Abstract
Genetic studies of calcium kidney stones evidenced the possible involvement of calcium-sensing receptor gene, vitamin D receptor gene and bicarbonate-sensitive adenylate cyclase gene, but it is uncertain which specific polymorphisms could be responsible. Thus, further studies are required to better assess the involvement of these or other genes and the interactions between different genes and between genes and environment. In addition to research in humans, the study of different strains of knock-out mice let us include the gene of phosphate reabsorption carrier NPT2, caveolin-1, protein NHERF-1, osteopontin and Tamm-Horsfall protein among the possible determinants. Further steps in the knowledge of calcium stone causes may be done using the instruments that the modern biotechnology and bioinformatics have made available to the researchers.
Keywords: kidney stones, genetics, calcium, oxalate.
Introduction
Current clinical and epidemiologic data support the inclusion of nephrolithiasis among complex diseases, such as diabetes, hypertension and ischemic heart disease. Complex diseases are the most prevalent disorders in modern Western society and the greatest burden for National Health Care Systems. In Italy nephrolitiasis is the leading cause of hospitalization for nephrologic and urologic diseases (~ 100.000 hospitalizations a year in the last decade), accounting for an annual expenditure of 250 million euros by the Italian Health Care System (data from the Italian Health Ministry website, http://www.ministerosalute.it/). As for other complex diseases, nephrolithiasis is likely caused by the interaction of multiple genetic and environmental factors (1).
This picture is sufficient to highlight the importance of prevention in kidney stone disease. The ability to identify individuals at risk of stones would allow implementation of measures to prevent kidney stones and their potential complications among susceptible individuals. However, no validated markers of susceptibility to kidney stones are presently available, and great hopes are placed in genetic research.
The importance of a genetic background in the development of nephrolithiasis has emerged from studies in North-American and European families showing a higher prevalence of kidney stones among first degree relatives of stone-forming patients then among first degree relatives of non-stone formers (2-5). The comparative study of monozygous and dizygous twins born in Vietnam showed a greater concordance for stone production among monozygotes than dizygotes (32.4% vs. 17.3%). This study estimated that in the population of Vietnam the proportion of kidney stone susceptibility attributable to genetic causes was 56%, while environmental causes explained 44% of the disease (6).
In the Western world 80% of kidney stones are made of calcium oxalate and calcium phosphate. Thus, calcium stones account for the majority of the economic and epidemiologic burden of nephrolithiasis. Thus, the present article focuses on research findings concerning calcium nephrolithiasis in animals and humans.
Studies on animals with kidney stones
Knock-out mice are created to lack the expression of a specific gene product through gene silencing. The assessment of the resulting phenotype provides important functional information about the silenced gene. Therefore, stone forming knock-out mice represent an important tool to understand which genes may be important for the development of calcium nephrolithiasis.
Five different strains of knock-out mice are known to develop phenotypes featuring calcium stones or kidney calcifications. The first is a knock-out strain for the carrier excreting oxalates into the intestinal lumen (slc26a13) (7). The absence of the intestinal oxalate carrier results in body accumulation of oxalate, increased urinary oxalate execretion, precipitation of calcium-oxalate in the urine and formation of kidney stones.
The second strain is knock-out for the caveolin-1 gene (cav-1). It lacks of caveolae, that are invaginations of the plasma membrane of kidney tubular cells. Caveolae are functional hot-points within the plasma membrane, rich in cholesterol and functional proteins like calcium-pump, vitamin D receptor and calcium-sensing receptors (8). In the absence of caveolae, proteins normally expressed on the plasma membrane remain free in the cytoplasm loosing their function. Knock-out mice for caveolin-1 do not express calcium pump on their plasma membrane, are hypercalciuric and develop tubular calcium deposits. The third strain of knock-out mice does not express NHERF-1, a protein modulating the function of renal carriers for uric acid, phosphate, sodium and calcium reabsorption (9). NHERF-1 silencing causes a sustained increase in calcium and urate excretion in the urine and the development papillary deposits of calcium.
