Abstract
15(S)-lipoxygenase-1 (15-LO-1) was present in the whole-cell homogenate of an acute human monocytic leukemia cell line (THP-1). Additionally, 15-LO-1 was detected on neutral lipid droplets isolated from THP-1 foam cells. To investigate if 15-LO-1 is active on lipid droplets, we used the mouse leukemic monocytic macrophage cell line (RAW 264.7), which are stably transfected with human 15-LO-1. The RAW 15-LO-1 cells were incubated with acetylated low density lipoprotein to generate foam cells. 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE], the major 15-LO-1 metabolite of arachidonic acid, was produced in the 15-LO-1 RAW but not in the mock transfected cells when incubated with arachidonic acid. Lipid droplets were isolated from the cells and incubated with arachidonic acid, and production of 15(S)-HETE was measured over 2 h. 15(S)-HETE was produced in the incubations with the lipid droplets, and this production was attenuated when the lipid droplet fraction was subjected to enzyme inactivation through heating. Efflux of 15(S)-HETE from cholesteryl ester-enriched 15-LO RAW cells, when lipid droplets are present, was significantly reduced compared with that from cells enriched with free cholesterol (lipid droplets are absent). We propose that 15-LO-1 is present and functional on cytoplasmic neutral lipid droplets in macrophage foam cells, and these droplets may act to accumulate the anti-inflammatory lipid mediator 15(S)-HETE.
Keywords: lipoxygenases, cholesterol, cholesteryl ester
Cytoplasmic lipid droplets were once regarded as inconsequential lipid storage depots. A new understanding of the dynamic nature of the droplets has emerged, and they have been redefined as metabolically active organelles (1, 2). Lipid droplets are now known to be intimately involved in many cellular processes, including lipolysis (3) and membrane trafficking (4). Previous studies on droplet proteins from various cell types have provided insight on possible metabolic roles for the droplets. For example, the presence of 15(S)-lipoxygenase-1 (15-LO-1) has been reported on neutral lipid droplets in a variety of cell types (5, 6). 15-LO-1 is part of a family of nonheme, iron-containing enzymes that catalyze the stereospecific oxidation of polyunsaturated fatty acids. 15-LO-1 is thought to be involved in atherosclerosis, although its exact role is not clear (7, 8). LDL and membrane steryl esters are substrates for human reticulocyte 15-LO-1 (9, 10); in fact, 15-LO-1 colocalizes with LDL in early atherosclerotic lesions (11, 12). In this study, we present evidence that human 15-LO-1 is present and functional on the cytoplasmic lipid droplets in macrophage foam cells.
MATERIALS AND METHODS
Materials
BSA (essentially fatty acid free), heat-inactivated FBS, gentamicin, and unesterified cholesterol [free cholesterol (FC)] were purchased from Sigma-Aldrich (St. Louis, MO). Organic solvents were obtained from Fisher Scientific (Pittsburgh, PA). Tissue culture flasks and plates were from Falcon (Lincoln, NJ) and Corning (Corning, NY). Tissue culture medium was obtained from GIBCO (Carlsbad, CA). Peroxidase-free arachidonic acid and the antibody to human 15-LO-1 were purchased from Cayman Chemicals (Ann Arbor, MI). Human LDL (1.019 < d < 1.063 g/ml) was isolated by sequential ultracentrifugation, dialyzed against 0.15 mol/l NaCl, and sterilized by 0.45 µmol/L filtration. LDL was acetylated with acetic anhydride (13).
Cell culture
THP-1 human monocytes (American Type Tissue Culture Collection, Manassas, VA) were grown in suspension at 37°C in 5% CO2 in bicarbonate-buffered RPMI containing 10% FBS (v/v), 50 µmol/l β-mercaptoethanol, and 50 µg/ml gentamycin at a cell density of 0.2–1.0 × 106/ml. Cells were plated at a density of 1.2 × 106/4 cm2 dish or 2.4 × 106/8 cm2 dish in RPMI with 10% FBS, 50 µmol/l β-mercaptoethanol, 50 µg/ml gentamicin, and 100 ng/ml phorbol myristate acetate for 3 days to become fully differentiated macrophages before use in experiments. RAW 264.7 (American Type Tissue Culture Collection) murine macrophages stably transfected to overexpress human 15-LO-1 (14) and mock-transfected cells were routinely grown in DMEM containing 10% FBS, 50 µg/ml gentamicin, and 500 µg/ml geneticin. To cholesterol enrich the macrophages, the appropriate media containing 1% FBS and acetylated LDL (acLDL; 100 µg protein/ml) was added to the cells for 48 h. Some incubations contained 100 μM arachidonic acid (added with BSA). The monolayers were then washed and equilibrated in MEM containing 0.2% BSA for 18 h.
