Abstract
In liver and intestine, transporters play a critical role in maintaining the enterohepatic circulation and bile acid homeostasis. Over the past two decades, there has been significant progress toward identifying the individual membrane transporters and unraveling their complex regulation. In the liver, bile acids are efficiently transported across the sinusoidal membrane by the Na+ taurocholate cotransporting polypeptide with assistance by members of the organic anion transporting polypeptide family. The bile acids are then secreted in an ATP-dependent fashion across the canalicular membrane by the bile salt export pump. Following their movement with bile into the lumen of the small intestine, bile acids are almost quantitatively reclaimed in the ileum by the apical sodium-dependent bile acid transporter. The bile acids are shuttled across the enterocyte to the basolateral membrane and effluxed into the portal circulation by the recently indentified heteromeric organic solute transporter, OSTα-OSTβ. In addition to the hepatocyte and enterocyte, subgroups of these bile acid transporters are expressed by the biliary, renal, and colonic epithelium where they contribute to maintaining bile acid homeostasis and play important cytoprotective roles. This article will review our current understanding of the physiological role and regulation of these important carriers.
Keywords: bile acids, cholesterol, nuclear receptors, cholestasis, enterohepatic circulation
Transporters have always assumed a prominent place in biology and human lore. Perhaps the most well known transporter is Charon, the ill-tempered old ferryman of Greek mythology whose task was to transport souls of the deceased across the river Acheron into Hades. Charon would ferry into Hades only those souls that would pay his toll while others were left to wander the shore for a hundred years before being delivered. This review focuses on another group of ferrymen: the Na+ taurocholate cotransporting polypeptide (NTCP), the bile salt export pump (BSEP), the apical sodium-dependent bile acid transporter (ASBT), and the organic solute transporter OSTα-OSTβ, the major bile acid transporters that control the fate of bile acids, either absorption and enterohepatic cycling or excretion and elimination from the body. The role of these transporters in maintaining bile acid homeostasis and their regulation under physiological and pathophysiological conditions is discussed. This article complements recent comprehensive reviews of bile acid chemistry and physiology (1), bile acid transporter structure and function (2, 3), bile acid synthesis (4), and bile acid signaling (5–7).
Bile acids are synthesized from cholesterol in the liver and secreted into the small intestine where they facilitate absorption of fat-soluble vitamins and cholesterol (1). The majority of bile acids are reabsorbed from the intestine and returned to the liver via the portal venous circulation. At the hepatocyte sinusoidal membrane, bile acids are extracted and resecreted into bile (8). This enterohepatic circulation of bile acids is an extremely efficient process; less than 10% of the intestinal bile acids escapes reabsorption and is eliminated in the feces. In the small intestine, bile acids are absorbed by both passive and active mechanisms (9). Whereas passive absorption occurs down the length of the intestine, active absorption of bile acids is restricted to the ileum (10, 11). In man and all other vertebrates examined to date, the ileal epithelium has developed an efficient transport system for the active reclamation of bile acids (1). The enterohepatic circulation maintains a bile acid pool size of approximately 4 mg in mice and 2 to 4 g in humans. This pool cycles multiple times per meal (12, 13) and as such, the intestinal bile acid absorbed may be as much as 20 mg/day in mice and 30 g/day in humans. Hepatic conversion of cholesterol to bile acid balances fecal bile acid excretion and this process represents a major route for elimination of cholesterol from the body (14, 15).
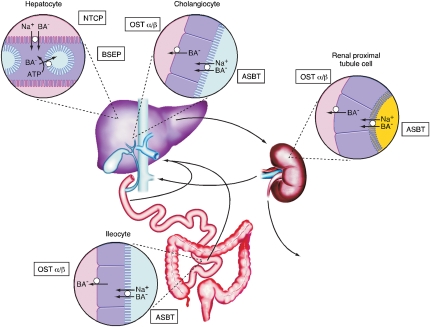
Evolution of an ileal active transport system and gallbladder improved the efficiency of intestinal lipid absorption by dissociating hepatic bile acid secretion from bile acid synthesis. This ensures a continuous supply of bile acids to be used repeatedly for lipid absorption during the digestion of a single meal or multiple meals throughout the day (16). Efficient intestinal reabsorption and hepatic extraction of bile acids also enables a very effective recycling and conservation mechanism that largely restricts these potentially cytotoxic detergents to the intestinal and hepatobiliary compartments. At a fundamental level, the bile acid enterohepatic circulation can be viewed as a series of storage chambers (gallbladder, small intestine), valves (sphincter of Oddi, ileocecal valve), mechanical pumps (canaliculi, biliary tract, small intestine), and chemical pumps (hepatocytic and enterocytic transporters). Over the past two decades, significant progress has been made to identify the major hepatic and intestinal transporters that function to maintain the enterohepatic circulation of bile acids (3, 17). These are summarized in Fig. 1.
Fig. 1.
Enterohepatic circulation of bile acids. Localization of the major transport proteins, NTCP, BSEP, ASBT, and OSTα-OSTβ of the enterohepatic circulation responsible for bile acid (BA) movement across hepatocytes, cholangiocytes, ileocytes (ileal enterocytes), and renal proximal tubule cells. In the liver, bile acids are efficiently extracted from portal blood by the Na+-taurocholate cotransporting polypeptide (NTCP; gene symbol SLC10A1) and resecreted across the canalicular membrane by the bile salt export pump (BSEP; gene symbol ABCB11). A fraction of the bile acids are absorbed by the epithelial cells lining the biliary tract (cholangiocytes) and sent back to the liver for resecretion into bile, a process termed cholehepatic shunting. The transcellular transport of bile acids across the biliary epithelium is mediated by the apical sodium bile acid transporter (ASBT, gene symbol SLC10A2) and the heteromeric transporter, OSTα-OSTβ, on the apical and basolateral membranes, respectively. Bile acids ultimately empty from the biliary tract into the small intestine where they are efficiently absorbed in the terminal ileum by the ASBT and OSTα-OSTβ and returned to the liver in the portal circulation. Bile acids that escape hepatic first-pass clearance are filtered by the kidney. Rather than be excreted in the urine, the bile acids are reabsorbed by the renal proximal tubule cells via the ASBT and OSTα-OSTβ and sent back to the liver for uptake and resecretion into bile. (Adapted with permission from Mosely RH: Bile secretion and cholestasis. In Kaplowitz N: Liver and Biliary Disease. 2nd ed. Philadelphia, Williams and Wilkins, 1996, p 194).
HEPATOCELLULAR TRANSPORT OF BILE ACIDS
Approximately 95% of the bile acids secreted into bile are derived from the recirculating pool. To maintain this process, liver parenchymal cells must transport bile acids efficiently from the portal blood into bile. This vectorial trans-hepatocellular movement of bile acids is a remarkably concentrative transport process that is driven by a distinct set of primary (ATP-dependent), secondary (Na+ gradient-dependent), and tertiary (OH− or HCO3−-dependent anion exchange) transport systems at the sinusoidal and canalicular plasma membranes (2, 3). Hepatocellular uptake of bile acids occurs against a presumed unfavorable electrochemical gradient (18, 19) via sodium-dependent and independent mechanisms. The uptake of conjugated (i.e., N-acyl amidated with taurine or glycine) bile acids, such as taurocholate, at the sinusoidal membrane is mediated predominantly (>75%) by secondary active Na+-dependent transport. In contrast to conjugated bile acids, Na+-dependent uptake accounts for less than half of the uptake of unconjugated bile acids (20–22). The Na+-dependent sinusoidal membrane uptake is mediated by the NTCP, whereas members of the organic anion transporting polypeptide (OATP) family are responsible for the Na+-independent transport.
After uptake, conjugated bile acids are shuttled across the hepatocyte to the canalicular membrane for secretion into bile; unconjugated bile acids follow a similar path after first undergoing N-acyl amidation to taurine or glycine (1). Whereas bile acid concentrations within the hepatocyte are presumed to be in the micromolar range, canalicular bile acid concentrations are as much as 1000-fold higher, necessitating active transport across the canalicular membrane (23). BSEP is the transporter responsible for canalicular transport of the major bile acid species (24), whereas secretion of sulfated bile acids or unusual bile acid such as tetra-hydroxylated forms is mediated by other ABC transporters, including the multidrug resistance protein (MRP)-2 (ABCC2) (25, 26) and multidrug resistance protein (MDR)-1 (ABCB1) (27).
An area of active investigation is the identification and analysis of transporters responsible for the sinusoidal efflux of bile acids and other organic solutes from the interior of the hepatocyte into the space of Disse. Whereas sinusoidal membrane bile acid transport is overwhelmingly in the direction of uptake under normal physiological conditions, bile acids can also be effluxed as an important protective mechanism to reduce bile acid overload under cholestatic conditions (28). Much of this work has focused on members of the MRP (ABCC) family of ATP-dependent transporters, in particular, MRP3 and MRP4 (29–31), and on the heteromeric transporter, OSTα-OSTβ (32, 33). MRP3 and MRP4 are expressed on the sinusoidal membrane of hepatocytes (29, 34) and have bile acid transport (30) or glutathione-bile acid cotransport activity (35). The expression of these transporters is induced under cholestatic conditions to promote bile acid efflux (3, 33, 36–40), thus lowering the concentration in the hepatocyte and decreasing the likelihood of apoptosis or necrosis (41). Our understanding of the relative contribution of the different efflux transporters is still developing; however, the most recent results point toward especially important roles of MRP4 and OSTα-OSTβ (33, 39, 42). The increased expression of these transporters is an important part of the adaptive response to conditions of bile acid overload that also includes downregulation of the major liver bile acid uptake transporters, NTCP, and members of the OATP family (28).
NTCP
NTCP (gene name SLC10A1) is the founding member of the SLC10 family of solute carrier proteins, which includes two bile acid carriers (SLC10A1/NTCP and SLC10A2/ASBT), one steroid sulfate transporter (SLC10A6/SOAT), and four orphan carriers (SLC10A3, SLC10A4, SLC10A5, SLC10A7) (43–45). NTCP, a 349 amino acid membrane glycoprotein, functions as an electrogenic sodium-solute cotransporter and moves 2 or more Na+ ions per molecule of solute (46, 47). NTCP's major physiological substrates include all the major glycine and taurine-conjugated bile acids (48–52). Depending on the structure of the bile acid and NTCP ortholog, unconjugated bile acids are moderate or weak substrates (50, 53, 54) and sulfated bile acids appear to be only weakly transported (55). In contrast to NTCP, members of the OATP family expressed on the hepatocyte sinusoidal membrane, such as Oatp1a1 (Slco1a1), efficiently transport unconjugated or sulfated bile acids, suggesting that these carriers participate in the hepatic uptake of those bile acid species in vivo (50, 56, 57). The high level of NTCP expression at the sinusoidal membrane of hepatocytes and NTCP's high affinity for conjugated bile acids promotes their efficient extraction from portal blood. Thus, NTCP functions to maintain the enterohepatic circulation of bile acids and keeps plasma concentrations at a minimum. Unlike the related ileal apical sodium bile acid transporter (ASBT or IBAT; SLC10A2), NTCP also interacts with a variety of drugs and steroids (51, 58–60). Although it is not yet clear how many of these compounds are competitive or noncompetitive inhibitors of NTCP versus actual substrates, this broader inhibitor profile supports a possible role for NTCP in the clearance of some drugs or drug metabolites.
The properties of NTCP satisfied all the functional criteria for hepatocytic Na+-coupled bile acid uptake, including: 1) preferential high affinity transport of conjugated bile acids; 2) kinetics for taurocholate transport similar to isolated hepatocytes; 3) electrogenic Na+-taurocholate uptake (46); 4) appropriate tissue-specific expression in the liver; 5) similar ontogeny for Na+-dependent bile acid uptake and NTCP expression in development (61); and 6) the finding that NTCP-specific antisense oligonucleotides decreased Na+-dependent taurocholate uptake by more than 90% in rat liver mRNA-injected Xenopus oocytes (62). Although the presence of additional Na+-dependent bile acid transporters, such as microsomal epoxide hydrolase, has been suggested (63), the current evidence indicates that NTCP accounts for most, if not all, hepatic sinusoidal membrane Na+-dependent bile acid transport (43). However, genetic evidence supporting a primary role of NTCP in hepatic sinusoidal bile acid uptake is still lacking. NTCP null mice have not yet been described. Polymorphisms that interfere with bile acid transport in vitro were reported as part of an analysis of human NTCP genotypes in different ethnic groups (64). Unfortunately, there was no description of the in vivo bile acid phenotype associated with those polymorphisms. In addition, whereas inherited disorders characterized by a relatively isolated elevated plasma bile acid level (hypercholanemia; part of the predicted phenotypes for a defect in hepatic bile acid uptake) have been described, mutations in genes other than NTCP, such as the tight junction proteins ZO-2 and claudin-1, were found to be responsible for these diseases (65, 66). These findings leave open the possibility that an isolated NTCP gene defect may be asymptomatic as hepatocytes also express Na+-independent bile acid transporters.
Regulation of NTCP expression and activity
Substrate, cytokines, liver injury, and hormones all regulate transcription of the NTCP gene (2, 67). Although the molecular mechanisms and transcription factors may differ, a common theme among these major regulatory pathways is the suppression of NTCP gene expression as an adaptive response to reduce bile acid entry into the hepatocyte. In fact, the short-term modulation of NTCP activity to match Na+-dependent bile acid uptake to the bile acid load under physiological conditions may be largely controlled by posttranscriptional mechanisms (68). Under pathophysiological conditions, numerous mechanisms exist to decrease NTCP transcription. As described below, bile acids feed back to downregulate NTCP gene transcription by FXR-dependent and independent mechanisms to prevent cytotoxic bile acid accumulation. NTCP transcription is also decreased with different forms of cholestatic liver disease, including those associated with inflammation, ethinylestradiol and pregnancy, obstruction, and drugs or toxins, again as part of a coordinated response to reduce the extent of liver injury (67).
