Abstract
Overall, the regulators (here the term is used in the broad sense including competent authorities, the national departments of health and the European commission) have a significant role in translating pharmacogenomics into clinical practice. The first objective is to establish the role of the genomic information that is available, and this should be data driven. Conduct of robust clinical trials that are sound both scientifically and from a regulatory perspective should be encouraged. Significant interaction between Academia, Pharma industry and the regulator is essential with the overall aim of improving public health. Conceptually, this would involve the triumvirate (Academia, industry and regulators) as an orchestra with the regulators perhaps taking the role of the conductor while the significant players would be those that generate data (Academia and industry). The regulators also need to ensure that clear guidance is available for use of the information and the tests with a significant level of uniformity between the ICH regions. The commercial availability of the test will have considerable impact on the use of pharmacogenomics, but this is currently beyond the scope of this paper.
Keywords: : pharmacogenomics, drug regulation, pharmacogenetic testing, regulatory guidance, clinical practise.
Introduction
The influence of inherited characteristics in determining the maximum safe and effective use of medicines is an area that has long been sought after. The fact that inherited characteristics affected individual response to medicines or other agents has been well known for nearly 50 years. The earliest example is probably the often repeated story of Pythagoras, a 6th century BC mathematician, and his injunction against fava beans although his reasons are poorly understood. A fair degree of discussion has taken place regarding the reasons for his injunctions and it is also hypothesised that his death was related to his inability (reluctance) to cross a field of beans (1).
“Pythagoras refused to walk through fields of fava beans and discouraged his disciples from eating them. He is said to have met his death in Crotonia in Ancient Italy. Pursued by the enemies, Pythagoras died among them because he would not flee across a bean field, he came to the edge of a bean field and, rather than set foot in it, was caught and killed”.
The association between haemolytic anaemia in those with G6PD deficiency with fava beans or medicines (a situation similar to favism) only became somewhat clearer in the 1950’s in relation to use of anti-malarial agents in troops being given primaquine for prophylaxis. Brown et al. in 1957 identified that primaquine precipitated haemolysis in susceptible individuals who inherited this x linked tendency (SNP at Xp28) and now nearly 400 variants have been identified. Thus, a number of examples are now available to show that inherited characteristics impact on drug response. A significant number of these are related to safety reports for established medicinal products involving drug metabolising enzymes.
Table I - .
List of Abbreviations.
| 6MP = 6-mercaptopurine |
| CISH = Chromogenic In Situ Hybridization |
| CYP2C9 = Cytochrome P450 2C9 enzyme |
| CYP2D6 = Cytochrome P450 enzyme 2D6 |
| EC = European Commission |
| EU = European Union |
| FISH = Fluorescent In Situ Hybridization |
| G6PD = Glucose 6-Phosphae Dehydrogenase |
| HLA = Human Leukocyte Antigen |
| ICH = International Conference on Harmonization |
| IHC = Immunohistochemistry |
| PGt = Pharmacogenetics |
| PGx = Pharmacogenomics |
| SNPs = Single Nucleotide Polymorphisms |
| TPMT = Thiopurine S-methyl-transferase enzyme |
| VKORC1 = Vitamin K epoxide Reductase Complex-subunit1 |
In one of the first examples of pharmacogenetics in oncology, Weinshilboum and Sladek identified polymorphic responses to the key anti-leukemic drug, 6-mercaptopurine (6-MP) in 1980, and polymorphism of the gene thiopurine S-methyl-transferase (TPMT; *2 and *3 alleles) remains one of the best understood examples of pharmacogenetic variation (2). Other examples include CYP2D6 polymorphism (CYP2D6*3) that affects a number of medicinal products (drugs) such as antipsychotics, selective serotonin uptake inhibitors, certain beta-blockers and perhexiline. These could involve SNPs (single nucleotide polymorphisms), haplotypes or HLA subtypes. While the tests for identifying these genotypes/polymorphisms have been avail- able for a number of years their use however has been variable and often limited. The European commission facilitated IPTS report on her-2 and TPMT tests in 4 different EU member states highlighted this and brought forth certain aspects regarding the use of pharmacogenetic tests or information in the clinics (3). The report was published in 2006 and explores the potential reasons for poor uptake of these tests. In order to understand the reasons for variability or inconsistency of clinical use of the available tests, we must examine the factors that have major influence on the use of these tests and the information they provide. The factors relate not only to the tests themselves, but also the available facilities to support the use of such tests and the perception of its utility, both by professionals or the consumer groups.
