Abstract
Bone remodelling is an active and dynamic process that relies on the correct balance between bone resorption by osteoclasts and bone deposition by osteoblasts. Moreover, these two functions must be tightly coupled not only quantitatively, but also in time and space. When the coupling is lost, the correct bone mass could be compromised, leading to several skeletal pathologies. Indeed, bone loss and osteoporosis are the result of an increased osteoclast function and/or a reduced osteoblast activity. In contrast, other pathologies are related to osteoclast failure to resorbe bone, such as osteopetrosis, a rare genetic disorder characterized by an increased bone mass and also linked to an impairment of bone marrow functions. Starting from these assumptions, it is necessary to more deeply understand the molecular mechanisms regulating bone cell functions. Indeed, recent studies evidenced a complex interplay between the immune and skeletal systems, which share several regulatory molecules including cytokines, receptors and transcription factors. These data allowed to more deeply understand the mechanisms underlying bone mass regulation and could open new avenue to identify target molecules for alterantive therapies more efficacious against bone diseases.
Keywords: osteoclast, osteoblast, bone remodelling, osteoimmunology
The bone remodelling process
Bone is a dynamic tissue, subjected to a continuous renewing during the life of each individual by the process of bone remodelling (1, 2). This physiological process is necessary:
-
-
to allow the substitution of primary bone, the infantile bone, with secondary bone which is more mechanically competent;
-
-
to remove ischemic or microfractured bone;
-
-
to guarantee a correct calcium homeostasis.
Bone remodelling relies on the correct function of two principal cells of the bone tissue: the osteoclasts, multinucleated cells that destroy the bone matrix, and the osteoblasts, having osteogenic functions. The osteocytes, another important cell type arising from the osteoblasts, are also involved in the remodelling process as they have a mechano-sensorial function (3).
A correct balance between bone resorption and osteogenic functions is mandatory to maintain a constant bone mass (1, 2).
As summarized in Figure1, bone remodelling is accomplished according to the following phases.
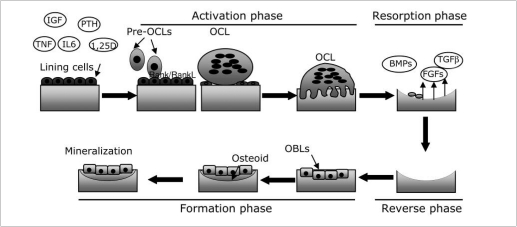
Figure 1.
Schematic representation of the bone remodelling process. Bone remodelling starts when different inputs led to activation of lining cells, which increase surface expression of RANKL. RANKL interacts with its receptor RANK (Receptor Activator of Nuclear κB) thus triggering osteoclast differentiation (Activation phase). Osteoclasts resorbe bone (Resorption Phase) thus allowing the release of factors usually stored in the bone matrix (BMPs, TGFβ, FGFs) that recruit osteoblasts in the reabsorbed area. Once recruited, osteoblasts produce the new bone matrix, and promote its mineralization (Formation phase), thus completing the bone remodelling process (Pre-OCLs = pre-osteoclasts; OCL = osteoclast; OBLs = osteoblasts).
Activation phase. Different inputs, such as a micro-fracture, an alteration of mechanical loading sensed by the osteocytes or some factors released in the bone microenvironment, including insulin growth factor-I (IGFI), tumour necrosis factor-α (TNF-α), parathyroid hormone (PTH) and interleukin-6 (IL-6), activate the lining cells which are quiescent osteoblasts. As a consequence, lining cells, increase their own surface expression of RANKL (Receptor Activator of Nuclear κB Ligand), which in turn interacts with its receptor RANK (Receptor Activator of Nuclear κB), expressed by pre-osteoclasts. RANKL/ RANK interaction triggers pre-osteoclasts fusion and differentiation toward multinucleated osteoclasts.
Resorption phase. Once differentiated, osteoclasts polarize, adhere to the bone surface and begin to dissolve bone. This function requires two steps: i) acidification of the bone matrix to dissolve the inorganic component, and ii) release of lysosomial enzymes, such as cathepins K, and of MMP9, both in charge for the degradation of the organic component of bone. Once accomplished their function, osteoclasts undergo to apoptosis. This is a physiological consequence needed to avoid an excessive bone resorption.
Reverse phase. The reverse cells, whose role has not been yet completely clarified, perform this phase. Indeed, it is known that they are macrophage-like cells with a likely function of removal of debris produced during matrix degradation.
Formation phase. Bone matrix resorption leads to the release of several growth factors herein stored, including bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs) and transforming growth factor β (TGF β), which are likely responsible for the recruitment of the osteoblasts in the reabsorbed area. Once recruited, osteoblasts produce the new bone matrix, initially not calcified (osteoid) and then they promote its mineralization, thus completing the bone remodelling process. Unbalance between the resorption and formation phases mirror an incorrect bone remodelling, which in turn affects the bone mass, eventually leading to a pathological condition.
