Abstract
Within the past several years, the relation between diet and health has been accepted by the mainstream nutrition community and in this connection interest in the physiological role of bioactive compounds present in plants has dramatically increased over the last decade.
The phytoestrogens are bioactive molecules present as nutritional constituents of widely consumed vegetables. Their name derives from the fact that they are able to bind to estrogen receptors and to induce an estrogenic/antiestrogenic response in target tissues. Natural estrogens are involved in a multiplicity of programmed events in target tissues as uterus, breast, pituitary gland and hormone responsive tumors. Phytoestrogens are present in many human foodstuffs including fruits (plum, pear, apple grape berries, …), vegetables (beans, sprouts, cabbage, spinach, soybeans, grains, hops, garlic, onion,…), wine, tea, and they have been identified in a number of botanical dietary supplements. They include a wide variety of structurally different compounds such as isoflavones, mainly found in soy, lignans found in grains, stilbenes found in the skin of grapes. Other less investigated compounds include flavones, flavans, isoflavanes and coumestans. The estrogenic or antiestrogenic activity of any chemicals depends on the ability of the compound to interact with the ERs (ERα , ERβ ).
This article reported the knowledge about the activity of phytoestrogens from a pharmacological point of view for their estrogenicity or antiestrogenicity.
Keywords: phytoestrogens, nutrition, bioactive compounds, soy, bone health
Introduction
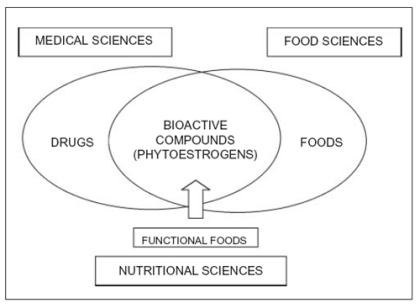
Within the past several years, the relation between diet and health has been accepted by the mainstream nutrition community, and in this connection interest in the physiological role of bioactive compounds present in plants has dramatically increased over the last decade. As a complex mixture of chemicals, foods provide essential nutrients, requisite calories, and other physiologically active constituents that may be useful for life and health. A new paradigm for “optimal nutrition” may be evolving that would identify physiologically active components that contribute to diseases prevention. In thus functional foods concept is unifying the medical, nutritional and food sciences (Fig.1). Collectively, plants contain several different families of natural bioactive products among which are compounds with weak estrogenic or antiestrogenic activity toward mammals. Of particular interest, in relation to human health, are these plant derived estrogens, or phytoestrogens, which embody several groups of non-steroidal estrogens widely distributed within the plant kingdom. Although in vitro and animal studies provide preliminary plausible mechanisms to explain how phytoestrogens act, the applications of diets rich in such compounds and their consequent biological effects still need to be fully examined, tested and confirmed through traditional scientific experimental pathway (TableI). Phytoestrogens are strikingly similar in chemical structure to the mammalian estrogen, estradiol, and bind to estrogen receptors (ERs) with the preference for ERβ (1). This suggests that these compounds may exert tissue specific effects, beside other non receptor mediated biological activities, as antioxidant capacity and antiproliferative/antiangiogenic effects.
Figure 1.
Phytoestrogens scientific research area.
Table I -.
Research needed to clarify possible roles of phytoestrogens in health.
|
Modified from Bidlack WR and Wang W. In “Modern Nutrition in Health and Disease”. Eds. Shils ME et al. 1999, 1823-1833.
Natural estrogens are involved in a multiplicity of programmed events in target tissues as uterus, breast, pituitary gland and hormone responsive tumors. The initiation of estrogen action by all of the estrogens is considered to be the same in each target tissue. Estrogen firstly bind to the nuclear ER, then an estrogenic ligand causes a conformational change that encourages dimerization and interaction with either specific DNA sequences or a protein-protein interaction with AP-1 or Sp1 sites in the promoter region of estrogen-responsive genes (2). These events herald the biological effects of estrogen in the specific target tissue or tumor. A small percentage (2-3%) of ERs are located on the cell membrane and contribute to non genomic effects of estrogen (3). Two ERs are currently known, ERα and ERβ. Although the two ERs can be localized within the same cell, they vary in tissue distributions and can have different effects on mixed agonist and antagonist molecules.
Phytoestrogens include a wide variety of structurally diverse compounds such as isoflavones, mainly found in soy, lignans found in grains, stilbenes found in the skin of grapes. Other less investigated compounds include flavones, flavans, isoflavanes and coumestans (4). The estrogenic or antiestrogenic activity of any chemicals depends on the ability of the compound to interact with the ERs (ERα, ERβ).
The aim of this article is to describe the substances that, up to date, has been validated from a pharmacological point of view for their estrogenicity or antiestrogenicity.
Phytoestrogens rich foods
Phytoestrogens are present in many human foodstuffs including fruits (plum, pear, apple grape berries…), vegetables (beans, sprouts, cabbage, spinaches, soybeans, grains, hops, garlic, onion, …), wine, tea, and have been identified in a number of botanical dietary supplements (TableII). Plants vary intra- and inter-species in the types and concentrations of phytoestrogens due to variables in plant growth, soil, weather conditions and the age of plant. Chemically phytoestrogens are phenolic phytochemicals or polyphenols. These are the largest category of phytochemicals and the most widely distributed in the plant kingdom (5).
Table II -.