With regard to substrate, NTCP transcription is downregulated in a complex fashion by bile acids via direct or indirect mechanisms that potentially involve farnesoid X receptor (FXR), small heterodimer partner (SHP), retinoic acid receptor alpha:retinoid X receptor alpha (RARα:RXRα), hepatocyte nuclear factor (HNF)-1α, HNF-4α, liver receptor homolog (LRH)-1, and forkhead box A2 (FOXA2) (HNF-3β) (69–76). FXR does not interact directly with the NTCP promoter but induces expression of other factors to indirectly repress NTCP expression. In the rat, bile acids act via FXR to induce the expression of SHP, which in turn can interfere with RXRα:RARα activation of the NTCP promoter. SHP may also be acting via HNF-4α and HNF-1α to repress rat NTCP expression (72, 73). In the mouse, NTCP expression is significantly reduced in HNF-4α null mice (77) and direct promoter analysis revealed HNF-4α strongly activates NTCP expression (78). HNF-4α, working in conjunction with peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), strongly activates NTCP expression and may be important for the increase in NTCP expression and liver sinusoidal bile acid uptake observed during fasting (79). In addition to the pathways involving RXRα:RARα and HNF-1α, several lines of evidence indicate that other mechanisms must exist for bile acid-dependent downregulation of NTCP. First, although HNF-1α and RXRα:RARα bind and activate the rat NTCP promoter, these factors do not appear to activate the mouse or human NTCP minimal promoters (73). Second, NTCP mRNA expression is still decreased by cholic acid feeding in SHP null mice, indicating that SHP-independent mechanisms must exist to mediate this repression of NTCP expression (80). The finding that the rat, mouse, and human NTCP promoters all include a consensus Foxa2 binding motif led to the suggestion that this may be a common pathway for NTCP regulation (73). However, recent studies argue against a direct role of Foxa2 in mediating the repression of NTCP by bile acids (81). Finally, some of the regulatory actions of bile acids are mediated through activation of the c-Jun N-terminal kinase (JNK) (82). JNK-dependent phosphorylation of RXRα has been shown to reduce RXRα:RARα binding and activation of the rat NTCP promoter (83), providing a potential mechanism to regulate NTCP expression independent of SHP.
With regard to inflammation, NTCP expression is rapidly downregulated following administration of lipopolysaccharide (LPS) or the inflammatory cytokines tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, or IL-6 (84–87). Under conditions of inflammation-induced cholestasis, IL-1β appears to play a major role in regulating NTCP expression (88), although there must be some redundancy because LPS still downregulated NTCP expression in IL-1β receptor and TNFα receptor1 knockout mice (89). In contrast to IL-β or TNFα, IL-6 does not appear to be important for downregulation of NTCP during the early stages of LPS-induced inflammation (85, 89). The downregulation of NTCP is mediated by FXR-independent mechanisms (90, 91) that potentially involve decreased RXRα expression, inactivation of RXRα by phosphorylation via mitogen-activated protein (MAP) kinase 4 and JNK (83, 92), and decreased nuclear levels of RXRα (93–95). In addition to affecting RXRα:RARα, LPS could downregulate NTCP expression by reducing HNF-1α expression and activity (86, 88). The regulation via HNF-1α may be secondary to LPS or IL-1β downregulation of HNF-4α (96), an important positive regulator of HNF-1α expression (97, 98).
In addition to regulation by substrate or under conditions of inflammation-induced cholestasis, NTCP is also downregulated in other forms of cholestatic liver injury, including obstructive, drug- or toxin-induced, and estradiol-induced cholestasis (67). In the early stages of obstructive cholestasis, NTCP transcription is downregulated by SHP-dependent mechanisms similar to those involved in the regulation by bile acids (90, 91, 99). At later stages, proinflammatory cytokines may contribute to the sustained downregulation of NTCP expression via RXRα:RARα-dependent mechanisms (91). NTCP expression is regulated during drug- or toxin-induced liver injury and during the liver regeneration that occurs in response to injury. After partial hepatectomy or in the carbon tetrachloride model of toxic liver injury, NTCP mRNA expression is rapidly decreased (100, 101). Inactivation of TNFα but not IL-1β prevented this downregulation of NTCP during liver regeneration, underscoring the central role of TNFα in the regulation of NTCP during this process (88).
With regard to hormones, one of the best-characterized mechanisms of transcriptional regulation is the induction by prolactin and growth hormone via recruitment of phosphorylated Stat5 to the NTCP promoter (102, 103). Glucocorticoids directly induce expression of NTCP via glucocorticoid receptor binding to the promoter in a mechanism that is enhanced by PGC-1α and suppressed by SHP (76). Finally, NTCP mRNA expression is decreased by estrogen and increased by thyroid hormone (104).
NTCP transport activity is also regulated posttranscriptionally. Cyclic AMP (cAMP) rapidly (within minutes) stimulates Na+-taurocholate cotransport in hepatocytes by increasing the transport maximum (Vmax) (105, 106). This increase in transport activity is due to cAMP-induced movement of NTCP from an intracellular compartment to the plasma membrane (68). The sinusoidal plasma membrane expression of NTCP is regulated by serine/threonine phosphorylation and dependent on microfilaments (107–109). NTCP is dephosphorylated at Serine 226 (110) in response to cAMP, leading to increased plasma membrane NTCP retention. The cAMP-induced dephosphorylation of NTCP is dependent on its ability to increase cytosolic Ca2+ and activate protein phosphatase 2B (PP2B), a calcium/calmodulin dependent serine/threonine protein phosphatase (111). In addition to this PP2B-mediated pathway, NTCP's microfilament-dependent insertion and retrieval from the plasma membrane is regulated by PI3-kinase (68). This PI3-kinase stimulated translocation is thought to be mediated in part via protein kinase C delta, as well as other signaling pathways (112–115).
BSEP
Functional evidence of ATP-dependent bile acid transport by the canalicular membrane existed since the early 1990s (23). After cloning and expression studies identified a novel canalicular ABC transporter closely related to the multidrug resistance protein (MDR1)/P-glycoprotein, this 160 kDa protein (originally named "sister of P-glycoprotein") was shown to transport conjugated bile acids efficiently and was subsequently renamed the bile salt export pump (BSEP; ABCB11) (24). The properties of BSEP satisfied all the functional criteria for hepatocytic canalicular bile acid export, including: 1) preferential high affinity transport of conjugated bile acids (116, 117); 2) appropriate tissue-specific expression in the liver; and 3) appropriate ontogeny of expression (118, 119). The role of BSEP as the major canalicular bile acid efflux pump was confirmed by the identification of ABCB11 mutations in patients with progressive familial intrahepatic cholestasis (PFIC) type 2, a hepatic cholestatic disorder characterized by biliary bile acid concentrations less than 1% of normal (120, 121). In addition to unconjugated, taurine-conjugated, and glycine-conjugated species of monovalent bile acids, human BSEP (but not rodent) also transports some sulfated bile acids such as taurolithosulfocholate (122). Whereas bile acids are the major physiological substrate, BSEP appears to be able to transport a limited number of drugs such as the HMG-CoA reductase inhibitor, pravastatin (123). Other drugs, such as cyclosprin, rifamycin, troglitazone, and glibenclamide, are not substrates but interact with BSEP and inhibit bile acid export. Direct inhibition of BSEP may be an important mechanism of hepatotoxicity for drugs inducing cholestasis (24, 117, 124).
Defects in BSEP are responsible for inherited forms of liver disease. Depending on the clinical course, BSEP deficiency is categorized as PFIC2 or benign recurrent intrahepatic cholestasis (BRIC) type 2. PFIC2 is associated with low bile acid secretion, failure to thrive, intractable pruritus, progressive cholestasis, and a significantly increased risk for hepatobiliary malignancy (121, 125). In patients with PFIC2, BSEP protein is typically absent or significantly reduced in liver biopsy specimens, suggesting that these mutations impair BSEP synthesis, cellular trafficking, or stability (121). BRIC2 is associated with milder forms of liver disease and with less severe BSEP defects such as missense mutations (126). However, it should be noted that some missense mutations cause severe forms of BSEP deficiency by altering premRNA splicing as described in a recent comprehensive analysis of the major BSEP sequence variants (127). The BSEP mutations associated with milder disease may also confer increased risk for development of acquired forms of cholestasis, including intrahepatic cholestasis of pregnancy (ICP) and drug-induced cholestasis (128, 129).
Regulation of BSEP expression and activity
BSEP mRNA expression is induced when hepatocyte bile acid levels are elevated, such as following dietary challenge with bile acids (130), or under certain cholestatic conditions (32, 131, 132). This is due to a direct activation of the human and rodent BSEP genes by bile acids acting via FXR (133, 134). In addition to identification of an FXR-responsive element in the BSEP promoter, inactivation of FXR in mice results in low levels of BSEP expression that are not induced by bile acid feeding (135, 136). The induction of BSEP expression is not a universal property of all species of bile acids and correlates with the FXR ligand specificity (137, 138). In fact, lithocholic acid may act as a partial FXR antagonist to reduce BSEP expression (139), thereby providing another potential mechanism for lithocholic acid's cholestatic effects (140). The FXR stimulation of BSEP expression appears to require recruitment of the chromatin-modifying enzymes, coactivator-associated arginine methyltransferase 1 (CARM1) and arginine methyltransferase (PRMT1), and subsequent chromatin remodeling (141, 142). Another potential regulator of BSEP is vitamin A. FXR functions as a heterodimer with RXR, and in vitro studies suggest that 9-cis retinoic acid activation of RXR inhibits the FXR-induced transcription of human BSEP (143). This observation was recently confirmed and diet studies in mice showed that the combination of vitamin A-deficiency and cholic acid feeding resulted in the highest levels of BSEP expression (144).
In addition to transcriptional regulation, there is considerable evidence for posttranscriptional regulation of the BSEP protein localization to the canalicular membrane (24). This short-term regulation enables the hepatocyte to rapidly modulate bile acid secretion in response to bile acid flux and to pathophysiological conditions such as cholestasis (68). Another rapidly developing area of study is the role of membrane lipid composition in the regulation of BSEP and other canalicular membrane transporters (145, 146). There is emerging evidence that the phosphatidylserine flippase, ATP8B1, plays a critical role in maintaining the phospholipid asymmetry and rigid cholesterol and sphingomyelin-rich exoplasmic leaflet of the canalicular membrane (147). In humans, ATP8B1 deficiency causes PFIC Type 1 (PFIC1) and BRIC Type 1 (BRIC1) (145). Among the mechanisms responsible for the cholestasis, inactivation of ATP8B1 results in a reduced cholesterol to phospholipid ratio for the canalicular membrane. This reduced cholesterol content is associated with a dramatic reduction in the Vmax for taurocholate transport by BSEP (146) but not reduced canalicular levels of BSEP protein (148).
INTESTINAL TRANSPORT OF BILE ACIDS
Bile acids are reclaimed through a combination of passive absorption in the proximal small intestine, active transport in the distal ileum, and passive absorption in the colon. Bile acids are actively transported in the terminal ileum by the well-characterized ASBT (SLC10A2) (43, 149). This sodium- and potential-driven transporter moves bile acids from the lumen of the small intestine across the apical brush border membrane. Bile acids are then shuttled to the basolateral membrane and effluxed into the portal circulation by OSTα-OSTβ (150, 151). Several observations support the concept that the terminal ileum is the major site of bile acid reabsorption in man and experimental animal models. These observations include the finding that there is little decrease in the intraluminal bile acid concentration prior to the ileum (9) and the appearance of bile acid malabsorption after ileal resection (152). Subsequent studies using in situ perfused intestinal segments to measure bile acid absorption (153–155) demonstrated that ileal bile acid transport is a high-capacity system sufficient to account for reabsorption of the biliary output of bile acids. The general consensus from these studies was that ileal active transport is the major route for conjugated bile acid uptake whereas the passive or facilitative absorption present down the length of the small intestine may be significant for unconjugated and some glycine-conjugated bile acids. However, the contribution of jejunal uptake to intestinal bile acid absorption in different species is still being debated (156).
In comparison to ileal absorption (described below), the mechanism(s) responsible for transport of bile acids in the proximal intestine and their quantitative significance are not as well defined. In the mouse, bile acid species are taurine-conjugated and hydrophilic thereby limiting their nonionic diffusion and necessitating the requirement for a carrier to mediate transport across the enterocyte apical brush border membrane. This was confirmed by analysis of ASBT null mice in which intestinal bile acid absorption was largely eliminated. A key observation was that feeding ASBT null mice a diet containing cholestyramine resulted in no further increase in fecal bile acid excretion. Because a bile acid binding resin would reduce alternative (nonASBT) passive or active mechanisms for intestinal bile acid absorption, these findings supported the conclusion that nonASBT mechanisms contribute little to intestinal bile acid absorption in the mouse (157).
In humans and many other species, the bile acid pool includes glycine conjugates, unconjugated bile acids, and more hydrophobic species (1). A fraction of the glycine conjugates and unconjugated bile acids are protonated under conditions of the acidic luminal surface pH in the intestine (158) and absorbed by passive diffusion across the apical brush border membrane (155). In addition to passive membrane diffusion, there is evidence for carrier-mediated transport of bile acids in the proximal small intestine. Studies using photoactivated bile acid derivatives specifically labeled an ∼87 kDa brush border membrane protein that was present along the entire length of rabbit small intestine (159). Elegant in vivo uptake and cis-inhibition studies using perfused jejunum demonstrated bile acid transport specificity consistent with a facilitative carrier (160, 161). In vitro studies using rat jejunal brush border membrane vesicles extended those findings and clearly demonstrated conjugated bile acid uptake operating through an anion exchange mechanism (162). The properties of this transport system are similar to those ascribed to the OATP family transporters and a candidate for the jejunal bile acid transporter was cloned from rat (163). This ∼80 kDa membrane glycoprotein, Oatp1a5 (Oatp3), exhibits expression properties, substrate specificity, and apical membrane localization consistent with the in vivo and in vitro studies of proximal small intestine (161, 162). Rodent Oatp1a5 is encoded as part of an OATP gene cluster and is syntenic with a region on human chromosome 12p12 that includes OATP1A2 (164). Human OATP1A2, which shares ∼72% amino acid identity with rodent Oatp1a5, is expressed at the apical brush border membrane of human small intestinal epithelial cells and transports bile acids as well as a variety of drugs (165, 166) (167). Although these findings are suggestive, it should be noted that the intestinal level of expression for Oatp1a5/OATP1A2 is very low compared with the ASBT (163, 168) and their contribution to intestinal bile acid absorption is thought to be small as compared with ASBT-mediated transport in the ileum (157, 169).
Regardless of the mechanism of bile acid uptake, these weak acids will ionize at the neutral pH of the cytosolic compartment, potentially trapping bile acids in the cell and necessitating the presence of efflux carriers. Recent studies have identified OSTα-OSTβ as a major mechanism responsible for intestinal basolateral membrane bile acid export (170, 171). Although highly expressed in terminal ileum, OSTα-OSTβ is also expressed at lower levels in proximal small intestine of rodents and humans (150, 151, 172) where it can serve to export bile acids that were passively absorbed across the apical brush border membrane of the enterocyte.