The bone remodelling players
To more deeply understand the causes of an altered bone remodelling it is necessary to know the mechanisms underlying the biology and function of bone cells. As already mentioned, two principal bone cells actively attend the bone remodelling, that is the osteoblasts and the osteoclasts, and an overview of their regulation and function will be done.
Regulation and function of osteoblasts
Osteoblastogenesis
Osteoblasts arise from mesenchymal stem cells (MSCs), which are pluripotent cells that following a specific program of gene expression may give rise to different tissue specific cells including osteoblasts, chondrocytes, fibroblasts, myocytes and adipocytes (4, 5).
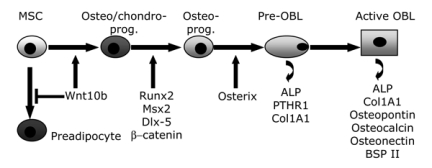
The initial step of osteoblastogenesis is the commitment of MSCs towards an osteo/chondro-progenitor (Figure2). As described in more detail later, the Wingless-int (Wnt) pathway and the BMPs play a key role in these early events. Indeed, a recent report (6) showed that Wnt10b not only shifts the commitment towards an osteo/chondro progenitor, but also inhibits preadipocyte commitment (Figure2). This is due to the suppression of the adipogenic transcription factors CCAAT enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) along with to an induction of transcription factors Runt-related transcription factor 2 (Runx2), distal-less homeobox 5 (Dlx5), and Osterix (Osx), the latter downstream of Runx2 (7). Conversely, high levels of Wnt signalling with the presence of Runx2 promote osteoblastogenesis at the expense of chondrocyte differentiation (8). Committed pre-osteoblasts are identifiable as they express Alkaline Phosphatase (ALP), one of the earliest markers of osteoblast phenotype. As the pre-osteoblasts cease to proliferate, a key signalling event occurs for development of the large cuboidal differentiated osteoblasts. The active osteoblast is highly enriched in ALP and secrete bone matrix proteins such as collagen I and several non-collagenous proteins including osteocalcin, osteopontin, osteonectin and bone sialoprotein II (BSPII). As a rule, ALP and the type 1 parathyroid receptor (PTH1R) are early markers of osteoblast progenitors that increase as osteoblasts mature and deposit matrix, but decline again as osteoblasts become osteocytes, whereas osteocalcin is a late marker that is up-regulated only in post-proliferative mature osteoblasts associated with mineralized osteoid (9).
Figure 2.
Schematic picture of the osteoblastogenesis process. Osteoblasts (OBL) arise from mesenchymal stem cells (MSC) which under proper stimuli are committed toward an osteo/chondro-progenitor (osteo/chondro-prog.), followed by an osteoprogenitor cell (Osteo-prog.), a pre-osteoblast (pre-OBL) expressing alkaline phosphatase (ALP) and an active, mature osteoblast which secretes bone matrix proteins.
a) Osteoblast regulation
A correct osteoblastogenesis relies on the activation of a complex network of pathways whose alteration causes several skeletal pathologies. In the next paragraphs we will focus on some of the principal mechanisms of osteoblast regulation.
Runt-related transcription factor 2 (Runx2). This transcription factor plays a key role in skeletal development as it is a master gene for osteoblast differentiation, driving the early steps of mesenchymal commitment toward the pre-osteoblast phenotype (10-12). Indeed, in Runx2 null mice lack of osteoblast differentiation results in the absence of bone formation, and chondrocytes of cartilages templates fail to undergo hypertrophy, while overexpression of a dominant-negative form of Runx2 in osteoblasts inhibits bone formation (13). Interestingly, Runx2 overexpression also leads to osteopenia, thus indicating that this factor at inappropriately high levels can inhibit the process of osteoblast maturation (14). In humans, haploinsufficiency of Runx2 causes cleidocranial dysplasia (CCD), an autosomal-dominant disease with abnormalities in bones formed by intramembranous ossification (15, 16).
Among the molecules able to regulate Runx2, BMPs, TGF β, PTH and FGFs promote its activation, while the transcription factor Twist is a negative regulator (17).
Osterix (Osx). This factor is downstream of Runx2 and, like the latter, is necessary for skeletal formation (18). To accomplish this function Osx needs to interact with activated NFAT2 (19).
Wnt/β-catenin signalling. Recent reports evidenced a pivotal role of this pathway in bone biology (20, 21). Indeed, the great interest for Wnt signalling in bone field came after the discovery that loss and gain-of-function mutations in the Low-density lipoprotein receptor-related protein 5 (LRP5), a putative Wnt co-receptor, led to the osteoporosis-pseudoglioma syndrome (22) and to high bone mass (HBM) (23, 24) respectively in humans. LRP5 is a transmembrane receptor, which interacts with the frizzled receptor. Binding of Wnt to frizzled and LRP5/6 receptor complex triggers a signal involving the proteins Disheveled (Dvl), Axin and Frat-1, thus inhibiting the activity of the Glycogen synthase kinase 3β (GSK3β) (25). This inhibition prevents β-catenin phosphorylation. Indeed, hypophosporylated β-catenin is more stable, thus accumulating in the cytoplasm. Upon reaching a certain concentration level, β-catenin translocates to the nucleus where it interacts with the Tcf/Lef family of transcription factors to regulate the expression of Wnt target genes. In contrast, in the absence of Wnt, GSK3β phosphorylates β-catenin, thus targeting the protein to proteasome ubiquitination (26, 27).
