Abstract
Oxygenation of the 5-lipoxygenase product 5S-hydroxyeicosatetraenoic acid by cyclooxygenase-2 yields a bicyclic di-endoperoxide. The di-endoperoxide contains two peroxides spanning from carbons 9 to 11 and 8 to 12, and two hydroxyls at carbons 5 and 15 of arachidonic acid (Schneider C., et al. 2006. Convergent oxygenation of arachidonic acid by 5-lipoxygenase and cyclooxygenase-2. J. Am. Chem. Soc. 128: 720). Here, we report that treatment of the di-endoperoxide with hematin or ferrous chloride results in cleavage of both peroxide O-O bonds and of the bonds between the carbons that carry the peroxide groups, producing the aldehydes 4-hydroxy-2E-nonenal (4-HNE), 8-oxo-5S-hydroxy-6E-octenoic acid, and malondialdehyde (MDA). The hematin- and ferrous iron-catalyzed transformation of the di-endoperoxide proceeded with a similar yield of products as the cleavage of the prostaglandin endoperoxide PGH2 to 12S-hydroxy-5Z,8E,10E-heptadecatrienoic acid and MDA. Chiral phase HPLC analysis of the 4-HNE cleavage product showed greater than 98% 4S and thus established the S configuration of the 15-carbon of the di-endoperoxide that had not previously been assigned. This transformation of the 5-lipoxygenase/cyclooxygenase-2 derived di-endoperoxide invokes the possibility of a novel pathway to formation of the classic lipid peroxidation products 4-HNE and MDA.
Keywords: 4-hydroxy-nonenal, malondialdehyde, lipid peroxide, aldehyde, prostaglandin
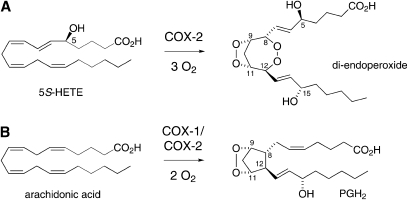
The 5-lipoxygenase (5-LOX) product 5S-hydroxy-eicosatetra-6E,8Z,11Z,14Z-enoic acid (5S-HETE) is an efficient substrate for oxygenation by the so-called “inducible” form of prostaglandin H synthase, cyclooxygenase-2 (COX-2) (1). 5R-HETE is far less efficient as a substrate for COX-2 and COX-1, the isozyme that is regarded as the “housekeeping” form, does not react with either enantiomer of 5-HETE. During the transformation of 5S-HETE, COX-2 consumes 3 mol of oxygen and forms a di-endoperoxide that is structurally reminiscent of the prostaglandin endoperoxide (PGH2) derived from double oxygenation of arachidonic acid by either COX isozyme (Fig. 1). The additional oxygen is incorporated as a peroxide that connects carbons 8 and 12 in place of the carbon-carbon bond that is part of the typical five-membered carbon ring of the prostaglandins (1).
Fig. 1.
Comparison of the reaction of COX-2 with (A) 5S-hydroxy-eicosatetra-6E,8Z,11Z,14Z-enoic acid (5S-HETE) and (B) arachidonic acid forming a di-endoperoxide product or the prostaglandin endoperoxide (PGH2), respectively. The relative configuration of the seven- or five-membered ring is the same in the di-endoperoxide and PGH2, respectively. The absolute configuration of carbons 8 and 12 is 8S and 12S in the di-endoperoxide whereas it is 8R and 12R in PGH2. Carbons 9 and 11 are 9S and 11R in both products. The configuration of C-15 of the di-endoperoxide had not been determined previously and was assigned in this study.
Identification of an unstable peroxide product similar to PGH2, but formed by consecutive action of 5-LOX and COX-2, has invoked the possibility of a cross-over of the leukotriene and prostaglandin biosynthetic pathways. The di-endoperoxide has been identified using in vitro biochemical transformation (1). Cross-over of the 5-LOX and COX-2 pathways has yet to be established to occur in vivo. The instability of the di-endoperoxide, however, impedes attempts aimed at direct detection of this product in cultured cells or animal tissue. The studies reported here were designed to predict and identify potential metabolites of the di-endoperoxide with the future goal of using this information for the detection of metabolites that are indicative of formation of the di-endoperoxide as consecutive oxygenation products of arachidonic acid by 5-LOX and COX-2.
The nonenzymatic transformations of the arachidonic acid-derived endoperoxide PGH2 include cleavage to malondialdehyde (MDA) and 12S-hydroxy-heptadecatri-5Z,8E,10E-enoic acid (HHT) as a minor reaction and rearrangement to the prostaglandins PGE2 and PGD2 and the so-called levuglandins as major reaction (2, 3). Cleavage of the carbon chain is accelerated by the presence of ferrous iron (4, 5), heme (6), and it is also catalyzed by the enzyme thromboxane synthase (4, 7). Here, we describe the nonenzymatic transformation of the 5S-HETE derived di-endoperoxide in the presence of heme and ferrous iron (Fe2+) in aqueous solution. In addition, chiral analysis of one of the transformation products, 4-HNE, 4-hydroxy-2E-nonenal (4-HNE), was used to determine the absolute configuration of the 15-carbon of the di-endoperoxide that had not been established as part of the original structural analysis (1).