Levels of isoflavones and lignans in various food sources. Values (in nanomoles per gram dry weight) were determined by isotope dilution gas chromatography mass spectrometry with selected ion monitoring. Mazur W, Adlercreutz H. 1998, modified.
| Plant species (Common name) | Genistein | Daidzein | Secoisolariciresinol | Metairesinol |
|---|---|---|---|---|
| Soybean | 993-3115 | 413-2205 | < 1-8 | < 1 |
| Kidney bean | < 1-19 | < 1-2 | 2-4 | < 1 |
| American groundnut | 4-30 | < 1 | < 1-2 | < 1 |
| Chicpea | 3-8 | < 1-8 | < 1 | 0 |
| Pea | < 1 | < 1 | < 1 | < 1 |
| Lentil | < 1 | < 1 | < 1 | < 1 |
| Kudzu root | 467 | 7283 | < 1 | < 1 |
| Flaxseed | 0 | 0 | 10-247 | 30 |
| Sunflower seed | < 1 | 6 | 2 | 17 |
| Peanut | 2 | 1 | 8 | < 1 |
| Wheat bran | < 1 | < 1 | 3 | 0 |
| Barley | < 1 | < 1 | 2 | 0 |
| Rye bran | 0 | 4 | 4 | 5 |
| Strawberry | 0 | 0 | 33 | < 1 |
| Cranberry | 0 | 0 | 29 | 0 |
| Bluberry | 0 | 0 | 23 | 0 |
| Rasberry | 0 | 0 | 4 | 0 |
| Red cabbage | < 1 | < 1 | 4 | < 1 |
| Broccoli | < 1 | < 1 | 11 | < 1 |
| Garlic | 0 | 0 | 11 | < 1 |
| Zucchini | 0 | 0 | 23 | < 1 |
| Carrot | 0 | 0 | 10 | < 1 |
| Breetroot | 0 | 0 | 3 | < 1 |
| Black tea | Trace | Trace | 73 | 12 |
| Green tea | Trace | Trace | 75 | 5 |
Polyphenols in plants show a multiple function, they act as antioxidants (protection against UV light), exert a protective action from insects, fungi, viruses and bacteria, and a visual attention-pollinator attraction (they are responsible of plant and flower colours) and they can also serve as feed-repellents and plant hormone controllers.
The study on phytoestrogens started in the ‘50s when it was realized that some plants derived substances could cause an estrogenic effect. Sheep that were grazing on pastures containing red clover had multiple fertility problems and it was shown that the clover present in this pastures had high amounts of isoflavones (6), in particular formononetin and biochanin A.
The phytoestrogens classification is controversial, anyway currently we can subdivide the phytoestrogens in four main classes: flavonoids (flavones and isoflavones), lignans, coumenstans and stilbenes (TableIII).
Table III -.
Main chemical categories of phytoestrogens.
| Lignans | Flavonols | Coumestans | Isoflavones | Stilbenes |
|---|---|---|---|---|
| Enterolactone | Quercetin | Cumestrol | Glycitein | Resveratrol |
| Enterodiol | Rutin | Genistein | ||
| Daidzein |
Flavonoids
They are the largest group of plant phenols including more than 4000 different compounds which are the most studied phytochemicals. The basic flavonoid structure allows a multitude of variations in chemical structure, giving rise to flavonols (quercetin, kaempferol, myricetin), flavones (apigenin, luteolin), flavanones (catechin, epicatechin), antocyanidins and isoflavonoids (glycitein, genistein, daidzein) (7). An important effect of flavonoids is the scavenging of oxygen-derived free radicals. The major source of isoflavonoids in the diet is from soy-based foods. The isoflavonoids from legumes, including genistein and daidzein, are the most studied phytoestrogens. They can exist as glycosides or as aglycones, the glucosides being readily hydrolized in the gut as their aglycones. The aglycones are easily transported across intestinal epithelial cells. Genistein has one-third the potency of estradiol when interacts with ERα, and one thousandth of the potency of estradiol when it interacts with ERβ as determined by expression of luciferase reporter gene construct in kidney cells that have been cotransfected with ERα and ERβ (8). Genistein may produce similar effects to estradiol in several different tissues as breast, ovarian, endometria, prostate, vascular , bone tissue and cell lines (9, 10). Furthermore, genistein induce also responses that are not associated with the ER, as the inhibition of tyrosine kinase and DNA topoisomerase (11). Such effect is produced even in the presence of the antiestrogen revealing a non genomic action that could explain a part of the difference between genistein and estradiol.
In vitro experimental systems also showed that flavonoids possess anti-inflammatory, antiallergic, antiviral and anticarcinogenic properties (12) and various of these molecules, notably isoflavonoids, are identified as phytoestrogens being able to bind estrogens receptors, and possess estrogenic or antiestrogenic activities (13, 14).
Lignans
They are constituens of higher plants, such as whole grains, legumes, vegetables and seeds with exceptionally high concentrations of lignans found in flaxseed. Although previously though to be present only in higher plants, mammalian lignans have been detected in the biological fluids of humans and animals. The chemical structure of plant lignans is very different from that of mammalian lignans and most of the changes occur in the colon, liver and small intestine. Enterolactone and enterodiol are metabolites of the plant lignans metairesinol e secoisolariciresinol, respectively (15). A clinical study showed that the excretion of the lignans, enterodiol and enterolactone, was significantly higher during a carotenoid (carrots and spinaches) and cruciferous (broccoli and cauliflowers) vegetable diet, than during a vegetable-free diet (16).
Coumestans
Legumes are the main source of coumestrol, the coumestan showing the highest estrogenic activity, and low level of coumestrol have been found also in brussel sprouts and spinaches, while the highest concentrations are reported in clover and in soybean sprouts.
Stilbenes
Recently, also the stilben resveratrol has been identified as a phytoestrogen. Resveratrol is a natural compound produced by some plants, such as grapevines, in response to injury. Peanuts are rich in resveratrol too. Polygonium cuspidatum roots, which have long been used in traditional oriental medicine, have been identified as the major active source of stilben phytoalexins. Trans resveratrol was firstly detected in grapevines in 1976 by Langcake and Pryce, who found that this compound was synthesized by leaf tissues in response to Botrytis cinerea fungal infection or exposure to ultraviolet light (17).
