Introduction
As the worldwide population ages, the population prevalence of osteoporosis is also increasing. About 30% of postmenopausal women in Europe and the United States have osteoporosis and it is estimated that over 40% of postmenopausal women with osteoporosis in Europe and the United States will experience at least one fragility fracture (1).
A worldwide estimate for new osteoporotic fractures in 2000 was 9 million: in which 1.6 million hip, 1.7 million forearm, and 1.4 million clinical vertebral fractures (2).
In the U.S.A. non vertebral fractures represent approximately 75% of osteoporotic fractures and account for 90% of osteoporosis related costs (3).
With regard to Italy, on the basis of ESOPO study, estimates of prevalent fracture among women aged 50-79 years are more than 1.600.000 (ISTAT 2001 data on Italian population) (4).
Consistently with international data the non vertebral fractures are most frequent type: 79% are non vertebral/non hip fractures (wrist, ankle, humerus, tibial plate, etc.), 13% are vertebral and 8% are hip fractures.
Osteoporosis fractures result in increased future fracture risk; after initial vertebral fracture, risk of subsequent, non-vertebral fractures increases by 2-3-fold (1). Furthermore, after a vertebral fracture, 1 in 4 women will re-fracture during the following year (5). Finally, postmenopausal women with a history of fracture at any site should be considered for further evaluation and treatment due to an approximate 2-fold increased risk of subsequent fractures (6).
The most serious consequences of osteoporosis are femoral fractures; from a clinical standpoint 24% of patient die within one year from occurrence and only 15% are able to return to unassisted ambulation (1) and 27% are forced to enter in nursing homes (7).
Impact of femur fractures is dramatic also from a social-economic standpoint: with regard to Italian data, cost for hospitalization following a femur fracture is € 400 million/year; taking into account also indirect costs (post-surgery rehabilitation, etc.) expenditure is over € 1 billion/year, with a 15% yearly increase trend (8).
Economic burden is similar to that of myocardial infarction for
Observational studies
Randomized controlled trials (RCTs) are the gold standard for determining drug efficacy and safety. RCTs are designed to minimize internal bias and to maximize treatment effect. However, RCT trial design creates shortfalls with regards to external validity of the outcomes.
Many patients with osteoporosis, however, cannot be included in standard RCTs because of co-morbidities and prior therapies, e.g. bone-active agents, steroids, etc. (9). Tightly controlled protocols may produce outcomes more favourable than in actual clinical practice (10).
Furthermore, RCTs are not always predictive for adverse event profile since the number of patients maybe too low to detect low frequency events; enrollment criteria maybe too strict and exclude high risk population; detailed patient instructions by dedicated personal may minimize the risk of wrong intake and there is limited follow-up.
The role of observational studies is to complement RCTs by expanding the clinical evidence. Observational studies can complement RCTs data of efficacy by demonstrating effectiveness across a range of patients and health care practices by showing that a drug indeed achieves its clinical effect in the real world.
Healthcare database studies can help address many questions such as insights into the disease; safety in actual clinical practice; treatment patterns; resource utilization and real world effectiveness - treatment comparisons. Examples include use of the General Practitioners Research Database in the UK to study risk of fracture after glucocorticoid use, comparative statin safety assessment, and use of databases to study under-diagnosis and treatment following fracture.
Over the past decade, large claims databases have become very common in North America and Europe and are used to conduct ‘effectiveness studies’.
RCTs and observational studies have consistent results in most cases; e.g. reduction in all causes of mortality following statin use. However, there are limitations in the use of health-care databases: chart review may not be available to validate codes or identify coding errors; we cannot demonstrate causation of the event to the disease; we cannot assess use of nonprescription products. Selection bias is possible since not all medical information is known and external variables are not controlled.
REAL study
The REAL study was a retrospective, observational study based on data retrieved from United States healthcare utilization records including 101 health plans (11).
Patients were women ≥65 years old newly treated with once weekly dosed risedronate (35 mg) or alendronate (35 or 70 mg). Patients prescribed bisphosphonates during 6 months before index drug initiation were excluded to select only new bisphosphonate users.
Patients were monitored until one of the following occurred:
-
-
12 months of bisphosphonate use
-
-
Bisphosphonate discontinuation, switching, or 15-day gap in treatment
-
-
Incident, non traumatic non-vertebral fracture.
A total of 12.215 risedronate users were followed for a mean of 226 days, with 63% completing 12 months of evaluation; 21,615 alendronate users (8% with 35 mg and 92% with 70 mg) were followed for a mean of 238 days, with 67% completing 12 months of evaluation.
Risedronate patients were older, used more concomitant medications, used more glucocorticoids, and had co-morbid rheumatoid arthritis significantly more than alendronate users (P ≤0.01).
