Abstract
Life expectancy in Italy is estimated to rise to 77.9 and 84.4 years in next years. Increased life expectancy is associated with a greater frailty of elderly people and an increased prevalence of chronic and degenerative illnesses such as cardiovascular diseases and osteoporosis. The impact of osteoporotic hip fractures in Italy is very similar to that of acute myocardial infarction (AMI), and there is a need for further epidemiological investigations concerning both the pathologies, as well as for a better understanding of possible mechanisms of their cosegregation. Actually, calcium metabolism is involved both in the development of osteoporosis and in the raise of cardiovascular risk. We have reviewed the most recent publications concerning epidemiological trends of both osteoporosis and acute myocardial infarction (AMI), and also the trials addressing cosegregation of these pathologies. According to the publications examined, in the Italian population (both ≥ 45 and > 65 years old), the number of hospitalizations following hip fracture and AMI are comparable. Both hip fractures and cardiovascular diseases represent in Italy a serious medical problem and a leading health cost driver, according to what has already been reported for many other Countries in the industrialized world, thus requiring a global clinical approach. Low calcium intake could represent one of the possible pathogenic paths underlining the association between hypertension and osteoporosis. Low calcium serum levels has been proved to enhance PTH and vitamin D3 production, which result in a remarkable lypogenesis performed by adypocites and switch on mechanisms leading to the raise of blood systolic pressure, the development of atherosclerotic plaques and cardiovascular events. Although many trials have suggested that bone mineral density may be included in the list of cardiovascular risk factors, more studies are needed in order to deeply investigate the causal relationships between calcium metabolism and cardiovascular diseases.
Keywords: osteoporosis, cardiovascular diseases, epidemiology
Introduction
Italy has one of the highest life expectancies in the world: according to the Italian National Institute for Statistics (ISTAT), life expectancy at birth increased at a rate of 4 months per year from 1950 to 2002, reaching 76.2 years for men and 82.6 years for women (1); life expectancy is estimated to rise to 77.9 and 84.4 years, respectively, by 2010 (1). High life expectancy combined with low natality has increased the elderly population, such that Italy represents an interesting international case study for aging-related diseases. In Italy, 18% of the population is actually over 65 years of age; within the next decade this age-group may exceed 22% of the population (2). Moreover, 4% of this group is already ≥ 80 years of age (1). Increased life expectancy is associated with a greater frailty of elderly people and an increased prevalence of chronic and degenerative diseases. Osteoporosis and its complications – especially hip fractures – represent a challenge for health professionals and decision makers in the twenty-first century. The World Health Organization (WHO) considers osteoporosis to be second only to cardiovascular diseases as a critical health problem (3). The Epidemiological Study on the Prevalence of Osteoporosis in Italy (ESOPO) reported a high prevalence of osteoporosis in 2000: 23% among all women, with age-specific rates ranging from 9% (40- to 49-year-olds) to 45% (70- to 79-year-olds), and almost 15% in men aged ≥ 60 years (4). Thus, we estimate that in Italy 4 million women and 800 thousand men are exposed to an increased risk of fracture that, as shown by several studies (5-12). The International Osteoporosis Foundation (IOF) estimated that, worldwide, hip fractures will occur in 18% of women and 6% of men, while 33% of women and 11% of men aged ≥ 80 years will experience a hip fracture due to osteoporosis (3). Hip fractures require a longer period of hospitalization than all other pathologies, with the only exception of psychiatric diseases (13). In developed countries, mortality from hip fractures is greater than that from gastric and pancreatic cancer; at the same time, the risk of experiencing a hip fracture is higher than that of developing breast, endometrial and ovarian cancer in women or prostatic cancer in men (3). The current mortality following hip fractures is similar to that for breast cancer, with a 5% acute mortality rate that increases to 15-25% within 1 year (2, 5, 14). Once hip fracture has occurred, the ability to walk is completely lost in 20% of cases, and only 30-40% of patients recover a degree of autonomy comparable to the period before the fracture (2, 14-18). Each year in Italy, almost 85,000 people suffer a hip fracture and 18,000 persons become completely disabled as a consequence of that (2). Therefore, the impact of hip fractures in Italy is very similar to that of acute myocardial infarction (AMI) as hospitalizations due to AMI are almost 110,000 per year (2), but there is a need for further epidemiological investigations concerning both the pathologies, as well as for a better understanding of possible mechanisms of their co-segregation.