ASBT
Bile acids are transported actively across the ileal brush-border membrane by the well-characterized ASBT (SLC10A2). The relationship between the hepatic, biliary, ileal, and renal Na+-bile acid cotransport systems was resolved with the cloning of the bile acid carriers from those tissues. The liver and ileum express distinct but related Na+ bile acid cotransporter genes, NTCP (SLC10A1) and ASBT (SLC10A2), whereas the ileal enterocyte, renal proximal tubule cell, and cholangiocyte all express the same Na+ bile acid cotransporter (ASBT) (2). The inwardly directed Na+ gradient maintained by the basolateral Na+/K+-ATPase as well as the negative intracellular potential provide the driving force for ASBT-mediated bile acid uptake (173). The properties of the ASBT satisfied all the functional criteria for ileal active bile acid uptake, including: 1) a strict sodium-dependence for bile acid transport (55); 2) specific transport of all the major monovalent species of bile acids with negligible uptake of other solutes (51); 3) specific intestinal expression in the terminal ileum; 4) similar ontogeny for rat ileal sodium-dependent taurocholate uptake and ASBT expression at fetal day 22 and postnatal day 17 (174); 5) targeted inactivation of the ASBT gene eliminates enterohepatic cycling of bile acid in mice (157); and 6) loss-of-function mutations in the human ASBT gene are associated with intestinal bile acid malabsorption (169).
Regulation of ASBT expression and activity
ASBT is expressed in tissues that serve to facilitate the enterohepatic circulation of bile acids, including the apical membrane of ileal enterocytes, proximal renal convoluted tubule cells, epithelial cells lining the biliary tract (cholangiocytes), and gallbladder epithelial cells (175–179). The molecular mechanisms controlling the tissue-specific expression of ASBT are not known. In the intestine, Na+-dependent bile acid transport activity and ASBT expression are found in villus but not crypt enterocytes (174, 180). Expression is largely restricted to the ileum in mice, hamster, rats, and humans (181–185). Regulation of ASBT expression along the longitudinal axis of the intestine is not fully understood although recent studies showed that the transcription factor GATA4 is essential for this process. Remarkably, intestine-specific inactivation of GATA4 in mice results in a dramatic induction of ASBT expression in proximal intestine (186, 187). After injury or resection of distal small intestine, therapeutic options for restoring bile acid absorption have been limited as compensatory increases in ASBT expression appear to occur only in those remaining intestinal regions that natively expressed ASBT (i.e., ileum) (185, 188, 189). However, the exciting finding that GATA4 is critical for establishing the functional gradient of bile acid absorption along the cephalocaudal axis of the small intestine reveals a novel opportunity for inducing proximal bile acid absorption by modulating the GATA4 pathway.
The enterohepatic circulation efficiently conserves bile acids, thereby maintaining bile flow and adequate intraluminal bile acid concentrations for micellular solubilization and absorption of lipids. Considering its central role in the enterohepatic circulation, inherited defects or dysfunctional regulation of the ASBT may play a role in the pathogenesis or clinical presentation of a variety of gastrointestinal disorders. For example, ASBT mutations were identified as a cause of primary bile acid malabsorption, a rare idiopathic disorder associated with interruption of the enterohepatic circulation of bile acids. Patients with primary bile acid malabsorption present with chronic diarrhea beginning in early infancy, steatorrhea, fat-soluble vitamin malabsorption, and reduced plasma cholesterol levels (169). Although dysfunctional mutations were not found in the ASBT gene from patients with adult-onset forms of idiopathic bile acid malabsorption (190), aberrant regulation of the ASBT may still contribute to the phenotype in a subset of those patients (172). Indeed, a recent genetic study identified an ASBT haplotype associated with significantly reduced ileal expression of ASBT mRNA and protein (191). Other disorders associated with intestinal bile acid malabsorption that could potentially involve the ASBT include hypertriglyceridemia (192, 193), idiopathic chronic diarrhea (194), chronic ileitis (195), gallstone disease (196, 197), postcholecystectomy diarrhea, Crohn's disease (198–202), and irritable bowel syndrome (203).
Substrate, cytokines, hormones, and sterols all regulate transcription of the ASBT gene. The regulation of ASBT expression by bile acids remains an area of controversy. Conflicting observations have been published, reporting that ASBT and/or ileal bile acid transport activity is induced, repressed, or unaffected by bile acids (204–208). Differences between experimental paradigms, methods for measuring ASBT protein or activity, species, and genetic background account for some of the discrepancies that have been observed (209, 210). The dose and mode of delivery of bile acid is also an important consideration, as cell models do not recapitulate the in vivo dynamic flux of bile acids. In addition, bile acids are cytotoxic in high concentrations or under conditions of static exposure, and can activate a variety of signaling pathways (6). As such, it is important to try to distinguish between "basal" feedback regulation of ASBT expression and more complex responses to protect the cell from bile acid-induced injury.
Some of the earliest support for regulation of ASBT by bile acids was obtained from intestinal perfusion studies in the guinea pig; those studies showed that the ileal bile acid transport capacity was decreased after bile acid feeding and increased following the administration of a bile acid binding resin (205). In the mouse and rabbit, bile acids can also repress ASBT expression, apparently by acting through FXR and SHP to antagonize LRH-1, a competence factor important for ASBT transcription (210, 211). A positive role for LRH-1 is supported by the finding that ASBT expression is decreased in ileum of intestine-specific LRH-1 null mice (75). In contrast, FXR is not essential for ASBT's basal expression as ASBT expression is not decreased in ileum of FXR null mice (212, 213). For humans, in vitro studies using Caco-2 cells or ileal biopsies have identified several different potential mechanisms for bile acid regulation of ASBT. In one mechanism, bile acids were found to act through the FXR-SHP pathway to antagonize RARα and decrease human ASBT transcription (214). Conversely, bile acids were also found to increase ASBT transcription through an FXR-independent pathway involving the epidermal growth factor receptor and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) signaling through an AP-1 element in the ASBT promoter (208). In vivo, several lines of evidence indirectly suggest that negative feedback regulation of intestinal bile acid transport is operational in humans, including the findings that retention of the bile acid analog 75Se-homocholic acid-taurine is increased in primary biliary cirrhosis (215) and intestinal ASBT expression is increased in patients with obstructive cholestasis (216). However, these are pathophysiological states and clearly more needs to be done to understand how bile acids regulate their own active intestinal absorption under physiological conditions. A new potential pathway for the bile acid-mediated repression of ASBT transcription has recently begun to emerge. Bile acids act through FXR to induce expression of FGF15/19, which feeds back in an autocrine or paracrine fashion to repress ASBT expression (217). In this model, the newly secreted FGF15/19 binds to its receptor, the FGFR4/β-klotho complex, expressed on basolateral surface of enterocytes and signals to stabilize the SHP protein by inhibiting its ubiquitin/proteosome-dependent degradation (218). The elevated levels of SHP could then antagonize LRH-1 or RARα to downregulate ASBT expression.
In addition to regulation by bile acids, ASBT mRNA expression appears to be negatively regulated by cholesterol. In vitro studies using Caco-2 cells showed that sterols such as 25-hydroxycholesterol downregulated ASBT mRNA expression and promoter activity (219). As elucidated using a variety of in vivo and in vitro approaches, the apparent underlying mechanism involves an indirect effect of SREBP-2 on HNF-1α-mediated stimulation of ASBT transcription (220). In general agreement with that work, the addition of cholesterol to a cholic acid-containing diet appeared to downregulate ASBT expression and intestinal bile acid absorption by an FXR-independent mechanism (221).
Ileal inflammation is associated with bile acid malabsorption (199), and ASBT expression is decreased in animal models of ileitis (222) and in ileal biopsies from patients with Crohn's disease (223). Investigation of the underlying mechanism revealed that cytokines decrease ASBT gene expression by acting through an activator-protein-1 (AP-1) site, which binds a c-jun/c-fos heterodimer (224, 225). In the setting of intestinal inflammation, c-fos is phosphorylated and translocates into the nucleus where binding of the downstream AP-1 element leads to transcriptional repression of ASBT expression (225). Corticosteroids have had a long-standing use in the treatment of inflammatory bowel disease. Exogenous corticosteroids can induce precocious expression of ASBT and also upregulate ASBT expression in mature animals (226). In humans, exogenous corticosteroid (Budesonide) treatment increased ASBT expression in ileal biopsies, and this effect is mediated through glucorticorticoid receptor binding to specific response elements in the human ASBT promoter (223). Thus, the beneficial effects of corticosteroids in inflammatory bowel disease may involve not only an effect on inflammatory cytokines but also a direct effect on ASBT expression (227). With regard to other nuclear receptors, ASBT gene expression is activated by peroxisome proliferator-activated receptor α (PPARα) (228) and by vitamin D nuclear receptor (VDR) (229) binding to their respective response elements in the ASBT promoter.
In addition to transcriptional regulation, ASBT is regulated posttranscriptionally by modulating protein stability or transporter activity. The ASBT protein resides on the plasma membrane in lipid rafts and depleting membrane cholesterol using methyl-β-cyclodextrin significantly reduced ASBT's association with rafts and taurocholate transport (230). The cholesterol-depleted cells exhibited no change in ASBT protein expression at the plasma membrane, suggesting that the decreased taurocholate uptake was due to reduced ASBT activity. Finally, proteosomal-mediated degradation of ASBT is stimulated by cytokines such as IL-1β (231). The downregulation of ASBT protein expression correlated with a decrease in the half-life of ASBT from ∼6 h to ∼3 h, and the enhanced degradation was blocked using a c-Jun N-terminal kinase inhibitor or proteasome inhibitors.
OSTα-OSTβ
The proteins responsible for bile acid export across the basolateral membrane of the ileal enterocyte, cholangiocytes, and renal proximal tubule cell have only recently been identified. In contrast to apical transport, little was known regarding the mechanism and regulation of bile acid export across the basolateral membrane of these epithelia. Several candidate ileal basolateral transporters had been partially characterized or implicated over the years, including a basolateral sodium-independent anion exchange activity (232), a bile acid photoprobe-labeled 54 kDa protein enriched in the ileal basolateral membrane fraction (233), an alternatively spliced form of the ASBT (234), and MRP3 (ABCC3) (235). However, none of these candidates fulfilled all the predicted criteria (236, 237) and the identity of the basolateral bile acid transporter remained an important missing link in our understanding of the enterohepatic circulation. The break-through in this area came with the elegant expression cloning of an unusual transporter, OSTα-OSTβ, from the little skate (Raja erinacea) by Ned Ballatori and coworkers (238). Subsequently, the human and mouse orthologs of skate OSTα-OSTβ were cloned and expressed in Xenopus oocytes where they transport bile acids as well as a variety of steroids (239). As with the skate, solute transport by the mammalian orthologs required coexpression of two different subunits: OSTα, a 340, amino acid polytopic membrane protein, and OSTβ, a 128 amino acid predicted type I membrane protein. Soon after their cloning, the mouse OSTα-OSTβ was identified as a candidate ileal basolateral bile acid transporter using a transcriptional profiling approach (150). Support for a role of OSTα-OSTβ in basolateral bile acid transport includes: 1) intestinal expression of OSTα and OSTβ mRNA that generally follows that of the ASBT with the highest levels in ileum (150, 151, 172), 2) appropriate cellular localization on the lateral and basal membranes of ileal enterocyte (150), 3) expression of OSTα-OSTβ on the basolateral membrane of hepatocytes, cholangiocytes, and renal proximal tubule cells, other membranes exhibiting bile acid efflux (151), 4) efficient transport of the major bile acid species (150, 151), and 5) expression of OSTα-OSTβ is positively regulated by bile acids (213, 240). Although the functional role of the individual subunits has not yet been determined, coexpression and assembly of both subunits into a complex is required for their trafficking to the plasma membrane and solute transport (150, 241).
No inherited defects have been reported for the OSTα or OSTβ genes in humans; however, targeted inactivation of the OSTα gene in mice resulted in impaired intestinal bile acid absorption and altered bile acid metabolism (170, 171). In contrast to ASBT null mice that exhibited the predicted sequelae associated with intestinal bile acid malabsorption (157, 242), the OSTα null mice exhibited a more complex phenotype. Studies using everted gut sacs (171) or intra-ileal administration of [3H]taurocholate (170) demonstrated a significant reduction in trans-ileal transport in OSTα null mice. However, fecal bile acid excretion was not increased as had been observed in ASBT null mice (157) or patients with ASBT mutations (169). These results were particularly perplexing because the whole body bile acid pool size was significantly decreased, a hallmark of intestinal bile acid malabsorption. Examination of the FGF15/19 signaling pathway provided a solution to this conundrum (Fig. 2). As predicted from this model, OSTα null mice had significantly increased FGF15 expression and reduced hepatic Cyp7a1 expression (170, 171). These findings further supported a central role of FGF15/19 in regulating hepatic bile acid synthesis (243, 244) as recently confirmed in the liver and intestine-specific FXR and LRH-1 null mice and reviewed by the late Dr. Roger Davis (75, 245, 246).
Fig. 2.
Model for differential regulation of hepatic bile acid synthesis. Bile acids are taken up by the ASBT and activate the nuclear receptor FXR to induce expression of OSTα-OSTβ and FGF15 in the ileal enterocyte. The bile acids are then released into the portal circulation via basolateral OSTα-OSTβ and reabsorbed by the hepatic sinusoidal (basolateral) transporter, NTCP. Bile acids are secreted across the apical (canalicular) membrane into the bile canaliculus via the BSEP and undergo another round of enterohepatic cycling. Ileal-derived FGF15 signals through its receptor, FGFR4, to repress Cyp7a1 expression and bile acid synthesis. A block in ileal brush border membrane uptake of bile acids results in downregulation of FXR target genes such as FGF15. The decreased FGF15 production and reduced return of bile acids to the liver leads to increased Cyp7a1 expression, increased hepatic conversion of cholesterol to bile acids, and reduced plasma cholesterol levels. This is the classical mechanism of action for the bile acid sequestrants. In contract, a block in ileal basolateral bile acid export leads to increased bile acid retention and increased FXR-mediated activation of FGF15 expression. Despite reduced return of bile acids in the enterohepatic circulation, the ileal-derived FGF15 signals to repress hepatic Cyp7a1 expression and bile acid synthesis. (From Davis and Attie (246); copyright 2008 National Academy of Sciences, USA).
In addition to demonstrating an important role for OSTα-OSTβ in bile acid transport and metabolism, this work had important physiological implications. Whereas blocking apical bile acid uptake using bile acid sequestrants or ASBT inhibitors dramatically reduces the ileal expression of FGF15 and increases hepatic Cyp7a1 expression (242), a block in basolateral bile acid transport increases FGF15 expression and reduces hepatic bile acid synthesis. This combination of reduced return of bile acids in the enterohepatic circulation and reduced bile acid synthesis may have therapeutic benefit in various forms of cholestatic liver disease. Conversely, the reduction in bile acid synthesis associated with inhibition of basolateral transport could predispose to elevated plasma cholesterol levels in marked contrast to inhibition of apical ileal bile acid transport (247, 248). Of particular interest with regard to human disease, this phenotype of decreased bile acid absorption coupled with an inability of the liver to synthesize additional bile acids to maintain a normal pool that was found in the OSTα null mice had been described by the late Z. Reno Vlahcevic (249) in a study of gallstone patients that was published in 1970. Whether OSTα-OSTβ or the FGF15/19 signaling pathway play a role in the pathophysiology of gallstone disease is an interesting question that is beginning to be explored (250).