EXPERIMENTAL PROCEDURES
Synthesis of 5S-HETE
Racemic 5-HETE was synthesized following the method of Corey and Hashimoto (8). One g of arachidonic acid was dissolved in 12.85 ml of tetrahydrofuran and 6.4 ml of aqueous potassium bicarbonate (1.24 g) and cooled to 0°C. Potassium iodide (1.64 g) and iodine (4.74 g) were added sequentially to the solution; the flask was wrapped with aluminum foil and stirred in the dark at 4°C for 15 h. The solution was poured into ice-cold sodium thiosulfate solution (120 g in 140 ml of water), and extracted three times with 20 ml of pentane/diethyl ether (3/2). The organic phase was washed with 3.5% Na2CO3 solution and with saturated NaCl. The organic phase was filtered over a 50 g silica bond cartridge and eluted with hexane-diethyl ether (3:1).
The collected iodo lactone was evaporated to dryness and dissolved in 10 ml of dry benzene. A solution of 0.2 g of 1,5-diazabicyclo[5.4.0]undec-5-ene in 2 ml of benzene was added and the solution was stirred at room temperature under argon overnight. After 17 h, 1.14 g of powdered CuSO4 × 5 H2O was added and the solution was stirred for an additional 30 min. The mixture was diluted with 12 ml of hexane and filtered rapidly through a 5 g silica bond cartridge eluted four times with 20 ml of diethyl ether-hexane (3:1). The δ-lactone of 5-HETE was evaporated under a stream of nitrogen and 3.5 ml of methanol and 0.43 g of triethylamine were added. The mixture was placed under argon and stirred at room temperature for 24 h. The solvent was evaporated and 5-HETE methyl ester (about 25% yield from arachidonic acid) was isolated using a 2 g silica bond cartridge eluted with hexane-diethyl ether (3:1). 5-HETE methyl ester was further purified by SP-HPLC using an Alltech Econosil Silica column (10 × 250 mm) eluted with a solvent of hexane-isopropanol (100:5, by vol) at a flow rate of 4 ml/min and UV detection at 250 nm.
5S-HETE methyl ester was resolved from 5R-HETE methyl ester by chiral phase HPLC using a Chiralpak AD column (10 × 250 mm) eluted with a solvent of hexane-methanol (100/2, by vol) at a flow rate of 3 ml/min and UV detection at 250 nm (9). The R enantiomer of 5-HETE methyl ester elutes before the S enantiomer. For hydrolysis to the free acid, a 4 mg aliquot of 5S-HETE methyl ester was dissolved in 2 ml of methanol and 100 μl of dichloromethane. One ml of 1 N potassium hydroxide was added and the solution was allowed to stand for 45 min. The organic solvents were evaporated under a stream of nitrogen and the solution was acidified with 1 N HCl to pH 3. 5S-HETE was extracted into dichloromethane, washed three times with water, and evaporated to dryness. A solution of 5 mg/ml of 5S-HETE in methanol was prepared and stored at −80°C until further use.
Synthesis of the di-endoperoxide
In a 7 ml glass vial, 80 μl of a 20 μM solution of purified recombinant human COX-2 (10) was added to 2 ml of 100 mM Tris-HCl pH 8.0 containing 500 μM phenol and 1 μM hematin. After 2 min of incubation in the absence of substrate to allow for reconstitution of the holoenzyme, 50 μg of unlabeled 5S-HETE and 150,000 cpm of [1-14C]5S-HETE were added. The reaction was allowed to proceed for 5 min and was terminated by the addition of 16 μl of glacial acetic acid dissolved in 50 μl of methanol. The entire reaction mixture was applied to a 1-cc (30 mg) Waters Oasis HLB cartridge, washed with water, and eluted with methanol. The di-endoperoxide product was isolated by RP-HPLC using a Waters Symmetry C18 column (4.6 × 250 mm) eluted with a gradient of 20% acetonitrile to 70% acetonitrile in 0.01% aqueous acetic acid in 20 min at a flow rate of 1 ml/min. Elution of the products was monitored at UV 206 nm. The isolated yield of the di-endoperoxide was calculated to be about 15%.
Transformation of the di-endoperoxide with hematin
For transformation of the di-endoperoxide with heme, 1 ml of 100 mM Tris-HCl buffer pH 8.0 containing 500 μM phenol and 1 μM hematin was used. Next, 40 μl of COX-2 solution was added (0.6 μM final concentration) followed by the addition of 20 μg of 5S-HETE. After 5 min at room temperature, 1 ml of a 500 μM solution of hematin was added and the reaction was continued for 1 h. The reaction mixture was either acidified with 1 N HCl to pH 3 and extracted or 1 ml of a solution of 2,4-dinitrophenylhydrazine (2,4-DNPH) (0.3 mg/ml 0.2 N HCl) was added and the reaction was incubated in the dark at room temperature for an additional 1 h followed by acidification to pH 3. The reaction mixtures were loaded on a 1-cc (30 mg) Waters Oasis HLB cartridge, washed extensively with water, and eluted with acetonitrile. For heme-catalyzed transformation of PGH2, the same protocol was followed except for using 20 μg of arachidonic acid instead of 5S-HETE. The transformation of 20 μg of 5S-HETE or arachidonic acid with 1 ml of 500 μM hematin in the absence of COX-2 was used as control reaction.