Wnt signalling is subjected to a fine tune regulation by several factors. Among them, the members of the secreted frizzled-related protein (sFRP) family and Wnt inhibitory factor 1 (Wif-1). These molecules are soluble decoy frizzled receptors that prevent interactions between Wnt and frizzled. A second group of inhibitors includes dickkopf (Dkk) and sclerostin (Sost) proteins, which bind to LRP5/6 receptors. Moreover, interaction of the Dkk/LRP complex with kremen internalises the complex for degradation, thus reducing the availability of Wnt receptors (28).
Bone Morphogenetic Proteins (BMPs) Except for BMP-1, all these proteins belong to the TGF-β superfamily. Identification of skeletal abnormalities in animals and patients with mutations in the BMP genes has highlighted the role of these proteins in bone metabolism (29-31). In vitro studies demonstrated that treatment with BMPs enhances the expression of ALP, parathyroid hormone related peptide (PTHrP) receptor type I, collagen I and osteocalcin (32) and stimulated the formation of mineralized bone-like nodules (33).
b) Osteoblast function
As already described, the principal function of the osteoblasts is to synthesize the proteins of the bone matrix and to attend the process of calcification. Indeed, several evidence reported a crucial role of osteoblasts in osteoclast biology by expressing and/or secreting key molecules that in turn regulate osteoclastogenesis and bone resorption (1). This latter issue will be showed in detail in the next paragraphs.
Regulation and function of osteoclasts
Osteoclastogenesis
Osteoclasts, the cells devoted to resorb the bone matrix, arise from the monocyte/macrophage lineage (34). They are multinucleated cells (from four up to twenty nuclei) formed by the fusion of mononuclear precursors (35). Starting from totipotent heamatopoietic stem cells, the transcription factor PU.1, along with the macrophage colony stimulating factor (M-CSF) drive the commitment of a common progenitor for macrophages and osteoclasts. In particular, M-CSF stimulates proliferation of osteoclast precursors and upregulates RANK expression, while PU.1 positively regulates the transcription of c-Fms, the M-CSF receptor (36). With the appearance of c-Fms and RANK receptors, the precursors become fully committed to an osteoclast lineage. RANKL pathway is mandatory for osteoclast differentiation and function, although it is not the only player for a correct osteoclastogenesis, as described below.
a) Osteoclast regulation
RANKL pathway. RANKL is a type II membrane protein belonging to the TNF superfamily, while its receptor RANK is a type I membrane protein. Osteotropic hormones and factors such as 1,25-dihydroxyvitamin D3(1,25(OH)2D3), PTH, prostaglandin E2 (PGE2) and IL-11 up-regulate the expression of RANKL on membrane surface of osteoblasts/stromal cells. RANKL interacts with its receptor RANK, located on the pre-osteoclast surface, which in turn activates the signalling by recruiting adaptor molecules belonging to the TNF-receptor associated factors (TRAF) family (Figure3A). Indeed, RANK cytoplasmic tail contains three binding sites for TRAF6 (37-39). This interaction is mandatory for osteoclast differentiation, as TRAF6 knock out mice develop osteopetrosis (40,41). The binding of TRAF6 to RANK induces the trimerization of TRAF6, leading to the activation of the transcription factor nuclear factor kappaB (NF-κB) and of the mitogen-activated kinases (MAPKs) (42) (Figure3A). NF-κB includes a family of dimeric transcription factors, which reside in the cytoplasm in non-stimulated conditions. However, they enter nucleus upon cell stimulation of various factors, including RANKL (43,44) and regulate transcription of several genes. Among them, it has been demonstrated that NFkB up-regulates the expression of another key molecule of osteoclast differentiation, that is the nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) transcription factor (45,46). This initial induction requires the interaction of NF-κB with NFATc2, which is recruited to the NFATc1 promoter independently of RANKL stimulation (47) (Figure3A).
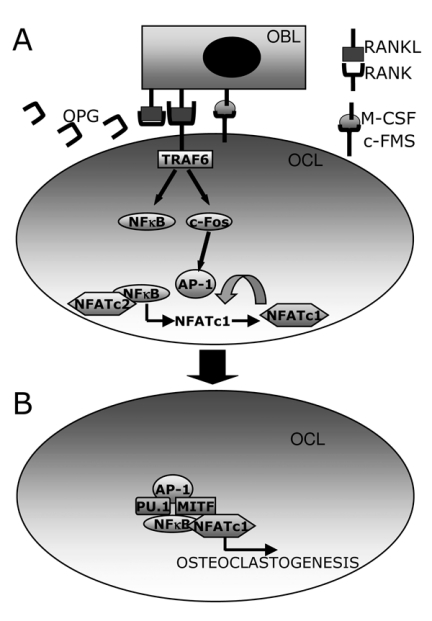
Figure 3.
