Abstract
Calcitonin (CT) is a polypeptide hormone with 32 aminoacids syntetized primarily by the thyroid.
Several evidences support the existence of nonthyroidal CT like peptide. The CT gene transcript also encodes a distinct peptide known as calcitonin gene related peptide (CGRP) which is a potent vasodilator and responsible for the stimulation of the glomerular filtration rate. In addition, a 37 aminoacid peptide amylin has been originally isolated by pancreatic β-cells. Amylin is able to inhibit insulin secretion, glucose transport into the skeletal musculature and gluconeogenesis. It is also able to inhibit gastric emptying. In the kidney it is able to modulate Calcium (Ca2+) excretion and increases renin activity. Finally, high affinity amylin receptors have been identified in the brain of the rat. The calcitonin receptor (CTR) is a member of a subfamily of the seven-transmembrane domain G-protein coupled receptor super family that includes several peptides. Members of this family have a similar structure with other seven-membrane-spanning domain G-protein coupled receptors.
The genetic contribution to osteoporosis susceptibility is well documented and many studies demonstrated that genetic factors play important roles in the regulation of bone metabolism. Restriction Fragment Length Polymorphisms (RFLPs) for the CTR gene have been described in the literature with a positive association with the lumbar bone mineral density (BMD), femoral neck BMD and with a lower incidence of vertebral fractures.
Keywords: calcitonin, calcitonin receptor, calcitonin receptor polymorphisms, osteoporosis
Calcitonin
The calcitonin (CT) is a polypeptide hormone first discovered by Coop and colleagues in the course of investigations to identify hormones that regulate serum levels of calcium (1). CT is able to decrease blood calcium levels by direct inhibition of mediated bone resorption and by enhancing calcium excretion by the kidney (2, 3). Human CT is a single-chain peptide of 32aminoacid residues. The molecular mass of the hormone is 3418 Da. There is a disulfile bridge connecting the cysteines at position 1 and 7 to form a 7 amino-acid ring structure at the amino terminus. The precursor of CT, pre-procalcitonin (Pre-ProCT) contains 141 aminoacids (4).
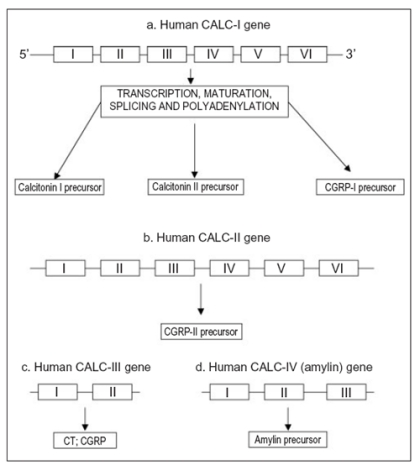
Several evidences support the existence of non-thyroidal CT-like peptide. Indeed, Davis et al. (5) demonstrated that prostate gland was able to produce a large amount of CT. In addition, CT is present in the central nervous system (6). The protein sequence for the CT has been determined in several species. It is highly conserved within the N-terminal loop region but demonstrates divergence in the rest of the sequence (7). The mature human CT originates from the calcitonin-I (CALC-I) gene on chromosome 11. The CT gene family consists of four known genes (CALC-I to CALC-IV) that contain nucleotide sequence homologies (8). CALC-I is the only gene that produces CT. However, both CALC-I and CALC-II genes produce the hormones CT gene-related peptide type I and II (CGRP-I and II). The CALC-III is able to produce both CT and CGRP and finally CALC-IV produces amylin (Fig.1) (8).
Figure 1.
The human calcitonin gene family.
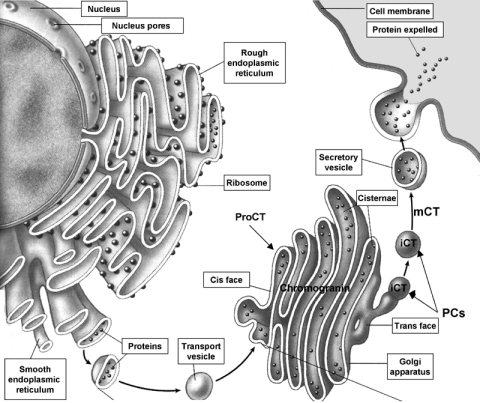
The biosynthetic secretory pathway for CT involves a complex series of progressive modifications: after the biosynthesis and folding of ProCT, subsequent proteolytic processing occurs both within the Golgi apparatus (9) and later within the secretory granules. It is likely that the cleavage of ProCT and consequent immature CT could be due to the action of a prohormone convertase enzymes (PCs). During early posttranscriptional processing, the nProCT segment may act as a signal for sorting its parent ProCT molecule to the nascent secretory vesicle of the regulatory pathway. Here, the prohormone undergoes further proteolytic cleavage to release CT (8). Chromogranin B may function as a helper protein to favor trans-Golgi sorting to the regulated secretory pathway (10). Within the newly formed secretion vesicles, the proteolytic cleavage releases immature CT. Then, as amidation proceeds, mature CT is produced and is progressively concentrated within the secretory vesicles (Fig.2) (8, 11).