Transformation of the di-endoperoxide with FeCl2
About 5 μg of the isolated [1-14C]labeled di-endoperoxide (≈15,000 cpm) was mixed immediately after collection off RP-HPLC with 1 ml of a freshly prepared solution of FeCl2 in water (20 mM). After 1 min, the products were loaded onto a Waters Oasis HLB cartridge, washed with water, and eluted with methanol.
Autoxidation of 5S-HETE
One mg of 5S-HETE was evaporated to dryness in a plastic tube and incubated at 37°C for 1 h. The entire autoxidation mixture was then injected using a Waters Symmetry C18 column (4.6 × 250 mm) eluted with a gradient of 20% acetonitrile to 70% acetonitrile in 0.01% aqueous acetic acid in 20 min followed by 10 min of isocratic elution at a flow rate of 1 ml/min. The peak eluting at 4.1 min (corresponding to 8-oxo-5-hydroxy-6E-octenoic acid) was collected, extracted into dichloromethane, and dissolved in CDCl3 for NMR analysis. The 1H and H,H-COSY NMR spectra were recorded using a Bruker AV-II-600 MHz spectrometer. Chemical shifts are calibrated to δ = 7.25 parts per million (ppm) for the remaining CHCl3 in the solvent. The isolated compound was further transformed to the methyl ester derivative with ethereal diazomethane, followed by treatment with methyloxime hydrochloride in pyridine to give the methoxime derivative, and finally, with N,O-bis(trimethylsilyl) trifluoroacetamide. The methyl ester, methoxime, trimethylsilyl (TMS) ether derivative was analyzed by GC-MS in the electron impact mode (70 eV) using a Thermo Finnigan Trace DSQ instrument equipped with a 30 m HP5 column (0.25 mm i.d., film thickness 0.25 μm). The temperature was programmed from 125°C (hold for 1 min) to 300°C at 20°C/min.
Synthesis of 1-pyrazole
One mg of 1,1,3,3-tetraethoxypropane was dissolved in 1 ml of 0.1 N HCl and incubated at 50°C for 1 h. The concentration of MDA in the solution was calculated assuming 100% conversion. To 100 μl of the solution, 1 ml of DNPH reagent (0.3 mg/ml 0.2 N HCl) was added and incubated in the dark at room temperature for 1 h. The solution was extracted using a Waters Oasis HLB cartridge as described above. A single product peak corresponding to DNPH-derivatized MDA (1-pyrazole) was detected using HPLC-diode array detector analysis.
HPLC analyses
Transformation of the di-endoperoxide and PGH2 was analyzed using a Waters Symmetry C18 column (4.6 × 250 mm; 5 μm) eluted with a gradient of 20% acetonitrile to 70% acetonitrile in 0.01% aqueous acetic acid in 20 min followed by 10 min of isocratic elution at a flow rate of 1 ml/min. Elution of the products was monitored using an Agilent 1200 diode array detector. For analysis of the transformation products of [1-14C]labeled di-endoperoxide, the effluent of the diode array detector was coupled on-line to a Radiomatic A100 radioactive flow detector. For detection of 1-pyrazole, the same HPLC-diode array system was used except the gradient was run from 20% acetonitrile to 80% acetonitrile in 0.01% aqueous acetic acid in 20 min followed by 5 min of isocratic elution and 5 min of washing with 100% methanol at a flow rate of 1 ml/min. LC-MS analysis of the 1-pyrazole was performed using a Thermo Finnigan LTQ ion trap mass spectrometer. The sample was resolved using a Waters Symmetry Shield RP-18 column (2.1 × 100 mm; 3 μm) eluted at 0.3 ml/min flow rate with a linear gradient of 5% acetonitrile to 95% acetonitrile in water (containing 10 mM NH4OAc each) within 10 min. The atmospheric pressure chemical ionization (APCI) interface was operated at a vaporizer temperature of 300°C and a tube lens voltage of −80 V. Negative ions were monitored over a range of 150–1200 mass units.
Chiral analysis of 4-HNE
The absolute configuration of 4-HNE was analyzed following a previously described method (11). The 4-HNE derived from the FeCl2-catalyzed transformation of the di-endoperoxide was collected using SP-HPLC and evaporated to dryness under a stream of nitrogen. The sample was dissolved in 10 μl of acetonitrile and treated with 20 μl of a solution of methoxyamine hydrochloride (10 mg/ml) in pyridine at room temperature overnight. In parallel, 10 μg of racemic 4-HNE was treated with methoxyamine hydrochloride in a similar way. The reaction mixtures were diluted with 500 μl of dichloromethane and washed three times with an equivalent volume of water. The organic solvent was evaporated and the syn and anti isomers were separated by RP-HPLC using a Waters Symmetry C18 column (4.6 × 250 mm) eluted with a solvent of acetonitrile-water-acetic acid (50:50:0.01, by vol) at a flow rate of 1 ml/min and UV detection at 235 nm. The later eluting isomer was analyzed by chiral phase HPLC using a Chiralpak AD column (4.6 × 250 mm) eluted with a solvent of hexane-ethanol (90:10, by vol) at a flow rate of 1 ml/min and UV detection at 235 nm.
