Abstract
Human meibum was targetly analyzed for the presence of intact wax esters (WEs) and related compounds by means of reverse-phase HPLC in combination with ion trap mass spectrometry. The major detected WEs were based on C18:n (n = 1–4) unsaturated FAs ranking in the following order of abundance: C18:1>C18:2>C18:3>C18:4. The major fatty alcohols (FAls) found in WE were of saturated nature and varied from C18:0 to C28:0. The three most abundant species were C18:1-FA esters of C24:0, C25:0, and C26:0-FAl. Typically, a major compound based on C18:1-FA and a saturated FAl was accompanied by a few related compounds based on a C18:2, C18:3, and C18:4-FA. Contrary to previous reports, no epoxy-WEs or epoxy-FAs were detected in fresh and 1-year-old meibum samples. More than 20 (O-acyl)-ω-hydroxy-FAs (OAHFAs) were observed. The main detected OAHFAs were based on very long-chain ω-hydroxy-FA (C30:1, C32:1, and C34:1) acylated through their ω-hydroxyls by a C18:1-FA. Due to their amphiphilic anionogenic nature, OAHFAs may be responsible for stabilization of the tear film lipid layer by creating an interface between the vast pool of strictly nonpolar lipids of meibum (WEs, cholesteryl esters, etc.) and the aqueous subphase beneath it, a role previously attributed to phospholipids.
Keywords: dry eye epoxides, high pressure liquid chromatography, ion trap mass spectrometry, lipids, Meibomian gland secretions, tear film lipid layer, tears, very long-chain fatty acids and alcohols
Meibum is a lipid-rich secretion that is produced by Meibomian glands located at the ocular lid margins of both upper and lower eyelids of various mammals and humans. Meibum is an intrinsic part of the human tear film (TF), the main role of which is to protect the ocular surface from dehydration. Among other proposed functions of TF are antimicrobial, lubricatory, and nutritional ones. For general information on meibum and TF, the reader is advised to read earlier comprehensive reviews published by various laboratories during the last few decades (1–8). In TF, meibum is believed to form its outermost layer, which retards evaporation of water from the bulk of TF and from the ocular surface beneath it (7). Yet another function of meibum is to form a hydrophobic barrier along the margins of the eyelids to contain TF at, and prevent it from leaking out of, the designated ocular surface area (2). These protective functions imply a very hydrophobic nature of meibum. Indeed, the major meibum components were identified as various wax esters (WEs) and cholesteryl esters (CEs) with long-chain and very long-chain FA (9–11). The information on acylglycerol content of meibum is more limited as no definite structural information on the putative meibomian acylglycerols is currently available (12, 13). Interestingly, lipidomic analyses of human meibum demonstrated that its composition is distinctively different from that of sebum. For example, free cholesterol, squalene, ceramides (Cers), and typical phospholipids were shown to be minor components of meibum (9–11, 14, 15), although they dominated in the skin (16, 17).
Meibum has been analyzed using various experimental techniques, discussion of which goes beyond the scope of this article. Recently, we published preliminary results on the lipid composition of human meibum and tears (9–11, 15). The major method employed in these studies was normal phase high performance liquid chromatography in combination with atmospheric pressure chemical ionization mass spectrometry (NP HPLC-MS). The main advantage of the NP HPLC is its ability to separate different classes of lipids. For example, mono-, di-, and triacylglycerols (MAGs, DAGs, and TAGs, respectively) elute as three distinctively different groups of HPLC peaks and so do other groups of lipids (free FAs and their amides and esters; simple Cer and acyl-Cer, etc.). However, under the conditions of NP HPLC, certain classes of lipids, mostly of a very hydrophobic nature, have very short retention times (ReTs) and tend to coelute. The coelution of various compounds of CE, WE, and TAG families observed in our earlier experiments with meibomian lipids made unambiguous characterization of these particular lipid classes somewhat difficult and necessitated elaborate fragmentation studies designed to overcome this problem (15). In this article, we concentrated on the lipidomic analysis of various species of WE and related compounds by using reverse-phase (RP) HPLC-MS, which was to provide better separation of very hydrophobic analytes detected in normal human meibum. This enabled us to compare them side-by-side with authentic lipid standards where available.
MATERIALS AND METHODS
Materials, reagents, and equipment
Authentic WE, FAl, and FA derivatives were purchased from Sigma-Aldrich (St. Louis, MO) and Nu-Chek Prep, Inc. (Elysian, MN). HPLC or spectroscopy grade solvents used for making lipid stock solutions and HPLC eluents were manufactured by Burdick and Jackson (Muskegon, MI) or Sigma-Aldrich. For RP HPLC experiments, a C18 Hypersil Gold column (2.1 × 150 mm, 5 μm; manufactured by ThermoFisher Scientific, Waltham, MA) was used. Chromatographic separation experiments were conducted on an Alliance 2695 HPLC Separations Module (Waters Corp., Milford, MA). The chromatograph was interfaced to an ion trap mass spectrometer (an LCQ Deca XP Max from ThermoFisher) equipped with an atmospheric pressure chemical ionization ion source and operated under an XCalibur software (from the same manufacturer).
Sample collection
The samples of normal human meibum were collected from both lower and upper eyelids of seven volunteers (three males and four females) using a platinum spatula, dried, and stored exactly as described earlier (9–11, 15). The samples were used for structural analyses of meibomian lipids only; no conclusions about the effects of gender, age, type of diet, etc. were to be made. Just before analyses, each sample was dissolved in an appropriate amount of a n-hexane:propan-2-ol (1:1, v/v) solvent mixture HP to make a ∼1 mg/ml sample stock solution. The study was approved by the UT Southwestern Medical Center Institutional Review Board and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice (GLP).
Epoxidation of MGS
Unsaturated compounds of meibomian gland secretion (MGS) (∼0.1 mg, dry weight) were epoxidized by incubating the sample for 15-min in 1 ml of 3% solution of peroxyacetic acid in solvent HP at 70°C under nitrogen. Then, the solvent was evaporated to dryness at 35°C under a stream of nitrogen.