Methods
We have reviewed the most recent publications concerning epidemiological trends of both osteoporosis and acute myocardial infarction (AMI), and also the trials addressing co-segregation of these pathologies. Actually, calcium metabolism is involved both in the development of osteoporosis and in the raise of cardiovascular risk. The studies selected in our metanalysis were the following:a) Piscitelli P., Iolascon G. et al. “Incidence and costs of hip fractures vs. acute myocardial infarction in the Italian population: a 4 years survey”, Osteoporosis International., 2007; 18: 211-219 (19);b) Varenna M. et al. “Unbalanced Diet to Lower Serum Cholesterol Level is a Risk Factor for Postmenopausal Osteoporosis and Distal Forearm Fracture”, Osteoporosis Int. 2001, 12: 296-301 (20);c) Kamycheva E et al., “Quartiles of serum PTH and serum Calcium as independent predictors of obesity in 4507 women aged 30 to 89 years”, Eur J Endocrinol, 2004 (21);d) Heaney RP et al., “Calcium and weight: clinical studies”, J Am Coll Nutr Vol. 21, No. 2, 152S155S, 2002 (22);e) Zemel MB, Shi H, Greer B, et al., “Regulation of adiposity by dietary calcium”, FASEB Journal 2000; 14:1132-1138 (23);f) Targher G. et al., “Early carotid atherosclerosis. Role of visceral fat accumulation”, Diabetes Care 2004; 27:2498-2500 (24);g) Kamycheva E et al., “Mean systolic blood pressure and daily calcium intake values by PTH quartiles in 1965 women aged 40-79 years”, Eur J Cardiovasc Prevention Rehab, 2004 (25);h) Zemel MB, “Regulation of adiposity and obesity risk by dietary calcium: mechanism and implications”, J Am Coll Nutr, J Am Coll Nutr, 2002, 21(2): 146S51S (26);i) Cappuccio FP, et al., High blood pressure and bone-mineral loss in elderly white women: a prospective study, Lancet, 354:971-75, 1999 (27);l) Browner WS, Pressman AR, Nevitt MC et al., “Association between low density and stroke in eldery women”, Stroke 1993; 24:940-46 (28);m) Mussolino ME et al., “Bone mineral density and mortality in women and men: the NHANES I epidemiologic follow-up study”, Ann Epidemiol 2003; 13:692-697 (29);n) Samelson EJ, “Metacarpal Cortical Area and Risk of Coronary Heart Disease”, The Framingham Study, Am J Epidemiol 2004; 159:589-595 (30);o) Jørgensen L et al., “Low bone mineral density is related to echogenic carotid artery plaques: a population-based study”, American Journal of Epidemiology 2004; 160:549-556 (31).
Results
According to the publications examined, in the Italian population (both ≥ 45 and > 65 years old), the number of hospitalizations following hip fracture and AMI are comparable (TableIandII) (19). Hip fractures Observed among adults aged ≥ 45 were 78,834 in 1999 and 86,719 in 2002, corresponding to an increase of 10.0% over 4 years (TableI). The majority of hip fractures occurred in patients ≥ 65 years of age or older (93.1%) and particularly in women (77.1%). Among women, 79.2% of fractures were experienced in patients ≥ 75 years of age. In the same population AMI resulted in the hospitalization of 86,100 patients in 1999 and 106,842 patients in 2002, corresponding to an increase of 24.0% over 4 years. The number of hospitalizations due to AMI in men approximates the number of hospitalizations for hip fractures in women; therefore, in 1999, AMI resulted only in 7,266 more hospitalizations than hip fractures (a difference of 9%); in the years 2000, 2001 and 2002, AMI resulted in 9,846 (11%), 13,425 (16%) and 20,123 (23%) additional hospitalizations, respectively, compared to hip fractures. Considering only elderly persons, direct costs of hospitalization for hip fractures were > 40% higher than those for AMI in each year analyzed. Overall direct costs sustained for the hospitalization of hip fractures and AMI in the period 19992002 were determined in the examined study as the sum of the weighted costs for each DRG. Direct costs of hip fractures were found to be higher and to grow faster than costs sustained for AMI (TableIII) (19).