RESULTS
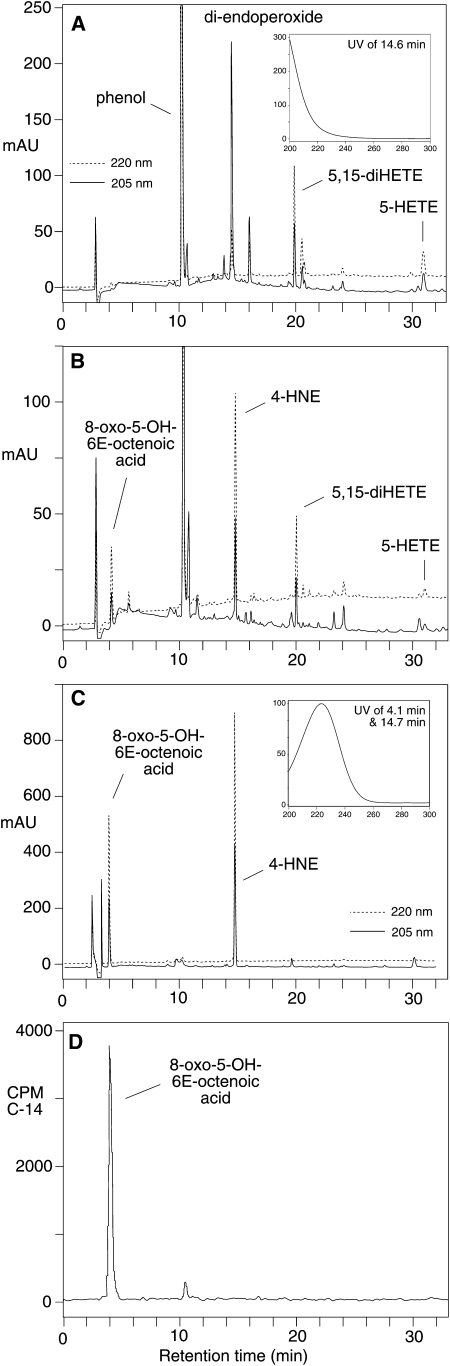
Oxygenation of 5S-HETE by COX-2 resulted in formation of the di-endoperoxide, which was detected using RP-HPLC analysis as described previously (Fig. 2A) (1). The di-endoperoxide (eluting at 14.6 min retention time) contained two isolated double bonds resulting in a UV chromophore with maximum absorbance around 200 nm and noticeable extension of the absorbance beyond 240 nm. A by-product of the reaction was identified as 5,15-diHETE by comparison of the UV spectrum and chromatographic retention time with an authentic standard. For transformation of the di-endoperoxide, the crude product mixture was treated with 500 μl of a 500 μM hematin solution. After 1 h of incubation, the products were extracted and reanalyzed by RP-HPLC. The di-endoperoxide was transformed quantitatively and two prominent peaks were formed with elution times of 4.1 min and 14.7 min (Fig. 2B). The UV spectra of both products were superimposable and indicative of a conjugated oxo-ene chromophore showing a maximum absorbance at 223 nm in RP-HPLC column solvent. When the HPLC-isolated [1-14C]labeled di-endoperoxide was treated with FeCl2 instead of hematin, the same peaks were detected using diode array detection (Fig. 2C). Analysis of the products using an on-line coupled radioactive flow detector showed that only the first peak at 4.1 min contained radioactivity whereas the second peak at 14.7 min was not radiolabeled (Fig. 2D). No significant other labeled or unlabeled products were detected.
Fig. 2.
RP-HPLC and UV analyses of the di-endoperoxide and the transformation reactions with heme and FeCl2. A: The di-endoperoxide (retention time 14.6 min) is the major product of the COX-2 catalyzed transformation of 5S-HETE. The UV spectrum (inset) shows the relative absorbance of the di-endoperoxide at 205 nm and 220 nm. The amount of COX-2 used was sufficient to achieve >95% conversion of the substrate. B: 8-Oxo-5-hydroxy-6E-octenoic acid and 4-hydroxynonenal (4-HNE) were the major products detected upon heme-catalyzed transformation of the crude reaction shown in panel A. C, D: The HPLC-purified [1-14C]labeled di-endoperoxide was treated with FeCl2 for 1 min. The products were extracted and analyzed using RP-HPLC with a diode array detector (C) and a radioactive flow detector connected on-line (D). The inset in C shows the identical UV spectra of 8-oxo-5-hydroxy-6E-octenoic acid and 4-HNE with λmax at 223 nm. Chromatograms recorded at 205 nm (solid line) and 220 nm (dashed line) are shown in A–C to illustrate the relative abundance of the products at the two wavelengths.