The fourth strain of knock-out mice lacks the proximal phosphate carrier gene (sodium-phosphate cotransport, Npt2a) (10). These mice develop a phenotype characterized by hypophosphatemia due to tubular phosphate loss, hypercalciuria, rickets and kidney stones. However, this strain is not a good model of human kidney stones because the same phenotype occurs in humans only after mutations of Npt2c carrier gene, a specific isoform of Npt2 phosphate carriers, different from Npt2a.
The fifth stone-forming knock-out mice strain does not express both osteopontin and Tamm-Horsfall protein and shows modifications of tubular phosphate and oxalate handling (11). Approximately 39.3% of these mice have papillary calcium deposits, whereas this prevalence decreases to 10-15% among mice lacking the expression of one of these two proteins. This observation suggests a synergistic effect of osteopontin and Tamm-Horsfall protein in inhibiting crystal adhesion to the tubular wall.
In addition to these knock-out mice models, a stone forming rat strain was selected by inbreeding generations of the most hypercalciuric Sprague-Dawley rats. These rats (GHS) were spontaneously (genetically) hypercalciuric and produced calcium-phosphate stones (12). GHS rats overexpress the vitamin D receptor in the intestine and have intestinal calcium hyperabsoption and low bone mass. Despite our knowledge of their phenotype, the primary defect leading to kidney stones in GHS rats is still unclear (13). A quantitative trait locus linked to kidney stones was identified studying rats bred from GHS females and normocalciuric Wistar-Kyoto rats. This trait locus was mapped on chromosome 1 and explained 7% of the phenotypic variance. The expression profile in rats at different generations has been studied by microarray bearing thousands of genes, but findings of these experiments are of difficult interpretation (14).
Studies in patients with kidney stones
Dent syndrome and familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC) are the most common among the many monogenic diseases including kidney stones in their phenotype (15, 16). Dent syndrome is due to mutations of either chloride channel 5 or phosphatidylinositol 4,5-bisphosphate 5-phosphatase genes (mapped on the chromosomes Xp11.22 and Xq26, respectively), while claudin 16 gene (chromosome 3q27) is responsible of FHHNC (17-19). Mutations of these genes are occasionally found in patients with calcium stones. However, epidemiologic data suggest that idiopathic kidney stones are likely to have a polygenic and heterogeneous background, although we still know very little about the underlying genes. Studies concerning the transmission pattern of kidney stones suggest that a small number of genes is likely to be involved. The model proposed by Goodman et al. established that no more than 3-4 genes contribute to stone production and acting on calcium, oxalate and citrate excretion (20). Another group of researchers hypothesized that one major gene explained the largest proportion of calcium excretion variance in stone patients (21). Although these studies suggest the contribution of major genes to the phenotype, the definition of a transmission model for kidney stones is far to be defined and a pattern without major genes but with multiple genes exerting small and additive effects cannot be excluded and could cause an increased susceptibility to kidney stones.
The vitamin D receptor (VDR) is located on chromosome 12q12-14 and its product modulates vitamin D stimulation on intestinal absorption of calcium, osteoblastic activity and other vitamin D targets. The study of 303 pairs of French-Canadian brothers showed that four of the six markers used to map the VDR gene were in linkage with kidney stones (22). The same chromosomal region was also found in linkage with kidney stones in four Indian families, even though findings with the same markers were not completely in agreement with those reported by the French-Canadian study (23).