Table I -.
Hospitalizations due to hip fractures (Italian hospitalization database 1999-2002) (19).
| Age | 1999 | 2000 | 2001 | 2002 | ||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| 45-64 | 2,520 | 3,633 | 2,527 | 3,259 | 2,550 | 3,584 | 2,510 | 3,405 |
| 65-74 | 3,573 | 9,854 | 3,611 | 9,589 | 3,716 | 9,618 | 3,715 | 9,879 |
| > 75 | 11,702 | 47,552 | 12,426 | 47,867 | 13,153 | 51,867 | 13,582 | 53,628 |
| Total | 17,795 | 61,039 | 18,564 | 60,715 | 19,419 | 65,069 | 19,807 | 66,912 |
Table II -.
Hospitalizations due to acute myocardial infarction (Italian hospitalization database 1999-2002) (19).
| Age | 1999 | 2000 | 2001 | 2002 | ||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| 45-64 | 25,220 | 14,646 | 25,357 | 14,765 | 27,359 | 15,279 | 28,594 | 15,673 |
| 65-74 | 17,519 | 17,838 | 17,996 | 17,925 | 18,909 | 18,637 | 20,109 | 19,149 |
| > 75 | 14,830 | 16,047 | 15,917 | 17,195 | 18,234 | 19,495 | 20,804 | 22,513 |
| Total | 57,569 | 28,531 | 59,240 | 29,885 | 64,502 | 33,411 | 69,507 | 37,335 |
Table III -.
Overall direct costs sustained for hospitalizations and rehabilitation following hip fractures vs. acute myocardial infarction in elderly Italian population (year 2002) (19).
| Hip fractures (2002) > 65 years old | Acute myocardial infarction (2002) > 65 years old | ||
|---|---|---|---|
| Hospitalization | 394,000,000 Euro | Hospitalization | 270,000,000 Euro |
| Rehabilitation | 335,000,000 Euro | Rehabilitation | 259,000,000 Euro |
| Direct costs (total) | 729,000,000 Euro | Direct costs (total) | 529,000,000 Euro |
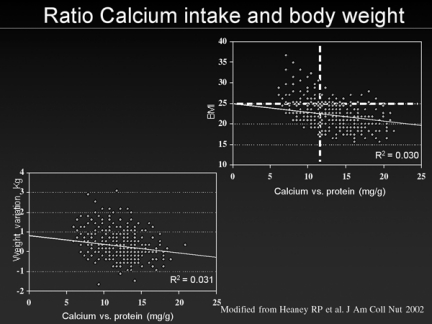
In the analysis of the selected clinical trials concerning co-segregation of osteoporosis and cardiovascular diseases, we found that women who were following diets with very low intake of milk and dairy products, in order to reduce their serum cholesterol levels, showed an increase in their body weight (20). These women also presented a higher prevalence of osteoporosis and their fractures’ incidence was almost the double than the value observed in a control group of women with no dietary restrictions. The difference between the two groups was influenced by the duration of the low cholesterol diet (20). Met-analyses of observational studies (21, 22) have demonstrated a reverse ratio between average daily calcium intake and body weight, as subjects with higher calcium intake tend to be slimmer than those people with dietary restriction (Fig.1). Actually, having abolished milk and dairy products from the diet results in a reduction both in cholesterol and calcium serum levels. As a consequence of that, the production of PTH rapidly increases, together with the serum levels of vitamin D3 (actually on their surfaces have been identified specific receptors for PTH and vitamin D3), thus unbalancing the physiologic equilibrium between lipolysis and lipogenesis inside the adipocytes. The final result of this process is the enhancement of fatty tissue formation (23), which has been demonstrated to increase cardiovascular risk, as visceral fat is responsible for the thickening of carotid walls, indicative of early atherosclerosis (24). Women with low serum calcium levels have higher serum PTH levels, and these values were found to be independent predictors of obesity in 4507 women aged 30 to 89 years in an international clinical trial (21).