Immunofluorescence
15-LO-1 or mock-transfected RAW macrophage foam cells were plated on glass coverslips and cholesterol enriched by incubation with acLDL (100 µg protein/ml) for 48 h. The cells were fixed in 3% paraformaldehyde using the method of DiDonato and Brasaemle (15) prior to immunofluorescent staining. Cells were incubated with 0.1% sodium borohydride for 2.5 min at room temperature to limit endogenous fluorescence. Primary and secondary antibodies were diluted in 5% BSA and 10% normal serum from which the secondary antibodies were raised. This solution was supplemented with 0.3% Triton X-100 (16, 17). Incubations were done at 37° for 3 h for the primary antibody and 1 h for the secondary antibody. Slides were cover-slipped with Mowiol (Calbiochem, San Diego, CA) before viewing using an inverted epifluorescence Nikon Elipse TE-2000-U or an inverted Bio-Rad Radiance 2000 confocal microscope.
Cell fractionation
To fractionate the cells and isolate cellular lipid inclusions, monolayers of macrophages containing inclusions were washed three times with PBS containing 100 mM ascorbic acid and scraped into 3 ml of PBS (3 ml per 100 mm dish) containing 20 μl/ml protease inhibitor cocktail (Sigma-Aldrich). The cells were disrupted using sonication (18). Some of the whole-cell homogenate was reserved and placed on ice for later use. Homogenates were centrifuged at 26,000 rpm in an SW40Ti rotor for 30 min. The floating lipid layer, cell pellet, and cytoplasm were removed with a syringe. The cell pellet and cytoplasm were reserved on ice for later use. The floating lipid layer was washed by dispersing it in PBS and reisolating as described above. This wash was repeated. The final lipid layer containing the droplets was removed and stored on ice until use. Acid phosphatase (Sigma kit; Sigma-Aldrich) and lactate dehydrogenase activity (Roche Diagnostics, Mannheim, Germany) were determined in the floating lipid layer in order to determine whether there was lysosomal or cytoplasmic contamination in the lipid fraction.
Assay for the production of 15(S)-hydroxyeicosatetraenoic acid
Aliquots of the whole-cell homogenate, lipid droplet layer, cell pellet, and cytoplasm were transferred into conical tubes. One set of samples was heated in boiling water for 5 min to serve as a heat-inactivated control. Arachidonic acid (100 µM final concentration) was added to each tube. The tubes were incubated at 37°C for 2 h. The reaction was stopped by extraction with chloroform and methanol (2:1, v/v).
Derivatization of lipids
Lipid samples were prepared by the method of Lee, Williams, and Blair (19). Briefly, a mixture of 12 heavy isotope internal standards ([2H4](9S)-hydroxyoctadecadienoic acid (HODE), [2H4]13(S)-HODE, [2H8](5S)-hydroxyeicosatetraenoic acid (HETE), [2H8]12(S)-HETE, [2H8]15(S)-HETE, [2H4]prostaglandin E2, [2H4]PGD2, and [2H4]Leukotriene B4 (1 ng each), and [2H6]20-HETE, [2H4]PGF2α (PGF2α), [2H4]11β-PGF2, and [2H4]8-iso-PGF2α (10 ng each; Cayman Chemicals, Ann Arbor, MI) were added to the isolated lipid droplet layer. The solution was extracted three times using the procedure of Bligh and Dyer (20). The antioxidant butylated hydroxytoluene (0.05%, w/v) was present during the extraction. The final extract was dried down under nitrogen and resuspended in 850 μl chloroform:methanol (8:1 v/v) followed by the addition of 700 μl 40% KOH. The tubes were flushed with nitrogen, capped, and incubated at 60°C for 30 min. At the end of the saponification period, 700 μl 50 mM phosphate buffer (pH 7.4) was added, and the pH of the solution was adjusted to 3 by the addition of 150 μl formic acid. The solution was extracted with diethyl ether and hexane (1:1, v/v), and the organic layer was evaporated to dryness under nitrogen. The sample was then derivatized with pentafluorobenzyl bromide. To do this, 100 μl pentafluorobenzyl bromide in acetonitrile (1:19, v/v) was added to the lipid extract followed by 100 μl of diisopropylethylamine in acetonitrile (1:9, v/v), and this solution was heated at 60°C for 60 min. The samples were allowed to cool, dried under nitrogen, and redissolved in 100 μl of hexane/ethanol (97:3, v/v).
MS
MS for the quantitative analysis of lipidomics profile was conducted on a triple-quadrupole Finnigan TSQ Quantum Ultra AM spectrometer (Thermo Fisher, San Jose, CA) equipped with an atmospheric pressure chemical ionization source in the negative ion mode. Operating conditions were as follows: spray voltage, 4.5 kV; vaporizer temperature, 450°C; and heated capillary temperature, 350°C. Nitrogen was used for the sheath gas and auxiliary gas set at 60 and 10 (in arbitrary units), respectively. collision induced dissociation was performed using argon as the collision gas at 1.5 mTorr in the second (rf-only) quadrupole, and the collision energy was set at 18 eV. An additional dc offset voltage was applied to the region of the second multipole ion guide (Q0) at 5 V to impart enough translational kinetic energy to the ions so that solvent adduct ions dissociate to form sample ions. For multiple reaction monitoring (MRM/MS) analysis, unit resolution was maintained for both parent and product ions. The following MRM/MS transitions were monitored: 15-HETEs (m/z 319 → 219) and [2H8]15(S)-HETE (m/z 327 → 226).