Schematization of the RANKL/RANK pathway. (A) RANKL expressed on the membrane surface of osteoblasts (OBL) interacts with RANK, expressed by pre-osteoclasts (OCL). This interaction recruits TRAF6 that activates NFκB and c-Fos, the latter dimerizing with c-Jun and forming the AP-1 complex. Both transcription factors cooperate to trigger transcription of NFATc1, which in turn promote its autoamplification. (B) NFATc1, AP-1, PU.1 and MITF cooperate to induce osteclast genes transcription.
Another crucial step for osteoclasts differentiation is the recruitment of the transcription factor complex AP-1, which is composed of the c-Fos, c-Jun and ATF proteins. In particular, c-Fos is specifically induced by RANK and is critical for osteoclastogenesis, as knock out c-Fos mice develop osteopetrosis due to the lack of osteoclasts (48). AP-1 activation along with a calcium-signal further induces NFATc1 transcription, thus allowing its autoamplification (47). In cooperation with AP-1, PU.1, NF-κB and MITF, NFATc1 regulates the transcription of several target genes involved in osteoclast differentiation and function. Among them, cathespin K, calcitonin receptor, tartrate-resistant acid phosphatase (TRAcP) (49,50), β3 integrin and osteoclast-associated receptor (OSCAR) (51) (Figure3B).
Osteoclast regulation by osteoblasts. The principal regulators of osteoclastogenesis are the osteoblasts. Indeed, the RANKL/ RANK signalling relies on the cell-cell interaction between the osteoblasts and the osteoclast precursors. Another key molecule secreted by osteoblasts which interfere with the RANKL pathway is osteoprotegerin (OPG) a decoy receptor for RANKL (52), which has an osteoprotective role. Indeed, OPG is a secreted protein having the same structure of RANK so that it binds to RANKL avoiding its interaction with RANK, with a consequent inhibition of osteoclastogenesis (Figure3A).
Osteoblasts trigger osteoclastogenesis also by expressing on their membrane surface the M-CSF, which interacts with its receptor c-Fms present on osteoclast precursors thus stimulating their proliferation and osteoclast differentiation (Figure3A).
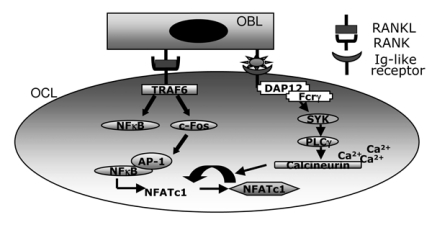
Osteoclast regulation by immune system. Several evidence has indicated a tight relationship between the immune system and bone, leading to a new interdisciplinary field, called osteoimmunology, focused to investigate the molecular mechanisms shared by these two tissues (53-55). These findings also point out that the RANKL pathway is necessary but not sufficient to trigger osteoclast differentiation. As described in Figure4, osteoblasts can regulate osteoclast differentiation by interacting with immunoglobulin (Ig)-like receptors, such as the osteoclast-associated receptor (OSCAR), whose ligand has not yet clearly identified. These receptors are associated with immunoreceptor tyrosine-based activation motif (ITAM)-harbouring adaptor molecules DAP12 and Fc-receptor common γ-sub-unit (FcRg). The role of the latter molecules in osteoclast regulation has been underlined by the evidence that mice deficient in both DAP12 and FcRγ have an osteotropic phenotype (56, 57). Phosphorylation of the ITAM sequence in DAP12 or FcRγ, resulting after RANK activation, allows the recruitment of splenocyte tyrosine kinases (SYK) and the consequent activation of the phospholypase Cγ (PLCγ), which in turn triggers calcium signalling. Calcium signalling promotes osteoclastogenesis by activating the CAMKIV (calcium/calmodulin-dependent protein kinase type IV), which concurs to c-Fos activation, and calcineurin, both cooperating to potentiate NFATc1 auto-amplification (53).
Figure 4.
Schematization of the immunological interplay between osteoclasts and osteoblasts. Osteoblast (OBL) interacts whit Immunoglobulin-like receptor (Ig-like receptor) expressed on pre-osteoclast (OCL) surface, thus allowing the phosphorylation of Dap12 or Fc Schematization of the immunological interplay between osteoclasts and osteoblasts. Osteoblast (OBL) interacts whit Immunoglobulin-like receptor (Ig-like receptor) expressed on pre-osteoclast (OCL) surface, thus allowing the phosphorylation of Dap12 or Fcrg and the subsequent activa-tion of the calcium signalling, which potentiates autoamplification of NFATc1.
Inflammatory cytokines and osteoclastogenesis. Several findings have demonstrated the involvement of some inflammatory cytokines produced by macrophages, such as interleukin (IL)1, TNFα and IL-6, in osteoclast differentiation and function (58, 44) thus further supporting the tight relationship between bone and immune system.