Regulation of OSTα-OSTβ expression and activity
Expression of both subunits is essential for function (150, 241) and as such, the two subunit genes appear to be regulated coordinately. Bile acid feeding (213), administration of a synthetic FXR agonist (213, 245, 251), or induction of cholestasis (33, 252) increases expression of OSTα and OSTβ mRNA and multiple groups identified functional FXR responsive elements in the mouse (213, 251) and human (240, 251) OSTα and OSTβ promoters. The promoters for OSTα and OSTβ include functional responsive elements for LRH-1 as well as FXR, providing a mechanism for both negative as well as positive regulation by bile acids (213). Whereas positive regulation by bile acids is dominant, this push-pull form of dynamic regulation would enable the cell to finely titrate expression of OSTα-OSTβ to match the flux of bile acids. The predominantly positive regulation would also ensure efficient export of bile acids thereby preventing cellular injury due to intracellular accumulation. In support of a potential role for LRH-1, ileal expression of OSTα and OSTβ was decreased in the intestine-specific LRH-1 null mice (75). In addition to being regulated by FXR, LXR potentially induces OSTα and OSTβ expression by operating via an inverted repeat-1 element shared with FXR (253).
RENAL BILE ACID TRANSPORT
A fraction (10% to 50%, depending on the bile acid species) of the bile acids returning in the portal circulation escapes hepatic extraction and spills into the systemic circulation. The binding of bile acids to plasma proteins reduces glomerular filtration and minimizes urinary excretion of bile acids. In healthy humans, the kidney filters approximately 100 µmol of bile acids each day. Remarkably, only 1 to 2 µmol is excreted in the urine because of a highly efficient tubular reabsorption (254). Even in patients with cholestatic liver disease in whom plasma bile acid concentrations are elevated, the 24-h urinary excretion of nonsulfated bile acids is significantly less than the quantity that undergoes glomerular filtration (254–257). Subsequent studies have shown that bile acids in the glomerular filtrate are actively reabsorbed from the renal tubules by a sodium-dependent mechanism (258, 259) and this process contributes to the rise in serum bile acid concentrations in patients with cholestatic liver disease.
As in the ileum, the renal proximal tubule epithelium expresses the ASBT as a salvage mechanism to conserve bile acids (55, 176). The overall pattern of bile acid membrane transporter expression appears to be similar for the ileal enterocyte and renal proximal tubule cells. In addition to expressing the ASBT on the apical surface, renal epithelial cells express OSTα-OSTβ (151) on the basolateral membrane thereby completing the circuit for efficient apical absorption from the tubule lumen and basolateral export into the systemic circulation.
In addition to the physiological implications, identification of the ASBT in kidney has therapeutic consequences. Potent inhibitors of the ileal apical sodium bile acid transporter have been developed as potential therapies for hypercholesterolemia (248, 260–262). Because the same transporter is expressed in the kidney, these inhibitors could be used to block renal reclamation of bile acids and increase urinary bile acid output. This would create a shunt for elimination of hepatotoxic bile acids; the predicted decrease in serum and hepatic bile acid concentrations may relieve the cholestasis-associated pruritus and slow the progression of hepatocellular degeneration. Although as yet untested, a variation of this therapeutic approach was originally suggested almost 30 years ago by Barbara Billing and colleagues (working with Dame Sheila Sherlock) (263, 264). However, the potential hepatoprotective effects of such an intervention must be carefully balanced against any risk of increased bile acid-induced kidney cell injury (265).
FUTURE RESEARCH
With the identification of all the major plasma membrane bile acid transporters that maintain the enterohepatic circulation, the focus is shifting toward how these carriers control the extracellular and intracellular levels of bile acids under different physiological and pathophysiological conditions. In addition to its role as a detergent to solubilize biliary and dietary lipids, bile acids activate a variety of nuclear receptors and signaling pathways (5, 6). There is a growing appreciation of the role of bile acids as "hormones" to modulate lipid and glucose metabolism (7). By controlling the flux of bile acids in the enterohepatic circulation, the bile acid transporters have an opportunity to modulate bile acid signaling and metabolic regulation. As such, it will be important to further understand the regulation of these carriers and their relationship to metabolism and human disease. Investigators have also begun to explore the effects of genetic single-nucleotide polymorphisms and epigenetic alterations on transporter expression (266) but much work still needs to be done. In addition to the bile acid binding resins and ursodeoxycholic acid, new compounds are in development that target bile acid signaling pathways, such as nor-ursodeoxycholic acid, synthetic FXR agonists, FXR modulators, and agonists for the bile acid-activated G-protein coupled receptors (5, 267). It will be important to understand how these compounds affect the bile acid transporters in animal models and patients. Other fertile areas for translational investigation include the interaction of drugs with BSEP as a mechanism for drug-induced cholestasis, the therapeutic utility of ASBT inhibitors to lower plasma cholesterol levels and improve insulin resistance, the utility of ASBT or OSTα-OSTβ inhibitors to relieve the hepatic bile acid burden in some forms of cholestatic liver disease, and the modulation of intestinal GATA4 activity to restore intestinal bile acid absorption following ileal disease or resection. So, although we have learned a great deal about the bile acid transporters, important questions remain about the role of these important ferrymen in human health and disease.
Footnotes
Abbreviations:
- ASBT
- apical sodium-dependent bile acid transporter
- BRIC
- benign recurrent intrahepatic cholestasis
- BSEP
- bile salt export pump
- FXR
- farnesoid X receptor
- HNF
- hepatocyte nuclear factor
- IL
- interleukin
- JNK
- Jun N-terminal kinase
- LPS
- lipopolysaccharide
- LRH
- liver receptor homolog
- MRP
- multidrug resistance protein
- NTCP
- Na+ taurocholate cotransporting polypeptide
- OATP
- organic anion transporting polypeptide
- OST
- organic solute transporter
- PFIC
- progressive familial intrahepatic cholestasis
- RAR/RXR
- retinoic acid receptor/retinoid X receptor
- SHP
- small heterodimer partner
- TNFα
- tumor necrosis factor alpha
This work was supported by the National Institutes of Health (DK-47987) and a Grant-in-Aid from the American Heart Association (0855100E) to P.A.D. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. A.R. is supported by a National Research Service Award (F32 DK-079576) from the National Institute of Diabetes and Digestive and Kidney Diseases.
REFERENCES
- 1.Hofmann A. F., Hagey L. R. 2008. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 65: 2461–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alrefai W. A., Gill R. K. 2007. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm. Res. 24: 1803–1823 [DOI] [PubMed] [Google Scholar]
- 3.Trauner M., Boyer J. L. 2003. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83: 633–671 [DOI] [PubMed] [Google Scholar]
- 4.Chiang J. Y.2009. Bile acids: regulation of synthesis. J Lipid Res. 50: 1955–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. 2008. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7: 678–693 [DOI] [PubMed] [Google Scholar]
- 6.Hylemon P. B., Zhou H., Pandak W. M., Ren S., Gil G., Dent P. 2009. Bile acids as regulatory molecules. J Lipid Res. 50: 1509–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. 2009. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89: 147–191 [DOI] [PubMed] [Google Scholar]
- 8.Dawson P. A., Shneider B. L., Hofmann A.F. 2006. Bile formation and the enterohepatic circulation. Physiology of the Gastrointestinal Tract. Johnson L. R., editor Elsevier Academic Press, Amsterdam: Chapter56: 1437–1462 [Google Scholar]
- 9.Dietschy J. M.1968. Mechanisms for the intestinal absorption of bile acids. J. Lipid Res. 9: 297–309 [PubMed] [Google Scholar]
- 10.Krag E., Phillips S. F. 1974. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J. Clin. Invest. 53: 1686–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiff E. R., Small N. C., Dietschy J. M. 1972. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J. Clin. Invest. 51: 1351–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulzebos C. V., Renfurm L., Bandsma R. H., Verkade H. J., Boer T., Boverhof R., Tanaka H., Mierau I., Sauer P. J., Kuipers F., et al. 2001. Measurement of parameters of cholic acid kinetics in plasma using a microscale stable isotope dilution technique: application to rodents and humans. J. Lipid Res. 42: 1923–1929 [PubMed] [Google Scholar]
- 13.Hofmann A. F., Molino G., Milanese M., Belforte G. 1983. Description and simulation of a physiological pharmacokinetic model for the metabolism and enterohepatic circulation of bile acids in man. Cholic acid in healthy man. J. Clin. Invest. 71: 1003–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietschy J. M., Turley S. D., Spady D. K. 1993. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 34: 1637–1659 [PubMed] [Google Scholar]
- 15.Dietschy J. M., Turley S. D. 2002. Control of cholesterol turnover in the mouse. J. Biol. Chem. 277: 3801–3804 [DOI] [PubMed] [Google Scholar]
- 16.Hofmann A. F., Schteingart C. D., Lillienau J. 1991. Biological and medical aspects of active ileal transport of bile acids. Ann. Med. 23: 169–175 [DOI] [PubMed] [Google Scholar]
- 17.Kullak-Ublick G. A., Stieger B., Meier P. J. 2004. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 126: 322–342 [DOI] [PubMed] [Google Scholar]
- 18.Weinman S. A., Maglova L. M. 1994. Free concentrations of intracellular fluorescent anions determined by cytoplasmic dialysis of isolated hepatocytes. Am. J. Physiol. 267: G922–G931 [DOI] [PubMed] [Google Scholar]
- 19.Lidofsky S. D., Fitz J. G., Weisiger R. A., Scharschmidt B. F. 1993. Hepatic taurocholate uptake is electrogenic and influenced by transmembrane potential difference. Am. J. Physiol. 264: G478–G485 [DOI] [PubMed] [Google Scholar]
- 20.Reichen J., Paumgartner G. 1976. Uptake of bile acids by perfused rat liver. Am. J. Physiol. 231: 734–742 [DOI] [PubMed] [Google Scholar]
- 21.Van Dyke R. W., Stephens J. E., Scharschmidt B. F. 1982. Bile acid transport in cultured rat hepatocytes. Am. J. Physiol. 243: G484–G492 [DOI] [PubMed] [Google Scholar]
- 22.Kouzuki H., Suzuki H., Ito K., Ohashi R., Sugiyama Y. 1998. Contribution of sodium taurocholate co-transporting polypeptide to the uptake of its possible substrates into rat hepatocytes. J. Pharmacol. Exp. Ther. 286: 1043–1050 [PubMed] [Google Scholar]
- 23.Stieger B., O'Neill B., Meier P. J. 1992. ATP-dependent bile-salt transport in canalicular rat liver plasma-membrane vesicles. Biochem. J. 284: 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stieger B., Meier Y., Meier P. J. 2007. The bile salt export pump. Pflugers Arch. 453: 611–620 [DOI] [PubMed] [Google Scholar]
- 25.Akita H., Suzuki H., Ito K., Kinoshita S., Sato N., Takikawa H., Sugiyama Y. 2001. Characterization of bile acid transport mediated by multidrug resistance associated protein 2 and bile salt export pump. Biochim. Biophys. Acta. 1511: 7–16 [DOI] [PubMed] [Google Scholar]
- 26.Nies A. T., Keppler D. 2007. The apical conjugate efflux pump ABCC2 (MRP2). Pflugers Arch. 453: 643–659 [DOI] [PubMed] [Google Scholar]
- 27.Lam P., Wang R., Ling V. 2005. Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry. 44: 12598–12605 [DOI] [PubMed] [Google Scholar]
- 28.Zollner G., Marschall H. U., Wagner M., Trauner M. 2006. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol. Pharm. 3: 231–251 [DOI] [PubMed] [Google Scholar]
- 29.Soroka C. J., Lee J. M., Azzaroli F., Boyer J. L. 2001. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology. 33: 783–791 [DOI] [PubMed] [Google Scholar]
- 30.Hirohashi T., Suzuki H., Takikawa H., Sugiyama Y. 2000. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J. Biol. Chem. 275: 2905–2910 [DOI] [PubMed] [Google Scholar]
- 31.Assem M., Schuetz E. G., Leggas M., Sun D., Yasuda K., Reid G., Zelcer N., Adachi M., Strom S., Evans R. M., et al. 2004. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J. Biol. Chem. 279: 22250–22257 [DOI] [PubMed] [Google Scholar]
- 32.Schaap F. G., van der Gaag N. A., Gouma D. J., Jansen P. L. 2009. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 49: 1228–1235 [DOI] [PubMed] [Google Scholar]
- 33.Boyer J. L., Trauner M., Mennone A., Soroka C. J., Cai S. Y., Moustafa T., Zollner G., Lee J. Y., Ballatori N. 2006. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G1124–G1130 [DOI] [PubMed] [Google Scholar]
- 34.Rius M., Nies A. T., Hummel-Eisenbeiss J., Jedlitschky G., Keppler D. 2003. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology. 38: 374–384 [DOI] [PubMed] [Google Scholar]
- 35.Rius M., Hummel-Eisenbeiss J., Hofmann A. F., Keppler D. 2006. Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G640–G649 [DOI] [PubMed] [Google Scholar]