LC
Normal phase chiral LC electron capture atmospheric pressure chemical ionization (ECAPCI)/MRM/MS analysis was conducted using a Waters Alliance 2690 HPLC system (Milford, MA) linked to the TSQ Quantum AM mass spectrometer. A Chiralpak AD-H column (250 × 4.6 mm inner diameter, 5 µm; Daicel Chemical Industries, West Chester, PA) was employed for gradient 1 with a flow rate of 1.0 ml/min. Solvent A was hexane, and solvent B was methanol/isopropanol (1:1, v/v). Gradient 1 was as follows: 2% B at 0 min, 2% B at 3 min, 3.6% B at 11 min, 8% B at 15 min, 8% B at 27 min, 50% B at 30 min, 50% B at 35 min, 2% B at 37 min, and 2% B at 45 min. Separations were performed at 30°C using a linear gradient.
Western blot analysis
Protein from monolayers was prepared for Western blot analysis as previously described (21). Protein from the floating lipid layer was prepared as follows. Lipids were extracted using the method of Bligh and Dyer (20). The interface containing droplets protein between the organic and aqueous layers of the extraction was collected. The proteins were dissolved in an SDS sample buffer containing the NuPage reducing agent (Invitrogen, Carlsbad, CA). Proteins were separated on a 3–8% Tris-acetate gel and transferred to nitrocellulose membranes and probed for 15-LO-1 as per the manufacturer's directions.
15(S)-HETE efflux
15-LO expressing RAW macrophages were incubated with 7 µg/ml arachidonic acid, +/−acLDL (100 µg/ml), and +/−CP113818 (ACAT inhibitor, 2 µg/ml) for 18 h. After this incubation, the monolayers were washed with MEM. Time zero cell monolayers were extracted with isopropanol to determine 15(S)-HETE in the monolayer before the efflux period. Phenol red-free media containing 1% BSA was added to the rest of the wells. Media were sampled after 2 h and filtered. 15(S)-HETE was quantitated using an ELISA kit (Cayman Chemicals, Ann Arbor, MI) as per the manufacturer's instructions. 15-HETE in the media was compared with total 15-HETE at time zero to determine the percentage release of 15-HETE.
Statistical analysis
Values are expressed as mean ± SD. Unpaired Student's t-test was used to determine statistical significance between groups (GraphPad Prism; GraphPad Software, San Diego, CA). The criterion for significance was set at P ≤ 0.05.
RESULTS
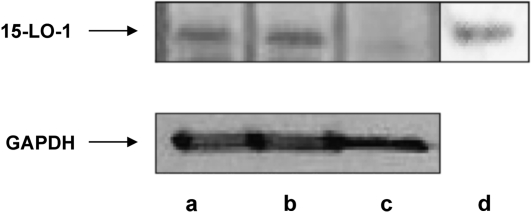
THP-1 human monocytes were differentiated into macrophages by incubation with phorbol myristate acetate as described in Materials and Methods. Cholesterol-enriched THP-1 macrophage foam cells were generated by incubation with acLDL. This treatment results in an increase in cellular unesterified cholesterol content as well as the production of cytoplasmic lipid droplets containing cholesteryl ester (CE) (22). These droplets were isolated from the foam cells by ultracentrifugation as described in Materials and Methods. Using this procedure, we determined that there was no detectable cytoplasmic or lysosomal contamination in the final droplet preparation measured by lactate dehydrogenase (data not shown) and acid phosphatase (data not shown) activity, respectively. As seen in the Western blot in Fig. 1, 15-LO-1 is present in THP-1 macrophage whole-cell homogenate (23) but not in THP-1 monocytes (Fig. 1). Additionally, we found 15-LO-1 protein on neutral lipid droplets isolated from THP-1 macrophage foam cells (Fig. 1).
Fig. 1.
15-LO-1 protein levels in THP-1 cells and cytoplasmic lipid droplets. Lane a, cholesterol normal THP-1; lane b, cholesterol-enriched THP-1; lane c, THP-1 monocytes; lane d, lipid droplets from cholesterol-enriched THP-1. Forty-five micrograms of cell protein was applied to lanes a–c. Protein from droplets isolated from two 100 mm plates of THP-1 macrophage foam cells was applied to lane d. 15-LO-1 protein levels were determined as described in Materials and Methods.