The biological activity of resveratrol was attested about fifteen years ago by the health benefits obtained by orally administered resveratrol. An experimental study suggested that an average drinker of wine may, in the long term, absorb a quantity of resveratrol sufficient to explain the beneficial effect of red wine on human health (18). Resveratrol possesses anticancer properties, antioxidant activity and some observations have challenged the protective effects of resveratrol against atherosclerosis then preventing cardiovascular diseases. As a phytoestrogen it may favourably influence several physiological processes and given that resveratrol has a different structure, also its mechanism of action might differ somewhat from that of other flavonoids (17, 18).
Health beneficial effects of phytoestrogens
In the last two decades many clinical and epidemiological studies have been performed to test the health effects of foods and supplements rich in phytoestrogens. A recent review summarizes the main results from scientific studies (19). The effects of phytoestrogens administration are evaluated on different clinical endpoints as listed in TableIV.
Table IV -.
Summary of phytoestrogen clinical studies.
| Clinical endpoints | Positive results | Total |
|---|---|---|
| Maintaining bone density | 11 | 15 |
| Relief menopause symptoms | 4 | 17 |
| Cardiovascular benefit | 25 | 38 |
| Cancer prevention | 7 | 13 |
| Hormon levels/menstrual cycle | 12 | 19 |
| Effect of hormones in men | 0 | 1 |
| Immune system | 1 | 1 |
| Neurological | 5 | 5 |
| Totals | 64 | 105 |
Totals are less than the sum of the studies since some studies examined several clinical endpoints. Modified from Cornwell et al. 2004 (ref.19).
Cardiovascular health
Estrogen may affect the vascular system both directly through the ERs located on vascular tissue, and indirectly through altering the lipoprotein profile (20). Initial epidemiologic studies showed that women taking hormone replace therapy (HRT) were 50% less likely to experience severe cardiovascular disease (CVD) than women not assuming HRT (21). Lately, a more comprehensive study performed by the Women’s Health Initiative has demonstrated a significant increase in CVD in the first year of HRT use, permaining is still elevated after 5 years of continued use (22-24). There are several clinical studies that have examined the effect of phytoestrogens on CVD. Isoflavonoids or soy/soy proteins and flaxseed have the ability to lower total cholesterol (25, 26), LDL cholesterol (25, 27) and to raise HDL cholesterol (28, 29). On the contrary, other studies showed no effect of isoflavonoids derived from soy or red clover on serum cholesterol levels or plasma lipids (30, 31). Although no clinical studies showed that resveratrol improves cardiovascular function, the consumption of red wine has been linked to the French Paradox, phenomenon observed in French people who have a diet similar to the North American diet with a significantly lower rate at CVD (32).
Cancer
There is a large body evidence coming from epidemiologic studies showing people who consume high amounts of isoflavonoids in their diets have lower rates of occurrence of several cancers including breast, prostate and colon cancer (33).
The protective effect of phytoestrogens on cancer may be due to their role in lowering circulating levels of unconjugated sex hormones. Estrogens mainly circulate as inactive conjugates of sex hormones binding globulin (SHBG) or albumin (34). Dietary supplementation with soy isoflavonoids or lignans produced an increase of the levels of SGHBG in postmenopausal women, lowering the serum levels of estradiol (35, 36). Furthermore, higher intakes of soy products and flaxseeds produced a significant decrease of the urinary excretion of genotoxic estrogen metabolites (37). Moreover, the role of phytoestrogens in preventing cancer in women may be related to changes in menstrual cycle lenght (38). Increases in menstrual cycle length together with eating a diet rich in phytoestroegens correlate with a decreased rate of hormone–dependent cancers development, including endometrial, ovarian, and breast cancer (39).
Menopausal symptoms
Epidemiological studies show that Asiatic women experience hot flashes less frequently than the Western women (20% versus 80%, respectively).
Diet has been included among the different reasons proposed to explain such proven difference. Indeed, Asian diet is known to be rich in phytoestrogens and even though only few clinical trials have been conducted to evaluate the role of these bioactive substances in regulating the menopause symptoms, most data from randomized studies indicate a significant drop in the severity and frequency of menopausal symptoms. However, women given placebos also demonstrated a decline in menopausal symptoms (40, 41). The variability in frequency and severity of the hot flashes and the high placebo-response rate make these clinical trials difficult to interpret and, currently, they are not sufficient to demonstrate the effectiveness of phytoestrogens in reducing menopausal symptoms. However, considering the lower than expected effectiveness of HRT (22), it is not surprising that many new studies, planned to show the benefit in the reduction of menopause symptoms with phytoestrogen supplementation, are in progress.
Soy and cognitive function
There is a clear evidence that treatment of postmenopausal women with mammalian estrogens improves memory and may alleviate the decline of cognitive function and the risk of dementia (42). A few studies have examined the effect of phytoestrogens on cognitive function. Data from a follow-up study of the cognitive functions showed improvement in pictures recall, sustained attention and ability to plan tasks, but on the other side these women did not demonstrate any improvement in mood or sleepiness, suggesting a specific action on frontal lobe functions (43). In summary, there are too few studies to evaluate whether phytoestrogens may exert some effects on cognitive abilities resulting in a better quality of life in postmenopausal women.