- 36.Marschall H. U., Wagner M., Zollner G., Fickert P., Diczfalusy U., Gumhold J., Silbert D., Fuchsbichler A., Benthin L., Grundstrom R., et al. 2005. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 129: 476–485 [DOI] [PubMed] [Google Scholar]
- 37.Akita H., Suzuki H., Sugiyama Y. 2001. Sinusoidal efflux of taurocholate is enhanced in Mrp2-deficient rat liver. Pharm. Res. 18: 1119–1125 [DOI] [PubMed] [Google Scholar]
- 38.Trauner M., Wagner M., Fickert P., Zollner G. 2005. Molecular regulation of hepatobiliary transport systems: clinical implications for understanding and treating cholestasis. J. Clin. Gastroenterol. 39: S111–S124 [DOI] [PubMed] [Google Scholar]
- 39.Zollner G., Wagner M., Fickert P., Silbert D., Gumhold J., Zatloukal K., Denk H., Trauner M. 2007. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int. 27: 920–929 [DOI] [PubMed] [Google Scholar]
- 40.Teng S., Piquette-Miller M. 2007. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br. J. Pharmacol. 151: 367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyer J. L.2005. Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology. 129: 735–740 [DOI] [PubMed] [Google Scholar]
- 42.Mennone A., Soroka C. J., Cai S. Y., Harry K., Adachi M., Hagey L., Schuetz J. D., Boyer J. L. 2006. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 43: 1013–1021 [DOI] [PubMed] [Google Scholar]
- 43.Hagenbuch B., Dawson P. 2004. The sodium bile salt cotransport family SLC10. Pflugers Arch. 447: 566–570 [DOI] [PubMed] [Google Scholar]
- 44.Geyer J., Wilke T., Petzinger E. 2006. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch. Pharmacol. 372: 413–431 [DOI] [PubMed] [Google Scholar]
- 45.Godoy J. R., Fernandes C., Doring B., Beuerlein K., Petzinger E., Geyer J. 2007. Molecular and phylogenetic characterization of a novel putative membrane transporter (SLC10A7), conserved in vertebrates and bacteria. Eur. J. Cell Biol. 86: 445–460 [DOI] [PubMed] [Google Scholar]
- 46.Hagenbuch B., Meier P. J. 1996. Sinusoidal (basolateral) bile salt uptake systems of hepatocytes. Semin. Liver Dis. 16: 129–136 [DOI] [PubMed] [Google Scholar]
- 47.Weinman S. A.1997. Electrogenicity of Na(+)-coupled bile acid transporters. Yale J. Biol. Med. 70: 331–340 [PMC free article] [PubMed] [Google Scholar]
- 48.Boyer J. L., Ng O. C., Ananthanarayanan M., Hofmann A. F., Schteingart C. D., Hagenbuch B., Stieger B., Meier P. J. 1994. Expression and characterization of a functional rat liver Na+ bile acid cotransport system in COS-7 cells. Am. J. Physiol. 266: G382–G387 [DOI] [PubMed] [Google Scholar]
- 49.Hagenbuch B., Meier P. J. 1994. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J. Clin. Invest. 93: 1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hata S., Wang P., Eftychiou N., Ananthanarayanan M., Batta A., Salen G., Pang K. S., Wolkoff A. W. 2003. Substrate specificities of rat oatp1 and ntcp: implications for hepatic organic anion uptake. Am. J. Physiol. Gastrointest. Liver Physiol. 285: G829–G839 [DOI] [PubMed] [Google Scholar]
- 51.Kramer W., Stengelin S., Baringhaus K. H., Enhsen A., Heuer H., Becker W., Corsiero D., Girbig F., Noll R., Weyland C. 1999. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J. Lipid Res. 40: 1604–1617 [PubMed] [Google Scholar]
- 52.Platte H. D., Honscha W., Schuh K., Petzinger E. 1996. Functional characterization of the hepatic sodium-dependent taurocholate transporter stably transfected into an immortalized liver-derived cell line and V79 fibroblasts. Eur. J. Cell Biol. 70: 54–60 [PubMed] [Google Scholar]
- 53.Mita S., Suzuki H., Akita H., Stieger B., Meier P. J., Hofmann A. F., Sugiyama Y. 2005. Vectorial transport of bile salts across MDCK cells expressing both rat Na+-taurocholate cotransporting polypeptide and rat bile salt export pump. Am. J. Physiol. Gastrointest. Liver Physiol. 288: G159–G167 [DOI] [PubMed] [Google Scholar]
- 54.Mita S., Suzuki H., Akita H., Hayashi H., Onuki R., Hofmann A. F., Sugiyama Y. 2006. Vectorial transport of unconjugated and conjugated bile salts by monolayers of LLC-PK1 cells doubly transfected with human NTCP and BSEP or with rat Ntcp and Bsep. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G550–G556 [DOI] [PubMed] [Google Scholar]
- 55.Craddock A. L., Love M. W., Daniel R. W., Kirby L. C., Walters H. C., Wong M. H., Dawson P. A. 1998. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am. J. Physiol. 274: G157–G169 [DOI] [PubMed] [Google Scholar]
- 56.Meng L. J., Wang P., Wolkoff A. W., Kim R. B., Tirona R. G., Hofmann A. F., Pang K. S. 2002. Transport of the sulfated, amidated bile acid, sulfolithocholyltaurine, into rat hepatocytes is mediated by Oatp1 and Oatp2. Hepatology. 35: 1031–1040 [DOI] [PubMed] [Google Scholar]
- 57.Meier P. J., Eckhardt U., Schroeder A., Hagenbuch B., Stieger B. 1997. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 26: 1667–1677 [DOI] [PubMed] [Google Scholar]
- 58.Kim R. B., Leake B., Cvetkovic M., Roden M. M., Nadeau J., Walubo A., Wilkinson G. R. 1999. Modulation by drugs of human hepatic sodium-dependent bile acid transporter (sodium taurocholate cotransporting polypeptide) activity. J. Pharmacol. Exp. Ther. 291: 1204–1209 [PubMed] [Google Scholar]
- 59.Mita S., Suzuki H., Akita H., Hayashi H., Onuki R., Hofmann A. F., Sugiyama Y. 2006. Inhibition of bile acid transport across Na+/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metab. Dispos. 34: 1575–1581 [DOI] [PubMed] [Google Scholar]
- 60.Leslie E. M., Watkins P. B., Kim R. B., Brouwer K. L. 2007. Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1)by bosentan: a mechanism for species differences in hepatotoxicity. J. Pharmacol. Exp. Ther. 321: 1170–1178 [DOI] [PubMed] [Google Scholar]
- 61.Boyer J. L., Hagenbuch B., Ananthanarayanan M., Suchy F., Stieger B., Meier P. J. 1993. Phylogenic and ontogenic expression of hepatocellular bile acid transport. Proc. Natl. Acad. Sci. USA. 90: 435–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagenbuch B., Scharschmidt B. F., Meier P. J. 1996. Effect of antisense oligonucleotides on the expression of hepatocellular bile acid and organic anion uptake systems in Xenopus laevis oocytes. Biochem. J. 316: 901–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Von Dippe P., Amoui M., Alves C., Levy D. 1993. Na(+)-dependent bile acid transport by hepatocytes is mediated by a protein similar to microsomal epoxide hydrolase. Am. J. Physiol. 264: G528–G534 [DOI] [PubMed] [Google Scholar]
- 64.Ho R. H., Leake B. F., Roberts R. L., Lee W., Kim R. B. 2004. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J. Biol. Chem. 279: 7213–7222 [DOI] [PubMed] [Google Scholar]
- 65.Carlton V. E., Harris B. Z., Puffenberger E. G., Batta A. K., Knisely A. S., Robinson D. L., Strauss K. A., Shneider B. L., Lim W. A., Salen G., et al. 2003. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat. Genet. 34: 91–96 [DOI] [PubMed] [Google Scholar]
- 66.Hadj-Rabia S., Baala L., Vabres P., Hamel-Teillac D., Jacquemin E., Fabre M., Lyonnet S., De Prost Y., Munnich A., Hadchouel M., et al. 2004. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 127: 1386–1390 [DOI] [PubMed] [Google Scholar]
- 67.Geier A., Wagner M., Dietrich C. G., Trauner M. 2007. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim. Biophys. Acta. 1773: 283–308 [DOI] [PubMed] [Google Scholar]
- 68.Anwer M. S.2004. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 39: 581–590 [DOI] [PubMed] [Google Scholar]
- 69.Karpen S. J., Sun A. Q., Kudish B., Hagenbuch B., Meier P. J., Ananthanarayanan M., Suchy F. J. 1996. Multiple factors regulate the rat liver basolateral sodium-dependent bile acid cotransporter gene promoter. J. Biol. Chem. 271: 15211–15221 [DOI] [PubMed] [Google Scholar]
- 70.Denson L. A., Sturm E., Echevarria W., Zimmerman T. L., Makishima M., Mangelsdorf D. J., Karpen S. J. 2001. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 121: 140–147 [DOI] [PubMed] [Google Scholar]
- 71.Denson L. A., Karpen S. J., Bogue C. W., Jacobs H. C. 2000. Divergent homeobox gene hex regulates promoter of the Na(+)-dependent bile acid cotransporter. Am. J. Physiol. Gastrointest. Liver Physiol. 279: G347–G355 [DOI] [PubMed] [Google Scholar]
- 72.Jung D., Kullak-Ublick G. A. 2003. Hepatocyte nuclear factor 1 alpha: a key mediator of the effect of bile acids on gene expression. Hepatology. 37: 622–631 [DOI] [PubMed] [Google Scholar]
- 73.Jung D., Hagenbuch B., Fried M., Meier P. J., Kullak-Ublick G. A. 2004. Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am. J. Physiol. Gastrointest. Liver Physiol. 286: G752–G761 [DOI] [PubMed] [Google Scholar]
- 74.Rausa F. M., Tan Y., Zhou H., Yoo K. W., Stolz D. B., Watkins S. C., Franks R. R., Unterman T. G., Costa R. H. 2000. Elevated levels of hepatocyte nuclear factor 3beta in mouse hepatocytes influence expression of genes involved in bile acid and glucose homeostasis. Mol. Cell. Biol. 20: 8264–8282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee Y. K., Schmidt D. R., Cummins C. L., Choi M., Peng L., Zhang Y., Goodwin B., Hammer R. E., Mangelsdorf D. J., Kliewer S. A. 2008. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol. Endocrinol. 22: 1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eloranta J. J., Jung D., Kullak-Ublick G. A. 2006. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol. Endocrinol. 20: 65–79 [DOI] [PubMed] [Google Scholar]
- 77.Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J. 2001. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21: 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geier A., Martin I. V., Dietrich C. G., Balasubramaniyan N., Strauch S., Suchy F. J., Gartung C., Trautwein C., Ananthanarayanan M. 2008. Hepatocyte nuclear factor-4alpha is a central transactivator of the mouse Ntcp gene. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G226–G233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dietrich C. G., Martin I. V., Porn A. C., Voigt S., Gartung C., Trautwein C., Geier A. 2007. Fasting induces basolateral uptake transporters of the SLC family in the liver via HNF4alpha and PGC1alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G585–G590 [DOI] [PubMed] [Google Scholar]
- 80.Wang L., Han Y., Kim C. S., Lee Y. K., Moore D. D. 2003. Resistance of SHP-null mice to bile acid-induced liver damage. J. Biol. Chem. 278: 44475–44481 [DOI] [PubMed] [Google Scholar]
- 81.Bochkis I. M., Rubins N. E., White P., Furth E. E., Friedman J. R., Kaestner K. H. 2008. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat. Med. 14: 828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta S., Stravitz R. T., Dent P., Hylemon P. B. 2001. Down-regulation of cholesterol 7alpha-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J. Biol. Chem. 276: 15816–15822 [DOI] [PubMed] [Google Scholar]
- 83.Li D., Zimmerman T. L., Thevananther S., Lee H. Y., Kurie J. M., Karpen S. J. 2002. Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J. Biol. Chem. 277: 31416–31422 [DOI] [PubMed] [Google Scholar]
- 84.Green R. M., Beier D., Gollan J. L. 1996. Regulation of hepatocyte bile salt transporters by endotoxin and inflammatory cytokines in rodents. Gastroenterology. 111: 193–198 [DOI] [PubMed] [Google Scholar]
- 85.Siewert E., Dietrich C. G., Lammert F., Heinrich P. C., Matern S., Gartung C., Geier A. 2004. Interleukin-6 regulates hepatic transporters during acute-phase response. Biochem. Biophys. Res. Commun. 322: 232–238 [DOI] [PubMed] [Google Scholar]
- 86.Trauner M., Arrese M., Lee H., Boyer J. L., Karpen S. J. 1998. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J. Clin. Invest. 101: 2092–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vee M. L., Lecureur V., Stieger B., Fardel O. 2009. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-alpha or interleukin-6. Drug Metab. Dispos. 37: 685–693 [DOI] [PubMed] [Google Scholar]
- 88.Geier A., Dietrich C. G., Voigt S., Kim S. K., Gerloff T., Kullak-Ublick G. A., Lorenzen J., Matern S., Gartung C. 2003. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 38: 345–354 [DOI] [PubMed] [Google Scholar]
- 89.Lickteig A. J., Slitt A. L., Arkan M. C., Karin M., Cherrington N. J. 2007. Differential regulation of hepatic transporters in the absence of tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and nuclear factor-kappaB in two models of cholestasis. Drug Metab. Dispos. 35: 402–409 [DOI] [PubMed] [Google Scholar]
- 90.Zollner G., Fickert P., Silbert D., Fuchsbichler A., Stumptner C., Zatloukal K., Denk H., Trauner M. 2002. Induction of short heterodimer partner 1 precedes downregulation of Ntcp in bile duct-ligated mice. Am. J. Physiol. Gastrointest. Liver Physiol. 282: G184–G191 [DOI] [PubMed] [Google Scholar]
- 91.Zollner G., Wagner M., Fickert P., Geier A., Fuchsbichler A., Silbert D., Gumhold J., Zatloukal K., Kaser A., Tilg H., et al. 2005. Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am. J. Physiol. Gastrointest. Liver Physiol. 289: G798–G805 [DOI] [PubMed] [Google Scholar]
- 92.Lee Y. K., Dell H., Dowhan D. H., Hadzopoulou-Cladaras M., Moore D. D. 2000. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol. Cell. Biol. 20: 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zimmerman T. L., Thevananther S., Ghose R., Burns A. R., Karpen S. J. 2006. Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. J. Biol. Chem. 281: 15434–15440 [DOI] [PubMed] [Google Scholar]
- 94.Ghose R., Zimmerman T. L., Thevananther S., Karpen S. J. 2004. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: a novel mechanism for reduced hepatic gene expression in inflammation. Nucl. Recept. 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geier A., Dietrich C. G., Voigt S., Ananthanarayanan M., Lammert F., Schmitz A., Trauner M., Wasmuth H. E., Boraschi D., Balasubramaniyan N., et al. 2005. Cytokine-dependent regulation of hepatic organic anion transporter gene transactivators in mouse liver. Am. J. Physiol. Gastrointest. Liver Physiol. 289: G831–G841 [DOI] [PubMed] [Google Scholar]