Figure 2.
The biosynthetic secretory pathway for CT. ProCT (ProCalcitonin) is cleaved into the Golgi apparatus by the action of a prohormone convertase enzymes (PCs). Consequently immature CT (iCT) present in the secretory vesicle, undergoes further proteolytic cleavage to release mature CT (mCT). Chromogranin B may function as a helper protein to favor trans-Golgi sorting to the regulated secretory pathway.
The biologically relevant effects of the CT appear in bone, kidney, central nervous system, respiratory tract and gastrointestinal and reproductive systems. Mature CT plays an important role in skeletal homeostasis, being a key modulator of bone resorption (8). It acts directly on osteoclast calcitonin receptor to inhibit bone resorption (1). The hormone inhibits bone resorption by inducing an acute quiescence of cell motility. The physiological actions of CT are mediated primarily by the ability of its receptor to couple to at least two signal transduction pathways.
Calcitonin-related peptide
CT is initially synthesized as a precursor containing 136 aminoacids and a leader sequence at its amino terminal region. The leader sequence is cleaved during transport of CT to the endoplasmic reticulum. The CT gene transcript also encodes a distinct peptide known as calcitonin gene-related peptide (CGRP). It is a potent vasodilator and responsible for the stimulation of the glomerular filtration rate (12). The tissue specificity of this trancript is regulated by alternative splicing of the CT gene (13). The pre-RNA of the CT/CGRP gene contain six exons and can be processed in two way with a regulatory event in the processing choice with inclusion or exclusion of the alternative 3’ terminal exon 4. In particular, in parafollicular cells of the thyroid, 95% of the CT/CGRP pre-RNA is processed to include exon 4 followed by polyadenylation in order to produce CT. In the brain, 99% of the CT/CGRP pre-RNA is processed to exclude the exon 4 and include exon 5 and 6 with usage of the exon 6 polyadenylation site to produce CGRP (7, 13).
In addition, a 37 aminoacid peptide amylin has been originally Calcitonin and calcitonin receptors isolated by pancreatic β-cells (14). Calcitonin gene-related peptide (CGRP) and amylin are homologous 37 aminoacid peptides the gene for which probably has a common ancestral origin. Amylin is able to inhibit insulin secretion, glucose transport into the skeletal musculature and gluconeogenesis (7). In addition, amylin is able to inhibit gastric emptying. In the kidney it is able to modulate calcium (Ca2+) excretion and increases renin activity (15, 16). Finally, high affinity amylin receptors have been identified in the brain of the rat (17).
Amylin and CGRP have comparable effects. In skeletal muscle the primary effects are the inhibition of glycogen synthesis and stimulation of glycogenolysis with a reduction of glucose uptake and increased glycolisis. In the liver they stimulate the glucose output as a result of gluconeogenesis. By these ways amylin and CGRP act as noncompetitive antagonist of insulin and produce insulin resistance (19). In addition CGRP is potent vasodilatator and its receptors are distributed throughout the vascular system. CGRP is also an abundant peptide in the nervous system suggesting a neuromodulatory role. Amylin and CGRP increase renin sectretion. CGRP is also able to inhibit bone resorption. There is also evidence that CGRP may act on osteoclast precursors (20). Amylin has a similar effect but only at 100 fold higher concentrations. On the other hand, animal studies demonstrated that both amylin and CGRP are able to stimulate the osteoblast activity (18). However, what role amylin and CGRP play in normal bone metabolism and bone pathology remains to be determined. The effects of both amylin and CGRP are, broadly, to increase bone formation and reduce bone resorption. The most recently discovered member of the calcitonin peptide family is the adrenomedullin (AM). It has been isolated for the first time from a human phecromocytoma and it is expressed in the adrenal medulla (20). It is clear that either activation or disruption of AM signaling might contribute to many pathological conditions including hypertension, congestive heart failure, pulmonary hypertension, neoplastic growth, and inflammatory diseases (21).