TNFα is able to directly stimulate osteoclastogenesis in the presence of M-CSF (59, 60) by stimulating the activation of NFκB mainly through TRAF2. Moreover, along with TGFβ it induces in vitro osteoclastogenesis even in the absence of RANK or TRAF6 (61).
IL-1 is not able alone to induce osteoclast differentiation, but it synergizes with RANKL to induce osteoclastogenesis and bone resorption, probably by stimulating TRAF6. Moreover, it indirectly promotes osteoclastogenesis by stimulating osteoblast secretion of PGE2 and RANKL (44).
Interferon-β (IFN-β) is another cytokine crucial in immune response that negatively affects osteoclatogenesis. Indeed, RANKL signalling induces IFN-β that in turn acts as a negative feedback regulator by inhibiting cFos expression (62).
b) Osteoclast function
Once differentiated, multinucleated osteoclasts need to adhere to the bone matrix and to polarize in order to resorb bone. Indeed, two principal domains can be identified on the osteoclast plasma membrane: the basolateral and the apical domains, which also differ for their function. In the apical domain, it is possible to identify a further membrane specialization, that is the ruffled border, characterized by several folding of the membrane and representing the resorbing organ (51).
One of the earliest events of osteoclast activity is to degrade the inorganic component of the bone matrix, that is the alkaline salts of bone mineral hydroxyapatite. This can be obtained by the release of propons into the area to be resorbed, called resorption lacuna (63, 64). Furthermore, this function also requires sealing the underlined bone matrix, which is obtained through a cytoskeletal rearrangement and the subsequent formation of the actin ring. This is a circumferential structure that surrounds the ruffled membrane and isolates the acidified resorptive microenvironment from the extracellular space. It is formed by several dynamic and dot-like structures called podosomes, each of them consisting of an actin core surrounded by the αvβ3integrin and associated cytoskeletal proteins such as vinculin, α-actinin and talin (65, 66).
As already told, the first step of bone resorption is the release of protons in the compartment between the osteoclast and the bone surface via an electrogenic proton pomp called vacuolar type ATPase (67, 68), which is present both in intracellular vesicles as well as in the ruffled border (67-69). This is a crucial step, as demonstrated by the fact that mutations in the a3 subunit of vacuolar ATPase cause osteopetrosis in humans (70, 71).
The production of protons is ensured by the activity of the carbonic anhydrase II (CA II) (72) which catalyses hydration of CO2thus forming carbonic acid (H2CO3). H2CO3in turn dissociates to protons (H+) and bicarbonate ions (HCO3–). H+are then secreted in the resorption lacuna, while HCO3–are extruded via an electroneutral chloride/bicarbonate exchanger in the basolateral membrane (73). Moreover, the chloride ion (Cl–) that enters the cell in exchange of HCO3- are transported into the resorptive microenvironment through a chloride channel (74), thus generating HCl (75). The functional importance of chloride channels was confirmed by the evidence that loss of the chloride channel isoform CIC-7 leads to osteopetrosis in human and mice (76).
Dissolution of mineral crystals allows the digestion of the bone matrix organic component that is performed by matrix metalloproteinases (MMPs) and lysosomial cathepsins. Among the latter, cathepsin K has a crucial role, as its deletion in mice leads to osteopetrosis (77, 78), while mutations in cathepsin K gene lead to pycnodysostosis (79, 80). As far as the MMPs is concerned, osteoclasts mainly produce the MMP-9 isoform and to a lesser extent the MMP-14 (81, 82).
Diseases associated to deregulation of osteoclasts
The correct balance between bone deposition and resorption is crucial for the proper maintenance of the bone mass. Moreover, a complex network of pathways attends the regulation of osteoclasts and osteoblasts activity, as demonstrated in the above paragraphs. Among the bone pathologies, we will briefly describe two opposite diseases, both due to an abnormal osteoclast function. These diseses also mirror the complexity of the mechanisms involved in bone mass regulation.
Rheumatoid arthritis
It is well known that this pathology is due to an inflammation of the synovial membrane with a subsequent destruction of the bone mediated by the osteoclasts (83). However the molecular mechanisms that induce abnormal osteoclast activation have only recently been clarified (53). Cells in the synovium include macrophages, fibroblasts, dendritic cells, plasma cells and most importantly, infiltrated CD4+T cells, which are a hallmark of the pathogenesis of arthritis (84, 85). Recent reports show that among the latter cells a specific subset, the interleukin-17 (IL17)-producing T helper cells (TH17 cells) have a crucial role in osteoclast activation (55). These cells do not produce IFNγ, which has an anti osteoclastogenesis activity, while they secrete large amounts of IL-17 that stimulates the expression of RANKL by synovial fibroblasts. IL-17 also acts on macrophages by stimulating their secretion of inflammatory cytokines including TNF, IL-1 and IL-6, which in turn trigger osteoclastogenesis and bone resorption directly or indirectly by stimulating expression of RANKL. Finally, TH17 cells per se express RANKL (55,86).