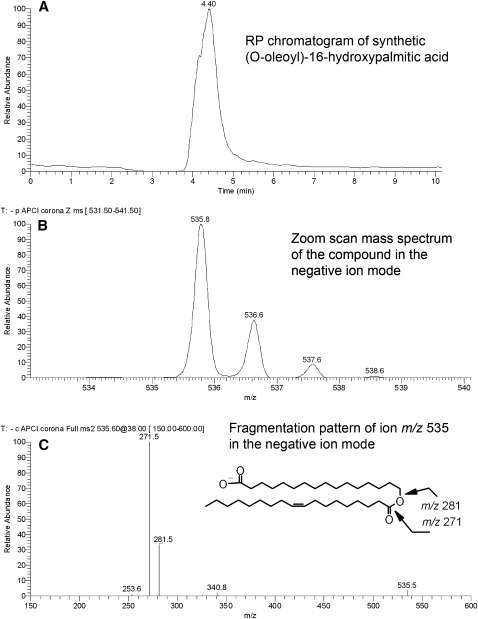
Synthesis of (O-oleoyl)-16-hydroxypalmitic acid
Ten μmoles (∼3 mg) of oleoyl chloride were dissolved in 2 ml of CHCl3 in a glass reaction vial and 10 μmoles (∼2.7 mg) of 16-hydroxypalmitic acid were added to the mixture. Then, the vial was sealed; the mixture was warmed up to 70°C in a dry block heater and kept at that temperature upon occasional vortexing for 30 min. The reaction product, (O-oleoyl)-16-hydroxypalmitic acid, was analyzed by RP HPLC-MS in the negative ion mode. The compound was detected as a prominent ion m/z 535.6 [(M-H)−; C34H63O4; theoretical mass-to-charge ratio 535.5]. Its retention time was about 4.4 min. In an MS2 experiment 535.6@50 V, it produced two prominent product ions, m/z 271 and 281, anions of 16-hydroxypalmitic and oleic acid, respectively.
Sample analyses using RP HPLC-MS
The samples were analyzed by RP HPLC-MS using a slightly modified version of a protocol described in our recent paper on CE in meibum (11). Dry meibum samples were dissolved in the solvent HP to yield ∼1 mg/ml stock sample solutions. A 2 ml HPLC-MS-certified vial with a silicon-free polytetrafluoroethylene cap was used. A C18 Hypersil Gold column was equilibrated with an acetonitrile:propan-2-ol:5 mM aqueous ammonium formate, 45:50:5 (v/v/v) solvent mixture at 35°C. The flow rate was maintained at 0.2 ml/min during the entire experiment. A sample aliquot (from 0.5 to 7 μl; larger injection volumes severely degrade the shapes of the HPLC peaks) was injected using an autoinjector and the elution started by linearly changing the eluent's composition from the original one to 5:90:5 over the course of 35 min. Then, the elution continued isocratically for another 10 min after which the solvent composition was changed back to the original 45:50:5 over the next 1 min and the column was reequilibrated for another 14 min with the initial solvent mixture.
The entire effluent was directed to the ion source of the mass spectrometer. As WEs easily form adducts with protons, ammonium, sodium, and potassium ions (9, 10), the detection was conducted in the positive ion mode. The following MS parameters were typically used: vaporization temperature 350°C, sheath gas (nitrogen) 40 arbitrary units; source voltage +5 kV; capillary temperature 350°C; capillary voltage between +2 and +10 V; tube lens offset −30 V. The activation time was set at 2 × 100 ms, whereas the scanning range was between m/z 205 and 2000. The ReT and MS signals of the WE species detected in meibum and tear samples were compared with those of authentic WE standards where available. When necessary, fragmentation of the analytes in MSn experiments was performed to verify their structural assignments. NP HPLC analyses of samples were performed exactly as described in our earlier publications (9, 10).
RESULTS
All seven samples of human meibum demonstrated very similar patterns with respect to the compounds discussed below. Our current results concern only structural characterization of the compounds; quantitation of the compounds would require the use of authentic lipid standards to generate corresponding calibration curves. For the vast majority of the meibum compounds, those standards are currently unavailable from any source. The experiments are in progress to address this issue.
Very long-chain wax esters
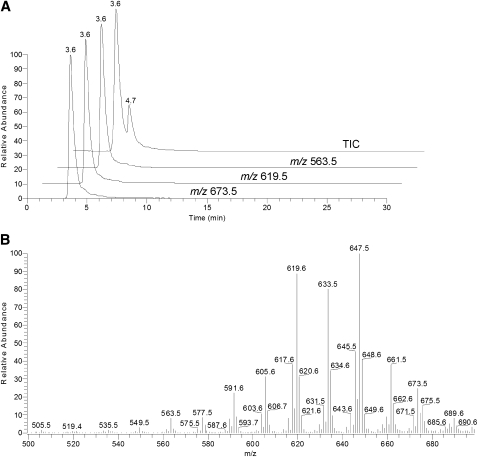
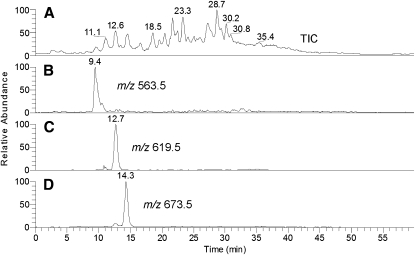
Due to the extremely complex composition of lipid samples extracted from human tissues, their total ion chromatograms (TIC) recorded in the m/z range of 205 to 2000 a.m.u. are often diffused and lack well-defined HPLC peaks. However, under the conditions of NP HPLC analysis, most of the detected compounds coelute forming one easily detectable but unresolved TIC HPLC peak with ReT of about 3.6 min (Fig. 1A, trace TIC). Note the complexity of the lipid profiles visible in a relatively narrow MS range where typical WE are observed (Fig. 1B). Extraction of a signal of a particular ion from TIC or running a selected ion monitoring (SIM) experiment dramatically improve the HPLC pattern, allowing one to clearly see the selected analytes in complex mixtures (11). Previously, we reported on the presence of a range of oleic acid-based WEs with m/z values between 535 and 673 or so in meibum (9, 10). However, extraction of the signals of these ions from TIC and plotting them as individual chromatograms (Fig. 1A, traces m/z 563.5, 619.5, and 673.5) demonstrated that they all coeluted. We speculated that separation of WEs might become possible under the conditions of RP HPLC. Thus, we used a previously described protocol that worked well for CEs (11). Although TIC RP HPLC of meibum produced a rather complex and diffused pattern (Fig. 2A), extraction of the individual ions of expected meibum WE resulted in a much clearer picture with sharp HPLC peaks of individual compounds (Figs. 2B–D).