Calcitonin receptors
The activities of CT are mediated by high affinity calcitonin receptors (CTRs). The CTR is a member of a subfamily of the seven-transmembrane domain G-protein coupled receptor superfamily that includes several peptides. Members of this family have a similar structure with other seven-membrane-spanning domain G-protein coupled receptors. Lin et al. cloned the porcine CTR (pCTR) cDNA for the first time in 1991 (22). This receptor was characterized by a long NH2-terminal domain that was extracellular. It was similar to parathyroid/parathyroid hormone-related peptide receptor and the secretin receptor (7). Subsequent cloning of the pCTR gene demonstrated it is approximately 70 kb in length and contains at least 14 exons, 12 of which encode the protein. The human CTR was cloned from an ovarian carcinoma cell line (BIN-67) (23). Different isoforms of CTR resulting from alternative splicing of the gene have been described in various animal species with differential tissue expression transcripts (24) and different signaling properties (25). Two different isoforms have been described in human giant cell tumor of bone (26). The first isoform, designed as GC-10, differs from the previously described ovarian-human CTR gene (26) in the 5’-region in that it lacks a 71-bp segment, while being almost identical in the 3’-region. The second human giant cell tumor-CTR cDNA variant, indicated as GC-2, lacks 71-bp 5’ insert but also 48 nucleotides encoding part of the first intracellular domain (26). It is likely that differential expression of CTR isoforms could be a mechanism of regulation of biological responses to CT. Shift in the predominant CTR isoform(s) could in part explain the variable responsiveness to CT in patients with high turnover metabolic bone disease (26). The CTR gene has been mapped to chromosome 7q21.3 (27).
Calcitonin and cell signalling
The principal mechanism of action of CT it due to the ability of its receptor to couple at least two signal transduction pathways. One of the most important pathways is coupled with the cAMP signal transduction. However, CTRs can also couple to the phospholipase C (PLC) enzyme pathway. The PLC pathway, as with the cAMP pathway, can be initiated by the coupling of receptors to multiple G-proteins. Activation of the PLC causes the release of Ca2+from intracellular stores and promotes an influx of external calcium (7).
In addition, CTRs are able to activate the phospholipase D (PLD) (7). Finally, Chen et al. demonstrated that CT when bound to CTRs stimulates Shc tyrosine phosphorylation of MAPK Erk1/2 (28).
Calcitonin activities
Bone
Osteoclasts are the major target for the action of CT. It is able to interfere with osteoclast differentiation from precursor cells and fusion of mononucleated precursors to form multinucleated cells in bone marrow cultures (29). CT plays an important role in skeletal homeostasis, being a key modulator on bone resorption (8). It acts directly on CTRs to inhibit bone resorption by inducing contraction and inhibits osteoclast motility (Q effect) occurring within 1 min and this is followed by a more gradual retraction of the osteoclasts (R effect) (7, 8, 30). Both cAMP and intracellular calcium (Ca2+) are second messengers for the Q and R effects, and both are G-protein mediated (8). CT inhibits also other components of the osteoclast, such as the release of acid phosphatase and the expression of carbonic anhydrase II (31, 32).
Kidney
The kidney is the principal site of CT degradation by neutral endopeptidase (NEP) (8). In normal men the effect of CT on the kidney is to stimulate diuresis and increases the fractional excretion rate of sodium and chloride. In addition, in urine a calcium and phosphate excretion increases. In hypercalcemic patients with metastatic bone disease, the administration of CT induces a rapid fall in serum calcium due primarily to inhibition of renal tubular reabsorption (7).
Central nervous system
CT has specific binding sites within the central nervous system. The intracerebral injection of CT suppresses food and water intake in rats (33). In the human, large doses of salmon CT reduce the serum concentrations of testosterone, LH and FSH probably acting via hypothalamic level (8, 34) and the chronic administration to humans with migraine headaches increases the levels of β-endhorfin, as well as ACTH and cortisol (8).
Breast cancer cell lines
It is known that cell proliferation was inhibited when human breast cancer cells in culture were treated with CT (35, 36). Autoradiographic and radioligand-binding techniques with iodinated CT have identified high-affinity CTRs in the human breast cancer cell lines T47D and MCF-7 (36). In a recent study Wang et al. have demonstrated that CTR mRNA is constantly expressed in normal ductal epithelium and in breast cancer. In addition a decrease of CTR expression was found more often in cases with lymph node metastasis suggesting that CTR might be of great potential significance in breast cancer progression (36).