The earlier eluting product (4.1 min retention time) was identified as 8-oxo-5S-hydroxy-6E-octenoic acid based on comparison to a reference compound that was prepared as follows: 8-oxo-5S-hydroxy-6E-octenoic acid was predicted to be formed upon cleavage of the 8,9-double bond during autoxidative transformation of 5S-HETE, analogous to the cleavage of 9S-hydroxy-10E,12Z-octadecadienoic acid to 12-oxo-9S-hydroxy-10E-dodecenoic acid (11). Autoxidation of 5S-HETE as a thin film gave the predicted peak at 4.1 min retention time. Using this route of synthesis, the product was formed with higher yield than via heme-catalyzed breakdown of the di-endoperoxide. Therefore, autoxidation of a 1 mg aliquot of 5S-HETE was used to generate sufficient material for spectroscopic characterization. 1H NMR analysis showed a doublet signal at 9.58 ppm for the aldehyde proton (H8) that was coupled to H7 with a coupling constant J7,8 = 7.9 Hz (δ 6.32 ppm; ddd; J7,5 = 1.5 Hz). H7 and H6 (δ 6.81 ppm; dd) form a trans double bond (J7,6 = 15.7 Hz). In the H,H-COSY spectrum, H6 showed a cross-peak with H5 (J = 4.6 Hz) that appeared as a multiplet signal at 4.47 ppm. For GC-MS analysis, the methyl ester, methoxime, TMS ether derivative was prepared. The electron impact analysis (70 eV) of the syn and anti isomers gave essentially the same mass spectra with major fragment ions at m/z 256 ([M-31]+, loss of OMe from the methyl ester or methoxime, respectively; 98% intensity) and m/z 186 ([CH-OTMS-CH-CH-CH = NOCH3]+; 95% intensity), respectively. The [M]+ peak at m/z 287 showed 5% intensity and the base peak was found at m/z 73, indicative of the TMS-derivative. The S-configuration of the 5-carbon is assumed to be unchanged from the 5S-HETE starting material (11). The later peak eluting at 14.7 min was identified as 4-HNE based on its identical UV spectrum and coelution with an authentic standard using both RP-HPLC and SP-HPLC (11).
Semiquantitative analysis of the formation of the two aldehyde products after 1 h of treatment with hematin gave about 10% molar yield of 4-HNE and 3–5% molar yield of the 8-oxo acid. Shorter reaction times resulted in incomplete transformation of the di-endoperoxide. Reaction times longer than 1 h resulted in a decline of the yield of the aldehydes, presumably due to an increased prevalence of secondary transformation reactions. In the absence of hematin or FeCl2, cleavage of the carbon chain was markedly reduced and the di-endoperoxide was predominantly transformed to two different products instead (M. Griesser and C. Schneider, unpublished observations).
The possibility that the aldehydes were derived from 5S-HETE directly or a reaction by-product, rather than from the di-endoperoxide, was excluded based on three observations. First, in all reactions, sufficient amount of COX-2 was used to achieve near complete conversion of 5S-HETE or arachidonic acid, respectively, prior to the addition of hematin or FeCl2 (cf. Fig. 2A). Second, when the HPLC-isolated di-endoperoxide was treated with FeCl2, the two aldehydes were detected as the almost exclusive products (cf. Fig. 2C). Third, when 20 μg of 5S-HETE were incubated in the absence of COX-2 with the hematin solution for 1 h, neither the aldehyde products nor any other abundant UV-active polar products were detected although only about 10% of the intact 5S-HETE was recovered (not shown). The absence of UV-detectable polar products indicated that 5S-HETE was transformed to products that lack UV absorbance or to products of lesser polarity than the starting material, possibly including dimeric or oligomeric products.
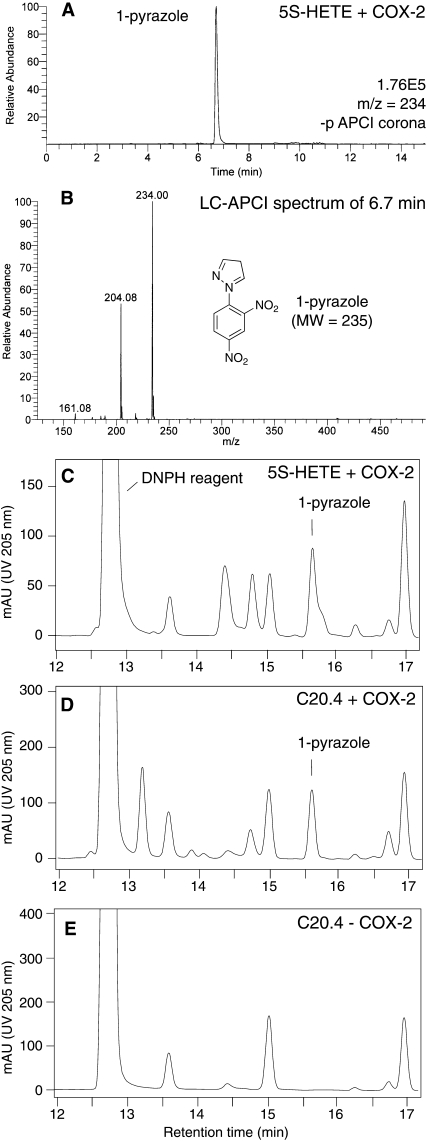
Cleavage of the 20-carbon di-endoperoxide to give the 9- and 8-carbon aldehydes, 4-HNE, and 8-oxo-5-hydroxy-6E-octenoic acid implicated that the 3-carbon aldehyde MDA was formed as the third product of heme-catalyzed transformation. MDA was detected as its 1-pyrazole derivative using the 2,4-DNPH reagent to trap the aldehyde (12). Incubation of 20 μg of 5S-HETE with COX-2 and the subsequent transformation with hematin was performed as described above and followed by immediate treatment of the crude reaction mixture with 2,4-DNPH. MDA was detected as the 1-pyrazole derivative of 2,4-DNPH using LC-MS with APCI ionization (Fig. 3A, B). The molar yield of MDA was between 5 and 10% as determined using RP-HPLC analyses (Fig. 3C). A similar amount of MDA was detected when 20 μg of arachidonic acid was oxygenated to PGH2 followed by treatment with heme and DNPH reagent (Fig. 3D). Thus, under the conditions used, the di-endoperoxide and PGH2 were about equally effective in formation of MDA when treated with hematin. Formation of MDA in either case was dependent on an endoperoxide intermediate because incubation of arachidonic acid with hematin and DNPH reagent without prior reaction with COX-2 failed to show formation of MDA (Fig. 3E). This excluded the possibility that MDA was derived from another unrelated transformation of arachidonic acid.