TABLE II - .
Possible Factors affecting PG testing in Clinics.
| A. Factors intrinsic to PGx information |
|
| B. Approach to Clinical trials—“A MINDSET” |
| C. Factors extrinsic to PGx Information |
|
Factors intrinsic to the PGx information
Factors that are intrinsic to the pharmacogenetic or genomic marker (or the test) could affect its clinical use. One such factor likely to have a major impact is the variability and lack of consistency in the results from studies or association of marker with disease or outcome. A large number association studies have found a relation between a single nucleotide polymorphisms or a set of polymorphisms (multiple gene profiles) and use of a particular agent, but confirmatory evidence of a true relationship that impacts clinical use has not been available in many cases. For example, the dosing strategy for warfarin users and the impact of CYP2C9 and VKORC1 polymorphisms has long been the subject of debate (4). A plethora of studies are available that show varied results of impact of these two interactions and more are being identified (CYP4F2 polymorphism) (5). The different results seen in the above reports suggest a varied level of interaction between polymorphisms and the dose of Warfarin. These studies were predominantly in achieving a stable daily maintenance dose. There are two concerns with the studies available so far; one is that majority of these were retrospective; second is that few studies have actually analysed the initiating dose algorithm. Retrospective studies selected patients who were already on a relatively stable Warfarin dose thus inadvertently excluding those who either had an event or poor stability as regards dose. This may have either overestimated or underestimated the contribution of the polymorphisms studied.
The size of the population the maker or test in question would be applicable is another factor that is important. Weinshilboum and colleagues (6) who first identified the mutations in TPMT and its impact on 6-mercaptopurine therapy recently raised a very important question; Is it appropriate to ask if the greatest impact of Pharmacogenetics / genomics will be obtained from applications to drugs that are used to treat relatively few patients? The answer of course is NO”. Therefore there is a need to identify those areas that have large impact on the usage of relatively common (in a large number of patients) medicines. Such an observation also relates to the regulatory and clinical mind set that requires need for confirmatory data in clinical trials of reasonable population size. Whilst this applies to majority of the day to day situations, in certain instances smaller proportions of common diseases could still have a significant market share, but the test for the marker would then have to be mandatory and have very clear, significant impact. Her-2 receptor status in breast cancer (early or late) is a good example (7); in spite of the fact that only 30% of breast cancers have Her-2 receptor positivity, the use of the test and test guided trastuzumab therapy have made significant impact. This is in spite of the complexities of the test that moved from Immunohistochemistry (IHC- assessing protein expression), FISH (fluorescent in situ hybridization) and more recently CISH (Chromogenic in situ hybridization) that assesses the number of gene copies. In contrast, the tests for TPMT polymorphisms are simpler but, affect a small percentage of the population (0.3% homozygotes and ~11% heterozygotes). This may explain why its uptake is variable; high among dermatologists, lower among gastroenterologists when deciding to commence azathioprine therapy. The factors that explain the difference between specialities is the physician education and guidance notes for the respective societies/ associations (especially in the UK) (8). The British dermatological society guidance insists on use of the test prior to initiating therapy with mercaptopurines or azathioprine while the society for gastroenterologists takes a more discussant approach even though use of azathioprine in inflammatory diseases of the bowel have been well known and increasing over the years. Furthermore, in oncology where its use was most anticipated and benefit deemed maximal, the uptake has been particularly small (IPTS study report; EN 22214-2006) (9). During the survey in 4 EU member states, a number of physicians expressed their lack of interest as due to the fact that the information TMPT testing afforded them was limited as it predicted only neutropenia but not other events such as platelet count for which standard monitoring was more predictable and moiré useful (10). Secondly, the population at risk was considered too small to affect their clinical practise.