Gastrointestinal system
In human, CT increases gastric acid and pepsin secretion and decreases pancreatic amylase and pancreatic polypeptide. At high concentrations, CT increases the net secretion of water and electrolytes form the human jejunum and ileum, and has been postulated that these effects may explain the presence of diarrhea seen in some patients affected by medullary thyroid carcinoma (8).
Reproductive system
Sperm contain a very high concentration of CT which is about 40 times that found in the serum. In vitro experiments showed that CT likely acts as an endogenous regulator of sperm fertilizing ability in vivo (7, 37). In addition, CT occurs in both the uterus and placenta. The intraplacental presence of CT receptors, suggest a role of CT in the regulation of placental function; however, small studies evaluated this possibility (8).
Respiratory system
9CT is found within the pulmonary neuroendocrine (PNE) cells, which are situated near the basement membrane and often extend to the lumen of the airway (8). CT affects transcellular and intracellular movements of calcium, and hence may exert and intrapulmonary paracrine action. In fact, CT is able to inhibit prostaglandin and thromboxane secretion (8) and increases the cartilagineous growth (8). Finally, CT blocks the bronchoconstrictor effects of bombesin-like peptides and substance P.
Calcitonin receptor polymorphisms and osteoporosis
Osteoporosis is a common skeletal disease characterized by an excessively fragile skeleton and susceptibility to fracture (38). It is a multifactorial disease and several environmental and genetic factors play an important role. The genetic contribution to osteoporosis susceptibility is well documented and many studies demonstrated that genetic factors play important roles in determining population variation of Bone Mineral Density (BMD) (39), bone quality (40), and osteoporotic fractures (41, 42). The genes that regulate BMD and bone fragility are potentially important targets for the design of new drugs that can be used to treat bone disease. Genetic markers will also be used as diagnostic tools in the assessment of individuals at risk of developing osteoporotic fractures and could be used to help target preventive therapies to those individuals who are at risk of fracture. In addition, genetic profiling could be useful to distinguish treatment responders from non-responders and to identify patients who might be at risk of developing unwanted side effects.
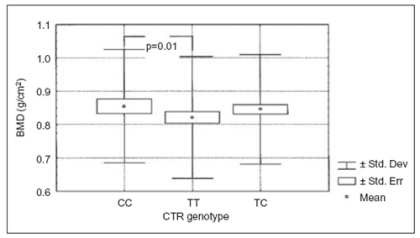
One of the gene that can be involved in this process is the CTR gene. A Restriction Fragment Length Polymorphism (RFLP) for the CTR gene has been for the first time described in 1998 using Taq I endonuclease (43). The authors found a positive association between this polymorphism and lumbar spine bone mineral density (LS-BMD). However, no significant variation of BMD among the genotypes was observed in the femoral neck BMD (43). In addition, Nakamura et al. described a polymorphic site at the CTR gene by Alu I restriction enzyme at the 1377th nucleotide expressing either proline (CC genotype) or leucine (TT genotype) (44). They observed that in the Japanese population the most represented CTR was the CC genotype and the less represented was the TT genotype. Conversely in the Italian population the TC and TT allelic variants represented the most frequent genotypes, underlying the importance of ethnic origin as a variable that should be always taken in consideration (45). In addition, Duncan's test used to compare the genotypes showed that TT genotype has significant lower lumbar BMD in comparison with CC genotype (Fig.3).
Figure 3.
Lumbar BMD values according to CTR genotypes. TT geno type had a significant lower lumbar BMD in comparison with CC genotype (Duncan’s test p=0.01).
Taboulet et al. studied the distribution of these alleles in a cohort of 215 postmenopausal Caucasian women suffering or not from osteoporotic fractures. They found that Rr genotype (the TC genotype indicated by Masi et al. and Nakamura et al.) was associated with a higher femoral neck BMD in comparison with RR and rr genotypes. In addition Rr genotype was associated with a lower incidence of vertebral fractures (46). The authors emphasize the importance of the high conservation of the proline residue in all species studied with the exception of the human isoforms. The absence of the proline residue could alter the secondary structure of the calcitonin receptor, more so as two other proline residues border this region. Progressive truncation of the C-terminal tail of the porcine receptor complex reduced the magnitude of adenylate cyclase responses (47). This underlines the importance of the CTR C-terminal domain and suggests that proline/leucine mutation could alter receptor biological activity (46). In addition, heterozygotes could produce both alleles of the receptor, resulting in advantage as compared with homozygotes, especially as this genetic effect accounts for 13% of the total variance of BMD in covariance analysis (46). Recently, this polymorphism has been studied in a group of postmenopausal women in Taiwan (48) confirming the association between TT genotype and low bone mineral density at the lumbar spine and femoral neck. A lower lumbar spine BMD was also found in subjects with TT genotype affected by Juvenile Idiopathic Arthritis (49).