Osteopetrosis
Osteopetrosis is a rare genetic disease characterized by an increase of the bone mass due to an inability of osteoclasts to resorb bone (87). According to the way of transmission and to the clinical manifestations, it can be classified into three forms with a wide range of severity: infantile malignant autosomal recessive osteopetrosis (ARO), intermediate autosomal recessive osteopetrosis (IRO), and autosomal dominant osteopetrosis (ADO). In the most severe forms, osteoclast failure does not allows enlargement of bone cavities, thus impairing development of bone marrow, leading to hematological failure. Closure of bone foramina causes cranial nerve compression with visual and hearing deterioration. Patients also present with osteosclerosis, short stature, malformations and brittle bones. This form is fatal in infancy, and is cured with hematopoietic stem cell transplantation, with a rate of success <50% and unsatisfactory rescue of growth and visual deterioration (87).
Loss-of-function mutations of various genes involved in osteoclast function are responsible for this disease. Among them, the TCIRG1 gene, which encode for the a3 subunit of the H+ATPase and accounting for >50% of cases (70, 71), the CLC7 (76, 88) and the OSTM1 genes, which have closely related function and account for approximately 10% of cases89-91). Further genes are implicated in rare forms with various severities and association with other syndromes and, recently, the RANKL gene was found to be mutated in a subset of patients lacking osteoclasts (92). Autosomal recessive osteopetrosis may also have intermediate severity, with a small number of cases due to loss-of-function mutations of the CAII (93) or the PLEKHM1 genes (94). Dominant negative mutations of the CLC7 gene cause the so-called Albers-Schönberg disease (95), which represents the most frequent and heterogeneous form of osteopetrosis, ranging from asymptomatic to intermediate/severe, thus suggesting additional genetic/environmental determinants affecting penetrance (96).
References
- 1.Zaidi M. Skeletal remodelling in health and disease. Nature Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MM. The new bone biology: pathologic, molecular and clinical correlates. Am J Med Genetics. 2006;140A:2646–2706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 3. Nijweide PJ, Burger EH, Klein Nulend J, et al. The osteocyte In: Bilezikian JP, Raisz LG, Rodan GA eds. Principles of bone biology London UK: Academic Press; 1996. 115 126 [Google Scholar]
- 4.Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi A, Khan AJ. Clonal osteogenic cell lines express myogenic and adipocytic developmental potential. Calcif Tissue Int. 1991;49:221–225. doi: 10.1007/BF02556122. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt 10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signalling promotes osteogenesis by directly stimulating RUNX2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 8.Glass DA II, Karsenty G. Minireview: In vivo analysis of Wnt signalling in bone. Endocrinology. 2007;148:2630–2634. doi: 10.1210/en.2006-1372. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458–466. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 10.Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 11.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 12.Olsen BR, Reginato AM, Wang W. Bone development. Ann Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 13.Ducy P, Starbuck M, Priemel M, et al. Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes and Development. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Toyosawa S, Furuichi T, et al. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001;155:157–166. doi: 10.1083/jcb.200105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B, Thirunavukkarasu K, Zhou L, et al. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 16.Mundlos S, Olsen BR. Heritable diseases of the skeleton. Part II: molecular insights into skeletal development-matrix components and their homeostasis. FASEB J. 1997;11:227–233. [PubMed] [Google Scholar]
- 17.Kaneki H, Guo R, Chen D, et al. TNF promotes RUNX2 degradation through up-regulation of SMURF1 and SMURF2 in osteoblasts. J Biol Chem. 2006;281:4326–4333. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 19.Koga T, Matsui Y, Asagiri M, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11:880–885. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 20.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signalling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Rawadi G, Roman-Roman S. Wnt signalling pathways: a new target for the treatment of osteoporosis. Exp Opin Ther Targets. 2005;9:1063–1077. doi: 10.1517/14728222.9.5.1063. [DOI] [PubMed] [Google Scholar]
- 22.Gong Y, Slee RB, Fuki N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 23.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. New Engl J Medicine. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 24.Van Wesenbeeck L, Cleiren E, Gram J, et al. Six novel missense mutations in the LDL receptor-related proteins (LRP5) gene in different conditions with increased bone density. Am J Human Genet. 2003;72:763–771. doi: 10.1086/368277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clevers H. Wnt/b-catenin signalling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Hay E, Faucheu C, Suc-Royer I, et al. Interaction between LRP5 and Frat1 mediates the activation of the Wnt canonical pathway. J Biol Chem. 2005;280:13616–13623. doi: 10.1074/jbc.M411999200. [DOI] [PubMed] [Google Scholar]