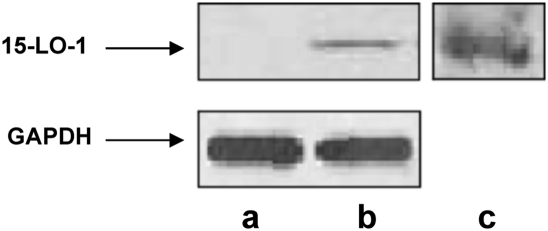
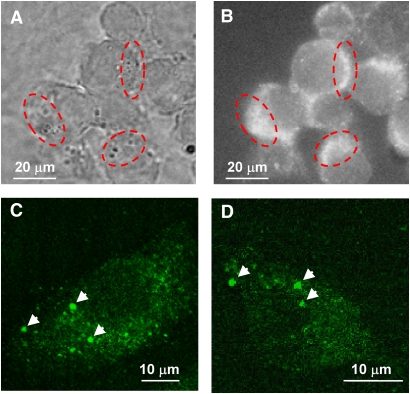
In an effort to determine if 15-LO-1 on the lipid droplet is functional, we used RAW (murine) macrophages, which stably express human 15-LO-1. Murine macrophages do not express 15-LO-1 and therefore provide a clean background for these studies. To generate foam cells, RAW macrophages transfected with human 15-LO-1 or mock-transfected cells were incubated with acLDL. Cytoplasmic lipid droplets were isolated from the cells, and the droplet proteins were analyzed by Western blot as described in Materials and Methods. We determined that 15-LO-1 was present in the 15-LO-1 RAW whole-cell homogenate and on the lipid droplets isolated from 15-LO-1 RAW macrophages but not in the mock-transfected RAW whole-cell homogenate (Fig. 2A–C). We extended these studies and evaluated whether 15-LO-1 was localized at neutral lipid droplets in 15-LO-1 RAW macrophage foam cells using immunofluorescent microscopy. 15-LO-1 macrophage foam cells were generated through incubation with acLDL. The procedure used for fixation and permeabilization of the cells prevents disruption of the droplet structure (15). Lipid droplets viewed under phase microscopy appeared as dark punctate structures asymmetrically distributed in the cells (Fig. 3A). 15-LO-1-expressing RAW macrophage foam cells incubated with anti-15-LO-1 and an FITC-conjugated secondary antibody exhibited fluorescent staining that matched the cellular location of the lipid droplets (Fig. 3B). Using confocal microscopy, we determined that individual lipid droplets exhibited immunoreactivity (Fig. 3C, D), indicating the presence of 15-LO-1 on the droplets. Mock-transfected RAW macrophage foam cells did not exhibit immunoreactivity (data not shown).
Fig. 2.
15-LO-1 protein levels in 15-LO-1 and mock-transfected RAW macrophages. Lane a, mock-transfected RAW macrophages (15 μg protein); lane b, 15-LO-1 RAW macrophages (15 μg protein); lane c, protein from lipid droplets isolated from two 100 mm plates of 15-LO-1 RAW macrophage foam cells. 15-LO-1 protein levels were determined as described in Materials and Methods.
Fig. 3.
Cellular localization of 15-LO-1 in 15-LO-1 RAW macrophage foam cells. Cells were incubated with acLDL to induce neutral lipid droplet formation. Lipid bodies were visualized by phase microscopy (A). 15-LO-1 was localized on lipid droplets using anti-15-LO-1 and an FITC-conjugated secondary antibody (b–d). Lipid bodies appear as dark punctate structures that are asymmetrically dispersed in the cell (A). 15-LO-1 staining appears at the same cellular location of the lipid droplets (B). The red-dashed circles indicate representative areas of overlap. 15-LO-1 staining of individual droplets (indicated by arrows) is apparent using confocal microscopy (C and D). C and D are micrographs of a single cell. Mock-transfected cells were used as a control (data not shown) and were absent of 15-LO-1 staining.
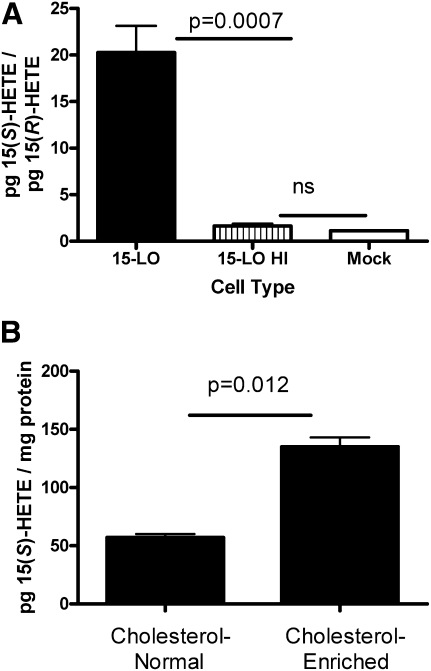
To test the activity of the 15-LO-1 in these cells, 15-LO-1 and mock-transfected RAW macrophages were homogenized as described in Materials and Methods and incubated in the presence of arachidonic acid. A portion of the 15-LO-1 RAW cell homogenate was heated in boiling water for 10 min to act as a heat-inactivated control (15-LO HI, Fig. 4A). 15(S)-HETE (enzymatic product) and 15(R)-HETE (nonenzymatic product) levels were measured in the cells by LC-ECAPCI/MRM/MS. The data in Fig. 4A are presented as a ratio of the enzymatic product [15(S)-HETE over the nonenzymatic product 15-(R)-HETE]. Assuming that 15(S)-HETE and 15(R)-HETE are produced by auto-oxidation in equivalent amounts, a ratio of 1 would indicate no enzymatic activity. The data in Fig. 4A demonstrate that 15(S)-HETE is produced in the RAW-expressing 15-LO-1 but not in the heat-inactivated 15-LO-1 RAW or mock-transfected cells. Additionally, 15(S)-HETE is present in 15-LO-1 RAW in the absence of exogenous arachidonic acid (Fig. 4B). Additionally, in 15-LO-1 RAW, 15(S)-HETE levels increase with cholesterol loading (Fig. 4B) in the absence of exogenous arachidonic acid.