A complete polymorphism scan of the human calcitonin receptor gene identified 11 polymorphic sites in the gene and confirmed the presence of the previously identified nucleotide T1340C (codon 447) polymorphism. Because a leucine to proline change has the potential for significant structural alteration, receptor genes encoding either leucine or proline at residue 447 were transiently expressed in COS-7 cells to determinate the binding and functional consequences of this polymorphism. The presence of this SNP (single nucleotide polymorphism) appears to have no statistically significant difference with the receptor’s ability to bind CT or signal when activated with the hormone (50). A study conducted in Italian men found that CC genotype was associated with lower BMD and with lower level of bone alkaline phosphatase and estradiol in comparison with TT genotype suggesting a depression of bone formation in these subjects (51). A correlation between CTR polymorphism and buccal margin bone loss has been observed in subjects undergoing to implants on maxillae and mandibles (52) and finally the CTR polymorphism has been indicated as an important genetic marker for severe periodontitis (53).
Recently a functional role of CTR polymorphism has been observed by Zofkaova et al. (54). In particular, the authors postulated that the 3β-hydroxysteroid dehydrogenase activity could be associated with C allele at least in C19 steroids. However, these data were observed in a relatively small number of postmenopausal women and need confirm (54). Research has recently begun to clarify the genes and genetic variants that predispose to osteoporosis and regulation of bone mass. CTR polymorphism together with other genes could be associated with the identification of genetic markers for assessment of fracture risk and the identification of novel molecular targets for the design of drugs that can be used to treat bone disease.
References
- 1.Coop DH, Camenon EC, Chenwy BA, Davidson GF, Henze G. Evidence for calcitonin – a new hormone from the parathyroid that lowers blood calcium. Endocrinology. 1962;70:638–649. doi: 10.1210/endo-70-5-638. [DOI] [PubMed] [Google Scholar]
- 2.Friedman J, Raisz LG. Thyrocalcitonin inhibitor of bone resorption in tissue culture. Science. 1965;150:1465–1467. doi: 10.1126/science.150.3702.1465. [DOI] [PubMed] [Google Scholar]
- 3.Warshawsky H, Goltzman D, Rouleau MF, Bergeron JM. Direct in vivo demonstration by radioautography of specific binding sites for calcitonin in skeletal and renal tissues of the rat. J Cell Biol. 1980;85:682–694. doi: 10.1083/jcb.85.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker KL, Nylèn ES, Choen R, Silva OL, Snider RH. Calcitonin: Structure, Molecular Biology, and Actions In “Principles of Bone Biology” (JP Bilezikian, LG Raisz, GA Rodan eds), Chapter 34 1996. 471 494 Academic Press; [Google Scholar]
- 5.Davis NS, DiSant’ A, Ewing JF, Mooney RA. The neuroendocrine prostate: characterization and quantization of calcitonin in the human gland. J Urology. 1989;142:884–888. doi: 10.1016/s0022-5347(17)38936-x. [DOI] [PubMed] [Google Scholar]
- 6.Fischer JA, Tobler PH, Henke H, Tschopp FA. Salmon and human calcitonin like peptides co-exist in the human thyroid and brain. J Clin Endocrinol Metab. 1983;57:1314–1316. doi: 10.1210/jcem-57-6-1314. [DOI] [PubMed] [Google Scholar]
- 7.Pondel M. Calcitonin and calcitonin receptor: bone and beyond. Int J Exp Path. 2000;81:405–422. doi: 10.1046/j.1365-2613.2000.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker L, Nylèn ES, Choen R, Silva OL, Snider RH. Calcitonin gene family of peptides In “Principles and Practice of Endocrinology and Metabolism” (KL Becker ed.) 2nd ed 1995. 474–483. JP Lippincott Co; Philadelphia: [Google Scholar]