Figure 1.
Correlation between calcium intake and body weight in Heaney RP et al. (22).
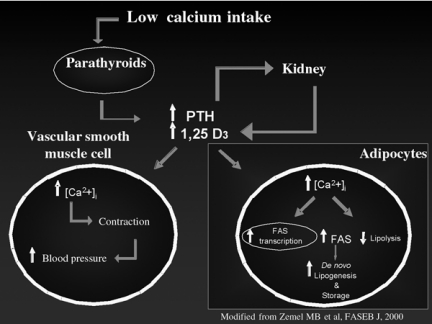
In addition to bone resorption and fatty tissue formation, the raise in PTH serum levels due to low calcium intake is also associated to an increase of systolic blood pressure. Actually, as observed for adypocites, also the arteriolar vascular muscle smooth cells have specific receptors for PTH and vitamin D3. The activity of these receptors due to the raise in PTH and vitamin D3 serum levels (following a low calcium intake) results in a higher contractility of arteriolar vessels (Fig.2), thus enhancing peripheral resistances and finally increasing systolic blood pressure of subjects with low calcium intake (25, 26). A study conducted on 1,965 women aged 40-79 years old has demonstrated that subjects with low calcium intake have both higher PTH serum levels and higher systolic blood pressure (25).
Figure 2.
Mechanisms leading to hypertension and lipogenesis enhancement following low calcium intake (23).
According to these data, there is a direct relationship between the raise of PTH serum levels and the increase in blood systolic pressure, with a reverse correlation with calcium intake (especially if the intake is < 400 mg/die) (25). Recent studies focusing on the correlation between hypertension and osteoporosis have observed that the incidence of hypertension and strokes is higher in people with low bone mineral density (27-29). A perspective study carried out on white women aged 6691 years old found that subjects having low bone mineral density were those included in the higher systolic blood pressure quartiles (27). The association between low bone mineral density and stroke has been suggested by a study conducted in elderly women, where the subjects included in the lowest calcaneus bone mineral density quartiles were associated to the highest stroke incidence rates (and possibly also with worse survival probabilities) (28). Moreover, in a 20 years follow up of 3501 patientis aged 45-74 from the U.S. NHANES I database (First National Health and Nutrition Examination Survey) it has been observed a significant reverse correlation between bone mineral density and mortality for all the causes (29). In a cohort of almost two-thousand patients from the Framingham study (1.236 women and 823 men aged 47-80 years old) monitored for 30 years, the incidence of coronary heart disease was higher in patients with worse values of metacarpal cortical area (30). Low bone mineral density seems also to have a reverse correlation with echogenic atherosclerotic carotid artery plaques, as confirmed by a cross-sectional population-based study carried out on 2.543 men and 2.726 post-menopausal women aged 55-74 years old (31).
Discussion
While BMD is a recognized predictor of osteoporotic fractures, it seems that this parameter should also be added to the our list of cardiovascular risk factors. The observation of a reverse correlation between daily calcium intake and body weight has been confirmed also in early post-menopausal women, in which the estrogens deficit could have a stronger role. Actually, the body weight of women with no more than 5 years of post-menopause and average daily calcium intake < 400 mg/die has been found to be higher than a control group where early post-menopausal subjects had a calcium intake > 750 mg/die (32). However, calcium metabolism and change in PTH serum levels have been demonstrated to be involved not only in lypogenesis performed by the adypocites, but also in the mechanisms leading to the raise of blood systolic pressure, the development of atherosclerotic plaques and cardiovascular events (24-31). Therefore, enhancing the bone mineral density could contribute to prevent cardiovascular diseases (30). American researchers have proposed to include the Coronary Artery Calcium Score (CACS) in the computation of the Framingham Risk Score (FRS), used for the evaluation of the risk of coronary heart disease (CHD) in order to improve our chance to predict cardiovascular events, at least in those patients whose 10-years FRS is >10% (33). Furthermore, determining both coronary artery calcium (CAC) and LDL serum levels has been proved to better predict the risk of first myocardial infarction than LDL evaluation alone, in patients receiving cholesterol-lowering therapies (34). According to these data, in order to assess the cardiovascular risk profile of individual patients, the attention of physicians should not exclude the evaluation of their calcium metabolism status and CAC in addition to LDL serum levels (34).