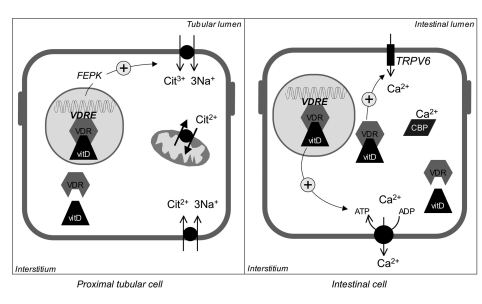
Another strategy was applied to study the association between VDR gene polymorphisms and kidney stones. Polymorphisms sited on intron 8 and exon 9, and at the transcription start codon were at first tested and found associated with kidney stones (24-26). Variant alleles at intron 8 and exon 9 polymorphisms correlated with the severity of the stone disease, being associated with early age of onset and higher frequency of stones (27). These findings may be explained by a lower urinary excretion of citrate observed in stone patients bearing variant alleles at these polymorphisms. Citrate metabolism may be influenced by VDR polymorphisms, as the VDR-vitamin D complex induces the transcription of phosphoenol-pyruvatecarboxyl-kinase gene. This kinase reduces citrate excretion by stimulating citrate reabsorption through the apical membrane carrier in proximal tubular cells (28). Through this mechanism (Figure 1), activating polymorphisms of the VDR gene could affect the clinical history of kidney stone patients. As it often occurs in complex diseases, these findings were not replicated in other studies (29, 30). This discrepancy may be accounted for by different environmental conditions or the effect of other genes masking the association between VDR and the phenotype (1). However, these studies have collected a significant amount of data suggesting that the VDR gene is involved in lithogenesis, although the mechanisms for this involvement is not yet defined.
Figure 1.
- In proximal tubular cells, the complex VDR/Vitamin D activates the transcription of phosphoenol-pyruvatecarboxyl-kinase (FEPK) gene and the function of citrate reabsorption carrier. Thus, vitamin D may decrease urinary citrate excretion. In intestinal cells, the complex VDR/Vitamin D causes a rapid non-genomic and a genomic activation of calcium absorption that occurs through apical calcium channels (TRPV6), and basolateral calcium pump. Calbindin (CBP) binds calcium ions in cytoplasm and carries them from the apical to the basolateral membrane.
The calcium sensing receptor (CaSR), mapped on chromosome 3q13.3-21, has been hypothesized to play a role in stone formation (31). CaSR is expressed throughout the nephron and modulates the function of different tubular regions. It is activated by the increase of calcium ions in the extracellular fluid that stimulates different G proteins and inhibits adenilate-cyclase to trigger intracellular pathways controlling tubular functions. CaSR activation inhibits calcium reabsorption in the ascending limb and water reabsorption in the collecting duct, while it increases phosphate reabsorption in the proximal tubule (32). The role of CaSR in stone production may be mediated by these tubular effects and was suggested by a case-control study that found an association of kidney stones with the polymorphisms of the 5’-untranslated region and first intron of CaSR gene in a large group of patients recruited in Italian stone centres. This large region contains CaSR gene promoters and polymorphic alleles of its sequence could modify the effect of promoters or transcription factors and, thus, gene expression. This case-control study involved a large number of individuals (over 500 patients and 600 controls) and it is currently the largest case-control study in the medical literature. CaSR polymorphisms were not related with intermediate phenotypes typically found in stone formers, like hypercalciuria and hypocitraturia, suggesting that CaSR could take part in the production of Randall plaque in papillary interstitium or calcium salt deposits in tubular lumen, the putative early events in lithogenesis (33, 34). It is, however, noteworthy that no linkage was observed between CaSR locus and kidney stones (35) in Franch-Canadian siblings.
In three families with hypercalciuric stone forming members, a linkage study identified 1q23.3-24 as a locus possibly involved in kidney stones (36). The subsequent association analysis in cases and controls recognized that variant alleles at six polymorphisms of bicarbonate-sensitive adenylate-cyclase gene (sAC) were more common in patients with stones (37). Despite these findings the activity of sAC and whether sAC is involved in stone formation or in hypercalciuria or both is still unknown.