- 9.Sossion WS, Fisher JM, Scheller RH. Sorting within the regulated secretory pathway occurs in the trans-Golgi network. J Cell Biol. 1990;110:1–12. doi: 10.1083/jcb.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huttner WB, Natori S. Helper proteins for neuroendocrine secretion. Curr Biol. 1995;5:242–245. doi: 10.1016/s0960-9822(95)00049-2. [DOI] [PubMed] [Google Scholar]
- 11.Treilhou-Lahille F, Pidoux E, Day F, Milhaud G, Moukhtar MS. Granular and extra-granular forms of immunoreactive calcitonin and normal rat “C” cells. Biol Cell. 1986;57:221–230. doi: 10.1111/j.1768-322x.1986.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 12.Gnadinger MP, Uehlinger DE, Weidmann P, Shaw SG, Muff R, Born W. Distinct renal and hemodynamic effects of calcitonin gene-related peptide and calcitonin. Am J Physiol. 1989;257:E843–E853. doi: 10.1152/ajpendo.1989.257.6.E848. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld MG, Amara SG, Evans RM. Alternative RNA processing determining neuronal phenotype. Science. 1984;225:1315–1320. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- 14.Westermark P, Wernstedt C, Wilander E, Sletten E. A novel peptide in the calcitonin gene-related peptide family as an amyloid fibril protein in endocrine pancreas. Biochem Biophys Res Commun. 1986;140:827–881. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 15.Blakely P, Vaughn DA, Fanestil DD. Amylin, calcitonin gene-related peptide, and adrenomedullin: effects on thiazide receptors and calcium. Am J Physiol. 1997;41:F410–F415. doi: 10.1152/ajprenal.1997.272.3.F410. [DOI] [PubMed] [Google Scholar]
- 16.Wookey PJ, Tikellis C, Du HC, Qin HF, Sexton PM, Cooper ME. Amylin binding in the rat renal cortex, stimulation of adenylyl cyclase and activation of plasma renin. Am K Physiol. 1996;270:F289–F294. doi: 10.1152/ajprenal.1996.270.2.F289. [DOI] [PubMed] [Google Scholar]
- 17.Beamount K, Kenney MA, Young AA, Rink TJ. High affinity amylin binding sites in rat brain. Mol Pharmacol. 1993;44:493–497. [PubMed] [Google Scholar]
- 18. Rei IR, Cornish J. Amylin and CGRP In “Principle of Bone Biology” Academic Press; (Bilezikian JP , Raisz LG, Rodan GA eds.) 1996. 35 495 505 [Google Scholar]
- 19.Mullins MW, Ciallella J, Rangnekar V, McGillis JP. Characterization of a calcitonin gene-related peptide (CGRP) receptor on mouse bone marrow cells. Regul Pept. 1993;49:65–72. doi: 10.1016/0167-0115(93)90385-l. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin a novel hypotensive peptide isolated from human pheocromocytoma. Biochem Biophys Res Commun. 1993;192 doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 21.Nagaya N, Mori H, Murakami S, Kangawa K, Kitamura S. Adrenomedullin: angiogenesis and gene therapy. Am J Physiol Regular Integr Comp Physiol. 2005;288:R1432–1437. doi: 10.1152/ajpregu.00662.2004. [DOI] [PubMed] [Google Scholar]
- 22.Lin HY, Harris TL, Flanery MS, et al. Expression cloning and characterization of a porcine renal calcitonin receptor. Science. 1991;254:1022–1024. [PubMed] [Google Scholar]
- 23.Bijovet OLM, Van der Sluys Veer J, De Vries HR, Van Koppen ATJ. Natriuretic effect of calcitonin in man. N Engl J Med. 1971;284:681–688. doi: 10.1056/NEJM197104012841301. [DOI] [PubMed] [Google Scholar]
- 24.Moore EE, Kuestner RE, Stroop SD, Grant GJ, Matthewes L, Brady CL, Sexton PM, Fidlay DM. Functionally different isoforms of the human calcitonin receptor result from alternative splicing of the gene transcript. Molecular Endocrinol. 1995;9:959–968. doi: 10.1210/mend.9.8.7476993. [DOI] [PubMed] [Google Scholar]
- 25.Nussenzveig DR, Thaw CN, Gershengorn MC. Inhibition of inositol phosphate second messenger formation by intracellular loop one of a human calcitonin receptor. Expression and mutational analysis of synthetic receptor genes. J Biol Chem. 1994;269:28123–28129. [PubMed] [Google Scholar]