The regulatory impact of pharmacogenetics or genomics is still a developing area as is their integration into clinical practice. There are a number of reasons for either of these. The main factors determining regulatory impact are likely to be whether this involves an established product or a new medicinal product; the developments so far have been for well established products and inclusion of a safety warning in the label has been considered an action of adequate magnitude. This appears to be the most common and the most feasible action from a regulatory perspective. A number of examples are available and include 6-mercaptopurines (TPMT polymorphism warning) in the EU, more recently carbamazapine and HLA-B-1502 for prediction of Steven Johnson Syndrome (SJS) in Han Chinese population. Warnings regarding certain other agents have been included in the US label by the FDA in their respective labels; for example, Atamoxitine and CYP2D6 polymorphism, irinotecan and UGT1A1*28 allele and, Warfarin and CYP2C9 or VKORC1. In the EU such a change to the existing label has occurred for two possible reasons; differences in national legislation between member states governing these nationally authorised products and possibly a view that the data were not sufficiently robust to recommend alteration in dosing strategy for these agents as yet.
In contrast to pharmacogenomics for long established products, newer agents face different challenges and the pharmacogenomic information is likely to have greater impact especially if the administration of or indication for a particular medicinal product was dependent on the genotype (i.e., linking the genotype with the indication). Two good examples of this are trastuzumab in HER-2 receptor positive patients only and more recently testing for HLA-B-5701 prior to abacavir use to treat HIV (although abacavir is not a newly approved product) (11).
These two serve as the best examples of use of pharmacogenetic information in clinical practise. In spite of the local issues with obtaining test results for HER-2 or HLA-B 5701, a high uptake of the test and the pharmacogenetic information is noted due to their interminable link with the use of the product. Both these situations have had a considerable clinical, social and economic impact. Use of both agents has increased albeit for different reasons. While systematic data on abacavir usage is yet to be collated, the EN2214 report confirms utility of HER-2 tests. Notwithstanding the act that Trastuzumab use is limited by HER-2 receptor status, the public and professional awareness of the benefit makes testing mandatory for all patients.
For abacavir, the knowledge that serious cutaneous reactions might be avoided using HLA-B 5701 appears to have prompted its use earlier in the treatment from being a third line agent.
Cost effectiveness of the test could be an important consideration in clinical practise although the cost of a test or the medical product is not regulatory consideration for majority of EU competent authorities. The clinical uptake of the pharmacogenetic/genomic test is therefore based on a number of other considerations which we will discuss subsequently.
The obvious message from the above experiences is that in order to achieve a high level of clinical utility for the pharmacogenetic test, the use of the product should be linked closely with the test and this is dictated by its link to the indication especially for new agents. This is currently the trend and the area that shows the highest level of development in this regard is oncology. Understandably, this involves tumour genetics more than patient genetics. A number of other fields also show promise. The need for substantiation of any claim in a robust clinical trial dictates the rate of development. The requirement for a confirmatory clinical trial is thus both scientific and regulatory.
Current approach to clinical trials
Historically, the clinical trials have governed the use of any medicinal product and this was evident in Avicenna’s The Canon of Medicine in 1025 where he detailed the rules and requirements of clinical trials (12). These still govern the current day clinical trials to a large extent (13). Over the last century, experiences with several agents have fine tuned the need for data before a medicinal product is authorised. Thalidomide in the 50-60s presented one of the worst examples of agents that was inadequately studied especially in first trimester pregnancy that led to the disaster of phocomelia and other malformations.