These demographic differences suggest that the risedronate cohort was at higher risk for subsequent fracture before treatment initiation.
Conversely, risedronate users had greater previous use of calcitonin or raloxifene, which may decrease fracture risk. In the six and twelve months “historical period” before bisphosphonate initiation clinical diagnosis of non vertebral fracture was similar between subsequent risedronate and alendronate users, while previous hip fracture had occurred significantly more frequently among patients later prescribed risedronate (P < 0.05).
Results
-
-
During the 12 months of observation after the start of bisphosphonate therapy, 507 subjects had non vertebral fractures. The site of non vertebral fracture was wrist (30%), hip (21%), leg (17%), pelvis (15%), humerus (14%), and clavicle (3%).
-
-
Fracture incidence was similar during the first 3 months of treatment. Fracture incidence was significantly lower with risedronate after 6 months.
-
-
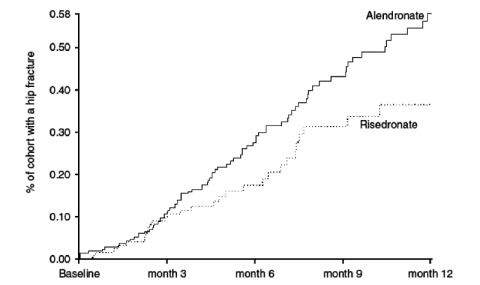
Non-vertebral fracture incidence with risedronate was 19% lower after 6 months and 18% lower after 12 months than with alendronate.
-
-
Hip fracture incidence with risedronate was 46% lower after 6 months and 43% lower after 12 months than with alendronate.
Significant differences at 6 and 12 months persisted after adjusting analyses for adherence, baseline fracture, and demographics. Excluding the small minority of patients treated with 35 mg alendronate also did not change outcome of analyses.
Conclusions
The REAL study results are generalizable to the real world clinical settings. Data were collected from more than 100 health plans in 34 states, providing a broad sample of patients and clinician practice patterns.
Results of the REAL study are consistent with efficacy timing (6 and 12 months) in RCTs for both risedronate (12) and alendronate (13).
As with all cohort studies, the interpretation of results may be limited by the non-randomized nature of the study design. However, these results are consistent with the results of analyses of clinical trials and show that patients treated with risedronate are better protected from non-vertebral and hip fractures during their first year of therapy than those treated with alendronate.
Superior protection from risedronate begins after 6 months of treatment initiation and leads to protection of more patients as compared to alendronate.
Figure 1.
Kaplan-Meier analysis for relative hip fractures incidence reduction.
References
- 1.Reginster J, et al. Osteoporosis: a still increasing prevalence. Bone. 2006;38(suppl 1):S4–S9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, et al. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Adachi JD, et al. Greater than 90% of managed care for fragility fractures are non vertebral in nature among women. Calcif Tissue Int. 2006;78(Suppl 1):S125. [Google Scholar]
- 4.Maggi S, et al. Quantitative heel ultrasound in a population-based study in Italy and its relationship with fractures history: the ESOPO study. Osteoporos Int. 2006;17:237–244. doi: 10.1007/s00198-005-1985-2. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay R, et al. One year outcomes and costs following a vertebral fracture. Osteoporos Int. 2005;16(1):78–85. doi: 10.1007/s00198-004-1646-x. [DOI] [PubMed] [Google Scholar]
- 6.Klotzbuecher CM, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Min Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, et al. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103:12S–19S. doi: 10.1016/s0002-9343(97)90022-x. [DOI] [PubMed] [Google Scholar]
- 8.Piscitelli P, et al. Incidence and cost of hip fractures compared to myocardial infarction in the Italian population: a 4-year survey. Osteoporosis International. 2007;18:211–9. doi: 10.1007/s00198-006-0224-9. [DOI] [PubMed] [Google Scholar]
- 9.Dowd R, et al. Study subjects and ordinary patients. Osteoporos Int. 2000;11:533–536. doi: 10.1007/s001980070097. [DOI] [PubMed] [Google Scholar]
- 10.Motheral BR, et al. The use of claims databases for outcomes research: rationale, challenges and strategies. Clin Ther. 1997;19(2):346–66. doi: 10.1016/s0149-2918(97)80122-1. [DOI] [PubMed] [Google Scholar]
- 11.Silverman SL, et al. Effectiveness of bisphosphonates on non vertebral and hip fractures in the first year of therapy: The risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18:25–34. doi: 10.1007/s00198-006-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington JT, et al. Risedronate rapidly reduces the risk of non vertebral fractures in women with postmenopausal osteoporosis. Calcif Tiss Int. 2004;74:129–135. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- 13.Black DM, et al. Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. J Clin Endocrinol Metab. 2000;85:4118–4124. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]