Fig. 1.
Normal phase chromatograms and MS spectrum of nonpolar lipids present in human meibum. Positive ion mode. A: Total ion chromatogram (TIC) and selected ion monitoring (SIM) traces of wax esters ions m/z 563, 619, and 673 extracted from the TIC. B: Mass spectrum of the HPLC peak with the retention time of 3.6 min. Positive ion mode.
Fig. 2.
Reverse-phase HPLC-MS analysis of human meibum in the positive ion mode. A: TIC of the whole meibum sample. B: SIM of ion m/z 563. C: SIM of ion m/z 619. D: SIM of ion m/z 673.
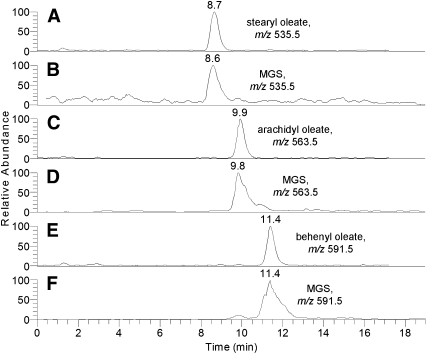
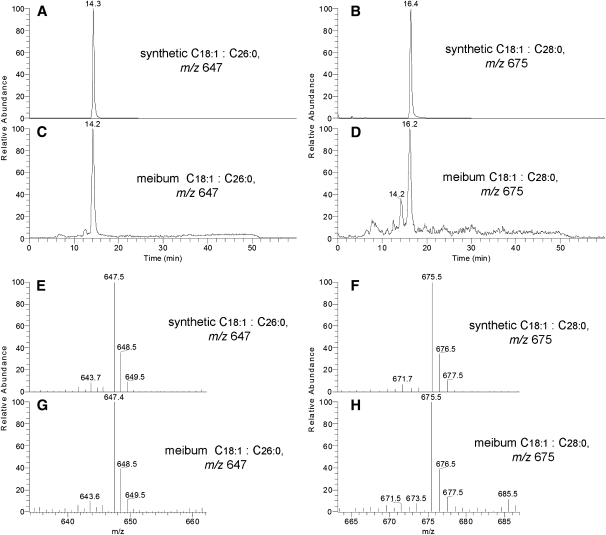
The ReT of some of the meibomian lipids were compared with those of corresponding authentic WE standards (Fig. 3). This, along with the observed m/z values of the parent compounds for the WE standards (Fig. 3A, C, E) and meibum sample (Fig. 3B, D, F), provided a solid basis for the correct identification of the analytes in human samples. At least three compounds detected in meibum matched standard WE, namely stearyl oleate, arachidyl oleate, and behenyl oleate. Currently, however, the longest oleic-acid WE available commercially is behenyl oleate (C40H78O2, isotopic molecular mass 590). To expand the range of WE standards, we synthesized in-house two new oleic acid-based WEs with C26:0 (hexacosanol) and C28:0 (octacosanol) FAl components. These compounds were produced in a reaction of oleoyl chloride with corresponding FAl (The details of the procedure are to be published elsewhere.). The newly synthesized compounds had the same ReT as the corresponding compounds from meibum (Fig. 4A–D), and produced the same mass spectra (Fig. 4E–H), which closely matched theoretical mass spectra of hexacosanyl oleate and octacosanyl oleate (not shown). Finally, fragmentation of the corresponding parent (M+H)+ ions in MS/MS experiments revealed reassuringly similar patterns of characteristic product ions for natural compounds and matching WE standards [not shown; see (10) for more details]. Thus, one can conclude that our preliminary data on the major unsaturated WEs found in human meibum was confirmed in our new RP HPLC-MS experiments.
Fig. 3.
Direct HPLC-MS comparison of selected authentic lipid standards and the corresponding wax esters (WEs) of human meibum. Positive ion mode. A: Authentic stearyl oleate, m/z 535. B: Meibum compound m/z 535. C: Authentic arachidyl oleate, m/z 563. D: Meibum compound m/z 563. E: Authentic behenyl oleate, m/z 591. F: Meibum compound m/z 591.
Fig. 4.
Direct HPLC-MS comparison of synthesized in-house very long-chain WEs and the corresponding compounds of human meibum. Positive ion mode. A: RP chromatogram of synthetic hexacosanyl oleate, m/z 647. B: RP chromatogram of synthetic octacosanyl oleate, m/z 675. C: RP chromatogram of a meibum compound m/z 647. D: RP chromatogram of a meibum compound m/z 675. E: MS spectrum of synthetic hexacosanyl oleate, peak ReT 14.3 min. F: MS spectrum of synthetic octacosanyl oleate, peak ReT 16.4 min. G: MS spectrum of meibum compound m/z 647, peak ReT 14.2 min. H: MS spectrum of meibum compound m/z 675, peak ReT 16.2 min.
However, oleic acid-based WEs (C18:1-WE) were not the only type of WE found in human meibum. Most of the major detected WE species were accompanied by a group of relatively minor but still visible compounds, which apparently differed from the major compound in terms of their unsaturation. As an example, a partial MS spectrum of three major WE species with m/z values of 619.6, 633.6, and 647.5 is presented in Fig. 5A. These three compounds were tentatively identified as C18:1-based esters of C24:0, C25:0, and C26:0 alcohols (10) with the total number of carbon atoms being 42, 43, and 44, respectively. From Fig. 5A, one can see that each of those C18:1-based WEs has an escort of less saturated homologs M-2, M-4, and M-6 (where M is the molecular mass of the main C18:1-based WE), presumably of C18:2, C18:3, and C18:4 nature (Fig. 5B–E). In all likelihood, the latter three are polyunsaturated linoleic, linolenic, and stearidonic acids, routinely found in mammals. These are considered essential dietary FAs vital for normal human metabolism.