- 96.Wang B., Cai S. R., Gao C., Sladek F. M., Ponder K. P. 2001. Lipopolysaccharide results in a marked decrease in hepatocyte nuclear factor 4 alpha in rat liver. Hepatology. 34: 979–989 [DOI] [PubMed] [Google Scholar]
- 97.Ktistaki E., Talianidis I. 1997. Modulation of hepatic gene expression by hepatocyte nuclear factor 1. Science. 277: 109–112 [DOI] [PubMed] [Google Scholar]
- 98.Odom D. T., Zizlsperger N., Gordon D. B., Bell G. W., Rinaldi N. J., Murray H. L., Volkert T. L., Schreiber J., Rolfe P. A., Gifford D. K., et al. 2004. Control of pancreas and liver gene expression by HNF transcription factors. Science. 303: 1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geier A., Zollner G., Dietrich C. G., Wagner M., Fickert P., Denk H., van Rooijen N., Matern S., Gartung C., Trauner M. 2005. Cytokine-independent repression of rodent Ntcp in obstructive cholestasis. Hepatology. 41: 470–477 [DOI] [PubMed] [Google Scholar]
- 100.Gerloff T., Geier A., Stieger B., Hagenbuch B., Meier P. J., Matern S., Gartung C. 1999. Differential expression of basolateral and canalicular organic anion transporters during regeneration of rat liver. Gastroenterology. 117: 1408–1415 [DOI] [PubMed] [Google Scholar]
- 101.Geier A., Kim S. K., Gerloff T., Dietrich C. G., Lammert F., Karpen S. J., Stieger B., Meier P. J., Matern S., Gartung C. 2002. Hepatobiliary organic anion transporters are differentially regulated in acute toxic liver injury induced by carbon tetrachloride. J. Hepatol. 37: 198–205 [DOI] [PubMed] [Google Scholar]
- 102.Ganguly T. C., O'Brien M. L., Karpen S. J., Hyde J. F., Suchy F. J., Vore M. 1997. Regulation of the rat liver sodium-dependent bile acid cotransporter gene by prolactin. Mediation of transcriptional activation by Stat5. J. Clin. Invest. 99: 2906–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao J., Gowri P. M., Ganguly T. C., Wood M., Hyde J. F., Talamantes F., Vore M. 2001. PRL, placental lactogen, and GH induce NA(+)/taurocholate-cotransporting polypeptide gene expression by activating signal transducer and activator of transcription-5 in liver cells. Endocrinology. 142: 4212–4222 [DOI] [PubMed] [Google Scholar]
- 104.Simon F. R., Fortune J., Iwahashi M., Qadri I., Sutherland E. 2004. Multihormonal regulation of hepatic sinusoidal Ntcp gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 287: G782–G794 [DOI] [PubMed] [Google Scholar]
- 105.Mukhopadhayay S., Ananthanarayanan M., Stieger B., Meier P. J., Suchy F. J., Anwer M. S. 1997. cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am. J. Physiol. 273: G842–G848 [DOI] [PubMed] [Google Scholar]
- 106.Grune S., Engelking L. R., Anwer M. S. 1993. Role of intracellular calcium and protein kinases in the activation of hepatic Na+/taurocholate cotransport by cyclic AMP. J. Biol. Chem. 268: 17734–17741 [PubMed] [Google Scholar]
- 107.Mukhopadhyay S., Webster C. R., Anwer M. S. 1998. Role of protein phosphatases in cyclic AMP-mediated stimulation of hepatic Na+/taurocholate cotransport. J. Biol. Chem. 273: 30039–30045 [DOI] [PubMed] [Google Scholar]
- 108.Mukhopadhyay S., Ananthanarayanan M., Stieger B., Meier P. J., Suchy F. J., Anwer M. S. 1998. Sodium taurocholate cotransporting polypeptide is a serine, threonine phosphoprotein and is dephosphorylated by cyclic adenosine monophosphate. Hepatology. 28: 1629–1636 [DOI] [PubMed] [Google Scholar]
- 109.Dranoff J. A., McClure M., Burgstahler A. D., Denson L. A., Crawford A. R., Crawford J. M., Karpen S. J., Nathanson M. H. 1999. Short-term regulation of bile acid uptake by microfilament-dependent translocation of rat ntcp to the plasma membrane. Hepatology. 30: 223–229 [DOI] [PubMed] [Google Scholar]
- 110.Anwer M. S., Gillin H., Mukhopadhyay S., Balasubramaniyan N., Suchy F. J., Ananthanarayanan M. 2005. Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J. Biol. Chem. 280: 33687–33692 [DOI] [PubMed] [Google Scholar]
- 111.Webster C. R., Blanch C., Anwer M. S. 2002. Role of PP2B in cAMP-induced dephosphorylation and translocation of NTCP. Am. J. Physiol. Gastrointest. Liver Physiol. 283: G44–G50 [DOI] [PubMed] [Google Scholar]
- 112.Webster C. R., Srinivasulu U., Ananthanarayanan M., Suchy F. J., Anwer M. S. 2002. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J. Biol. Chem. 277: 28578–28583 [DOI] [PubMed] [Google Scholar]
- 113.McConkey M., Gillin H., Webster C. R., Anwer M. S. 2004. Cross-talk between protein kinases Czeta and B in cyclic AMP-mediated sodium taurocholate co-transporting polypeptide translocation in hepatocytes. J. Biol. Chem. 279: 20882–20888 [DOI] [PubMed] [Google Scholar]
- 114.Sarkar S., Bananis E., Nath S., Anwer M. S., Wolkoff A. W., Murray J. W. 2006. PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic. 7: 1078–1091 [DOI] [PubMed] [Google Scholar]
- 115.Schonhoff C. M., Gillin H., Webster C. R., Anwer M. S. 2008. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology. 47: 1309–1316 [DOI] [PubMed] [Google Scholar]
- 116.Noe J., Stieger B., Meier P. J. 2002. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology. 123: 1659–1666 [DOI] [PubMed] [Google Scholar]
- 117.Byrne J. A., Strautnieks S. S., Mieli-Vergani G., Higgins C. F., Linton K. J., Thompson R. J. 2002. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 123: 1649–1658 [DOI] [PubMed] [Google Scholar]
- 118.Tomer G., Ananthanarayanan M., Weymann A., Balasubramanian N., Suchy F. J. 2003. Differential developmental regulation of rat liver canalicular membrane transporters Bsep and Mrp2. Pediatr. Res. 53: 288–294 [DOI] [PubMed] [Google Scholar]
- 119.Gao B., St Pierre M. V., Stieger B., Meier P. J. 2004. Differential expression of bile salt and organic anion transporters in developing rat liver. J. Hepatol. 41: 201–208 [DOI] [PubMed] [Google Scholar]
- 120.Strautnieks S. S., Bull L. N., Knisely A. S., Kocoshis S. A., Dahl N., Arnell H., Sokal E., Dahan K., Childs S., Ling V., et al. 1998. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat. Genet. 20: 233–238 [DOI] [PubMed] [Google Scholar]
- 121.Strautnieks S. S., Byrne J. A., Pawlikowska L., Cebecauerova D., Rayner A., Dutton L., Meier Y., Antoniou A., Stieger B., Arnell H., et al. 2008. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 134: 1203–1214 [DOI] [PubMed] [Google Scholar]
- 122.Hayashi H., Takada T., Suzuki H., Onuki R., Hofmann A. F., Sugiyama Y. 2005. Transport by vesicles of glycine- and taurine-conjugated bile salts and taurolithocholate 3-sulfate: a comparison of human BSEP with rat Bsep. Biochim. Biophys. Acta. 1738: 54–62 [DOI] [PubMed] [Google Scholar]
- 123.Hirano M., Maeda K., Hayashi H., Kusuhara H., Sugiyama Y. 2005. Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J. Pharmacol. Exp. Ther. 314: 876–882 [DOI] [PubMed] [Google Scholar]
- 124.Horikawa M., Kato Y., Tyson C. A., Sugiyama Y. 2003. Potential cholestatic activity of various therapeutic agents assessed by bile canalicular membrane vesicles isolated from rats and humans. Drug Metab. Pharmacokinet. 18: 16–22 [DOI] [PubMed] [Google Scholar]
- 125.Knisely A. S., Strautnieks S. S., Meier Y., Stieger B., Byrne J. A., Portmann B. C., Bull L. N., Pawlikowska L., Bilezikci B., Ozcay F., et al. 2006. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 44: 478–486 [DOI] [PubMed] [Google Scholar]
- 126.van Mil S. W., van der Woerd W. L., van der Brugge G., Sturm E., Jansen P. L., Bull L. N., van den Berg I. E., Berger R., Houwen R. H., Klomp L. W. 2004. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 127: 379–384 [DOI] [PubMed] [Google Scholar]
- 127.Byrne J. A., Strautnieks S. S., Ihrke G., Pagani F., Knisely A. S., Linton K. J., Mieli-Vergani G., Thompson R. J. 2009. Missense mutations and single nucleotide polymorphisms in ABCB11 impair bile salt export pump processing and function or disrupt pre-messenger RNA splicing. Hepatology. 49: 553–567 [DOI] [PubMed] [Google Scholar]
- 128.Pauli-Magnus C., Meier P. J. 2006. Hepatobiliary transporters and drug-induced cholestasis. Hepatology. 44: 778–787 [DOI] [PubMed] [Google Scholar]
- 129.Dixon P. H., van Mil S. W., Chambers J., Strautnieks S., Thompson R. J., Lammert F., Kubitz R., Keitel V., Glantz A., Mattsson L. A., et al. 2009. Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut. 58: 537–544 [DOI] [PubMed] [Google Scholar]
- 130.Wolters H., Elzinga B. M., Baller J. F., Boverhof R., Schwarz M., Stieger B., Verkade H. J., Kuipers F. 2002. Effects of bile salt flux variations on the expression of hepatic bile salt transporters in vivo in mice. J. Hepatol. 37: 556–563 [DOI] [PubMed] [Google Scholar]
- 131.Zollner G., Fickert P., Silbert D., Fuchsbichler A., Marschall H. U., Zatloukal K., Denk H., Trauner M. 2003. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J. Hepatol. 38: 717–727 [DOI] [PubMed] [Google Scholar]
- 132.Wagner M., Fickert P., Zollner G., Fuchsbichler A., Silbert D., Tsybrovskyy O., Zatloukal K., Guo G. L., Schuetz J. D., Gonzalez F. J., et al. 2003. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 125: 825–838 [DOI] [PubMed] [Google Scholar]
- 133.Ananthanarayanan M., Balasubramanian N., Makishima M., Mangelsdorf D. J., Suchy F. J. 2001. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 276: 28857–28865 [DOI] [PubMed] [Google Scholar]
- 134.Plass J. R., Mol O., Heegsma J., Geuken M., Faber K. N., Jansen P. L., Muller M. 2002. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology. 35: 589–596 [DOI] [PubMed] [Google Scholar]
- 135.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 102: 731–744 [DOI] [PubMed] [Google Scholar]
- 136.Zollner G., Fickert P., Fuchsbichler A., Silbert D., Wagner M., Arbeiter S., Gonzalez F. J., Marschall H. U., Zatloukal K., Denk H., et al. 2003. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J. Hepatol. 39: 480–488 [DOI] [PubMed] [Google Scholar]
- 137.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368 [DOI] [PubMed] [Google Scholar]
- 138.Lew J. L., Zhao A., Yu J., Huang L., De Pedro N., Pelaez F., Wright S. D., Cui J. 2004. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J. Biol. Chem. 279: 8856–8861 [DOI] [PubMed] [Google Scholar]
- 139.Yu J., Lo J. L., Huang L., Zhao A., Metzger E., Adams A., Meinke P. T., Wright S. D., Cui J. 2002. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J. Biol. Chem. 277: 31441–31447 [DOI] [PubMed] [Google Scholar]
- 140.Hofmann A. F.2004. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab. Rev. 36: 703–722 [DOI] [PubMed] [Google Scholar]
- 141.Ananthanarayanan M., Li S., Balasubramaniyan N., Suchy F. J., Walsh M. J. 2004. Ligand-dependent activation of the farnesoid X-receptor directs arginine methylation of histone H3 by CARM1. J. Biol. Chem. 279: 54348–54357 [DOI] [PubMed] [Google Scholar]
- 142.Rizzo G., Renga B., Antonelli E., Passeri D., Pellicciari R., Fiorucci S. 2005. The methyl transferase PRMT1 functions as co-activator of farnesoid X receptor (FXR)/9-cis retinoid X receptor and regulates transcription of FXR responsive genes. Mol. Pharmacol. 68: 551–558 [DOI] [PubMed] [Google Scholar]
- 143.Kassam A., Miao B., Young P. R., Mukherjee R. 2003. Retinoid X receptor (RXR) agonist-induced antagonism of farnesoid X receptor (FXR) activity due to absence of coactivator recruitment and decreased DNA binding. J. Biol. Chem. 278: 10028–10032 [DOI] [PubMed] [Google Scholar]
- 144.Hoeke M. O., Plass J. R., Heegsma J., Geuken M., van Rijsbergen D., Baller J. F., Kuipers F., Moshage H., Jansen P. L., Faber K. N. 2009. Low retinol levels differentially modulate bile salt-induced expression of human and mouse hepatic bile salt transporters. Hepatology. 49: 151–159 [DOI] [PubMed] [Google Scholar]
- 145.Oude Elferink R. P., Paulusma C. C., Groen A. K. 2006. Hepatocanalicular transport defects: pathophysiologic mechanisms of rare diseases. Gastroenterology. 130: 908–925 [DOI] [PubMed] [Google Scholar]
- 146.Paulusma C. C., de Waart D. R., Kunne C., Mok K. S., Elferink R. P. 2009. Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content. J. Biol. Chem. 284: 9947–9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Folmer D. E., Elferink R. P., Paulusma C. C. 2009. P4 ATPases - lipid flippases and their role in disease. Biochim Biophys Acta. 1791: 628–635 [DOI] [PubMed] [Google Scholar]
- 148.Cai S. Y., Gautam S., Nguyen T., Soroka C. J., Rahner C., Boyer J. L. 2009. ATP8B1 deficiency disrupts the bile canalicular membrane bilayer structure in hepatocytes, but FXR expression and activity are maintained. Gastroenterology. 136: 1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shneider B. L.2001. Intestinal bile acid transport: biology, physiology, and pathophysiology. J. Pediatr. Gastroenterol. Nutr. 32: 407–417 [DOI] [PubMed] [Google Scholar]
- 150.Dawson P. A., Hubbert M., Haywood J., Craddock A. L., Zerangue N., Christian W. V., Ballatori N. 2005. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 280: 6960–6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ballatori N., Christian W. V., Lee J. Y., Dawson P. A., Soroka C. J., Boyer J. L., Madejczyk M. S., Li N. 2005. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 42: 1270–1279 [DOI] [PubMed] [Google Scholar]
- 152.Hofmann A. F., Poley J. R. 1972. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 62: 918–934 [PubMed] [Google Scholar]