Other studies have identified the polymorphisms of osteopontin (38), urokinase (39), interleukin-1 receptor (40), epidermal growth factor (41) and E-cadherin genes (42) as polymorphisms involved in calcium stone formation. The first two compounds are salt precipitation inhibitors, while the interleukin-1 receptor could be involved in the tubular inflammation that takes part to stone formation. Findings of these studies were not replicated in subsequent research.
Conclusions
We have reviewed the most relevant studies on the genes that are potentially involved in the pathogenesis or may be considered markers of stone susceptibility. Although these studies have collected interesting information about the mechanisms of stone formation, we are, unfortunately, still unable to provide reliable markers of susceptibility. Polymorphisms of VDR and CaSR genes may be involved in the nephrolithiasis, but it is uncertain which specific polymorphisms could be responsible. Thus, further studies are required to better assess the involvement of these or other genes and the interactions between different genes and between genes and environment. Only the cooperation between stone centers will ensure the collection of an adequate number of cases to identify groups of patients with a specific intermediate phenotype and to warrant data replicability. On the other hand, biotechnology progress today allows genotyping a large number of DNA samples in a short time and at a relatively low cost thanks to microarrays technology that can also be applied to the study of gene expression. Modern biotechnology requires specific and complex genetic-statistical analyses that can be performed with dedicated bioinformatics expertise and workstations. The establishment of large samples of carefully phenotyped patients and adequate biotechnological facilities will allow our research focusing on a relatively small number of loci with the potential of developing screening tests capable of identifying individuals with an increased susceptibility to calcium kidney stones.
References
- 1.Colhourn HM, McKeigue PM, Smith JD. Problems of reporting genetics associations with complex outcomes. Lancet. 2003;361:865–872. doi: 10.1016/s0140-6736(03)12715-8. [DOI] [PubMed] [Google Scholar]
- 2.Ljunghall S. Family history of renal stones in a population study of stone formers and healthy subjects. Br J Urol. 1979;51:249–52. doi: 10.1111/j.1464-410x.1979.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 3.Resnick M, Pridgen DB, Goodman HO. Genetic predisposition to formation of calcium oxalate renal calculi. N Engl J Med. 1968;278:1313–1318. doi: 10.1056/NEJM196806132782403. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri A, Mandressi A, Luongo P, et al. Familial aggregation of renal calcium stone disease. J Urol. 1988;138:478–481. doi: 10.1016/s0022-5347(17)42497-9. [DOI] [PubMed] [Google Scholar]
- 5.Curhan GC, Willett WC, Rimm EB, et al. Family history and risk of kidney stones. J Am Soc Nephrol. 1997;8:1568–1573. doi: 10.1681/ASN.V8101568. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb DS, Fischer ME, Keich Y, Goldberg J. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kid Int. 2005;67:1053–61. doi: 10.1111/j.1523-1755.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Z, Asplin JR, Evan AP, et al. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38:474–8. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 8.Cao G, Yang G, Timme TL, et al. Disruption of the caveolin-1 gene impairs renal calcium reabsorption and leads to hypercalciuria and urolithiasis. Am J Pathol. 2003;162:1241–1248. doi: 10.1016/S0002-9440(10)63920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham R, Brazie M, Kanumuru SEX, et al. Sodium-hydrogen exchanger regulatory factor-1 interacts with mouse urate transporter 1 to regulate renal proximal tubule uric acid transport. J Am Soc Nephrol. 2007;18:1419–1425. doi: 10.1681/ASN.2006090980. [DOI] [PubMed] [Google Scholar]