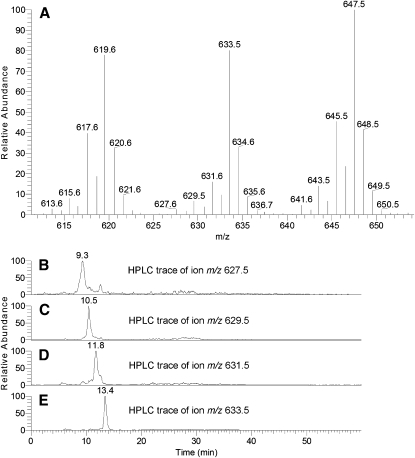
Fig. 5.
Expanded MS spectrum of human meibum WEs (A) and SIM RP chromatograms of a family of homologous WEs differing in the degree of their unsaturation (B through E). Ion m/z 627 – C18:4 (stearidonic acid)-based ester of C25:0 (pentacosanol) alcohol. Ion m/z 629 – C18:3 (linolenic acid)-based ester of pentacosanol. Ion m/z 631 – C18:2 (linoleic acid)-based ester of pentacosanol. Ion m/z 633 – C18:1 (oleic acid)-based ester of pentacosanol.
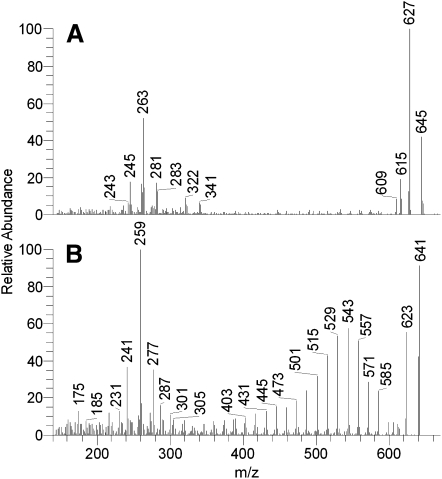
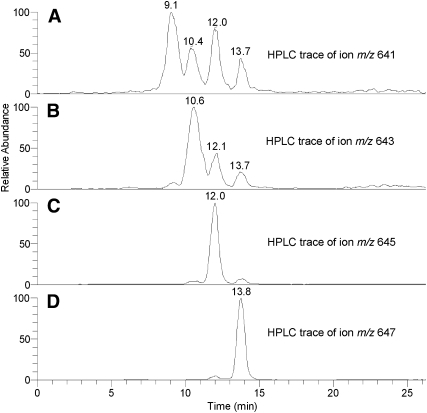
The homologous ions were fragmented in MS2 experiments in order to verify their structures. As an example, let's consider a family of ions: m/z 641.5 (I), 643.5 (II), 645.5 (III), and 647.5 (IV). Fragmentation of these ions produced a repeatable pattern of product ions: m/z 277, 259, and 241 (I), 279, 261, and 243 (II), 281, 263, and 245 (III), and 283, 265, and 247 (IV) (Fig. 6). The product ions of meibum ion m/z 647.5 were identical to those of authentic hexacosanyl oleate, ion m/z 283 was that of a proton adduct of oleic acid, whereas ions m/z 265 and 247 were its mono- and di-dehydrated products. Similarly, ion m/z 281 and its product ions belonged to linoleic acid, ion m/z 279, to linolenic acid, and 277, to stearidonic acid. Interestingly, with the increase in the degree of unsaturation, the compounds became progressively less stable, revealing more fragmentation of their FA chains (compare the spectra of diene III and tetraene I, Figs. 6A and 6B). Thus, one can conclude that the meibum compounds that produced ions m/z 641, 643, 645, and 647 belong to the family of hexacosanyl esters of those C18:n FAs differing in the number of their double bonds (n = 1–4). Similar results were obtained for other families of ions presented in Figs. 1B and 5A. Interestingly, single ion monitoring experiments revealed another correlation, this time between the number of double bonds in the molecules and the number of HPLC peaks detected for each ion (Fig. 7). Thus, monoene IV produced one prominent HPLC peak, diene III, two peaks, triene II, three peaks, and tetraene I, four clearly visible components. One possible explanation of these observations is that polyunsaturated compounds I–III are present in several isoforms, differing in either location, or geometry (cis trans) of their double bonds whereas monoene IV does not have any isoforms. Note that the fragmentation patterns of the corresponding isoforms of each ion (641, 643, and 645) were almost identical, e.g., four peaks of tetraene I produced very similar, if not identical, product ions depicted in Fig. 6B. Experiments are in progress to elucidate their structures in more details.
Fig. 6.
Effects of the degree of unsaturation on the fragmentation spectra of WEs. A: Fragmentation spectrum of ion m/z 645 (linoleic acid-based ester of hexacosanol; positive ion mode). Note the formation of a product ion m/z 281 (a proton adduct of linoleic acid), and its dehydration products 263 and 245. Indicative is the absence of prominent ions in the range of m/z 400 to 600. B: Fragmentation spectrum of ion m/z 641 (stearidonic acid-based ester of hexacosanol; positive ion mode). Note the formation of a product ion m/z 277 (a proton adduct of stearidonic acid), and its dehydration products 259 and 241. Indicative is the presence of a large number of prominent ions in the range of m/z 400 to 600 caused by the lesser stability of PUFA under the conditions of MS/MS.
Fig. 7.
The number of double bonds in the series of homologous WEs correlates with the number of the detected HPLC peaks. A: SIM RP HPLC trace of ion m/z 641 (positive ion mode; four isomers detected; all have similar fragmentation patterns). B: SIM RP HPLC trace of ion m/z 643 (three isomers detected). C: SIM RP HPLC trace of ion m/z 645 (one major, and two minor isomers detected). D: SIM RP HPLC trace of ion m/z 647 one major isomer detected).