A nutritional model: soy-foods and bone health
Soy intake is part of the regular diet of the Asian populations. Observations that soy consuming populations have lower hip fracture rate have given rise to the hypothesis that the intake of soy–proteins and/or soy–derived isoflavonoid phytoestrogens may be protective for bone health (44). Researchers have long recognized that Asian women, consuming traditional diet, enjoy better cardiovascular and bone health than their counterparts in Western societies. Nutrition epidemiologists believe that this may partly be the result of the soy rich diet. The scientific interest of isoflavones began with epidemiological studies of elderly women in Asian countries who consume high levels of isoflavones from soy products. The studies showed a positive correlation between a lower prevalence of hip fractures and the intake of soy–food (45). However, this type of association is inconclusive, as Asian women have different hip geometry than Caucasian women (46). Further epidemiological studies, conducted in Japan and in Hong Kong to directly examine the linkage between dietary soy–foods intake and lumbar spine bone mineral density (BMD) in Asian women, showed a significantly greater BMD in women consuming the highest level of dietary isoflavones compared to those who consumed the least (47). These results do not demonstrate how this protective effects of soy and/or their isoflavones is related to the length of exposure that, usually, for the majority of Asians is over the entire life span (48).
Various studies on animal model, using ovariectomized female rats fed with soy-foods or soy derivatives have shown comparable bone-sparing effects of 17β-estradiol and soy protein isolate (49), genistein (50) or daidzein (51).
Clinical trials on the effects of soy isoflavones on bone are too limited and have shown mixed results (52, 53). Studies have used both soy protein isolate with isoflavones and isolated isoflavones, but the published clinical studies have been of short duration with only one 12-months trial. The 6 months studies demonstrated promising effects of soy protein isolates containing isoflavones on spine BMD in peri- and postmenopausal women, but they were too short to adequately evaluate the impact of an intervention on BMD (52). However, the 12 months trial provided confirmations of the positive effect of genistein on bone (53), although no consistent changes in biochemical markers of bone turnover have been reported.
None of the studies in both animals and humans have assessed the effects of soy isoflavones on calcium metabolism. Such information is essential in ascertaining the mode of action of isoflavones on the skeleton. In addition to the estrogenic role of isoflavones in bone health, it has been also demonstrated that many soy foods are a good source of calcium and soy proteins are less calciuric than animal proteins. Although soybeans contain both oxalates and phytates, which are inhibitors of calcium absorbtion, calcium bioavailability from soy beans has been shown to be equivalent to that from milk (54). An exception is represented by calcium-fortified soy beverage from which calcium absorption was reported to be 75% of that from cows milk (55). In comparison to animal proteins, soy proteins contain less sulfur-containing aminoacids. One study found that subjects consuming animal protein-based diets excreted 150 mg of urinary calcium per day in comparison with about 100 mg of those subjects consuming a soy protein-based diet (56).
In conclusion, the habitual intake of soy, as traditional Asian food, appears to be beneficial to bone health, though the effective or optimal dosage is still unclear. The soy-bone action could be mediated through its estrogenic or calcium conserving effects. In addiction, soy intake also contributes to an increase in high quality protein intake. Therefore, this traditional dietary practice should be encouraged and preserved in Asian populations.
Although European countries have no soy consumption tradition, another plant estrogen group, known as lignans, seems to have a positive impact on bone health in European countries, as well as in the vegetarian population. This is due to the relatively high levels of these compounds in certain grains, especially in rye, as well as in flaxseed that are part of the daily diet in these populations. Interestingly, there is a striking similarity between the European vegetarian and Asian-Japanese populations, exhibiting a lower incidence of osteoporosis compared to the non-vegetarian populations (57).
A pharmacological model: phytoestrogens and bone health
Most of the studies suggest that phytoestrogens are somewhat effective in maintaining BMD in postmenopausal women (58-65). Moreover, recent results from the Women’s Health Initiative Study, showing an unexpected lack of cardioprotective effects of HRT (22), pushed the research for alternative and natural strategies for managing and preventing osteoporosis. The last full review (66) about the dietary phytoestrogens and their effect on bone justifies the bone-conserving properties of isoflavones by the following bodies of experimental evidences showing tight similarities with an experimental pharmacological model from in vitro studies of cultured bone cells through animal models of osteoporosis to epidemiological and intervention studies on humans.
Most of the in vitro studies with human and animal osteoblasts and osteoblast-like cell-lines have shown that daidzein and genistein have a stimulatory effect on protein synthesis and on alkaline phosphatase release (67, 68). This effect is blocked by the addition of actinomycin or cycloheximide, suggesting that these isoflavones influence transcriptional or translational events (66). More recently, genistein has been found to stimulate the production of osteoprotegerin (OPG) by human osteoblasts, providing a further mechanism for the bone-sparing effects of soy isoflavones. Besides ER-dependent processes, genistein and daidzein both suppress osteoclast activity by several mechanisms, including apoptosis, activation of protein tyrosine phosphatase, inhibition of cytokines, changes in intracellular Ca++, membrane depolarization and antioxidant activity, much like many other polyphenols (69, 72). In some cases the antioxidant effect occurs in the nM range. Furthermore a positive synergy between phytoestrogens and other antioxidants has been also described (73).
A number of observational and dietary intervention studies confirm the general findings from in vitro effects of phytoestrogens on human bone cells in culture. Thus far, observational or epidemiologic studies (74, 75) and dietary intervention studies (76, 81) have shown significant relationships between phytoestrogens and surrogate markers of bone turnover such as urinary calcium, magnesium and phosphorous, hydroxyproline, and collagen cross-links and serum markers including bone specific alkaline phosphatase, osteocalcin, insulin-like growth factor I (IGFI), and interleukin 6 (TablesVaandVb). Most of the observational studies have been performed in women living in Countries where the indigenous population have relatively high phytoestrogen intake, mostly related to the soy protein foods.
Table V -.