- 10.Chau H, El-Maadawy S, McKee MD, Tenenhouse HS. Renal calcification in mice homozygous for the disrupted type IIa Na/Pi cotransporter gene Npt2. J Bone Miner Res. 2003;18:644–657. doi: 10.1359/jbmr.2003.18.4.644. [DOI] [PubMed] [Google Scholar]
- 11.Mo L, Liaw L, Evan AP, Sommer AJ, Lieske JC, Wu XR. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am J Physiol Renal Physiol. 2007;293:F1935–1943. doi: 10.1152/ajprenal.00383.2007. [DOI] [PubMed] [Google Scholar]
- 12.Bushinsky DA, Favus MJ. Mechanism of hypercalciuria in genetic hypercalciuric rats: inherited defect in intestinal calcium transport. J Clin Invest. 1988;82:1585–1591. doi: 10.1172/JCI113770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M, Sessler NE, Tembe V, et al. Response of genetic hypercalciuric rats to a low calcium diet. Kidney Int. 1993;43:189–196. doi: 10.1038/ki.1993.31. [DOI] [PubMed] [Google Scholar]
- 14.Hoopes RR Jr, Middleton FA, Sen S, et al. Isolation and confirmation of a calcium excretion quantitative trait locus on chromosome 1 in genetic hypercalciuric stone-forming congenic rats. J Am Soc Nephrol. 2006;17:1292–1304. doi: 10.1681/ASN.2005080828. [DOI] [PubMed] [Google Scholar]
- 15.Scheinman SJ, Cox JPD, Lloyd SE, et al. Isolated hypercalciuria with mutation in CLCN5: relevance to idiopathic hypercalciuria. Kidney Int. 2000;57:232–239. doi: 10.1046/j.1523-1755.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- 16.Kutluturk F, Temel B, Uslu B, et al. An unusual patient with hypercalciuria, recurrent nephrolithiasis, hypomagnesemia and G227R mutation of Paracellin-1. Horm Res. 2006;66:175–181. doi: 10.1159/000094253. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd SE, Pearce SHS, Fisher SE, et al. A common molecular basis for three inherited kidney stone diseases. Nature. 1996;379:445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- 18.Hoopes RR, Shrimpton AE, Knohl SJ, et al. Disease with mutations in OCRL1. Am J Hum Genet. 2005;76:260–267. doi: 10.1086/427887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller D, Kausalya J, Claverie-Martin F, et al. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet. 2003;73:1293–1301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman HO, Brommage R, Assimos DG, Holmes RP. Genes in idiopathic calcium oxalate stone disease. World J Urol. 1997;15:186–194. doi: 10.1007/BF02201856. [DOI] [PubMed] [Google Scholar]
- 21.Loredo-Osti JC, Roslin NM, Tessier J, et al. Segregation of urine calcium excretion in families ascertained for nephrolithiasis: evidence for a major gene. Kidney Int. 2005;68:966–971. doi: 10.1111/j.1523-1755.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 22.Scott P, Ouimet D, Valiquette L, et al. Suggestive evidence for a susceptibility gene near the vitamin D receptor locus in idiopathic calcium stone formation. J Am Soc Nephrol. 1999;10:1007–1013. doi: 10.1681/ASN.V1051007. [DOI] [PubMed] [Google Scholar]
- 23.Khullar M, Relan V, Singh SK. VDR gene and urinary calcium excretion in nephrolithiasis. Kidney Int. 2006;69:943. doi: 10.1038/sj.ki.5000176. [DOI] [PubMed] [Google Scholar]
- 24.Nishijima S, Sugaya K, Naito A, et al. Association of vitamin D receptor gene polymorphism with urolithiasis. J Urol. 2002;167:2188–91. [PubMed] [Google Scholar]