There are a number of more recent examples such as cerivastatin, troglitazone, etc, all of which provided further impetus and support to the current ‘mindset’ regarding clinical trials. Perforce, in order to address major questions such as efficacy and safety, clinical trials have become larger, with calculation of statistical power a priori and they assume massive proportions in certain fields in order to address the outcome data. In clinical practise a similar situation prevails with some flexibility for the prescribers world wide. The practise is determined by data generated during the clinical trials. The doses are often determined using body weight or body surface area. Such practise has many limitations; although agent selection might be somewhat individual, it is based on the physician’s experience, available clinical trial data, and prior knowledge of the risk of adverse reaction. These are frequently determined by trials using groups of patients defined by a phenotype (disease characteristic or symptom), and the need for statistical evidence of effect and a comparator population. Over the last 18-19 years, 39 drugs have been withdrawn from the market after registration including cerivastatin, troglitazone, rofecoxib and latest of course being lumiracoxib. Each of these agents followed the general principles prevalent; i.e., clinical trails of certain size using phenotypic characteristics (symptoms) and safety data that were generated during these trials but proved to be inadequate subsequently.
Such events have a significant impact on several fronts; the Pharma industry, regulators and academia/clinicians. The Pharma industry R&D has to bear the major brunt of the cost of development including a potential loss 10-15 years from discovery to market of a new agent. The regulators are affected by the impact such a withdrawal has on the robustness of the approval process and could suffer loss of public confidence and impact public health. The Academia/clinicians that contributed to the development by scientific input during development will be hampered by lack of newer of better agents. Most important of all, the public health impact is enormous. Data from several regions have shown that hospitalisations due to adverse reactions have major impact on health spending (14, 15). If the anticipation in the future is that pharmacogenetics/genomic information would provide the necessary basis to reduce such events and also limit the high rate of attrition during the development process, then a huge effort and drive are needed to develop pharmacogenomics. This could only occur with a collaborative and combined effort between the stakeholders; Academia/clinicians, Pharma industry and the regulators.
Questions faced by Regulators
As the field of genetics/genomics has emerged and evolved, its applications have impacted significantly on the development, approval and use of drugs at a rate not seen before 2000 and it is even more obvious since the human genome project. Historically the regulation of medicines has been governed by two major facts: i) regulators or regulatory agencies do not generate their own data (in broad terms) unlike industry or academia, and ii) regulation rarely precedes scientific development.
The table III lists some common questions faced by regulators. As detailed before consistency of association and causality are important considerations and this leads to the stringent requirement for confirmatory data/evidence. Often this is the elusive step in pharmacogenomics and hence a concerted effort is needed from academia/industry in generation of such data. The second aspect is the applicability of the findings in the clinical area. From purely scientific view point often a link may be found between a SNP or a set of SNPs. If the link is tenuous and offers limited guidance towards therapy such pharmacogenomic information however exiting scientifically, is unlikely to find its way into clinical practise. The use of EGFR receptor status may be an example here. Although the link between EGFR status and some cancers is well known, data to confirm that ‘therapy directed against this impacts clinically meaningful endpoints’ is not available yet. Moreover, if the test required to identify the ‘genomic marker or pharmacogenomic information’ is extremely complex and needs very special centres of excellence to perform, the clinical utility of that particular test is likely to be small. For example FISH testing for HER-2; while IHC can be used by most pathological services, few are geared towards performing FISH testing routinely. Added to the complexity is the question of how to convey this information to the general physicians; the product literature is most obvious way in addition to educational seminars/symposia. These would have to be simple and clear as physician expertise varies especially with regard to pharmacogenomic information. Last but not the least is the fact that in EU and the three ICH regions have differences in legislation. What is applicable in one region or EU member state may not easily applicable in another. This relates to legal requirements for sample collection and storage, ethical principles, dissemination of certain personal information. All these are likely to affect the translation of pharmacogenomics into the clinics.
Table III - .