Fig. 3.
Analysis of malondialdehyde (MDA) formed by heme-catalyzed transformation of the di-endoperoxide and PGH2. A: LC- atmospheric pressure chemical ionization (APCI) analysis of a reaction of cyclooxygenase-2 (COX-2) with 20 μg of 5S-HETE followed by treatment with heme (1 h) and 2,4-dinitrophenylhydrazine (2,4-DNPH) reagent (1 h). The ion trace of m/z 234 for the 1-pyrazole derivative of 2,4-DNPH, recorded in negative ion mode, is shown. B: LC-APCI mass spectrum of the peak at 6.7 min retention time in A. The base peak shows the molecular ion [M-H]- of the 1-pyrazole of 2,4-DNPH. The signal at m/z 204 is due to loss of a fragment of 30 mass units, possibly [N2H2] or [NO], from the parent ion. C: The same reaction of 20 μg of 5S-HETE with COX-2 followed by treatment with heme and 2,4-DNPH reagent was analyzed using RP-HPLC with diode array detection. The back shoulder of the 1-pyrazole peak is due to an unidentified compound. D: A similar amount of the 1-pyrazole derivative was detected when 20 μg of arachidonic acid were reacted with COX-2 followed by treatment with heme and DNPH reagent. E: The 1-pyrazole derivative was not detected when arachidonic acid was treated with heme and DNPH reagent in the absence of COX-2. Equal aliquots of the transformation reactions were injected. The chromatograms shown in panels C–E were recorded at UV 205 nm. The chromatographic conditions used for A and C–E, respectively, are described in the Experimental Procedures.
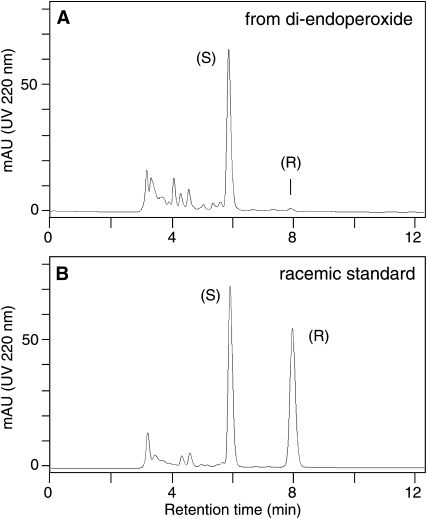
The 4-HNE cleavage product is derived from the methyl end of the di-endoperoxide and, therefore, the absolute configuration of the 4-hydroxyl group is equivalent to the configuration of the 15-carbon of the di-endoperoxide. The configuration of this chiral center of the di-endoperoxide had not been established previously (1). To enable analysis of the absolute configuration using chiral phase HPLC, the 4-HNE formed by hematin-catalyzed transformation of the di-endoperoxide was isolated and reacted to its methoxime derivatives (syn and anti isomers). The later eluting double bond isomer was isolated using RP-HPLC and then resolved into its enantiomers using a Chiralpak AD column.The 4-HNE was greater than 98% of the S-configuration, providing evidence that the 15-carbon of the di-endoperoxide has the S-configuration (Fig. 4).
Fig. 4.
Chiral-phase HPLC analysis of 4-HNE derived from the di-endoperoxide. The 4-HNE derived from the di-endoperoxide (A) and a racemic standard (B) were derivatized with methoxyamine hydrochloride and analyzed by chiral phase HPLC using a Chiralpak AD column as described in Experimental Procedures. The elution order of the 4-HNE enantiomers on the chiral column had been established previously using CD spectroscopy of the methoxime derivatives (11).
DISCUSSION
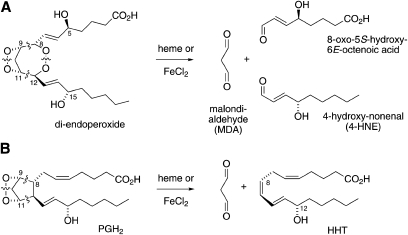
Heme-catalyzed transformation of the 5-LOX/COX-2 derived di-endoperoxide of arachidonic acid resulted in chain cleavage between the carbons carrying the peroxide groups and also between the O-O bonds of both peroxide groups (Fig. 5A). The resulting aldehyde fragments were identified as 8-oxo-5-hydroxy-6E-octenoic acid, 4-HNE, and MDA. The equivalent transformation of the arachidonic acid-derived prostaglandin endoperoxide PGH2 gives MDA and the 17-carbon fragment, HHT (Fig. 5B). Cleavage of the 8,9 and 11,12 carbon bonds and of the 9,11-peroxide occurs similarly in both the di-endoperoxide and PGH2. However, whereas in PGH2, C-8 and C-12 are connected directly as part of the cyclopentane (prostane) ring, in the di-endoperoxide, C-8 and C-12 are connected through the second peroxide group and thus, cleavage of the 8,12-peroxide leads to a further fragmentation of the carbon chain. The lack of a five-membered carbon-ring precludes the possibility that the di-endoperoxide is transformed into analogs of the levuglandins or isoketals that are formed by cleavage of either the 9,10 or 10,11 carbon bond of PGH2 while the 8,12 carbon bond stays intact (3, 13).