Fig. 4.
15(S)-HETE is produced in 15-LO-1 RAW cells. A: Mock-transfected or 15-LO-1 RAW macrophage cell homogenates were incubated in the presence of arachidonic acid for 2 h. A portion of the 15-LO-1 RAW homogenate was heated in boiling water for 10 min to act as a heat-inactivated control (15-LO HI). Lipids were extracted and analyzed for 15(S)-HETE and 15(R)-HETE content as described in Materials and Methods (n = 4 from four independent experiments). B: 15-LO-1 RAW macrophages were incubated in the presence or absence of acLDL for 24 h. Cellular lipids were extracted and analyzed for 15(S)-HETE as described in Materials and Methods. n = 3 from a representative experiment. Experiment was repeated three times.
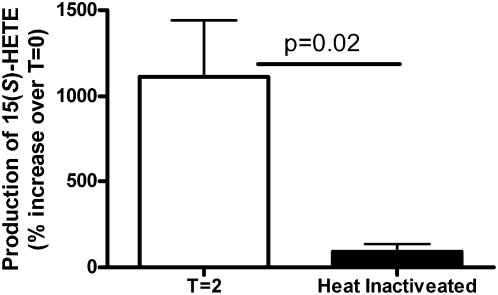
To investigate if the 15-LO-1 present in the lipid droplets from these cells was active, we fractionated the cells into the floating lipid droplet layer, cytoplasm, and the cell pellet. The individual fractions and whole-cell homogenate were incubated in the presence of arachidonic acid for 2 h. The amount of 15(S)-HETE in each fraction was determined by LC-ECAPCI/MRM/MS analysis before (time 0 h) and after (time 2 h) the addition of the arachidonic acid. Heat-inactivated controls were run for each fraction to account for any auto-oxidation that might have occurred. The data presented in Table 1 represent the 15(S)-HETE produced (time 2 h minus time 0 h) during the 2 h incubation with arachidonic acid. Heat-inactivated control values were subtracted from the data presented in Table 1 to account for any auto-oxidation. As expected, the majority of the 15-LO-1 activity was associated with the cytosolic fraction. There was, however, measurable production of 15(S)-HETE in the droplet fraction (∼2% of the total activity) and a small amount produced in the cell pellet (Table 1). Additionally, we determined that the production of 15(S)-HETE in the droplet fraction was inhibited when the fraction was subjected to enzyme inactivation through heating (Fig. 5).
TABLE 1.
Production of 15(S)-HETE in 15-LO-1 RAW macrophages
| 15(S)-HETE (ng) | 15(S)-HETE (% of Total) | |
|---|---|---|
| Whole-cell homogenate | 23,784.8 ± 1320.2 | 100% |
| Droplets | 446 ± 149 | 1.9 ± 0.7% |
| Cytoplasm | 20,956.9 ± 922.5 | 88.4 ± 8.8% |
| Cell pellet | 184 ± 78 | 0.8 ± 0.4% |
15-LO-1 RAW macrophage cells were cholesterol enriched by incubation with acLDL. The cells were homogenized and fractionated as described in Materials and Methods. The cell fractions were incubated with arachidonic acid (100 μM) for 2 h. 15(S)-HETE levels were measured by LC-ECAPCI/MRM/MS as described in Materials and Methods. Before incubation with arachidonic acid, there was 12,025 ± 1,080 ng 15(S)-HETE present in the whole-cell homogenate.
Fig. 5.
Enzyme inactivation through heating inhibits 15(S)-HETE production in cytoplasmic lipid droplets. Cytoplasmic lipid droplets isolated from 15-LO-1 RAW macrophage foam cells were incubated for 2 h in the presence of arachidonic acid (100 μM). One aliquot of the droplets was heated in boiling water for 5 min to inactivate enzymatic activity before the addition of arachidonic acid. 15(S)-HETE was measured by GC-MS as described in Materials and Methods.