Intervention studies with phytoestrogens and bone health in womenincluding findings on BMD and BMC.
| Reference | Subjects | Intervention (protein type and daily isoflavone level) | Study length (months) | Findings BMD or BMC endpoint by DXA |
|---|---|---|---|---|
| Dalais et al., 1998 | Postmenopausal n = 45 | Soy grits 45 g Wheat kibble 45 g Flaxseed 45 g | 3 | All 3 groups had increased in BMC. Soy group 5.2%, Flax group 5.2% and Control group 4.0% |
| Potter et al., 1998 | Postmenopausal n = 66 | Casein 40 g/d Soy 40 g/56 mg Soy 40 g/90 mg Ca supplemented | 6 | + 2.2% increase in lumbar spine BMD in soy 90 mg isoflavone group. The 56 mg isoflavone group unchanged No changes in other sites. |
| Clifton-Bligh et al., 2001 | Postmenopausal n = 46 | Clover-derived tablets 28.5, 57 and 85.5 mg | 6 | BMD at proximal radio and ulna increased 4.1% with 57 mg/d and 3.0% with 85.5 mg/d.The 18.5 mg/d remained unchanged. No increase in endometrial thickness was seen in any group. |
| Anderson et al., 2002 | Young healthy Adult women n = 27, age 21-25 y | Isoflavone-rich diet, 90 mg, compared with control diet | 12 | No effect of soy diet on BMD or BMC in healthy, menstruating women |
Modified from Setchell KDR et al., 2003 (68).
BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; uDpy, urinary deoxypiridinoline; PTH. parathyroid hormone; BAP, bone-specific alkaline phosphatase; IGF-I, insulin-like growth factor; ERT, estrogen replacement therapy; NTx, crosslinked N-telopeptides of type I collagen; OC osteocalcin; Ca, calcium; ALP, alkaline phosphatase.
Table VI -.
Intervention studies with phytoestrogens and bone health in women including findings on bone markers.
| Reference | Subjects | Intervention (protein type and daily isoflavone level) | Study length | Findings Bone markers endpoints |
|---|---|---|---|---|
| Wong, 2000 | Postmenopausal n = 6 Open pilot study Mean age 55.4 y | Soy isoflavones 160 mg/d | 6 wk | Resorption markers: uDpy -7 ± 41%, Urinary Ca concentration -12 ± 46% s PTH +33 ± 60% Urinary Ca excretion -5 ± 29% Bone formation markers: sOC 9 ± 15% |
| Serum BAP -16 ± 23%, IGF-I -8 ± 27% Changes are of similar magnitude to those reported for ERT. | ||||
| Scheiber, 2001 | Postmenopausal n = 42 Mean age 55.5 y Open pilot study | Whole soy foods, 60 mg isoflavones/d No Ca supplementation | 3 mo | Resorption markers: serum ALP unchanged, urinary NTx decreased 13.9% Formation markers: serum OC increased 10.3%. |
| Lu et al., 2002 | Postmenopausal n = 12 Age range: 49-66 y | Soy milk 1.08 L/d ≈ 112 mg isoflavones | 4 mo | Increase in uDpy 21% and serum BAP 18%, increase in OC 34%. Values returned to prediet level 4 mo after diet. No estrogenic effects on uterus. No changes in serum levels of PTH, follicle-stimulating hormone or estradiol. |
Modified from Setchell KDR et al., 2003 (68).
BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; uDpy, urinary deoxypiridinoline; PTH. parathyroid hormone; BAP, bone-specific alkaline phosphatase; IGF-I, insulin-like growth factor; ERT, estrogen replacement therapy; NTx, crosslinked N-telopeptides of type I collagen; OC osteocalcin; Ca, calcium; ALP, alkaline phosphatase.
Conclusions
Since it has now been established that lifestyle and particularly nutritional habits significantly contribute to the occurrence of different rates of degenerative disorders, such as cardiovascular diseases, osteoporosis, cognitive disability, and cancer, in recent years Eastern and Western governments and industries have started to invest in the evaluation of the health effects of phytochemicals contained in the diet. This aspect is becoming a main feature of preventive medicine since life expectation is growing in the Western World population.
Up to date, it is not still completely clear if the foods rich in duce extract compounds from this foodstuffs, researchers may such compounds may really be protective on human health take advantage to plan experimental studies and clinical trials and, in the event of, at which daily regimen they should be as-in which these molecules can be tested in relation to biomarksumed to exert a real pharmacological action. Moreover, non ers and other functional parameters connected with human estrogenic compounds are coexisting with phytoestrogens in pathophysiology. Consequently, the possibility of testing the the same plant-derived food and the role that they may play on purified phytochemical compounds, both in in vitro and in vivo both activity and bioavailability of phytoestrogen themselves experimental models, may offer the opportunity to corroborate has not been elucidated. Since at present industries can pro-data obtained from epidemiological studies.
Unfortunately, due to the great diversity of phytoestrogen compounds, including different bioavailability, pharmacokinetics, pharmacological properties and metabolic fates, it is quite complex to define, assess and understand their precise effects on human health since large and time extended intervention studies are needed.
Osteoporosis represents one of the human disease models more largely studied for the potential therapeutic effects of phytoestrogens. Currently, the results from the few studies on bone health are seductive but too few to draw definitive conclusions. Supporting data from in vitro and in vivo studies of models of osteoporosis strongly shows bone-sparing effects from dietary phytoestrogens. Such data may justify large-scale clinical dietary intervention studies of phytoestrogens in early future.
Finally, the preliminary data in this field suggest to include phytoestrogen rich foods in the health foods sector and push scientists to isolate the active molecules to be used as new potential drugs.