Fig. 5.
Transformation of the di-endoperoxide (A) and PGH2 (B) by heme or FeCl2.
The lipid aldehyde 4-HNE has gained significance and notoriety as one of the major cytotoxic products formed during lipid peroxidation (14) but it can also serve as a bona fide signaling molecule (15, 16). The chemical mechanisms that account for the formation of 4-HNE from polyunsaturated fatty acids during lipid peroxidation have been interrogated for some time and there have been suggestions for several pathways (17). 4-HNE is derived from different fatty acid precursors, including linoleic and arachidonic acids, and it is likely a common end point of multiple chemical reactions rather than the product of one single pathway. A recent review of mechanisms that are involved in formation of 4-HNE suggested cleavage of a 1,2-diperoxy moiety as a possibility of carbon chain fission and aldehyde generation (18). The di-endoperoxide contains two such 1,2-peroxide moieties and the aldehydes we have identified result from the predictable cleavage sites (Fig. 5). It is a remarkable structural coincidence that 4-HNE is formed as one of the cleavage products. The question, whether the di-endoperoxide could be a precursor for formation of 4-HNE (and MDA) during lipid peroxidation in vivo, remains to be addressed. Although the di-endoperoxide has been identified originally as an enzymatic product (1), there is also the mechanistic possibility of formation during autoxidation of arachidonic acid or 5-HETE. Studies on the autoxidative transformation of arachidonic acid have shown that essentially all eicosanoids formed enzymatically can likewise be produced by nonenzymatic pathways [e.g., (19, 20)], and the same might hold true for the di-endoperoxide.
The other large cleavage product, 8-oxo-5-hydroxy-6E-octenoic acid, is a γ-hydroxylated α,β-unsaturated aldehyde like 4-HNE (Fig. 5). The same compound, when esterified to the phospholipid backbone, has been identified as one of the targets recognized by the CD36 scavenger receptor (21, 22). As discussed above, if there were autoxidative formation of the di-endoperoxide, it would likely occur in the membrane with the carboxyl group esterified to the phospholipid headgroup. Breakdown of the esterified di-endoperoxide would result in the phospholipid aldehyde as identified by Podrez et al. (21). Again, it will be interesting to see whether the di-endoperoxide could be an intermediate in the formation of this biologically active aldehyde.
One of the goals of this study was to generate and identify products that could serve as indicators of the cross-over of the 5-LOX and COX-2 pathways in vivo by using the model of heme-catalyzed transformation of the di-endoperoxide. Because the three major products formed, 4-HNE, MDA, and 8-oxo-5-hydroxy-6E-octenoic acid, can be derived through other pathways of enzymatic and/or nonenzymatic lipid peroxidation as well, they do not serve this purpose. Nevertheless, we attempted to compare the levels of 4-HNE in intact activated murine RAW264.7 cells treated with and without 5S-HETE but could not see a difference (data not shown). ESI-LC-MS analyses of 8-oxo-5-hydroxy-6E-octenoic acid in cells were hampered by the unexpectedly low sensitivity for detection of this compound.
We used the transformation to 4-HNE as a strategy to indirectly determine the absolute configurations of the 15-carbon of the di-endoperoxide. Although oxygenation at the 15-carbon in prostaglandin biosynthesis occurs with very high precision in the S-configuration (23, 24), there are several well-documented examples where COX-2 catalyzes a specific oxygenation in the 15R-configuration. For example, aspirin-treated COX-2 specifically forms 15R-HETE (25) and certain mutations in the active site lead to formation of 15R-configuration prostaglandins (26) and other 15R-oxygenated products (10). In the latter case, inversion of the oxygenation at C-15 was likely dependent on the conformation of the reacting intermediate in the active site (10). It was, therefore, an open question whether the third oxygenation in the biosynthesis of the di-endoperoxide from 5S-HETE would occur in the R- or S-configuration. Our results provide evidence that the configuration of C-15 of the di-endoperoxide is >98% S.