- 153.Marcus S. N., Schteingart C. D., Marquez M. L., Hofmann A. F., Xia Y., Steinbach J. H., Ton-Nu H. T., Lillienau J., Angellotti M. A., Schmassmann A. 1991. Active absorption of conjugated bile acids in vivo. Kinetic parameters and molecular specificity of the ileal transport system in the rat. Gastroenterology. 100: 212–221 [DOI] [PubMed] [Google Scholar]
- 154.Aldini R., Roda A., Montagnani M., Polimeni C., Lenzi P. L., Cerre C., Galletti G., Roda E. 1994. Hepatic uptake and intestinal absorption of bile acids in the rabbit. Eur. J. Clin. Invest. 24: 691–697 [DOI] [PubMed] [Google Scholar]
- 155.Aldini R., Montagnani M., Roda A., Hrelia S., Biagi P. L., Roda E. 1996. Intestinal absorption of bile acids in the rabbit: different transport rates in jejunum and ileum. Gastroenterology. 110: 459–468 [DOI] [PubMed] [Google Scholar]
- 156.Gui X., Carraway R. E. 2004. Involvement of mast cells in basal and neurotensin-induced intestinal absorption of taurocholate in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 287: G408–G416 [DOI] [PubMed] [Google Scholar]
- 157.Dawson P. A., Haywood J., Craddock A. L., Wilson M., Tietjen M., Kluckman K., Maeda N., Parks J. S. 2003. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J. Biol. Chem. 278: 33920–33927 [DOI] [PubMed] [Google Scholar]
- 158.Lucas M.1983. Determination of acid surface pH in vivo in rat proximal jejunum. Gut. 24: 734–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kramer W., Girbig F., Gutjahr U., Kowalewski S., Jouvenal K., Muller G., Tripier D., Wess G. 1993. Intestinal bile acid absorption. Na(+)-dependent bile acid transport activity in rabbit small intestine correlates with the coexpression of an integral 93-kDa and a peripheral 14-kDa bile acid-binding membrane protein along the duodenum-ileum axis. J. Biol. Chem. 268: 18035–18046 [PubMed] [Google Scholar]
- 160.Aldini R., Roda A., Montagnani M., Cerre C., Pellicciari R., Roda E. 1996. Relationship between structure and intestinal absorption of bile acids with a steroid or side-chain modification. Steroids. 61: 590–597 [DOI] [PubMed] [Google Scholar]
- 161.Amelsberg A., Schteingart C. D., Ton-Nu H. T., Hofmann A. F. 1996. Carrier-mediated jejunal absorption of conjugated bile acids in the guinea pig. Gastroenterology. 110: 1098–1106 [DOI] [PubMed] [Google Scholar]
- 162.Amelsberg A., Jochims C., Richter C. P., Nitsche R., Folsch U. R. 1999. Evidence for an anion exchange mechanism for uptake of conjugated bile acid from the rat jejunum. Am. J. Physiol. 276: G737–G742 [DOI] [PubMed] [Google Scholar]
- 163.Walters H. C., Craddock A. L., Fusegawa H., Willingham M. C., Dawson P. A. 2000. Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. Am. J. Physiol. Gastrointest. Liver Physiol. 279: G1188–G1200 [DOI] [PubMed] [Google Scholar]
- 164.Hagenbuch B., Meier P. J. 2004. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 447: 653–665 [DOI] [PubMed] [Google Scholar]
- 165.Glaeser H., Bailey D. G., Dresser G. K., Gregor J. C., Schwarz U. I., McGrath J. S., Jolicoeur E., Lee W., Leake B. F., Tirona R. G., et al. 2007. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin. Pharmacol. Ther. 81: 362–370 [DOI] [PubMed] [Google Scholar]
- 166.Dresser G. K., Bailey D. G., Leake B. F., Schwarz U. I., Dawson P. A., Freeman D. J., Kim R. B. 2002. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin. Pharmacol. Ther. 71: 11–20 [DOI] [PubMed] [Google Scholar]
- 167.Maeda T., Takahashi K., Ohtsu N., Oguma T., Ohnishi T., Atsumi R., Tamai I. 2007. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol. Pharm. 4: 85–94 [DOI] [PubMed] [Google Scholar]
- 168.Meier Y., Eloranta J. J., Darimont J., Ismair M. G., Hiller C., Fried M., Kullak-Ublick G. A., Vavricka S. R. 2007. Regional distribution of solute carrier (SLC) mRNA expression along the human intestinal tract. Drug Metab Dispos. 35: 590–594 [DOI] [PubMed] [Google Scholar]
- 169.Oelkers P., Kirby L. C., Heubi J. E., Dawson P. A. 1997. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J. Clin. Invest. 99: 1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ballatori N., Fang F., Christian W. V., Li N., Hammond C. L. 2008. Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G179–G186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rao A., Haywood J., Craddock A. L., Belinsky M. G., Kruh G. D., Dawson P. A. 2008. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc. Natl. Acad. Sci. USA. 105: 3891–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Balesaria S., Pell R. J., Abbott L. J., Tasleem A., Chavele K. M., Barley N. F., Khair U., Simon A., Moriarty K. J., Brydon W. G., et al. 2008. Exploring possible mechanisms for primary bile acid malabsorption: evidence for different regulation of ileal bile acid transporter transcripts in chronic diarrhoea. Eur. J. Gastroenterol. Hepatol. 20: 413–422 [DOI] [PubMed] [Google Scholar]
- 173.Weinman S. A., Carruth M. W., Dawson P. A. 1998. Bile acid uptake via the human apical sodium-bile acid cotransporter is electrogenic. J. Biol. Chem. 273: 34691–34695 [DOI] [PubMed] [Google Scholar]
- 174.Shneider B. L., Dawson P. A., Christie D. M., Hardikar W., Wong M. H., Suchy F. J. 1995. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J. Clin. Invest. 95: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wong M. H., Oelkers P., Craddock A. L., Dawson P. A. 1994. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J. Biol. Chem. 269: 1340–1347 [PubMed] [Google Scholar]
- 176.Christie D. M., Dawson P. A., Thevananther S., Shneider B. L. 1996. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am. J. Physiol. 271: G377–G385 [DOI] [PubMed] [Google Scholar]
- 177.Alpini G., Glaser S. S., Rodgers R., Phinizy J. L., Robertson W. E., Lasater J., Caligiuri A., Tretjak Z., LeSage G. D. 1997. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 113: 1734–1740 [DOI] [PubMed] [Google Scholar]
- 178.Lazaridis K., Pham L., Tietz P., Marinelli R., deGroen P., Levine S., Dawson P., LaRusso N. 1997. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J. Clin. Invest. 100: 2714–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chignard N., Mergey M., Veissiere D., Parc R., Capeau J., Poupon R., Paul A., Housset C. 2001. Bile acid transport and regulating functions in the human biliary epithelium. Hepatology. 33: 496–503 [DOI] [PubMed] [Google Scholar]
- 180.Kapadia C. R., Essandoh L. K. 1988. Active absorption of vitamin B12 and conjugated bile salts by guinea pig ileum occurs in villous and not crypt cells. Dig. Dis. Sci. 33: 1377–1382 [DOI] [PubMed] [Google Scholar]
- 181.Saeki T., Matoba K., Furukawa H., Kirifuji K., Kanamoto R., Iwami K. 1999. Characterization, cDNA cloning, and functional expression of mouse ileal sodium-dependent bile acid transporter. J. Biochem. 125: 846–851 [DOI] [PubMed] [Google Scholar]
- 182.Hakansson P., Andersson I., Nystrom S., Lofgren L., Amrot L. F., Li H. 2002. Ontogenetic development and spatial distribution of the ileal apical sodium-dependent bile acid transporter and the ileal lipid-binding protein in apoE knockout and C57BL/6 mice. Scand. J. Gastroenterol. 37: 1089–1096 [DOI] [PubMed] [Google Scholar]
- 183.Stelzner M., Hoagland V., Somasundaram S. 2000. Distribution of bile acid absorption and bile acid transporter gene message in the hamster ileum. Pflugers Arch. 440: 157–162 [DOI] [PubMed] [Google Scholar]
- 184.Stelzner M., Somasundaram S., Kearney D. 2000. Distribution of bile acid transport capacities in the human ileum (abstract). Gastroenterology. 118: A77 [Google Scholar]
- 185.Coppola C. P., Gosche J. R., Arrese M., Ancowitz B., Madsen J., Vanderhoof J., Shneider B. L. 1998. Molecular analysis of the adaptive response of intestinal bile acid transport after ileal resection. Gastroenterology. 115: 1172–1178 [DOI] [PubMed] [Google Scholar]
- 186.Bosse T., Piaseckyj C. M., Burghard E., Fialkovich J. J., Rajagopal S., Pu W. T., Krasinski S. D. 2006. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol. Cell. Biol. 26: 9060–9070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Battle M. A., Bondow B. J., Iverson M. A., Adams S. J., Jandacek R. J., Tso P., Duncan S. A. 2008. GATA4 is essential for jejunal function in mice. Gastroenterology. 135: 1676–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Al-Ansari N., Xu G., Kollman-Bauerly K., Coppola C., Shefer S., Ujhazy P., Ortiz D., Ma L., Yang S., Tsai R., et al. 2002. Analysis of the effect of intestinal resection on rat ileal bile acid transporter expression and on bile acid and cholesterol homeostasis. Pediatr. Res. 52: 286–291 [DOI] [PubMed] [Google Scholar]
- 189.Tsuchiya T., Kalogeris T. J., Tso P. 1996. Ileal transposition into the upper jejunum affects lipid and bile salt absorption in rats. Am. J. Physiol. 271: G681–G691 [DOI] [PubMed] [Google Scholar]
- 190.Montagnani M., Love M. W., Rossel P., Dawson P. A., Qvist P. 2001. Absence of dysfunctional ileal sodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathic bile acid malabsorption. Scand. J. Gastroenterol. 36: 1077–1080 [DOI] [PubMed] [Google Scholar]
- 191.Renner O., Harsch S., Schaeffeler E., Schwab M., Klass D. M., Kratzer W., Stange E. F. 2009. Mutation screening of apical sodium-dependent bile acid transporter (SLC10A2): novel haplotype block including six newly identified variants linked to reduced expression. Hum. Genet. 125: 381–391 [DOI] [PubMed] [Google Scholar]
- 192.Love M. W., Craddock A. L., Angelin B., Brunzell J. D., Duane W. C., Dawson P. A. 2001. Analysis of the ileal bile acid transporter gene, SLC10A2, in subjects with familial hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 21: 2039–2045 [DOI] [PubMed] [Google Scholar]
- 193.Duane W. C., Hartich L. A., Bartman A. E., Ho S. B. 2000. Diminished gene expression of ileal apical sodium bile acid transporter explains impaired absorption of bile acid in patients with hypertriglyceridemia. J. Lipid Res. 41: 1384–1389 [PubMed] [Google Scholar]
- 194.Schiller L. R., Hogan R. B., Morawski S. G., Santa Ana C. A., Bern M. J., Norgaard R. P., Bo-Linn G. W., Fordtran J. S. 1987. Studies of the prevalence and significance of radiolabeled bile acid malabsorption in a group of patients with idiopathic chronic diarrhea. Gastroenterology. 92: 151–160 [DOI] [PubMed] [Google Scholar]
- 195.Meihoff W. E., Kern F., Jr 1968. Bile salt malabsorption in regional ileitis, ileal resection and mannitol-induced diarrhea. J. Clin. Invest. 47: 261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vitek L., Carey M. C. 2003. Enterohepatic cycling of bilirubin as a cause of ‘black’ pigment gallstones in adult life. Eur. J. Clin. Invest. 33: 799–810 [DOI] [PubMed] [Google Scholar]
- 197.Holzer A., Harsch S., Renner O., Strohmeyer A., Schimmel S., Wehkamp J., Fritz P., Stange E. F. 2008. Diminished expression of apical sodium-dependent bile acid transporter in gallstone disease is independent of ileal inflammation. Digestion. 78: 52–59 [DOI] [PubMed] [Google Scholar]
- 198.Nyhlin H., Merrick M., Eastwood M. 1994. Bile acid malabsorption in Crohn's disease and indications for its assessment using SeHCAT. Gut. 35: 90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Krag B., Krag B. 1976. Regional ileitis (Crohn's disease): I. Kinetics of bile acid absorption in the perfused ileum. Scand. J. Gastroenterol. 11: 481–486 [PubMed] [Google Scholar]
- 200.Farivar S., Fromm H., Schindler D., McJunkin B., Schmidt F. 1980. Tests of bile-acid and vitamin B12 metabolism in ileal crohn's disease. Am. J. Clin. Pathol. 73: 69–74 [DOI] [PubMed] [Google Scholar]
- 201.Tougaard L., Giese B., Pedersen B., Binder V. 1986. Bile acid metabolism in patients with Crohn's disease in terminal ileum. Scand. J. Gastroenterol. 21: 627–633 [DOI] [PubMed] [Google Scholar]
- 202.Fujisawa T., Kimura A., Ushijima K., Nakashima E., Inoue T., Yamashita Y., Kato H. 1998. Intestinal absorption of ursodeoxycholic acid in children and adolescents with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 26: 279–285 [DOI] [PubMed] [Google Scholar]
- 203.Camilleri M., Nadeau A., Tremaine W. J., Lamsam J., Burton D., Odunsi S., Sweetser S., Singh R. 2009. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 21: 734–e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Higgins J. V., Paul J. M., Dumaswala R., Heubi J. E. 1994. Downregulation of taurocholate transport by ileal BBM and liver BLM in biliary-diverted rats. Am. J. Physiol. 267: G501–G507 [DOI] [PubMed] [Google Scholar]
- 205.Lillienau J., Crombie D. L., Munoz J., Longmire-Cook S. J., Hagey L. R., Hofmann A. F. 1993. Negative feedback regulation of the ileal bile acid transport system in rodents. Gastroenterology. 104: 38–46 [DOI] [PubMed] [Google Scholar]
- 206.Stravitz R. T., Sanyal A. J., Pandak W. M., Vlahcevic Z. R., Beets J. W., Dawson P. A. 1997. Induction of sodium-dependent bile acid transporter messenger RNA, protein, and activity in rat ileum by cholic acid. Gastroenterology. 113: 1599–1608 [DOI] [PubMed] [Google Scholar]
- 207.Arrese M., Trauner M., Sacchiero R. J., Crossman M. W., Shneider B. L. 1998. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology. 28: 1081–1087 [DOI] [PubMed] [Google Scholar]
- 208.Duane W. C., Xiong W., Wolvers J. 2007. Effects of bile acids on expression of the human apical sodium dependent bile acid transporter gene. Biochim. Biophys. Acta. 1771: 1380–1388 [DOI] [PubMed] [Google Scholar]
- 209.Hofmann A. F.1999. Regulation of ileal bile acid transport: desirability of measuring transport function as well as transporter activity. Hepatology. 29: 1335–1337 [DOI] [PubMed] [Google Scholar]
- 210.Chen F., Ma L., Dawson P. A., Sinal C. J., Sehayek E., Gonzalez F. J., Breslow J., Ananthanarayanan M., Shneider B. L. 2003. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J. Biol. Chem. 278: 19909–19916 [DOI] [PubMed] [Google Scholar]