- 27.Baron R, Rawadi G. Minireview: targeting the Wnt/β-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 28.Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 29.Kingsley DM, Bland AE, Grubber JM, et al. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- 30.Storm EE, Huynh TV, Copeland NG, et al. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JT, Kilpatrick MW, Lin K, et al. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet. 1997;17:58–64. doi: 10.1038/ng0997-58. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita T, Ishii H, Shimoda K, et al. Subcloning of three osteoblastic cell lines with distinct differentiation phenotypes from the mouse osteoblastic cell line KS-4. Bone. 1996;19:429–436. doi: 10.1016/s8756-3282(96)00255-4. [DOI] [PubMed] [Google Scholar]
- 34.Walker DG. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975;190:784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- 35.Udagawa N, Takahashi N, Akatsu T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci. USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arai F, Miyamoto T, Ohneda O, et al. Commitment and differentiation of osteoclast precursor cells by sequential expression of c-Fms and receptor activator of nuclear kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galibert L, Tometsko ME, Anderson DM, et al. The involvement of multiple tumour necrosis factor receptor (TNFR)-associated factors in the signalling mechanisms of receptor activator of NF-κB, a member of the TNFR superfamily. J Biol Chem. 1998;273:34120–34127. doi: 10.1074/jbc.273.51.34120. [DOI] [PubMed] [Google Scholar]
- 38.Darnay BG, NI J, Moore PA, et al. Activation of NF-κB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and activation of NF-κB inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem. 1999;274:7724–7731. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- 39.Gohda J, Akiyama T, Koga T, et al. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005;24:790–799. doi: 10.1038/sj.emboj.7600564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lomaga MA, Yeh WC, Sarosi I, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signalling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naito A, Azuma S, Tanaka S, et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4:353–362. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi N, Kadono Y, Naito A, et al. Segregation of TRAF6-mediated signalling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takayanagi H, Ogasawara K, Hida S, et al. T cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 44.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Matsuo K, Xing L, et al. Over-expression of activated NFATc1 plus RANKL rescues the osteoclastogenesis defect of NF-κB p50/p52 double knockout splenocytes. J Bone Miner Res. 2004;19:S2. [Google Scholar]
- 46.Takatsuna H, Asagiri M, Kubota T, et al. Inhibition of RANKL-induced osteoclastogenesis by (-)-DHMEQ, a novel NF-κB inhibitor, through downregulation of NFATc1. J Bone Miner Res. 2005;20:653–662. doi: 10.1359/JBMR.041213. [DOI] [PubMed] [Google Scholar]
- 47.Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang ZQ, Ovitt C, Grigoriadis AE, et al. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- 49.Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signalling for terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 50.Matsuo K, Galson DL, Zhao C, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279:26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 51.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 53.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nature. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 54.Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Ann Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 55.Sato K, Takayanagi H. Osteoclast, rheumatoid arthritis and osteoimmunology. Curr Opin Rheumatol. 2006;18:419–426. doi: 10.1097/01.bor.0000231912.24740.a5. [DOI] [PubMed] [Google Scholar]
- 56.Koga T, Inui M, Inoue K, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 57.Mocsai A, Humphrey MB, Van Ziffle JA, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor γ chain (FcRg) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suda T, Takahashi N, Udagawa N, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 59.Azuma Y, Kaji K, Katogi R, et al. Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi K, Takahashi N, Jimi E, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim N, Kadono Y, Takami M, et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takayanagi H, Kim S, Matsuo K, et al. RANKL maintains bone homeostasis through c-Fos dependent induction of interferon-β. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 63.Baron R, Neff L, Louvard D, et al. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985;101:2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuukkanen J, Väänänen HK. Omeprazole, a specific inhibitor of H+-K+-ATPase, inhibits bone resorption in vitro. Calcif Tissue Int. 1986;38:123–125. doi: 10.1007/BF02556841. [DOI] [PubMed] [Google Scholar]
- 65.Teti A, Marchisio PC, Zallone AZ. Clear zone in osteoclast function: role of podosomes in regulation of bone-resorbing activity. Am J Physiol. 1991;261:C1–C7. doi: 10.1152/ajpcell.1991.261.1.C1. [DOI] [PubMed] [Google Scholar]