Conclusion
Both hip fractures and cardiovascular diseases represent in Italy a serious medical problem and a leading health cost driver, according to what has already been reported for many other Countries in the industrialized world, thus requiring a global clinical approach (19, 35-37). Low calcium intake could represent, even in post-menopausal women, one of the possible pathogenic paths underlining the association between hypertension and osteoporosis. Low calcium serum levels has been proved to enhance PTH and vitamin D3 production, which result in a remarkable lypogenesis performed by adypocites and switch on mechanisms leading to the raise of blood systolic pressure, the development of atherosclerotic plaques and cardiovascular events. Although many trials have suggested that bone mineral density may be included in the list of cardiovascular risk factors, more studies are needed in order to deeply investigate the causal relationships between calcium metabolism and cardiovascular diseases. Until indisputable data are available, all the measures aimed to enhance bone mineral density in individual patients should be limited to the prevention of osteoporotic fractures.
References
- 1.National Institute for Statistics; Rome: 2002. Annuario statistico italiano. [Google Scholar]
- 2.Italian Senate; Rome: 2002. Italian Senate Health Commission. Official Report of the Survey on Osteoporosis. [Google Scholar]
- 3.International Osteoporosis Foundation; Lyon; 2002. Osteoporosis in the European Community: a call for action. [Google Scholar]
- 4.Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 5.Adami S, Giannini S, Giorgino R, et al. The effect of age, weight, and lifestyle factors on calcaneal quantitative ultrasound:the ESOPO study. Osteoporos Int. 2003;14:198–207. doi: 10.1007/s00198-002-1352-5. [DOI] [PubMed] [Google Scholar]
- 6.Browner WS, Pressman AR, Nevitt MC, Cummings SR. Mortality following fractures in older women. The study of osteoporotic fractures. Arch Intern Med. 1996;156:1521–1525. [PubMed] [Google Scholar]
- 7.Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 8.Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European prospective Osteoporosis Study (EPOS) Osteoporos Int. 1998;8:291–297. doi: 10.1007/s001980050067. [DOI] [PubMed] [Google Scholar]
- 9.Pluijm SM, Tromp AM, Smit JH, et al. Consequences of vertebral deformities in older men and women. J Bone Miner Res. 2000;15(8):1564–1572. doi: 10.1359/jbmr.2000.15.8.1564. [DOI] [PubMed] [Google Scholar]
- 10.Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 11.Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women. Arch Intern Med. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 12.Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporosis Int. 2000;11:556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 13.Meyer HE, Tverdal A, Falch JA, Pedersen JI. Factors associated with mortality after hip fracture. Osteoporos Int. 2000;11:228–232. doi: 10.1007/s001980050285. [DOI] [PubMed] [Google Scholar]
- 14.Papaioannou A, Adachi JD, Parkinson W, et al. Lengthy hospitalization associated with vertebral fractures despite control for comorbid conditions. Osteoporos Int. 2001;12:870–874. doi: 10.1007/s001980170039. [DOI] [PubMed] [Google Scholar]
- 15.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248–1250. doi: 10.1136/bmj.307.6914.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagsten B, et al. Health-related quality of life and selfreported ability concerning ADL and IADL after hip fracture: a randomized trial. Acta Orthop. 2006;77(1):114–119. doi: 10.1080/17453670610045786. [DOI] [PubMed] [Google Scholar]