- 26.Gorn AH, Rudolph SM, Flannery MR, Morton CC, Weromowicz S, Wang JT, Krane SM, Goldring SR. Expression of two human skeletal calcitonin receptor isoforms cloned from a giant tumor of bone. J Clin Invest. 1995;95:2680–2691. doi: 10.1172/JCI117970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorn AH, Lin HY, Yamin M, Auron PE, Flannery MR, Tapp DR, Manning CA, Lodish HF, Krane SF, Goldring SR. Cloning, characterization, and expression of a human calcitonin receptor from an ovarian carcinoma cell line. J Clin Invest. 1992;90:1726–1735. doi: 10.1172/JCI116046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Shyu JF, Santhanagopal A, et al. The calcitonin receptor stimulates Shc tyrosine phosphorylation and Erk 1/2 activation: involvement of Gi, protein kinase C, and calcium. J Biol Chem. 1998;273:19808–19816. doi: 10.1074/jbc.273.31.19809. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Yamana H, Yoshiki S, et al. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988;122:1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- 30.Chamber TJ, Hall TJ. Cellular and molecular mechanisms in the regulation and function of osteoclasts. Vitam Horm. 1991;46:41–86. doi: 10.1016/s0083-6729(08)60682-2. [DOI] [PubMed] [Google Scholar]
- 31.Zaidi M, Bax B, Shankar VS, Moonga BS, Simon S, Alam B, Gaines AS, Das RE, Pazianas M, Huang CL. Dimensional analysis of osteoclastic bone resorption and the measurement of biologically active calcitonin. 1994;79:387–399. doi: 10.1113/expphysiol.1994.sp003773. [DOI] [PubMed] [Google Scholar]
- 32.Zheng MH, Fan Y, Wysocki S, Wood DJ, Papadimitriou JM. Carbonic acid anhydrase II gene transcription in cultured osteoclasts from neonatal rats: Effects of calcitonin. Cell Tissue Res. 1994;276:7–13. doi: 10.1007/BF00354778. [DOI] [PubMed] [Google Scholar]
- 33.Chait A, Suaudeau C, De Beaurpaire R. Extensive brain mapping of calcitonin-induced anorexia. Brain Res Bull. 1995;36:467–472. doi: 10.1016/0361-9230(94)00223-n. [DOI] [PubMed] [Google Scholar]
- 34.Mulder H. Calcitonin-testosteron inter-relationship. A classic feedback system? Neth J Med. 1993;42:209–211. [PubMed] [Google Scholar]
- 35.Lacroix M, Siwek B, Body JJ. Breast cancer cell response to calcitonin: modulation by growth-regulating agents. Eur J Pharmacol. 1998;344:279–286. doi: 10.1016/s0014-2999(97)01578-1. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Nakamura M, Mori I, Takeda K, Nakamura Y, Utsunomiya H, Yoshimura G, Sakurai T, Kakudo K. Calcitonin receptor gene and breast cancer: quantitative analysis with laser capture microdissection. Breast Cancer Res Treat. 2004;83:109–117. doi: 10.1023/B:BREA.0000010703.59483.c0. [DOI] [PubMed] [Google Scholar]
- 37.Fraser LR. Dibutyrl cyclic AMP decreases capacitation time in vitro in mouse spermatozoa. J Reprod Fert. 1981;62:63–72. doi: 10.1530/jrf.0.0620063. [DOI] [PubMed] [Google Scholar]
- 38. Anonymous Consensus development conference. Diagnosis, prophylaxis and treatment of osteoporosis Am J Med 1993. 94 646 650 [DOI] [PubMed] [Google Scholar]
- 39.Shen H, Liu Y, Liu P, Recker RR, Deng HW. Nonreplication in genetic studies of complex diseases – lessons learned from studies of osteoporosis and tentative remedies. J Bone Miner Res. 2005;20:365–376. doi: 10.1359/JBMR.041129. [DOI] [PubMed] [Google Scholar]
- 40.Deng HW, Deng XT, Conway T, Xu FH, Heaney RR. Determination of bone size of hip, spine, wrist in human pedigrees by genetic and lifestyle factors. J Clin Densitiom. 2002;5:45–56. doi: 10.1385/jcd:5:1:045. [DOI] [PubMed] [Google Scholar]
- 41.Deng HW, Chen WM, Recker S, Stegman MR, Li JL, Davies KM, Zhou Y, Deng H, Heaney R, Recker RR. Genetic determination of Colles’ fracture and differential bone mass in women with and without Colles’ fracture. J Bone Miner Res. 2000;15:1243–1252. doi: 10.1359/jbmr.2000.15.7.1243. [DOI] [PubMed] [Google Scholar]