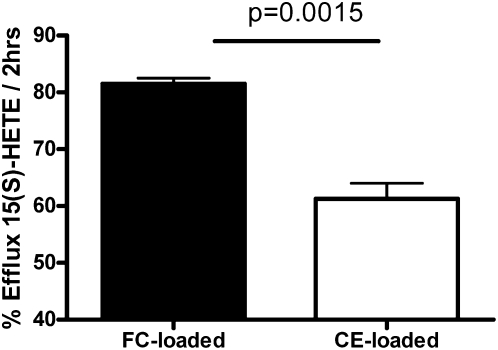
To determine the effect of 15(S)-HETE deposition when present in the lipophillic environment of neutral lipid droplets, fractional efflux of 15(S)-HETE was determined in cholesterol normal (control), free cholesterol (FC)-enriched (no cytoplasmic lipid droplets present), and CE-enriched (with cytoplasmic lipid droplets) 15-LO-1 expressing RAW macrophages. RAW macrophages were incubated with arachidonic acid [to add substrate for the production of 15(S)-HETE], +/−acLDL (to cholesterol enrich the cells), and +/−ACAT inhibitor (to prevent CE production). After this incubation, the media were changed, and new media containing 1% BSA were added for 2 h. FC-induced cellular toxicity was assessed under these cholesterol enrichment conditions by LDH release and cellular protein levels. We measured no changes in LDH release (cholesterol normal, 0.11 ± 0.03 LDH release in arbitrary units (AU) per well; FC enriched, 0.12 ± 0.01 AU per well; CE enriched, 0.11 ± 0.02 AU per well; n = 4 for each treatment) and protein levels (cholesterol normal, 180 ± 2 µg protein/well; FC enriched, 173 ± 10 µg protein/well; CE enriched, 186 ± 11 µg protein/well; n = 4 for each treatment), indicating no toxicity due to FC loading using these conditions. Figure 6 demonstrates that fractional efflux of 15(S)-HETE was lower when cells contain cytoplasmic lipid droplets, indicating that 15(S)-HETE may be sequestered in cytoplasmic lipid droplets.
Fig. 6.
Effect of cellular lipid composition on 15(S)-HETE fractional efflux. 15-LO-1 RAW macrophages were incubated with 7 µg/ml arachidonic acid, +/−acLDL (100 µg/ml), and +/−CP113818 (ACAT inhibitor, 2 µg/ml) for 18 h. These treatments result in cholesterol normal, FC enrichment, and CE enrichment. 15(S)-HETE in time zero cell monolayers was determined at this point. Efflux media containing 1% BSA was added to the rest of the wells. Media were sampled after 2 h and filtered. 15(S)-HETE was quantitated using an ELISA kit (Cayman Chemicals, Ann Arbor, MI) as per the manufacturer's instructions. Efflux was calculated as described in Materials and Methods. Data presented are from a representative experiment (n = 3). Experiment was repeated three times.
DISCUSSION
This study found human reticulocyte 15-LO-1 in THP-1 macrophage cells but not in the undifferentiated monocyte. These data agrees with other studies employing THP-1 and primary human cells (23, 24). Additionally, 15-LO-1 is present on the neutral lipid droplets isolated from THP-1 macrophage foam cells. We ensured that the 15-LO-1 protein is not fortuitously present in the isolated droplet fraction by measuring cytosolic contamination in the fraction and found none. Additionally, we have shown that the 15-LO-1 protein colocalizes with neutral lipid droplets in 15-LO-1-expressing RAW foam cells. 15-LO-1 is commonly thought of as cytoplasmic protein; however, it has been shown to translocate from the cytosol to membranes (25), is present on neutral lipid droplets in other cell types, and is involved in processes such as spermatogenesis and inflammation (5, 6).
To determine if the 15-LO-1 we detected on the macrophage neutral lipid droplets is functional, we utilized RAW murine macrophage cells, which overexpress human 15-LO-1. Murine macrophages do not constitutively express 15-LO-1 (8) and therefore provide a clean background for studying human 15-LO-1 function. As expected, 15(S)-HETE was produced in the 15-LO-1 RAW when the cells were incubated with arachidonic acid. The level of 15(S)-HETE detected in the mock RAW was equivalent to the level of 15(R)-HETE in the mock RAW and is due to nonenzymatic oxidation of the exogenously added arachidonic acid. Interestingly, the amount of 15(S)-HETE in the 15-LO-1 RAW increased with cholesterol loading via acLDL. These data are consistent with that of Habenicht et al. (26) who showed that LDL delivers arachidonic acid for prostaglandin biosynthesis in fibroblasts.
15(S)-HETE was produced in an isolated droplet fraction when incubated with arachidonic acid (27), indicating that 15-LO-1 is functioning on the droplet. This observation was further confirmed by the fact that inactivating 15-LO-1 through heating attenuated the production of 15(S)-HETE in an isolated droplet fraction. The 15-LO-1 activity measured in the droplet fraction accounted for 2% of the total cellular 15-LO-1 activity. This is not surprising considering the protein resides primarily in the cytosol, in which 88% of the total 15-LO-1 activity was detected.