- 17.Di Monaco M, et al. Muscle mass and functional recovery in women with hip fracture. Am J Phys Med Rehabil. 2006;85(3):209–215. doi: 10.1097/01.phm.0000200387.01559.c0. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann S, et al. The lower extremity gain scale: a performance-based measure to assess recovery after hip fracture. Arch Phys Med Rehabil. 2006;87(3):430–436. doi: 10.1016/j.apmr.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Piscitelli P, Iolascon G, et al. Incidence and costs of hip fractures vs. acute myocardial infarction in the Italian population: a 4 years survey. Osteoporosis International. 2007;18:211–219. doi: 10.1007/s00198-006-0224-9. [DOI] [PubMed] [Google Scholar]
- 20.Varenna M, Binelli L, Zucchi F, Ghiringhelli D, Sinigaglia L. Unbalanced Diet to Lower Serum Cholesterol Level is a Risk Factor for Postmenopausal Osteoporosis and Distal Forearm Fracture. Osteoporosis Int. 2001;12:296–301. doi: 10.1007/s001980170119. [DOI] [PubMed] [Google Scholar]
- 21.Kamycheva E, et al. Quartiles of serum PTH and serum Calcium as independent predictors of obesity in 4507 women aged 30 to 89 years. Eur J Endocrinol. 2004 [Google Scholar]
- 22.Heaney RP, et al. Calcium and weight: clinical studies. J Am Coll Nutr. 2002;21(2):152S–155S. doi: 10.1080/07315724.2002.10719213. [DOI] [PubMed] [Google Scholar]
- 23.Zemel MB, Shi H, Greer B, et al. Regulation of adiposity by dietary calcium. FASEB Journal. 2000;14:1132–1138. [PubMed] [Google Scholar]
- 24.Targher G, et al. Early carotid atherosclerosis in healthy men. role of visceral fat accumulation. Diabetes Care. 2004;27:2498–2500. doi: 10.2337/diacare.27.10.2498. [DOI] [PubMed] [Google Scholar]
- 25.Kamycheva E, et al. Mean systolic blood pressure and daily calcium intake values by PTH quartiles in 1965 women aged 40-79 years. Eur J Cardiovasc Prevention Rehab. 2004 [Google Scholar]
- 26.Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanism and implications. J Am Coll Nutr. 2002;21(2):146S–51S. doi: 10.1080/07315724.2002.10719212. [DOI] [PubMed] [Google Scholar]
- 27.Cappuccio FP, et al. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Lancet. 1999;354:971–75. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- 28.Browner WS, Pressman AR, Nevitt MC, et al. Association between low density and stroke in eldery women. Stroke. 1993;24:940–46. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- 29.Mussolino ME, et al. Bone mineral density and mortality in women and men: the NHANES I epidemiologic follow-up study. Ann Epidemiol. 2003;13:692–697. doi: 10.1016/s1047-2797(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 30.Samelson EJ. Metacarpal Cortical Area and Risk of Coronary Heart Disease, The Framingham Study. Am J Epidemiol. 2004;159:589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- 31.Jørgensen L, et al. Low bone mineral density is related to echogeniccarotid artery plaques: a population-based study. American Journal of Epidemiology. 2004;160:549–556. doi: 10.1093/aje/kwh252. [DOI] [PubMed] [Google Scholar]
- 32.Varenna M, Binelli L, Zucchi F, Casari S, Sinigaglia L. Role of dietary calcium intake in influencing body weight, bone mass and prevalence of osteoporosis in early postmenopausal women. 5th International Symposium on Nutritional Aspects of Osteoporosis; Lausanne. May 14-17 2003. [Google Scholar]
- 33.Greenland P, et al. Coronary artery calcium score combined with framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 34.Raggi P., et al. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24:1272–1277. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 35.Piscitelli P, et al. Incidence and costs of hip fractures vs. acute myocardial infarction in the population of Local Health Authorities ASL Lecce/1 and ASL Lecce/2: a 2 years survey. Italian Journal of Public Health Year 4. 2006;3(2):75–77. [Google Scholar]
- 36.Piscitelli P, Iolascon G, Gimigliano R, Guida G, et al. Femoral fractures and orthopaedic surgery: a four years survey in Italy. Journal of Orthopaedics and Traumatology. 2005;6:203–206. [Google Scholar]
- 37.Piscitelli P, et al. Disease management approach for osteoporosis: potential advantages for the incidence and costs of hip fractures in Italy if adopting a global clinical approach. Journal of Bone and Joint Surgery - British Volume. 87-B((Suppl II)):197. [Google Scholar]