- 42.Ioannidis JP, Ralston SH, Bennet ST, Brandi ML, Grindberg D, Karassa FB, Lang van Merus JB, Mosekilde L, Scollen S, Albagha OM, Carey AH, Am D, Enjuanes A, can Leeuwen JP, Mavilia C, Masi L, McGigan FE, Nogues X, Reid PDM, Schuit SC, Sherlock RE, Uitterlinden AG. GENOMOS Study. Differential genetic effects of ESR1 gene polymorphisms on osteoporotic outcomes. JAMA. 2004;292:2105–2114. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- 43.Masi L, Becherini L, Colli E, Gennari L, Mansani R, Falchetti A, Becorpi AM, Cepollaro C, Gonnelli S, Tanini A, Brandi ML. Polymorphisms of the calcitonin receptor gene are associated with bone mineral density in postmenopausal Italian women. Biochemical Biophysical Research Communications. 1998;248:190–195. doi: 10.1006/bbrc.1998.8880. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura M, Zhang Z, Shan L, Hisa T, Sasaki M, Tsukino R, Yokoi T, Kaname A, Kakudo K. Allelic variants of human calcitonin receptor in the Japanese population. Hum Genet. 1997;140:2924–2927. doi: 10.1007/s004390050307. [DOI] [PubMed] [Google Scholar]
- 45.Masi L, Becherini L, Gennari L, Colli E, Mansani R, Falchetti A, Cepollaro C, Gonnelli S, Tanini A, Brandi ML. Allelic variants of human calcitonin receptor: distribution and association with bone mass in postmenopausal Italian women. Biochemical Biophysical Research Communications. 1998;245:622–626. doi: 10.1006/bbrc.1998.8445. [DOI] [PubMed] [Google Scholar]
- 46.Taboulet J, Frenkian M, Frendo JL, Feingold N, Julliene A, Vernjoul MC. Calcitonin receptor polymorphism is associated with a decreased fracture risk in post-menopausal women. Hum Mol Genet. 1998;13:2129–2133. doi: 10.1093/hmg/7.13.2129. [DOI] [PubMed] [Google Scholar]
- 47.Findlay DM, Houssami S, Lin HY, Myers DE, Brady CL, Darcy PK, Ikeda K, Martin TJ, Sexton PM. Truncation of the porcine calcitonin receptor cytoplasmatic tail inhibits internalization and signal transduction but increases receptor affinity. Mol Endocrinol. 1994;8:1691–1700. doi: 10.1210/mend.8.12.7708057. [DOI] [PubMed] [Google Scholar]
- 48.Tsai FJ, Chen WC, Chen HY, Tsai CH. The AluI calcitonin receptor gene polymorphism (TT) is associated low bone mineral density and susceptibility to osteoporosis in postmenopausal women. Gynecol Obstet Invest. 2003;55:82–87. doi: 10.1159/000070179. [DOI] [PubMed] [Google Scholar]
- 49.Masi L, Cimaz R, Simonini G, Bindi G, Stagi S, Gozzini A, Malentacchi C, Brandi ML. Association of low bone mineral mass with vitamin D receptor gene and calcitonin receptor gene polymorphisms in juvenile idiopathic arthritis. J Rheumatol. 2002;29:2225–2231. [PubMed] [Google Scholar]
- 50.Wolfe L.A. IIIa, Fling ME, Xue Z, Armour S, Kerner SA, Way J, Rimele T, Cox RF. In vitro characterization of a human calcitonin receptor gene polymorphism. Mutation Res. 2003;522:93–105. doi: 10.1016/s0027-5107(02)00282-8. [DOI] [PubMed] [Google Scholar]
- 51.Braga V, Sangalli M, Malerba G, Mottes M, Mirandola S, Gatti D, Rossini M, Zam M, Adami S. Relationschip amog VDR (Bsm I and Fok I), COLIA1 and CTR polymorphisms with bone mass, bone turnover markers and sex hormones in men. Calcif Tissue Int. 2002;70:457–462. doi: 10.1007/s00223-001-1088-9. [DOI] [PubMed] [Google Scholar]
- 52.Nosaka Y, Tachi Y, Shimpuku H, Kawamura T, Ohura K. Association of calcitonin receptor gene polymorphism with early margin bone loss around endosseous implants. Int J Oral Maxillofac Implants. 2002;17:38–43. [PubMed] [Google Scholar]
- 53.Suzuki A, Ji G, Numabe Y, Ishii K, Maramatsu M, Kamoi K. Large scale investigation of genomic markers for severe periodontitis. Odontology. 2004;92:43–47. doi: 10.1007/s10266-004-0035-4. [DOI] [PubMed] [Google Scholar]
- 54.Zofkaova I, Zajickova K, Hill M. Postmenopausal serum androstenedione levels are associated with the calcitonin receptor gene polymorphism T1377C. A pilot study. J Endocrinol Invest. 2004;27:442–444. doi: 10.1007/BF03345288. [DOI] [PubMed] [Google Scholar]