When plotted as extracted chromatograms, corresponding M, M-2, M-4, and M-6 ion families produced a repeating pattern of HPLC peaks whose ReT differed by 1 to 2 min. As an example, individual chromatograms of a family of ions with m/z 627.5, 629.5, 631.5, and 633.5 are presented in Fig. 5B–E. Note that similar patterns were observed for other ion families as well.
The incremental changes in the ReT of WE standards obeyed a simple equation, one similar to that one employed in a preceding paper on cholesteryl esters found in meibum (11):
where k1 = 13.58, k2 = (−0.90), and k3 = 0.02 are experimental constants; A, a number of carbon atoms in the WE molecule; B, number of double bonds in the WE molecule; r2 >0.999. Using this equation, one can verify whether an unknown compound belongs to the WE families described in this paper because it is unlikely that unrelated isobaric compounds would obey equation 1 with the same coefficients k1 and k2 as a WE would. All observed WEs of meibum obeyed equation 1 (not shown).
Epoxides of wax esters
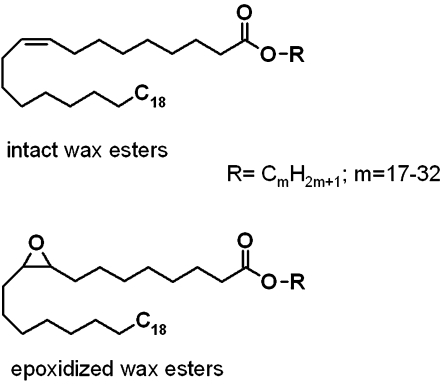
Epoxy-lipids, in particular 9,10-epoxy-C18:0- and 11,12-epoxy-C20:0 FA, were reported earlier to be present in combined CE/WE fractions of human meibum in noticeable quantities (18). The epoxy compounds were detected and identified gas chromatographically after transesterification of an entire meibum sample (18). Uncharacterized mixture of epoxy-DAG was also reported by Sullivan et al. (12). However, none of the epoxy-containing lipid species have ever been detected and structurally characterized in its intact unmodified form. In an attempt to further characterize WE species present in human samples, molecular masses of a series of hypothetical epoxy-WEs of a general formula CnH2n-2O3 were computed (Scheme 1 and Table 1). These compounds were assumed to be based on 9,10-epoxy-C18:0 and 11,12-epoxy-C20:0 FA. Their FAl components ranged from a relatively short C17:0 to a very long chain C32:0.
Scheme. 1.
Chemical structures of intact and epoxidized WEs.
TABLE 1.
Major intact WEs of human meibum and their epoxidized derivatives detected in the positive ion modea
| Intact WEs Found in Meibum, General Formula CnH2n-2O2 [10] |
Epoxidized WEs, General Formula CnH2n-2O3 |
||||||
|---|---|---|---|---|---|---|---|
| n | Compound, FA:FAl | Molecular Formula | m/z (H adduct) | Compound, epFA:FAl | Molecular Formula | m/z (H adduct) | Detected in Meibum, Yes/No |
| 35 | C18:1:C17:0 | C35H68O2 | 521.53 | epC18:0:C17:0 | C35H68O3 | 537.52 | no |
| 36 | C18:1:C18:0 | C36H70O2 | 535.54 | epC18:0:C18:0 | C36H70O3 | 551.54 | no |
| 37 | C18:1:C19:0 | C37H72O2 | 549.56 | epC18:0:C19:0 | C37H72O3 | 565.55 | no |
| 38 | C18:1:C20:0 | C38H74O3 | 563.58 | epC18:0:C20:0 | C38H74O3 | 579.57 | no |
| 39 | C18:1:C21:0 | C39H76O2 | 577.59 | epC18:0:C21:0 | C39H76O3 | 593.59 | no |
| 40 | C18:1:C22:0 | C40H78O2 | 591.61 | epC18:0:C22:0 | C40H78O3 | 607.60 | no |
| 41 | C18:1:C23:0 | C41H80O2 | 605.62 | epC18:0:C23:0 | C41H80O3 | 621.62 | no |
| 42 | C18:1:C24:0 | C42H82O2 | 619.64 | epC18:0:C24:0 | C42H82O3 | 635.63 | no |
| 43 | C18:1:C25:0 | C43H84O2 | 633.65 | epC18:0:C25:0 | C43H84O3 | 649.65 | no |
| 44 | C18:1:C26:0 | C44H86O2 | 647.67 | epC18:0:C26:0 | C44H86O3 | 663.66 | no |
| 45 | C18:1:C27:0 | C45H88O2 | 661.69 | epC18:0:C27:0 | C45H88O3 | 677.68 | no |
| 46 | C18:1:C28:0 | C46H90O2 | 675.70 | epC18:0:C28:0 | C46H90O3 | 691.70 | no |
| 47 | C18:1:C29:0 | C47H92O2 | 689.72 | epC18:0:C29:0 | C47H92O3 | 705.71 | no |
| 48 | C18:1:C30:0 | C48H94O2 | 703.73 | epC18:0:C30:0 | C48H94O3 | 719.73 | no |
| 49 | C18:1:C31:0 | C49H96O2 | 717.75 | epC18:0:C31:0 | C49H96O3 | 733.74 | no |
| 50 | C18:1:C32:0 | C50H98O2 | 731.76 | epC18:0:C32:0 | C50H98O3 | 747.76 | no |
Only 9,10-epoxy-C18:0-based WEs are shown.