Questions commonly faced by regulators.
| 1. Consistency of findings or results?!! |
| 2. Applicability of findings in the clinical area |
| 3. What is the Impact on practice?!! |
| 4. How to convey/enforce |
| 5. Legal/regulatory aspects |
| National differences especially in EU |
| Differences between the 3 ICH regions |
Regulatory contribution in translation of pharmacogenomics
The regulators tend to have a unique perspective of the issues albeit it follows scientific development. The role of the regulator is to interpret the available data in the context of achieving consistency, conforming to legal definitions and limitations, and finally protection of public health. This spans across the EU as a whole and the three ICH regions. It is evident that the field of pharmacogenomics is developing rapidly but there is a need for consistency in both data generation and interpretation of results. Clarity regarding definitions of the reference terminology is the first issue that needed to be established (16). The recently published ICH topic E15 paper provides guidance on definitions that have been agreed between the three ICH regions and guidance was open for comments/suggestions. This was only achievable with collaboration between regulators, academia and industry. It is thus obvious that the crucial role of the regulator in bringing forward pharmacogenetics and genomics is to establish close and periodic links with academia and industry in a scientific forum that opens debate to facilitate understanding of the areas of development, and provides a unified framework for scientific discussion.
Table IV - .
Regulatory role in translation of PGx.
| 1. Interact with Academia/ Industry |
| 2. Working parties and collaboration at ICH |
| 3. Guidelinesgenerate |
| and promote use of guidelines |
| 4. Educational aspects |
| assessors/physicians/industry/public |
| 5. Role for other agencies such as departments of health/EC/EU |
| Initiate/ fund research |
| Legislation if required. |
Such a framework would require constitution of working parties that include both regulators and academics with periodic input from industry. This framework which has served well for usual scientific advice and other aspects, but would need to be adopted in the context of pharmacogenomics data submissions. In Europe, the constitution of the pharmacogenomic working party of the CHMP is a major step forward. The working party has its own work plan and interacts with other agencies worldwide (the three regions); Americas (FDA), the EU (EMEA) and Japan (MHLW). The joint VGDS meetings between EMEA (PGx Working party) and FDA provide a significant platform for interaction between agencies. For the industry this is a unique opportunity to gain insight from at least two regulatory bodies on a common platform. This interactive framework is considered crucial from both regulatory and academic view points as it provides an informal opportunity to discuss recent developments and provide scientific and regulatory advice on several fronts; the potential role for markers, possible and appropriate end points for studies of various size and complexity.
The regulator (and the working party) also has the main task of generating guidelines, reflection papers and position papers relevant to pharmacogenomics. The EMEA website has a list of documents available thus far (17). These guidelines and reflection papers (position papers) should and do provide an overview of the current understanding and view points. The regulators take lead in the generation of relevant guidelines that take into account the current state of knowledge, the need for guideline, the existing national regulatory and legal requirements, and in Europe, any differences between the member states in relation to the requirements. For example in certain member states, the sample collection for pharmacogenomic testing is controlled due to local legislation and this may have a bearing on overall impact of any guidance note relating to sample collection and storage. It is imperative that the documents include discussion on data submissions whether for informal discussion or for preliminary advice on marketing authorisations. The latter is in the remit of scientific advice groups in the respective regulatory agencies.
Table V - .
Other factors impacting translation of PGx.