References
- 1.Cassidy A. Potential risks and benefits of phytoestrogen-rich diets. Int J Vitam Nutr Res. 2003;Mar doi: 10.1024/0300-9831.73.2.120. [DOI] [PubMed] [Google Scholar]
- 2.Jordan VC, Mc Gregor Schafer J, Levenson AS, et al. Molecolar classification of estrogens. Cancer Res. 2001;September 15(61):6619–23. [PubMed] [Google Scholar]
- 3.Chen DB, Bird IM, Zheng J, et al. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acutet activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–25. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 4.Mueller SO. Overview of in vitro tools to assess the estrogenic and antiestrogenic activity of phytoestrogens. J Chromatog B. 2002;777:155–65. doi: 10.1016/s1570-0232(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 5. UCLA Center for Human Nutrition Phytoestrogens at http: //www.cellinteractive.com/ucla/natural_remedies/phytoestrogens.html 26/04/2004
- 6.Rossiter RC, Beck AB. Physiological and ecological studies on the estrogenic isoflavones in subterranean clover (tifolium subterraneum) I. Effects of temperature. Aust J Agrc.Res. 1996;17:29–37. [Google Scholar]
- 7. UCLA Center for Human Nutrition – Flavonoids and health at http://www.cellinteractive.com/ucla/natural_remedies/flavonoids.html 26/04/2004.
- 8.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Gutkowska J, Marcinkiewicz M, et al. Genistein supplementation stimulates the oxytocin system in the aorta of ovariectomized rats. Cardiovasc Res. 2003;57:186–194. doi: 10.1016/s0008-6363(02)00655-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhou S, Turgeman G, Harris Stephen E, et al. Estrogens activate bone morphogenetic protein-2 gene trascription in mouse mesenchymal stem cells. Mol Endocrinol. 2003;17:56–66. doi: 10.1210/me.2002-0210. [DOI] [PubMed] [Google Scholar]
- 11.Jonas J, Plant T, Gilon P, et al. Multiple effects and stimulation of insulin secretion by the tyrosine kinase inhibitor genistein in normal mouse islets. Br J Pharmacol. 1995;114:872–880. doi: 10.1111/j.1476-5381.1995.tb13285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nijveldt RJ., van Nood E., van Hoorn DEC, et al. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 13.Van der Woude H, Gliszczynska-Swiglo A, Struijs K, et al. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003;Oct 8, 200(1):41–7. doi: 10.1016/s0304-3835(03)00412-9. [DOI] [PubMed] [Google Scholar]
- 14.Prouillet C, Mazière JC, Mazière C, et al. Stimulatory effect of naturally occurring flavonols querceetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblast through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67:1307–13. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Tham DM, Gardner CD, Haskell WL. Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological and mechanistic evidence. J Clin Endocrinol Metab. 1998;83:2223–35. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 16.Kirkman LM, Lampe JW, Campbell DR, et al. Urinary lignan and isoflavonoid excretion in man and women consuming vegetable and soy diet. Nutr Cancer. 1995;24:1–12. doi: 10.1080/01635589509514388. [DOI] [PubMed] [Google Scholar]
- 17.Frémont L. Biological effects of resveratrol. Life Sciences. 2000;66(8):663–73. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 18.Bertelli A, Bertelli AA, Gozzini A, et al. Plasma and tissue resveratrol concentrations and pharmacological activity. Drugs Exp Clin Res. 1998;24(3):133–8. [PubMed] [Google Scholar]
- 19.Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Rubanyi GM, Johns A, Kaiser K. Effect of estrogen on endothelial function and angiogenesis. Vasc Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 21.Lissin LW, Cook JP. Phytoestrogens and cardiovascular health. J Am Coll Card. 2000;35:1403–1410. doi: 10.1016/s0735-1097(00)00590-8. [DOI] [PubMed] [Google Scholar]
- 22.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Iniative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 23.Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health related quality of life. New Engl J Med. 2003;348:1835–7. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 24.Manson JE, Hsia J, Johnson KC, et al. The Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. New Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 25.Lemay A, Dodin S, Kadri N, et al. Flaxseed dietary supplement versus hormone replacement thrapy in hypercholesterolemic menopausal women. Obstet Gynecol. 2002;100:495–504. doi: 10.1016/s0029-7844(02)02123-3. [DOI] [PubMed] [Google Scholar]
- 26.Teede HJ, Dalais FS, Kotsopoulos D, et al. Dietary soy has both beneficial and potentially adverse cardioascular effects: a placebo controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–60. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- 27.Jayagopal V, Albertazzi P, Kilpatrick ES, et al. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25-990-4 doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- 28.Potter S, Baum J, Teng H, et al. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998;68:1375–9. doi: 10.1093/ajcn/68.6.1375S. [DOI] [PubMed] [Google Scholar]
- 29.Sanders TAB, Dean TS, Grainger D, et al. Moderate intakes of intact soy protein rich in isoflavones compared with ethanol-extracted soy protein increase HDL but do not influence transforming groeth factor beta(1) concentrations and hemostatic risk factors for coronary heart disease in healthy subjects. Am J Nutr. 2002;76:373–377. doi: 10.1093/ajcn/76.2.373. [DOI] [PubMed] [Google Scholar]
- 30.Howes JB, Sullivan D, Lai N, et al. The effect of dietary supplementation with isoflavones from red clover on the lipoprotein profiles of post menopausal women with mild to moderate hypercholesterolemia. Atherosclerosis. 2000;152:143–7. doi: 10.1016/s0021-9150(99)00437-2. [DOI] [PubMed] [Google Scholar]