Footnotes
Abbreviations:
- APCI
- atmospheric pressure chemical ionization
- COX-2
- cyclooxygenase-2
- 2,4-DNPH
- 2,4-dinitrophenylhydrazine
- 5S-HETE
- 5S-hydroxy-eicosatetra-6E,8Z,11Z,14Z-enoic acid
- HHT
- 12S-hydroxy-heptadecatri-5Z,8E,10E-enoic acid
- 4-HNE
- 4-hydroxy-2E-nonenal
- 5-LOX
- 5-lipoxygenase
- MDA
- malondialdehyde
- PGH2
- prostaglandin H2
This work was supported by awards R01GM076592 and P50GM015431 from the National Institute of General Medical Sciences and the Department of Defense Breast Cancer Research Program (BC063074). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
REFERENCES
- 1.Schneider C., Boeglin W. E., Yin H., Stec D. F., Voehler M. 2006. Convergent oxygenation of arachidonic acid by 5-lipoxygenase and cyclooxygenase-2. J. Am. Chem. Soc. 128: 720–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamberg M., Samuelsson B. 1967. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 242: 5344–5354 [PubMed] [Google Scholar]
- 3.Salomon R. G., Miller D. B. 1985. Levuglandins: isolation, characterization, and total synthesis of new secoprostanoid products from prostaglandin endoperoxides. Adv. Prostaglandin Thromboxane Leukot. Res. 15: 323–326 [PubMed] [Google Scholar]
- 4.Hamberg M., Samuelsson B. 1974. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc. Natl. Acad. Sci. USA. 71: 3400–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammarberg T., Hamberg M., Wetterholm A., Hansson H., Samuelsson B., Haeggstrom J. Z. 2009. Mutation of a critical arginine in microsomal prostaglandin E synthase-1 shifts the isomerase activity to a reductase activity that converts prostaglandin H2 into prostaglandin F2α. J. Biol. Chem. 284: 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nugteren D. H., Hazelhof E. 1973. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim. Biophys. Acta. 326: 448–461 [DOI] [PubMed] [Google Scholar]
- 7.Diczfalusy U., Falardeau P., Hammarstrom S. 1977. Conversion of prostaglandin endoperoxides to C17-hydroxy acids catalyzed by human platelet thromboxane synthase. FEBS Lett. 84: 271–274 [DOI] [PubMed] [Google Scholar]
- 8.Corey E. J., Hashimoto S. 1981. A practical process for large-scale synthesis of (S)-5-hydroxy-6-trans-8,11,14,cis-eicosatetraenoic acid (5-HETE). Tetrahedron Lett. 22: 299–302 [Google Scholar]
- 9.Schneider C., Boeglin W. E., Brash A. R. 2000. Enantiomeric separation of hydroxy-eicosanoids by chiral column chromatography: effect of the alcohol modifier. Anal. Biochem. 287: 186–189 [DOI] [PubMed] [Google Scholar]
- 10.Schneider C., Boeglin W. E., Brash A. R. 2004. Identification of two cyclooxygenase active site residues, leucine-384 and glycine-526, that control carbon ring cyclization in prostaglandin biosynthesis. J. Biol. Chem. 279: 4404–4414 [DOI] [PubMed] [Google Scholar]
- 11.Schneider C., Tallman K. A., Porter N. A., Brash A. R. 2001. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 276: 20831–20838 [DOI] [PubMed] [Google Scholar]
- 12.Bakalova R., Mileva M., Kotsev C., Bardarov V., Ribarov S. 2000. Determination of malondialdehyde in biological samples by solid-phase extraction and high-performance liquid chromatography. Methods Find. Exp. Clin. Pharmacol. 22: 267–269 [DOI] [PubMed] [Google Scholar]
- 13.Brame C. J., Salomon R. G., Morrow J. D., Roberts L. J., 2nd 1999. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J. Biol. Chem. 274: 13139–13146 [DOI] [PubMed] [Google Scholar]
- 14.Benedetti A., Comporti M., Esterbauer H. 1980. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 620: 281–296 [DOI] [PubMed] [Google Scholar]
- 15.Nakashima I., Liu W., Akhand A. A., Takeda K., Kawamoto Y., Kato M., Suzuki H. 2003. 4-hydroxynonenal triggers multistep signal transduction cascades for suppression of cellular functions. Mol. Aspects Med. 24: 231–238 [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi S., Sharma A., Patrick B., Sharma R., Awasthi Y. C. 2007. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 12: 4–10 [DOI] [PubMed] [Google Scholar]
- 17.Porter N. A., Pryor W. A. 1990. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic. Biol. Med. 8: 541–543 [DOI] [PubMed] [Google Scholar]
- 18.Schneider C., Porter N. A., Brash A. R. 2008. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J. Biol. Chem. 283: 15539–15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao L., Zackert W. E., Hasford J. J., Danekis M. E., Milne G. L., Remmert C., Reese J., Yin H., Tai H. H., Dey S. K., et al. 2003. Formation of prostaglandins E2 and D2 via the isoprostane pathway: a mechanism for the generation of bioactive prostaglandins independent of cyclooxygenase. J. Biol. Chem. 278: 28479–28489 [DOI] [PubMed] [Google Scholar]
- 20.Roberts L. J., 2nd, Fessel J. P. 2004. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem. Phys. Lipids. 128: 173–186 [DOI] [PubMed] [Google Scholar]
- 21.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Febbraio M., Hajjar D. P., et al. 2002. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 277: 38517–38523 [DOI] [PubMed] [Google Scholar]
- 22.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Gugiu B., Fox P. L., et al. 2002. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 277: 38503–38516 [DOI] [PubMed] [Google Scholar]
- 23.Nugteren D. H., Van Dorp D. A., Bergstrom S., Hamberg M., Samuelsson B. 1966. Absolute configuration of the prostaglandins. Nature. 212: 38–39 [DOI] [PubMed] [Google Scholar]
- 24.Schneider C., Pratt D. A., Porter N. A., Brash A. R. 2007. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 14: 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtzman M. J., Turk J., Shornick L. P. 1992. Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J. Biol. Chem. 267: 21438–21445 [PubMed] [Google Scholar]
- 26.Schneider C., Boeglin W. E., Prusakiewicz J. J., Rowlinson S. W., Marnett L. J., Samel N., Brash A. R. 2002. Control of prostaglandin stereochemistry at the 15-carbon by cyclooxygenases-1 and 2. A critical role for serine 530 and valine 349. J. Biol. Chem. 277: 478–485 [DOI] [PubMed] [Google Scholar]