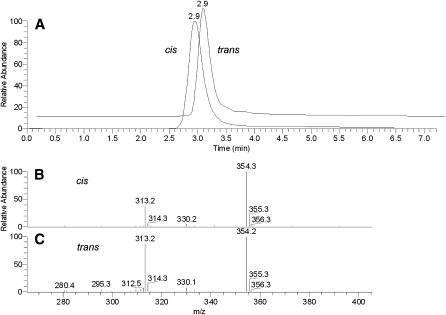
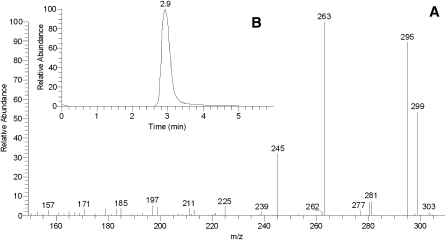
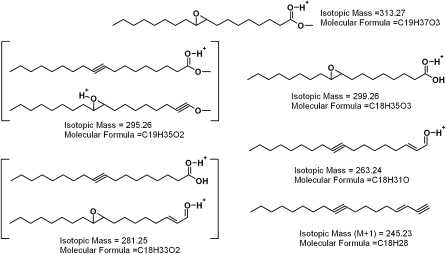
First, two samples of authentic cis- and trans-9,10-epoxy-C18:0 FA methyl esters were tested (Fig. 8A). Both the compounds coeluted with a short ReT of 2.9 min. Their mass spectra (Fig. 8B, C) were virtually indistinguishable from each other and produced a series of major ions with m/z 313 (M + H)+, 330 (M + NH4)+ (weak), and 354 (M + H+ CH3CN)+. The only noticeable difference between the spectra was the relative intensities of signals m/z 313 and 354: for the cis-isomer, the ratio was found to be ∼40:100 whereas the trans-isomer produced the signals in a 90:100 ratio. Thus, methyl esters of epoxy-FAs were easily detectable by HPLC-MS and so should be epoxy-WEs. In an MS2 experiment, ion m/z 313 (M+H+) produced a series of prominent ions: m/z 299 (M + H – CH2)+, 295 (M + H – H2O)+, 281 (M + H – H2O – CH2)+, 263 (M + H – 2H2O – CH2)+, and 245 (M + H – 3H2O – CH2)+, among others (Fig. 9). Their tentative assignments are presented in Scheme 2. Ion m/z 299 is of especial importance as it allows us to directly detect the epoxidized FA moiety of esters.
Fig. 8.
RP HPLC-MS analysis of cis- and trans-9,10-epoxy stearic acids in the positive ion mode. A: Chromatograms of the compounds demonstrated no difference in the ReT. B: Mass spectrum of cis-9,10-epoxy stearic acid. C: Mass spectrum of trans-9,10-epoxy stearic acid.
Fig. 9.
Fragmentation spectrum of cis-9,10-epoxy stearic acid taken in the positive ion mode (A) and the TIC of the compound (B).
Scheme. 2.
Fragmentation pattern of trans-9, 10-epoxy-stearic acid methyl ester.
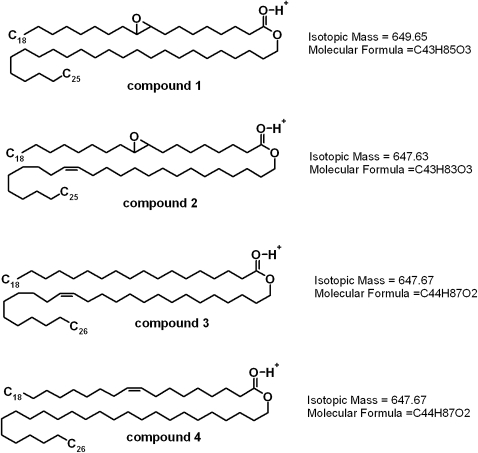
As an example, let's consider a series of four related WEs which, according to previous reports (9, 10), were likely to be found in meibum. Two of these compounds are based on 9,10-epoxy-C18:0 FA (structures 1 and 2, Scheme 3) and two on C18:0 and C18:1 FA (structures 3 and 4). Theoretically, compound 1 [an epoxidized derivative of a naturally occurring C18:1:C25:0 WE (10)] should produce a major MS signal m/z 649 and two weaker isotopic peaks 650 and 651 in the ratio 100:48:12. Compounds 2–4 should have given similar triplet of ions with m/z 647, 648, and 649 in the same ratio.
Scheme. 3.
A family of related intact and epoxidized WEs.
When extracting signals m/z 647 and 649 from TIC of meibum, there was only one major HPLC peak formed by ion m/z 647 detected (Fig. 4C); its MS spectrum (Fig. 4G) was very similar to those of compounds 2, 3, and 4 with the m/z 647, 648, and 649 signal ratio being 100:41:10. Ion m/z 649, in contrast, did not produce any major HPLC peaks. Thus, no evidence of the presence of the epoxy-WE (compound 1) was obtained. Further selection between compounds 2 to 4 had to be made on the bases of their fragmentation patterns and ReT. First, in an MS2 experiment m/z 647@38V→product ions, an authentic hexacosanyl oleate (compound 4) produced a clear signal of protonated oleic acid with m/z 283 and so did a meibum compound with m/z 647 (10). Second, their ReTs were found to be identical (Fig. 4A, C). On the other hand, a hypothetical epoxy-WE (compound 2) should have produced a product ion m/z 299 (a proton adduct of free epoxy-stearic acid), which was not the case. Instead, the fragments of a meibomian lipid with m/z 647 matched those of chemically synthesized compound 4. Also, the ReT of compound 2 should have been shorter than that of compounds 3 and 4 because of the former's higher hydrophilicity. Compound 3, on the other hand, should have produced an FA ion with m/z 285 (a proton adduct of stearic acid). Again, the presence of such ion was not observed in the fragmentation spectra of ion m/z 647. Thus, the presented data are indicative of compound 4 as the most probable structure of the corresponding meibomian WE with m/z 647. Similar approaches and rationale were applied to evaluate other WEs found in meibum, and the results confirmed our earlier conclusions about their structures. An interesting result of our experiments is a lack of observed epoxy-WE in meibum, both fresh and one year old.