- 31.Dewell A, Hollenbeck CB, Bruce B. The effects of the soy derived phytoestrogens on serum lipids and lipoproteins in moderately hypercholesterolemic postmenopausal women. J Clin Endocrinol Metab. 2002;87:118–21. doi: 10.1210/jcem.87.1.8155. [DOI] [PubMed] [Google Scholar]
- 32.Asby J, Tinwell H, Pennie W, et al. Partial and weak estrogenicity of the red wine constituent resveratrol 4: consideration of its superagonist activity in MCF-7 cells and its suggestedcardiovascular protective effects. J Appl Toxicol. 1999;19:39–45. doi: 10.1002/(sici)1099-1263(199901/02)19:1<39::aid-jat534>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.American Institute for Cancer Research. World Cancer Research Fund; Washinghton: 1997. Food Nutrition and the Prevention of Cancer. A Global Perspective. [DOI] [PubMed] [Google Scholar]
- 34.Dotsch J, Dorr HG, Wildt L. Endocrine disruptors Part 1. Ed. Metzler M. Berlin Heidelberg: Springer-Verlag; 2001. Exposure in endogenous estrogens during lifetime; pp. 83–99. [Google Scholar]
- 35.Hutchins AM, Martini MC, Olson BA, et al. Flaxseed compsumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer. 2001;39:58–65. doi: 10.1207/S15327914nc391_8. [DOI] [PubMed] [Google Scholar]
- 36.Berrino F, Bellati C, Secreto, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: the diet and androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev. 2001;10:25–33. [PubMed] [Google Scholar]
- 37.Xu X, Duncan AM, Merz BE, et al. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;7:1101–1108. [PubMed] [Google Scholar]
- 38.Nagata C, Takatsuka N, Inaba S, et al. Effect of soymilk consumption on serum estrogen concentrations in premenopausal Japanese women. J Natl Cancer Inst. 1998;90:1830–1835. doi: 10.1093/jnci/90.23.1830. [DOI] [PubMed] [Google Scholar]
- 39.Tsourounis C. Clinical effects of phytoestrogens. Clin Obst Gynecol. 2004;44:836–842. doi: 10.1097/00003081-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Tice JA, Ettinger B, Ensrud K. Phytoestrogen supplements for the treatment of hot flashes: the isoflavone clover extract (ICE) study: a randomizzed controlled trial. JAMA. 2003;290:207–214. doi: 10.1001/jama.290.2.207. [DOI] [PubMed] [Google Scholar]
- 41.Nikander E, Kikkinen A, Metsa-Heikkila M, et al. A randomized placebo-controlled crossover trial with phytoestrogens in treatment of menopause in breast cancer patients. Obst Gynecol. 2003;101:1213–1220. doi: 10.1016/s0029-7844(03)00232-1. [DOI] [PubMed] [Google Scholar]
- 42.Yaffe K, Sawaya G, Lieberburg I, et al. Estrogen therapy in postmenopausal women: Effects on cognitive function and dementia. JAMA. 1998;279:688–95. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 43.Duffy R, Wiseman H, File SE. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol Biochem Behav. 2003;75:721–29. doi: 10.1016/s0091-3057(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 44.Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70:439S–50S. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 45. Lacey JM, Anderson JJB. Older women in Japan and the United States: physical and nutritional comparisons In Takahashi ed. Bone Morphometry: Prooceedings of the Fifth International Congress Nishimura, Japan 1991 562 5 [Google Scholar]
- 46.Nakamura T, Turner CH, Yoshikawa T, et al. Do variations in hip geometry explain differences in hip fracture risk between Japanese and white Americans? J Bone Miner Res. 1994;9(7):1071–6. doi: 10.1002/jbmr.5650090715. [DOI] [PubMed] [Google Scholar]
- 47.Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clinical Endocrinol Metabol. 2001;11:5217–21. doi: 10.1210/jcem.86.11.8040. [DOI] [PubMed] [Google Scholar]
- 48. Cai DJ, Spence LA, Weaver CM. Soy isoflavones and bone health In New SA and Bonjour JP, eds Nutritional aspects of bone health Cambridge: The Royal Society of Chemistry; 2003. 421 438 [Google Scholar]
- 49.Ajrmandi BH, Alekel L, Hollis BW, et al. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996;126:161–167. doi: 10.1093/jn/126.1.161. [DOI] [PubMed] [Google Scholar]
- 50.Fanti P, Monier-Faugere MC, Geng Z, et al. The phytoestrogen genistein reduces bone loss in short term ovariectomized rats. Osteoporosis Int. 1998;8:274–281. doi: 10.1007/s001980050065. [DOI] [PubMed] [Google Scholar]
- 51.Ishida H, Uesugi T, Hirai K, et al. Preventive effects of the plants isoflavones, daidzeine and genisteine on bone loss in ovariectomized rats fed a calcium-deficient diet. Biol Pharm Bull. 1998;21:62–66. doi: 10.1248/bpb.21.62. [DOI] [PubMed] [Google Scholar]
- 52.Alekel L, St. German A, Peterson CT, et al. Isoflavone-rich soy protein isolate exerts significant bone-sparing in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;729:844–852. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- 53.Morabito N, Crisafulli A, Vergara C, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17:1904–1912. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- 54.Poneros AG, Erdman JW Jr. Bioavailability of calcium from tofu, tortillas, nonfat dry milk and mozzarella cheese in rats, effect of supplemental ascorbic ascorbic acid. J Food Sci. 1993;58:382–384. [Google Scholar]
- 55.Heaney RP, Dowell MS, Rafferty K, et al. Bioavailability of the calcium in fortified soy imitation milk some observations on method. Am J Clin Nutr. 2000;71:1166–1169. doi: 10.1093/ajcn/71.5.1166. [DOI] [PubMed] [Google Scholar]
- 56.Breslau NA, Brinkley L, Hill KD, et al. Relationship of animal protein rich diet to kidney stone formation and calcium metabolism. J Clin Endocrin Meter. 1988;66:140–146. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 57.Brouns F. Soy isoflavones: a new and promising ingredient for the health foods sector. Food Res Int. 2002;35:187–93. [Google Scholar]