| – Awareness within the medical profession |
| – Lack of consistent PGx education at medical schools, |
| – Lack of integration of PGx knowledge into practice, |
| – Recognition of the effect of PGx on healthcare. |
Educational opportunities
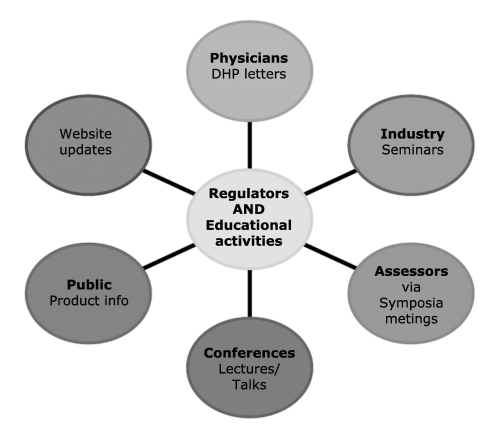
The educational aspect of the regulatory work should involve various levels. Education of assessors within the regulatory agency (ies) is the prime target regarding both current state of knowledge but also the approach to assessment of technical details. More importantly, increasing awareness of physicians/ clinicians to the recent developments is another major aspect. This is achieved through seminars or symposia but more importantly using the DHP (‘Dear Health Professional’) communications as a means of disseminating developments is occupying a significant role. This aspect would be crucial for agents such as warfarin that have been long established on the market but information about genotype or phenotype dependent dosing is recent and still emerging. For newer agents that are authorised based on pharmacogenomic information, there are two aspects; one is to link the product use closely with the genomic information or trait and the second is dissemination of such crucial information. the most effective way of dissemination of information. A close or interminable link between use of the product and the genomic information would automatically make consideration of the test (or genomic information) mandatory for most clinicians. The two classical examples of this are trastuzumab (Herceptin) and Abacavir as discussed before.
Figure 1.
A few pathways for regulators to promote awareness of pharmacogenomics.
Dissemination of information through public assessment reports that detail data available and the scientific consideration behind the decision process is another area which could have considerable impact in clinical use of pharmacogenomics. Several agencies have made provisions for such reports to be made available. In the EU, European public assessments reports (EPARs) are available for all centrally authorised products. The websites could also be used to post warning information or updates relating to established products and for announcements regarding newly approved chemical entities (medical products) that would highlight the main aspects. In the UK for example, these updates/warnings have been in use for some time- “Drug Safety update” and this could serve as model for future communications. This is seen as an important aspect of regulatory work in the current climate.
Medical, nursing and pharmacy education
One major lacuna that has been recognised in limiting awareness of pharmacogenomics among health care professionals including trainees is the lack of consistent programme in the medical schools, universities or schools of pharmacy. Therefore there is a need to incorporate some form of teaching and training in pharmacogenomics in the curriculum if pharmacogenomics were to become a useful clinical tool. This clearly requires effort from not just regulatory agencies that authorise medicinal products but from other governmental bodies, university boards and those that determine the overall curriculum. Educating health professionals (clinicians, nurses, and pharmacists etc) will need a significant effort for a number of bodies; universities, regulatory agencies, hospitals and overall for the health departments and finally policy makers such as EC as this will need a number of factors to be considered such as policies, achieving uniformity, and finally funding.
Role for other bodies (such as health departments or EC)
Any effort to enhance the profile of pharmacogenomics, will involve significant funding issues at various levels; first is establishing the need for development in a particular area, defining the area and possible route map, funding studies or trials to establish the utility and finally impact research (on clinical use, cost-effectiveness, social impact and overall impact on health service delivery). Applying these principles specifically, the need for such intervention appears to have been recognised as evidenced by the SACGHS report (18) and the EC commissioned report EN 22214.
In Europe, several examples of a centrally funded projects that assess the impact of pharmacogenomics on prescribing and use of test are available. For example, the EN2214 assessed use of HER-2 and TPMT testing. It assessed the clinical use, cost effectiveness and overall impact on practise of these two tests. From a scientific view point, the more recently publicised GENOMOS study (19) in osteoporosis is a good example although this primarily involved assessment of genomics variations in osteoporosis rather than a particular therapeutic intervention. The more recent example of the study of anticoagulant dosing (Warfarin) and the association with recognised genotypes of CYP2C9 or VKORC1, the EU-PACT study is another. These efforts involve clinicians and academics achieving a high level of interaction to develop pharmacogenomics. Similar efforts are underway at national levels including the UK.
Ackowledgements
The author wishes to recognise the contribution of the members of the Pharmacogenomic working party to this venture and expresses sincere thanks to all of them.
The views expressed here are that of the author and does not necessarily reflect views or policies of any organisation.
