 10.1093/ndt/14.9.2178. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrino ED, Biltz RM. The composition of human bone in uremia. Medicine. 1965;44:397–418. doi: 10.1097/00005792-196509000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Block GA, Raggi P, Bellasi A, et al. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 13.Slatopolsky E, Robson AM, Elkan I, et al. Control of phosphate excretion in uremic man. J Clin Invest. 1968;47:1865–1874. doi: 10.1172/JCI105877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slatopolsky E, Gradowska L, Kashemsant C. The control of phosphate excretion in uremia. J Clin Invest. 1966;45:672–677. doi: 10.1172/JCI105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craver L, Marco MP, Martinez I, et al. Mineral metabolism parameters throughout chronic kidney disease stages 1-5--achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–1176. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- 16.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2006;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Jerosch-Herold M, Jacobs J, et al. Coronary Artery Calcification and Myocardial Perfusion in Asymptomatic Adults: The MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2006;48:1018–1026. doi: 10.1016/j.jacc.2006.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pletcher MJ, Tice JA, Pignone M, et al. Using the Coronary Artery Calcium Score to Predict Coronary Heart Disease Events: A Systematic Review and Meta-analysis. Archives of Internal Medicine. 2004;164:1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 19.Davies MR, Lund RJ, Hruska KA. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J Am Soc Neph. 2003;14:1559–1567. doi: 10.1097/01.asn.0000068404.57780.dd. [DOI] [PubMed] [Google Scholar]
- 20.Davies MR, Lund RJ, Mathew S, et al. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Neph. 2005;16:917–928. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 21.Mathew S, Lund R, Strebeck F, et al. Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol. 2007;18:122–130. doi: 10.1681/ASN.2006050490. [DOI] [PubMed] [Google Scholar]
- 22.Towler DA, Bidder M, Latifi T, et al. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 23.Demer LL. A skeleton in the atherosclerosis closet. Circulation. 1995;92:2029–2032. doi: 10.1161/01.cir.92.8.2029. [DOI] [PubMed] [Google Scholar]
- 24.Moe SM, Duan D, Doehle BP, et al. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 25.Fischer JW, Steitz SA, Johnson PY, et al. Decorin Promotes Aortic Smooth Muscle Cell Calcification and Colocalizes to Calcified Regions in Human Atherosclerotic Lesions. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:2391–2396. doi: 10.1161/01.ATV.0000147029.63303.28. [DOI] [PubMed] [Google Scholar]
- 26.Shanahan CM, Cary NRB, Metcalfe JC, et al. High expression of genes for calcification-regulating proteins in human atherosclerotic placques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2007.11.031. http://dx.doi.org/10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boström K, Watson KE, Horn S, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhore CR, Cleutjens J, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary LR, Hofmeister AM, Hruska KA. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone. 2004;34:402–411. doi: 10.1016/j.bone.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Mathew S, Tustison K, Sugatani T, et al. The mechanism of phosphorus as a cardiovascular risk factor in chronic kidney disease. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007070760. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:e10–e17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Yang HY, Giachelli CM. Role of the Sodium-Dependent Phosphate Cotransporter, Pit-1, in Vascular Smooth Muscle Cell Calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 34.Beck GR Jr., Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck GR Jr. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem. 2003;90:234–243. doi: 10.1002/jcb.10622. [DOI] [PubMed] [Google Scholar]
- 36.Villa-Bellosta R, Bogaert YE, Levi M, et al. Characterization of Phosphate Transport in Rat Vascular Smooth Muscle Cells: Implications for Vascular Calcification. Arterioscler Thromb Vasc Biol. 2007;27:1030–1036. doi: 10.1161/ATVBAHA.106.132266. [DOI] [PubMed] [Google Scholar]
- 37.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 38.Salusky IB, Ramirez JA, Oppenheim WL, et al. Biochemical markers of renal osteodystrophy in pediatric patients undergoing CAPD/CCPD. Kidney Int. 1994;45:253–258. doi: 10.1038/ki.1994.31. [DOI] [PubMed] [Google Scholar]
- 39.Hercz G, Pei Y, Greenwood C, et al. Aplastic osteodystrophy without aluminum: The role of “suppressed” parathyroid function. Kidney Int. 1993;44:860–866. doi: 10.1038/ki.1993.323. [DOI] [PubMed] [Google Scholar]
- 40.Salusky IB, Goodman WG, Kuizon BD. Implications of intermittent calcitriol therapy on growth and secondary hyperparthyroidism. Pediatr Nephrol. 2000;14:641–645. doi: 10.1007/s004670000352. [DOI] [PubMed] [Google Scholar]
- 41.Mathew S, Lund RJ, Strebeck F, et al. Effects of paricalcitol therapy in the adynamic bone disorder. 2005;16:32A. [Google Scholar]
- 42.Hendy GN, Hruska KA, Mathew S, et al. New insights into mineral and skeletal regulation by active forms of vitamin D. Kidney Int. 2006;69:218–223. doi: 10.1038/sj.ki.5000091. [DOI] [PubMed] [Google Scholar]
- 43.Panda DK, Miao D, Bolivar I, et al. Inactivation of the 25-hydroxyvitamin D 1a-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- 44.Wang M, Hercz G, Sherrard DJ, et al. Relationship between intact 1-84 parathyroid hormone and levels for bone turnover in patients on chronic maintenance dialysis. Am J Kidney Dis. 1995;26:836–844. doi: 10.1016/0272-6386(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 45.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham J, Sprague S, Cannata-Andia J, et al. Osteoporosis in chronic kidney disease. Am J Kidney Dis. 2004;43:566–571. doi: 10.1053/j.ajkd.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Stehman-Breen C. Osteoporosis and chronic kidney disease. Semin Nephrol. 2004;24:78–81. doi: 10.1053/j.semnephrol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Rix M, Andreassen H, Eskildsen P, et al. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56:1084–1093. doi: 10.1046/j.1523-1755.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 49.Bonyadi M, Waldman SD, Liu D, et al. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci USA. 2003;100:5840–5845. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stehman-Breen C. Bone Mineral Density Measurements in Dialysis Patients. Semin Dial. 2001;14:228–229. doi: 10.1046/j.1525-139x.2001.00057-2.x. [DOI] [PubMed] [Google Scholar]
- 51.Stehman-Breen C, Sherrard D, Walker A, et al. Racial differences in bone mineral density and bone loss among end-stage renal disease patients. Am J Kidney Dis. 1999;33:941–946. doi: 10.1016/s0272-6386(99)70430-0. [DOI] [PubMed] [Google Scholar]
- 52.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36:1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]