- 58.Dalais FS, Rice GE, Wahlqvist ML, et al. Effects of dietary phytoestrogens in postmenopausal women. Climateric. 1998;1:124–9. doi: 10.3109/13697139809085527. [DOI] [PubMed] [Google Scholar]
- 59.Kaardinal AFM, Morton MS, Bruggemann-Rotgans IEM, et al. Phytoestrogen excretion and rate of bone loss in postmenopausal women. Eur J Clin Nutr. 1998;52:850–5. doi: 10.1038/sj.ejcn.1600659. [DOI] [PubMed] [Google Scholar]
- 60.Alekel DL, Germain AS, Peterson CT, et al. Isoflavone rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;72:844–52. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- 61.Ho SC, Chan SG, Yi Q, et al. Soy intake and the maintainance of peak bone mass in Hong Kong Chinese women. J Bone Miner Res. 2001;16:1363–9. doi: 10.1359/jbmr.2001.16.7.1363. [DOI] [PubMed] [Google Scholar]
- 62.Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001;86:5217–21. doi: 10.1210/jcem.86.11.8040. [DOI] [PubMed] [Google Scholar]
- 63.Chiechi LM, Secreto G, D’Amore M., et al. Efficacy of soy rich diet in preventing postmenopausal osteoporosis: the Menfis randomized trial. Maturitas. 2002;42:295–300. doi: 10.1016/s0378-5122(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 64.Kim MK, Chung BC, Yu VY, et al. Relationships of urinary phyto-oestrogen excretion to BMD in postmenopausal women. Clin Endocrinol. 2002;56:321–8. doi: 10.1046/j.1365-2265.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 65.Morabito N, Crisafulli A, Vergara C, et al. Effects of genistein and hormone-replacement therapy on bone loss in early post nopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17:1904–12. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- 66.Setchell KDR, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evince from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003;78:593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- 67.Sugimoto E, Yamaguchi M. Anabolic effect of genistein in osteoblastic MC3T3-E1 cells. Int J Mol Med. 2000;5(5):515–20. doi: 10.3892/ijmm.5.5.515. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguchi M, Sugimoto E. Stimulatory effect of genistein and daidzein on protein synthesis in osteoblastic MC3T3-E1 cells: activation of aminoacyl-tRNA synthetase. Mol Cell Biochem. 2000;214(1-2):97–102. doi: 10.1023/a:1007199120295. [DOI] [PubMed] [Google Scholar]
- 69.Blair HC, Jordan SE, Peterson TG, et al. Variable effects of tyrosine kinase inhibitors on avian osteoclastic activity and reduction of bone loss in ovariectomized rats. J Cell Biochem. 1996;61:629–37. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C629::AID-JCB17%3E3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 70.Williams JP, Jordan SE, Barnes S, et al. Tyrosyne kinase inhibitor effects on avian osteoclastic acid transport. Am J Clin Nutr. 1998;68(suppl):1369S–74S. doi: 10.1093/ajcn/68.6.1369S. [DOI] [PubMed] [Google Scholar]
- 71.Okamoto F, Okabe K, Kajiya H. Genistein, a soybean isoflavone, inhibits inward rectifier K+ channels in rat osteoclasts. Jpn J Physiol. 2001;51:501–9. doi: 10.2170/jjphysiol.51.501. [DOI] [PubMed] [Google Scholar]
- 72.Gao Yh, Yamaguchi M. Suppressive effects of genistein on rat bone osteoclasts: apoptosis is reduced through Ca++ signaling. Biol Pharm Bull. 1999;22:805–9. doi: 10.1248/bpb.22.805. [DOI] [PubMed] [Google Scholar]
- 73.Gao Yh, Yamaguchi Suppressive effect of genistein on rat bone osteoclasts: involvement of protein kinase inhibition and protein tyrosine phosphatase activation. Int J Mol Med. 2000;5:261–7. doi: 10.3892/ijmm.5.3.261. [DOI] [PubMed] [Google Scholar]
- 74.Chin-Dusting JP, Fisher LJ, Lewis TV, et al. The vascular activity of some isoflavone metabolites: implications for cardioprotective role. Brit J Pharmacol. 2001;133:595–605. doi: 10.1038/sj.bjp.0704088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel RP, Boersma B, Crawford JH, et al. Antioxidant mechanisms of isoflavones in lipid systems: paradoxical effects of peroxyl radical scavanging. Free Rad Biol Med. 2001;31:1570–81. doi: 10.1016/s0891-5849(01)00737-7. [DOI] [PubMed] [Google Scholar]
- 76.Horiuchi T, Onouchi T, Takahashi M, et al. Effect of soy protein on bone metabolism in postmenoausal women. Osteoporosis. 2000;11:721–4. doi: 10.1007/s001980070072. [DOI] [PubMed] [Google Scholar]
- 77.Somekawa Y, Chiguchi M, Ishibashi T, et al. Soy intakerelated to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol. 2001;97:109–15. doi: 10.1016/s0029-7844(00)01080-2. [DOI] [PubMed] [Google Scholar]
- 78. Pansini F, Bonaccorsi G, Albertazzi P., et al. Soy phytoestrogens and bon In: Proceedings of the North American Menopause Society 1997. 44 (abstr)
- 79.Wangen KE, Duncan AM, Merz-Demlow BE, et al. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85:3043–9. doi: 10.1210/jcem.85.9.6787. [DOI] [PubMed] [Google Scholar]
- 80.Wong WW. Effects of soy isoflavones on blood lipids, blood pressure and biochemical markers of bone metabolism in postmenopausal women. J Nutr. 2000;130:686S. (abstr) [Google Scholar]
- 81.Arjmandi BH, Khalil DA, Lucas EA, et al. Soy protein with its isoflavones improves bone markers in middle aged and elderly-women. FASEB J. 2001;15:A728. (abstr) [Google Scholar]
- 82.Scheiber M, Liu J, Subbiah MT, et al. Dietary soy supplementation reduces LDL oxidation and bone turnover in healthy postmenopausal women. Menopause. 2001;8:384–92. doi: 10.1097/00042192-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 83.Cook A, Pennington G. Phytoestrogen and multiple vitamin/mineral effects on bone mineral density in early postmenopausal women: a pilot study. J Women Health. 2002;11:53–60. doi: 10.1089/152460902753473462. [DOI] [PubMed] [Google Scholar]