The oxidized products of LO actions on polyunsaturated fatty acids such as eicosanoids affect a multitude of cellular processes, including inflammation and induction of cholesterol transport proteins ABCA1 and ABCG1 (28, 29). Bioactive eicosanoids are not thought to be stored within cells and are generated as needed (30). This study has shown functional human reticulocyte 15-LO-1 on the neutral lipid droplets in macrophage foam cells, consistent with Bozza and Bandeira-Melo (5), who demonstrated that leukocyte lipid bodies are a site for eicosanoid production. The major eicosanoid product of 15-LO-1, 15(S)-HETE, as well as minor products of the enzyme [e.g., 12(S)-HETE and 13(S)-HODE] are potent lipid mediators that have been shown to impact many cellular processes (31, 32, 24, 30, 33). It is possible that droplet-associated 15-LO-1 produces products such as 15(S)-HETE and sequesters the eicosanoids in the droplet. In this model, the lipophilic environment within a lipid droplet could provide a mechanism for the cell to store 15(S)-HETE and related metabolites. The fact that fractional efflux of 15(S)-HETE, regardless of whether it is present as a free fatty acid or as the fatty acyl chain of CE, is diminished when cellular lipid droplets are present supports this hypothesis.
In summary, the 15-LO-1 protein is present and functioning on steryl ester lipid droplets within macrophage foam cells. The cellular role of 15(S)-HETE produced at the lipid droplet warrants further investigation.
Acknowledgments
We thank Dr. Colin D. Funk (Queen's University, Kingston, Canada) for the pcDNA3 plasmid containing the human 15-LO-1 gene. We thank Kevin J. Yu and Kristine M. De Bolt for excellent technical assistance.
Footnotes
Abbreviations:
- 15-LO-1
- 15(S)-lipoxygenase-1
- acLDL
- acetylated low density lipoprotein
- AU
- arbitrary unit
- CE
- cholesteryl ester
- ECAPCI
- electron capture atmospheric pressure chemical ionization
- FC
- free cholesterol
- 15(S)-HETE
- 15(S)-hydroxyeicosatetraenoic acid
- HODE
- hydroxyoctadecadienoic acid
- MRM
- multiple reaction monitoring
This project was supported by National Institutes of Health PPG Grant HL-22633, National Institutes of Health Grants RO1CA091016 and P30ES013508, and National Heart, Lung, and Blood Institute Grant HL-19737. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the National Heart, Lung, and Blood Institute.
REFERENCES
- 1.Beckman M.2006. Great balls of fat. Science. 311: 1232–1234 [DOI] [PubMed] [Google Scholar]
- 2.Martin S., Parton R. G. 2006. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7: 373–378 [DOI] [PubMed] [Google Scholar]
- 3.Brasaemle D. L.2007. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lypolysis. J. Lipid Res. 48: 2547–2559 [DOI] [PubMed] [Google Scholar]
- 4.Ozeki S., Cheng J., Tauchi-Sato K., Hatano N., Taniguchi H., Fujimoto T. 2005. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J. Cell Sci. 118: 2601–2611 [DOI] [PubMed] [Google Scholar]
- 5.Bozza P. T., Bandeira-Melo C. 2005. Mechanisms of leukocyte lipid body formation and function in inflammation. Mem. Inst. Oswaldo Cruz. 100: 113–120 [DOI] [PubMed] [Google Scholar]
- 6.Fisher K. A., Van Leyen K., Lovercamp K. W., Manandhar G., Sutovsky M., Feng D., Safranski T., Sutovsky P. 2005. 15-Lipoxygenase is a component of the mammalian sperm cytoplasmic droplet. Reproduction Res. 130: 213–222 [DOI] [PubMed] [Google Scholar]
- 7.Kuhn H.2004. Lipoxygenases in the cardiovascular system. Circ. Res. 94: 1527–1529 [DOI] [PubMed] [Google Scholar]
- 8.Wittwer J., Hersberger M. 2007. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids. 77: 67–77 [DOI] [PubMed] [Google Scholar]
- 9.Belkner J., Wiesner R., Rathman J., Barnett J., Sigal E., Kühn H. 1993. Oxygenation of lipoproteins by mammalian lipoxygenases. Eur. J. Biochem. 213: 251–261 [DOI] [PubMed] [Google Scholar]
- 10.Cathcart M. K., McNally A. K., Chisolm G. M. 1991. Lipoxygenase-mediated transformation of human low-density lipoprotein to an oxidized and cytotoxic complex. J. Lipid Res. 32: 63–70 [PubMed] [Google Scholar]