- 211.Li H., Chen F., Shang Q., Pan L., Shneider B. L., Chiang J. Y., Forman B. M., Ananthanarayanan M., Tint G. S., Salen G., et al. 2005. FXR-activating ligands inhibit rabbit ASBT expression via FXR-SHP-FTF cascade. Am. J. Physiol. Gastrointest. Liver Physiol. 288: G60–G66 [DOI] [PubMed] [Google Scholar]
- 212.Kok T., Hulzebos C. V., Wolters H., Havinga R., Agellon L. B., Stellaard F., Shan B., Schwarz M., Kuipers F. 2003. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J. Biol. Chem. 278: 41930–41937 [DOI] [PubMed] [Google Scholar]
- 213.Frankenberg T., Rao A., Chen F., Haywood J., Shneider B. L., Dawson P. A. 2006. Regulation of the mouse organic solute transporter alpha-beta, Ostalpha-Ostbeta, by bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G912–G922 [DOI] [PubMed] [Google Scholar]
- 214.Neimark E., Chen F., Li X., Shneider B. L. 2004. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 40: 149–156 [DOI] [PubMed] [Google Scholar]
- 215.Lanzini A., De Tavonatti M. G., Panarotto B., Scalia S., Mora A., Benini F., Baisini O., Lanzarotto F. 2003. Intestinal absorption of the bile acid analogue 75Se-homocholic acid-taurine is increased in primary biliary cirrhosis, and reverts to normal during ursodeoxycholic acid administration. Gut. 52: 1371–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hruz P., Zimmermann C., Gutmann H., Degen L., Beuers U., Terracciano L., Drewe J., Beglinger C. 2006. Adaptive regulation of the ileal apical sodium dependent bile acid transporter (ASBT) in patients with obstructive cholestasis. Gut. 55: 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sinha J., Chen F., Miloh T., Burns R. C., Yu Z., Shneider B. L. 2008. Beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G996–G1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Miao J., Xiao Z., Kanamaluru D., Min G., Yau P. M., Veenstra T. D., Ellis E., Strom S., Suino-Powell K., Xu H. E., et al. 2009. Bile acid signaling pathways increase stability of small heterodimer partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 23: 986–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Alrefai W. A., Sarwar Z., Tyagi S., Saksena S., Dudeja P. K., Gill R. K. 2005. Cholesterol modulates human intestinal sodium-dependent bile acid transporter. Am. J. Physiol. Gastrointest. Liver Physiol. 288: G978–G985 [DOI] [PubMed] [Google Scholar]
- 220.Thomas C., Landrier J. F., Gaillard D., Grober J., Monnot M. C., Athias A., Besnard P. 2006. Cholesterol dependent downregulation of mouse and human apical sodium dependent bile acid transporter (ASBT) gene expression: molecular mechanism and physiological consequences. Gut. 55: 1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Miyata M., Matsuda Y., Nomoto M., Takamatsu Y., Sato N., Hamatsu M., Dawson P. A., Gonzalez F. J., Yamazoe Y. 2009. Cholesterol feeding prevents hepatic accumulation of bile acids in cholic acid-fed farnesoid X receptor (FXR)-null mice: FXR-independent suppression of intestinal bile acid absorption. Drug Metab. Dispos. 37: 338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stelzner M., Somasundaram S., Khakberdiev T. 2001. Systemic effects of acute terminal ileitis on uninflamed gut aggravate bile acid malabsorption. J. Surg. Res. 99: 359–364 [DOI] [PubMed] [Google Scholar]
- 223.Jung D., Fantin A. C., Scheurer U., Fried M., Kullak-Ublick G. A. 2004. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 53: 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen F., Ma L., Al-Ansari N., Shneider B. 2001. The role of AP-1 in the transcriptional regulation of the rat apical sodium-dependent bile acid transporter. J. Biol. Chem. 276: 38703–38714 [DOI] [PubMed] [Google Scholar]
- 225.Chen F., Ma L., Sartor R. B., Li F., Xiong H., Sun A. Q., Shneider B. 2002. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology. 123: 2005–2016 [DOI] [PubMed] [Google Scholar]
- 226.Nowicki M., Shneider B., Paul J., Heubi J. 1997. Glucorticoids up-regulate taurocholate transport by the ileal brush border membrane. Am. J. Physiol. 273: G197–G203 [DOI] [PubMed] [Google Scholar]
- 227.Kwon R. S., Carey M. C. 2004. Do steroids ameliorate bile acid malabsorption in Crohn's disease? Gut. 53: 10–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jung D., Fried M., Kullak-Ublick G. A. 2002. Human apical sodium-dependent bile salt transporter gene (SLC10A2) is regulated by the peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 277: 30559–30566 [DOI] [PubMed] [Google Scholar]
- 229.Chen X., Chen F., Liu S., Glaeser H., Dawson P. A., Hofmann A. F., Kim R. B., Shneider B. L., Pang K. S. 2006. Transactivation of rat apical sodium-dependent bile acid transporter and increased bile acid transport by 1alpha,25-dihydroxyvitamin D3 via the vitamin D receptor. Mol. Pharmacol. 69: 1913–1923 [DOI] [PubMed] [Google Scholar]
- 230.Annaba F., Sarwar Z., Kumar P., Saksena S., Turner J. R., Dudeja P. K., Gill R. K., Alrefai W. A. 2008. Modulation of ileal bile acid transporter (ASBT) activity by depletion of plasma membrane cholesterol: association with lipid rafts. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G489–G497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xia X., Roundtree M., Merikhi A., Lu X., Shentu S., Lesage G. 2004. Degradation of the apical sodium-dependent bile acid transporter by the ubiquitin-proteasome pathway in cholangiocytes. J. Biol. Chem. 279: 44931–44937 [DOI] [PubMed] [Google Scholar]
- 232.Weinberg S. L., Burckhardt G., Wilson F. A. 1986. Taurocholate transport by rat intestinal basolateral membrane vesicles. Evidence for the presence of an anion exchange transport system. J. Clin. Invest. 78: 44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lin M. C., Weinberg S. L., Kramer W., Burckhardt G., Wilson F. A. 1988. Identification and comparison of bile acid-binding polypeptides in ileal basolateral membrane. J. Membr. Biol. 106: 1–11 [DOI] [PubMed] [Google Scholar]
- 234.Lazaridis K. N., Tietz P., Wu T., Kip S., Dawson P. A., LaRusso N. F. 2000. Alternative splicing of the rat sodium/bile acid transporter changes its cellular localization and transport properties. Proc. Natl. Acad. Sci. USA. 97: 11092–11097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kruh G. D., Belinsky M. G. 2003. The MRP family of drug efflux pumps. Oncogene. 22: 7537–7552 [DOI] [PubMed] [Google Scholar]
- 236.Belinsky M. G., Dawson P. A., Shchaveleva I., Bain L. J., Wang R., Ling V., Chen Z. S., Grinberg A., Westphal H., Klein-Szanto A., et al. 2005. Analysis of the in vivo functions of Mrp3. Mol. Pharmacol. 68: 160–168 [DOI] [PubMed] [Google Scholar]
- 237.Zelcer N., van de Wetering K., de Waart R., Scheffer G. L., Marschall H. U., Wielinga P. R., Kuil A., Kunne C., Smith A., van der Valk M., et al. 2006. Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J. Hepatol. 44: 768–775 [DOI] [PubMed] [Google Scholar]
- 238.Wang W., Seward D. J., Li L., Boyer J. L., Ballatori N. 2001. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc. Natl. Acad. Sci. USA. 98: 9431–9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Seward D. J., Koh A. S., Boyer J. L., Ballatori N. 2003. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J. Biol. Chem. 278: 27473–27482 [DOI] [PubMed] [Google Scholar]
- 240.Landrier J. F., Eloranta J. J., Vavricka S. R., Kullak-Ublick G. A. 2006. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G476–G485 [DOI] [PubMed] [Google Scholar]
- 241.Li N., Cui Z., Fang F., Lee J. Y., Ballatori N. 2007. Heterodimerization, trafficking and membrane topology of the two proteins, Ost alpha and Ost beta, that constitute the organic solute and steroid transporter. Biochem. J. 407: 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jung D., Inagaki T., Gerard R. D., Dawson P. A., Kliewer S. A., Mangelsdorf D. J., Moschetta A. 2007. FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J. Lipid Res. 48: 2693–2700 [DOI] [PubMed] [Google Scholar]
- 243.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C. L., McDonald J. G., Luo G., Jones S. A., Goodwin B., Richardson J. A., et al. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2: 217–225 [DOI] [PubMed] [Google Scholar]
- 244.Davis R. A., Attie A. D. 2008. Deletion of the ileal basolateral bile acid transporter identifies the cellular sentinels that regulate the bile acid pool. Proc. Natl. Acad. Sci. USA. 105: 4965–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kim I., Ahn S. H., Inagaki T., Choi M., Ito S., Guo G. L., Kliewer S. A., Gonzalez F. J. 2007. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 48: 2664–2672 [DOI] [PubMed] [Google Scholar]
- 246.Davis R. A.2008. Resolving the mechanism of bile acid negative-feedback regulation, a Journal of Lipid Research tradition. J. Lipid Res. 49: 2–3 [DOI] [PubMed] [Google Scholar]
- 247.Bhat B. G., Rapp S. R., Beaudry J. A., Napawan N., Butteiger D. N., Hall K. A., Null C. L., Luo Y., Keller B. T. 2003. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. J. Lipid Res. 44: 1614–1621 [DOI] [PubMed] [Google Scholar]
- 248.Root C., Smith C. D., Sundseth S. S., Pink H. M., Wilson J. G., Lewis M. C. 2002. Ileal bile acid transporter inhibition, CYP7A1 induction, and antilipemic action of 264W94. J. Lipid Res. 43: 1320–1330 [PubMed] [Google Scholar]
- 249.Vlahcevic Z. R., Bell C. C., Jr., Buhac I., Farrar J. T., Swell L. 1970. Diminished bile acid pool size in patients with gallstones. Gastroenterology. 59: 165–173 [PubMed] [Google Scholar]
- 250.Renner O., Harsch S., Strohmeyer A., Schimmel S., Stange E. F. 2008. Reduced ileal expression of OSTalpha-OSTbeta in non-obese gallstone disease. J. Lipid Res. 49: 2045–2054 [DOI] [PubMed] [Google Scholar]
- 251.Lee H., Zhang Y., Lee F. Y., Nelson S. F., Gonzalez F. J., Edwards P. A. 2006. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J. Lipid Res. 47: 201–214 [DOI] [PubMed] [Google Scholar]
- 252.Zollner G., Wagner M., Moustafa T., Fickert P., Silbert D., Gumhold J., Fuchsbichler A., Halilbasic E., Denk H., Marschall H. U., et al. 2006. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G923–G932 [DOI] [PubMed] [Google Scholar]
- 253.Okuwaki M., Takada T., Iwayanagi Y., Koh S., Kariya Y., Fujii H., Suzuki H. 2007. LXR alpha transactivates mouse organic solute transporter alpha and beta via IR-1 elements shared with FXR. Pharm. Res. 24: 390–398 [DOI] [PubMed] [Google Scholar]
- 254.Stiehl A.1974. Bile salt sulphates in cholestasis. Eur. J. Clin. Invest. 4: 59–63 [DOI] [PubMed] [Google Scholar]
- 255.Stiehl A., Earnest D. L., Admirant W. H. 1975. Sulfation and renal excretion of bile salts in patients with cirrhosis of the liver. Gastroenterology. 68: 534–544 [PubMed] [Google Scholar]
- 256.Raedsch R., Lauterburg B. H., Hofmann A. F. 1981. Altered bile acid metabolism in primary biliary cirrhosis. Dig. Dis. Sci. 26: 394–401 [DOI] [PubMed] [Google Scholar]
- 257.Rudman D., Kendall F. E. 1957. Bile acid content of human serum. I. Serum bile acids in patients with hepatic disease. J. Clin. Invest. 36: 530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wilson F. A., Burckhardt G., Murer H., Rumrich G., Ullrich K. J. 1981. Sodium-coupled taurocholate transport in the proximal convolution of the rat kidney in vivo and in vitro. J. Clin. Invest. 67: 1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Weiner I. M., Glasser J. E., Lack L. 1964. Renal excretion of bile acids: taurocholic, glycocholic, and colic acids. Am. J. Physiol. 207: 964–970 [DOI] [PubMed] [Google Scholar]
- 260.Wess G., Kramer W., Enhsen A., Glombik H., Baringhaus K. H., Boger G., Urmann M., Bock K., Kleine H., Neckermann G., et al. 1994. Specific inhibitors of ileal bile acid transport. J. Med. Chem. 37: 873–875 [DOI] [PubMed] [Google Scholar]
- 261.Higaki J., Hara S., Takasu N., Tonda K., Miyata K., Shike T., Nagata K., Mizui T. 1998. Inhibition of ileal Na+/bile acid cotransporter by S-8921 reduces serum cholesterol and prevents atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol. 18: 1304–1311 [DOI] [PubMed] [Google Scholar]
- 262.Tollefson M. B., Vernier W. F., Huang H. C., Chen F. P., Reinhard E. J., Beaudry J., Keller B. T., Reitz D. B. 2000. A novel class of apical sodium co-dependent bile acid transporter inhibitors: the 2,3-disubstituted-4-phenylquinolines. Bioorg. Med. Chem. Lett. 10: 277–279 [DOI] [PubMed] [Google Scholar]
- 263.Summerfield J. A., Cullen J., Barnes S., Billing B. H. 1977. Evidence for renal control of urinary excretion of bile acids and bile acid sulphates in the cholestatic syndrome. Clin. Sci. Mol. Med. 52: 51–65 [DOI] [PubMed] [Google Scholar]
- 264.Corbett C. L., Bartholomew T. C., Billing B. H., Summerfield J. A. 1981. Urinary excretion of bile acids in cholestasis: evidence for renal tubular secretion in man. Clin. Sci. (Lond.). 61: 773–780 [DOI] [PubMed] [Google Scholar]
- 265.Morgan W. A., Nk T., Ding Y. 2008. The use of high performance thin-layer chromatography to determine the role of membrane lipid composition in bile salt-induced kidney cell damage. J. Pharmacol. Toxicol. Methods. 57: 70–73 [DOI] [PubMed] [Google Scholar]
- 266.Imai S., Kikuchi R., Kusuhara H., Yagi S., Shiota K., Sugiyama Y. 2009. Analysis of DNA methylation and histone modification profiles of liver-specific transporters. Mol. Pharmacol. 75: 568–576 [DOI] [PubMed] [Google Scholar]
- 267.Fickert P., Wagner M., Marschall H. U., Fuchsbichler A., Zollner G., Tsybrovskyy O., Zatloukal K., Liu J., Waalkes M. P., Cover C., et al. 2006. 24-norursodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 130: 465–481 [DOI] [PubMed] [Google Scholar]