- 25.Relan V, Khullar M, Singh SK, Sharma SK. Association of vitamin D receptor genotypes with calcium excretion in nephrolithiatic subjects in northern India. Urol Res. 2004;32:236–240. doi: 10.1007/s00240-004-0414-x. [DOI] [PubMed] [Google Scholar]
- 26.Soylemezoglu O, Ozkaya O, Gonen S, et al. Vitamin D receptor gene polymorphism in hypercalciuric children. Pediatr Nephrol. 2004;19:724–727. doi: 10.1007/s00467-004-1490-4. [DOI] [PubMed] [Google Scholar]
- 27.Rendina D, Mossetti G, Viceconti R, et al. Association between vitamin D receptor gene polymorphisms and fasting idiopathic hypercalciuria in recurrent stone-forming patients. Urology. 2004;64:833–838. doi: 10.1016/j.urology.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Mossetti G, Vuotto P, Rendina D, et al. Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med. 2003;253:194–200. doi: 10.1046/j.1365-2796.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- 29.Vezzoli G, Soldati L, Proverbio MC, et al. Polymorphism of vitamin D receptor gene start codon in patients with calcium kidney stones. J Nephrol. 2002;15:158–164. [PubMed] [Google Scholar]
- 30.Chen HY, Hsu CD, Wu JY, Tsai FJ. No association of vitamin D receptor gene BsmI polymorphisms with calcium oxalate stone formation. Mol Urol. 2001;5:7–10. doi: 10.1089/109153601750124203. [DOI] [PubMed] [Google Scholar]
- 31.Bushinsky DA. Nephrolithiasis: site of the initial solid phase. J Clin Invest. 2003;111:602–605. doi: 10.1172/JCI18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium. 2004;35:229–237. doi: 10.1016/j.ceca.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Evans AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kok DJ, Khan SR. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int. 1994;46:847–854. doi: 10.1038/ki.1994.341. [DOI] [PubMed] [Google Scholar]
- 35.Petrucci M, Scott P, Ouimet D, et al. Evaluation of the calcium-sensing receptor gene in idiopathic hypercalciuria and calcium nephrolithiasis. Kidney Int. 2000;58:38–42. doi: 10.1046/j.1523-1755.2000.00138.x. [DOI] [PubMed] [Google Scholar]
- 36.Reed BY, Heller HJ, Gitomer WL, Pak CY. Mapping a gene defect in absorptive hypercalciuria to chromososme 1q23.3-q24. J Clin Endocrinol Metab. 1999;84:3907–3913. doi: 10.1210/jcem.84.11.6155. [DOI] [PubMed] [Google Scholar]
- 37.Reed BY, Gitomer WL, Heller HJ, et al. Identification and characterization of a gene with base substitutions associated with the absorptive hypercalciuria phenotype and low spine bone density. J Clin Endocrinol Metab. 2002;87:1476–1485. doi: 10.1210/jcem.87.4.8300. [DOI] [PubMed] [Google Scholar]
- 38.Gao B, Yasui T, Itoh Y, et al. Association of osteopontin gene haplotypes with nephrolithiasis. Kidney Int. 2007;72:592–598. doi: 10.1038/sj.ki.5002345. [DOI] [PubMed] [Google Scholar]
- 39.Tsai FJ, Lin CC, Lu HF, Chen HY, Chen WC. Urokinase gene 3’-UTR T/C polymorphism is associated with urolithiasis. Urology. 2002;59:458–461. doi: 10.1016/s0090-4295(01)01576-x. [DOI] [PubMed] [Google Scholar]
- 40.Chen WC, Wu HC, Chen HY, et al. Interleukin-1beta gene and receptor antagonist gene polymorphisms in patients with calcium oxalate stones. Urol Res. 2001;29:321–324. doi: 10.1007/s002400100193. [DOI] [PubMed] [Google Scholar]
- 41.Chen WC, Chen HY, Wu HC, et al. Vascular endothelial growth factor gene polymorphism is associated with calcium oxalate stone disease. Urol Res. 2003;31:218–222. doi: 10.1007/s00240-003-0325-2. [DOI] [PubMed] [Google Scholar]
- 42.Tsai FJ, Wu HC, Chen HY, et al. Association of E-cadherin gene 3-UTR C/T polymorphism with calcium oxalate stone disease. Urol Int. 2003;70:278–281. doi: 10.1159/000070135. [DOI] [PubMed] [Google Scholar]