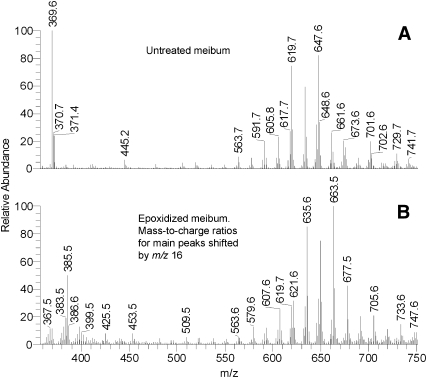
To verify whether meibum WEs could be converted to the corresponding epoxy-derivatives and to generate their samples for further in-depth bioanalytical studies, an aliquot of MGS solution in the HP solvent was treated with peroxyacetic acid as described in Materials and Methods. Then, both treated and untreated samples were analyzed by HPLC-MS side by side. Indeed, epoxidation of meibum lipids with peroxyacetic acid dramatically changed their mass spectra (Fig. 10). Note that the mass-to-charge ratios of the major meibum components increased synchronously by 16 units, which is what one would expect of formation of their mono-epoxy derivatives. For example, an ion m/z 369 became ion m/z 385, 619 became 635, 633 became 649, 647 became 663.5, etc. Although signals of the epoxides may be isobaric to some (M+1) and (M+2) isotopic peaks of normal meibum components, these newly formed epoxides were obviously not present in the intact untreated meibum. Corroborating this observation were missing product ions of epoxy-FA in MS2 experiments discussed above (Figs. 4, 8, 9).
Fig. 10.
Human meibum before (A) and after (B) epoxidation with peroxyacetic acid.
Very long-chain (O-acyl)-ω-hydroxy fatty acids
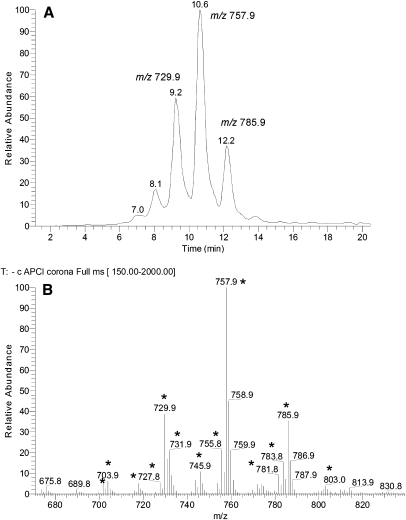
Another group of WE-related but poorly characterized compounds was identified in meibum. These compounds were detected in the negative ion mode as a series of HPLC peaks with ReT between 8 and 12 min with ions m/z 729.9, 757.9, and 785.9 being the major ones (Fig. 11). A range of related compounds with varying degrees of unsaturation, chain lengths, and locations of their hydroxy groups were detected as well. As these compounds were visible as anions, they either: 1) had an easily ionizable acidic group(s) in their structures; 2) formed adducts with acidic components of the HPLC eluent (i.e., formate), or 3) formed inorganic anion adducts (most likely, Cl−). However, the last two possibilities were ruled out as the signals were detected in various tested solvents using various MS ion sources (atmospheric pressure chemical ionization, electrospray ionization, and photoionization). Thus, the most likely explanation was the acidic nature of the compounds.
Fig. 11.
RP HPLC-MS analysis of human meibum in the negative ion mode. A: TIC chromatogram. B: Expanded MS spectrum of the peaks shown in (A). Major compounds are labeled with asterisk.
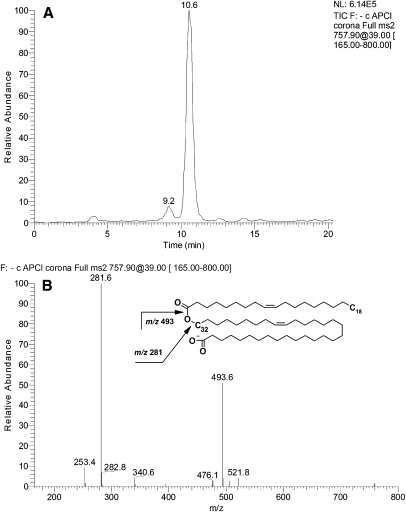
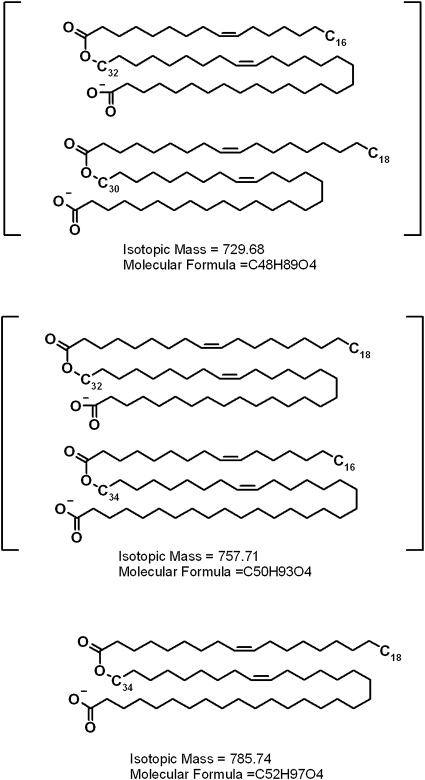
To determine their structures, the compounds m/z/ 729, 757, and 785 were fragmented in MS2 experiments (Fig. 12). All three major compounds produced a strong signal of a product ion m/z 281. The other major product ions had m/z values of 465, 493, and 521 for compounds m/z 729, 757, and 785, respectively. Interestingly, compound m/z 729 showed another pair of product ions, 253 and 493, which apparently added up to the same parent ion as 281 and 465. This was a clear sign of the presence of at least two isobaric compounds in the mixture. We hypothesized that these novel compounds might be related to the relatively poorly characterized families of di- and triesters of various kinds postulated by Nicolaides and Santos (19), specifically, the (O-acyl)-ω-hydroxy FA (OAHFA) family. Interestingly, OAHFAs were not listed in the latter publication. To test this hypothesis, we needed a standard for such compound to be analyzed by HPLC and fragmented the same way the meibum compounds were. Unfortunately, to the best of our knowledge, no such standard was/is available from any manufacturer. Thus, we synthesized a related, albeit shorter, compound, (O-oleoyl)-16-hydroxypalmitic acid, in-house. Under the conditions of RP-HPLC analysis, its ReT was an expectedly short ∼4.2–4.5 min, which reflected its lower hydrophobicity compared with its meibum counterparts. However, in a negative ion mode MS2 experiment, (O-oleoyl)-16-hydroxypalmitic acid fragmented exactly like the meibum compounds did, producing just two major fragments, m/z 281 and 271 (Fig. 13). These product anions were clearly those of oleic acid and 16-hydroxypalmitic acid. This finding allowed us to assign the following structures to the meibum compounds: (O-oleoyl)-30-hydroxy-triacontenoic and (O-palmitoleoyl)-32-hydroxy-dotriacontenoic acid (both m/z 729), (O-oleoyl)-32-hydroxy-dotriacontenoic acid and (O-palmitoleoyl)-34-hydroxy-tetratriacontenoic acid (both m/z 757), and (O-oleoyl)-34-hydroxy-tetratriacontenoic acid (m/z 785) (Scheme 4). The other members of the OAHFA family varied by the carbon chain length, the number of double bonds, and possibly, their location (Table 2). Experiments are in progress to elucidate their structure in more detail.