- 11.Kühn H., Belkner J., Zaiss S., Fährenklemper T., Wohlfeil S. 1994. Involvement of 15-lipoxygenase in early stages of atherogenisis. J. Exp. Med. 179: 1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Glass C. K., Sigal E., Witztum J. L., Steinberg D. 1990. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc. Natl. Acad. Sci. USA. 87: 6959–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu S. K., Goldstein J. L., Anderson R. G. W., Brown M. S. 1976. Degradation of cationized low-density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc. Natl. Acad. Sci. USA. 73: 3178–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu P., Oe T., Blair I. A. 2008. Determination of cellular redox status by stable isotope dilution liquid chromatograph of glutathione and glutathione disulfide. Rapid Commun. Mass Spectrom. 22: 432–440 [DOI] [PubMed] [Google Scholar]
- 15.DiDonato D., Brasaemle D. L. 2003. Fixation methods for the study of lipid bodies by immunofluorescence microscopy. J. Histochem. Cytochem. 51: 773–780 [DOI] [PubMed] [Google Scholar]
- 16.Dobbs L. G., Williams M. C., Gonzalez R. 1988. Monoclonal antibodies specific to apical surfaces of rat liver type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim. Biophys. Acta. 970: 146–156 [DOI] [PubMed] [Google Scholar]
- 17.Madesh M., Antonsson B., Srinivasula S., Alnemri E., Hajnoczky G. 2002. Rapid kinetics of tBid-induced cytochrome c and Smac/DIABLO release and mitochondrial depolarization. J. Biol. Chem. 277: 5651–5659 [DOI] [PubMed] [Google Scholar]
- 18.Adelman S. J., Glick J. M., Phillips M. C., Rothblat G. H. 1984. Lipid composition and physical state effects on cellular cholesteryl ester clearance. J. Biol. Chem. 259: 13844–13850 [PubMed] [Google Scholar]
- 19.Lee S. H., Williams M. V., Blair I. A. 2003. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 17: 2168–2176 [DOI] [PubMed] [Google Scholar]
- 20.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 21.Zimetti F., Weibel G. K., Duong M-N., Rothblat G. H. 2006. Measurement of cholesterol bidirectional flux between cells and lipoproteins. J. Lipid Res. 47: 605–613 [DOI] [PubMed] [Google Scholar]
- 22.Kellner-Weibel G., Yancey P. G., Jerome W. G., Walser T., Mason R. P., Phillips M. C., Rothblat G. H. 1999. Crystalization of free cholesterol in model macrophage foam cells. Arterioscler. Thromb. Vasc. Biol. 19: 1891–1898 [DOI] [PubMed] [Google Scholar]
- 23.Stachowska E., Dziedziejko V., Safranow K., Jakubowska K., Olszewska M., Machalinski B., Chlubek D. 2007. Effect of conjugated linoleic acids on the activity of mRNA expression of 5- and 15-lipoxygenases in human macrophages. J. Agric. Food Chem. 55: 5335–5342 [DOI] [PubMed] [Google Scholar]
- 24.Conrad D. J., Kuhn H., Mulkins M., Highland E., Sigal E. 1992. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc. Natl. Acad. Sci. USA. 89: 217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinckmann R., Schnurr K., Heydeck D., Rosenbach T., Kolde G., Kühn H. 1998. Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood. 91: 64–74 [PubMed] [Google Scholar]
- 26.Habenicht A. J., Salbach P., Goerig M., Zeh W., Janssen-Timmen U., Blattner C., King W. C., Glomset J. A. 1990. The LDL receptor pathway delivers arachidonic acid for eicosanoid formation in cells stimulated by platelet-derived growth factor. Nature. 345: 634–636 [DOI] [PubMed] [Google Scholar]
- 27.Bryant R. W., Bailey M., Schewe T., Rapoport S. M. 1982. Positional specificity of a reticulocyte lipoxygenase. J. Biol. Chem. 257: 6050–6055 [PubMed] [Google Scholar]
- 28.Li A. C., Glass C. K. 2004. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J. Lipid Res. 45: 2161–2173 [DOI] [PubMed] [Google Scholar]
- 29.Kliewer S. A., Sundseth S. S., Jones S. A., Brown P. J., Wisely G. B., Koble C. S., Devchand P., Wahli W., Willson T. M., Lenhard J. M., et al. 1997. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA. 94: 4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus A. J.1984. The eicosanoids in biology and medicine. J. Lipid Res. 25: 1511–1516 [PubMed] [Google Scholar]
- 31.Wen Y., Gu J., Vandenhoff G. E., Liu X., Nadler J. L. 2008. Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am. J. Heart Circ. Physiol. 294: 1933–1938 [DOI] [PubMed] [Google Scholar]
- 32.Nagelin M. H., Srinivasan S., Lee J., Nadler J. L., Hedrick C. C. 2008. 12/15-lipoxygenase activity increases the degradation of macrophage ATP-binding cassette transporter G1. Arterioscler. Thromb. Vasc. Biol. 28: 1811–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ternowitz T., Fogh K., Kragballe K. 1998. 15-Hydroxyeicosatetraenoic acid (15-HETE) specifically inhibits LTB4-induced chemotaxis of human neutrophils. Skin Pharmacol. 1: 93–99 [DOI] [PubMed] [Google Scholar]