- 66.McHugh KP, Hodivala-Dilke K, Zheng MH, et al. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blair HC, Teitelbaum SL, Ghiselli R, et al. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 68.Väänänen HK, Karhukorpi EK, Sundquist K, et al. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol. 1990;111:1305–1311. doi: 10.1083/jcb.111.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bekker PJ, Gay CV. Biochemical characterization of an electrogenic vacuolar proton pump in purified chicken osteoclast plasma membrane vesicles. J Bone Miner Res. 1990;5:569–579. doi: 10.1002/jbmr.5650050606. [DOI] [PubMed] [Google Scholar]
- 70.Frattini A, Orchard PJ, Sobacchi C, et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nature Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 71.Kornak U, Schulz A, Friedrich W, et al. Mol Genet. 2000;9:2059–2063. doi: 10.1093/hmg/9.13.2059. [DOI] [PubMed] [Google Scholar]
- 72.Sundquist KT, Leppilampi M, Järvelin K, et al. Carbonic anhydrase isoenzymes in isolated rat peripheral monocytes, tissue macrophages, and osteoclasts. Bone. 1987;8:33–38. doi: 10.1016/8756-3282(87)90129-3. [DOI] [PubMed] [Google Scholar]
- 73.Teti A, Blair HC, Teitelbum SL, et al. Cytoplasmic pH regulator and chloride/bicarbonate exchange in avian osteoclasts. J Clin Invest. 1989;83:227–233. doi: 10.1172/JCI113863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blair HC, Schlesinger PH. Purification of a stilbene sensitive chloride channel and reconstitution of chloride conductivity into phospholipid vesicles. Biochem Biophys Res Commun. 1990;171:920–925. doi: 10.1016/0006-291x(90)90771-e. [DOI] [PubMed] [Google Scholar]
- 75.Schlesinger PH, Blair HC, Teitelbaum SL, et al. Characterization of the osteoclast ruffled border chloride channel and its role in bone resorption. J Biol Chem. 1997;272:18636–18643. doi: 10.1074/jbc.272.30.18636. [DOI] [PubMed] [Google Scholar]
- 76.Kornak U, Kasper D, Bosl MR, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 77.Saftig P, Hunziker E, Wehmeyer O, et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci. USA. 1998;95:13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gowen M, Lazner F, Dodds R, et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14:1654–1663. doi: 10.1359/jbmr.1999.14.10.1654. [DOI] [PubMed] [Google Scholar]
- 79.Gelb BD, Shi GP, Chapman HA, et al. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273:1236–1238. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- 80.Johnson MR, Polymeropoulos MH, Vos HL, et al. A nonsense mutation in the cathepsin K gene observed in a family with pycnodysostosis. Genome Res. 1996;6:1050–1055. doi: 10.1101/gr.6.11.1050. [DOI] [PubMed] [Google Scholar]
- 81.Andersen TL, del Carmen Ovejero M, Kirkegaard T, et al. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 2004;3:1107–1119. doi: 10.1016/j.bone.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 82.Linsuwanont-Santiwong B, Takagi Y, Ohya K, et al. Expression of MT1-MMP during deciduous tooth resorption in odontoclasts. J Bone Miner Metab. 2006;24:447–453. doi: 10.1007/s00774-006-0714-z. [DOI] [PubMed] [Google Scholar]
- 83.Broomley M, Woolley DE. Chondroclasts and osteoclasts at subchondral sites of erosion in rheumatoid joint. Arthritis Rheum. 1984;27:968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- 84.Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 85.Takayanagi H, Huji T, Miyazaki T, et al. Suppression of arthritic bone destruction by adenovirus-mediated csk gene transfer to synoviocytes and osteoclasts. J Clin Invest. 1999;104:137–146. doi: 10.1172/JCI6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takayanagi H. Inflammatory bone destruction and osteoimmunology. Curr Opin Rheumatol. 2006;18:419–426. doi: 10.1097/01.bor.0000231912.24740.a5. [DOI] [PubMed] [Google Scholar]
- 87. Whyte MP. Osteopetrosis. In: Royce PM, Steinamann B, eds. Connective tissue and its heritable disorders: medical, genetic and molecular aspects New York: Wiley-Liss Inc. 2002. 753 770 [Google Scholar]
- 88.Frattini A, Pangrazio A, Susani L, et al. Chloride channel CICN7 mutations are responsible for severe recessive, dominant and intermediate osteopetrosis. J Bone Miner Res. 2003;18:1740–1747. doi: 10.1359/jbmr.2003.18.10.1740. [DOI] [PubMed] [Google Scholar]
- 89.Lange PF, Wartosch L, Jentsch TJ, et al. CIC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature. 2006;440:220–223. doi: 10.1038/nature04535. [DOI] [PubMed] [Google Scholar]
- 90.Chalhoub N, Benachenhou N, Raiapurohitam V, et al. Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med. 2003;9:399–406. doi: 10.1038/nm842. [DOI] [PubMed] [Google Scholar]
- 91.Pangrazio A, Poliani PL, Megarbane A, et al. Mutation in OSTM1 (grey lethal) define a particularly severe form of autosomal recessive osteopetrosis with neural involvement. J Bone Miner Res. 2006;21:1098–1105. doi: 10.1359/jbmr.060403. [DOI] [PubMed] [Google Scholar]
- 92.Sobacchi C, Frattini A, Guerrini MM, et al. Osteoclast-poor human osteopetrosis due to mutation in the gene encoding RANKL. Nat Genet. 2007;39:960–962. doi: 10.1038/ng2076. [DOI] [PubMed] [Google Scholar]
- 93.Bolt RJ, Wennink JM, Verbeke JI, et al. Carbonic anhydrase type II deficiency. Am J Kidney Dis. 2005;46:A50,e71–3. [PubMed] [Google Scholar]
- 94.Van Wesenbeeck L, Odgren PR, Coxon FP, et al. Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J Clin Invest. 2007;117:919–930. doi: 10.1172/JCI30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Albers-Schönberg HE. Röntgenbilder einer seltenen Knockenerkrankung. Munch Med Wochenschr. 1904;5:365–368. [Google Scholar]
- 96.Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. doi: 10.1016/j.bone.2007.08.029. [DOI] [PubMed] [Google Scholar]