References
- 1.Meletis J, Konstantopoulos K. Favism - from the ‘avoid fava beans’ of Pythagoras to the present . Haema. 2004;7(1):17–21. ICID:7603. [Google Scholar]
- 2.Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurinemethyltransferase activity. Am J Hum Genet. 1980;32:651–662. [PMC free article] [PubMed] [Google Scholar]
- 3.Woelderink A, Ibarreta D, Hopkins MM, Rodriguez-Cerezo E., Pharmacogenomics J. The current clinical practice of pharmacogenetic testing in Europe: TPMT and HER2 as case studies. Pharmacogenomics. 2006;6:3–7. doi: 10.1038/sj.tpj.6500341. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz UI, Ritchie MD, Bradford Y, et al. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008 Mar 6;358(10):999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell M, Award T, Johnson JA, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. Apr 15;11(8):4106–12. doi: 10.1182/blood-2007-11-122010. Epub 2004 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Kane DJ, Weinshilboum RM, Moyer TP. Pharmacogenomics and reducing the frequency of adverse events. Pharmacogenomics. 2003;4(1):1–4. doi: 10.1517/phgs.4.1.1.22588. [DOI] [PubMed] [Google Scholar]
- 7.http://www.emea.europa.eu/humandocs/Humans/EPAR/herceptin/ herceptin.htm http://www.emea.europa.eu/humandocs/Humans/EPAR/herceptin/ herceptin.htm
- 8.Payne K, Newman W, Fargher E, et al. TPMT testing in rheumatology; any better than routine monitoring. Rheumatology. 2007 Jan;46(5):727–9. doi: 10.1093/rheumatology/kel427. Epub 2007 Jan 25. [DOI] [PubMed] [Google Scholar]
- 9.IPTS report for European commission on HER-2 and TPMT testing in EU ftp://ftp.jrc.es/pub/EURdoc/eur22214en.pdf. ftp://ftp.jrc.es/pub/EURdoc/eur22214en.pdf
- 10.Colombel JF, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppresion during azathioprine therapy. Gas troenterology. 2000;118:1025–30. doi: 10.1016/s0016-5085(00)70354-4. [DOI] [PubMed] [Google Scholar]
- 11.http://www.emea.europa.eu/humandocs/PDFs/EPAR/Ziagen/ 101999en8b.pdf http://www.emea.europa.eu/humandocs/PDFs/EPAR/Ziagen/ 101999en8b.pdf
- 12.http://en.wikipedia.org/wiki/The_Canon_of_Medicine http://en.wikipedia.org/wiki/The_Canon_of_Medicine
- 13.Craig Braiter D, Walter JD. Clinical pharmacology in the Middle Ages: Principles that presage the 21st century. Clinical Pharmacology & Therapeutics. 2000;67(5):447–450. doi: 10.1067/mcp.2000.106465. [448] [DOI] [PubMed] [Google Scholar]
- 14.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998 Apr 15;279(15):1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 15.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004 Jul 3;329(7456):15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.emea.europa.eu/pdfs/human/ich/43798606en.pdf. Nov, 2007. http://www.emea.europa.eu/pdfs/human/ich/43798606en.pdf ICH, E-15.
- 17.Pharmacogenomics. http://www.emea.europa.eu/htms/human/humanguidelines/multidiscipline.htm. http://www.emea.europa.eu/htms/human/humanguidelines/multidiscipline.htm
- 18.http://www4.od.nih.gov/oba/SACGHS/reports/SACGHS_PGx_Report.pdf. http://www4.od.nih.gov/oba/SACGHS/reports/SACGHS_PGx_Report.pdf The report for the Secretary of State, DHSS, USA.
- 19.Ralston RH, Uitterlinden AG, Brandi ML, et al. Large-scale evidence for the effect of the COLIA1 Sp1 polymorphism on osteoporosis outcomes: the GENOMOS study. PLoS Med. 2006;3(4):e90. doi: 10.1371/journal.pmed.0030090. Epub 2006 Feb 21. Erratum in: PLoS Med. 2006 May;3(5):e90. [DOI] [PMC free article] [PubMed] [Google Scholar]