Fig. 12.
Structural analysis of compound m/z 757 in the negative ion mode. A: SIM of ion m/z 757 showed the presence of one major and one minor RP HPLC peak. B: Fragmentation pattern of ion m/z 757, its proposed structure, and identified fragments.
Fig. 13.
RP HPLC-MS analysis of an authentic (O-oleoyl)-16-hydroxypalmitic acid. A: SIM of ion m/z 535 showed the presence of one major RP HPLC peak (ReT ∼4.4 min). B: Zoom scan MS spectrum of the compound. C: Fragmentation pattern of ion m/z 535, its proposed structure, and identified fragments: m/z 281 (oleate) and 271 (16-hydroxypalmitate).
Scheme. 4.
A family of major very long chain (O-acyl)-ω-hydroxy FA detected in human meibum. The exact, cis, trans geometry of the double bonds of ω-hydroxy-FA moieties and their localization are yet to be established.
TABLE 2.
Ten major (O-acyl)-ω-hydroxy FA of human meibum detected in the negative ion mode
| Detected m/z, (M-H)− | Relative Intensity of MS Signals | OAHFA Anion, (M-H)−, CnH2n-7O4 | Proposed Structures, (O-FA1)-(ω−hydroxy-FA2)a | Theoretical m/z, (M-H)− |
|---|---|---|---|---|
| 701.8 | 0.058 | C46H85O4 | 701.6 | |
| 703.9 | 0.063 | C46H87O4 | 703.7 | |
| 729.8 | 0.387 | C48H89O4 | (O-C16:1)-ωC32:1 (O-C18:1)-ωC30:1 | 729.7 |
| 743.8 | 0.067 | C49H91O4 | 743.7 | |
| 745.8 | 0.107 | C49H93O4 | 745.7 | |
| 755.7 | 0.214 | C50H91O4 | 755.7 | |
| 757.8 | 1b | C50H93O4 | (O-C16:1)-ωC34:1 (O-C18:1)-ωC32:1 | 757.7 |
| 781.7 | 0.091 | C52H93O4 | 781.7 | |
| 783.8 | 0.154 | C52H95O4 | 783.7 | |
| 785.8 | 0.355 | C52H97O4 | (O-C18:1)-ωC34:1 | 785.7 |
FA1, a shorter chain FA; FA2, a very long-chain ω-hydroxy FA; structures confirmed by HPLC and MS/MS analyses.
Most intense signal.
DISCUSSION
Earlier, we presented our initial results on the characterization of the major nonpolar lipid species found in human meibum (9–11, 15) and aqueous tears (15). These results confirmed independent observations from a number of research laboratories on the presence of large amounts of CEs and WEs in human meibum (18–23). However, we could not concur on the reported findings on oleamide (24), Cer, and other typical polar lipids (25, 26). If these compounds in question are in fact present in normal meibum, their molar ratio to other meibum lipids (primarily, WE and CE) is extremely small and it seems to be unlikely that they could possibly play any structural role in the normal TF. Recently, we discussed in detail CE found in human meibum (11). Thus, the focus of this article is on the second major group of lipids in meibum, namely WE and related compounds.
To characterize WE, we chose RP HPLC. Unlike the NP HPLC technique used to characterize lipid classes [Fig. 1 and (9, 10)], RP HPLC was used to separate the individual members of the WE class based on their hydrophobicity, i.e. chain length, unsaturation, degree of oxidation, etc. Indeed, RP HPLC had provided separation of various CE species (11) and was effective in separating WE as well (Fig. 2). A commendable separation of WE species achieved in these experiments, the ability to simultaneously monitor all of the compounds of interest using their characteristic m/z values and plot them as individual chromatograms retrospectively, and the ability to compare the ReT of meibum compounds with the ReT of authentic lipid standards (where available) (Figs. 2–5, 7), plus the MSn capabilities of the ion trap mass spectrometer (Figs. 6, 9, 10) made it possible to characterize WE and related compounds to a much better degree than before. It became clear that unsaturated WEs are predominantly based on a series of C18:n-based FA (n = 1–4) with FAl moiety ranging from ∼C17:0 to C32:0 (Table 1). Note that WEs can be grouped in sub-families in different ways based on either their chain length or unsaturation. Figs. 1–5 clearly demonstrate the astounding complexity of this class of compounds alone. However, three more factors need to be discussed: the locations of the double bonds, their cis,trans geometry, and branching. The location of the double bonds could not be evaluated in our current experiments and would require chemical modification of the samples, as described by Thomas et al. (27), or sample hydrolysis or transesterification with subsequent silylation and ozonation of the hydrolyzate followed by gas-liquid chromatography (GLC) or GC-MS (28). The experiments are in progress to address these issues. In any case, it seems likely that a change in the location of a double bond in a molecule would have some impact on the ReT of the isomeric compound. The cis,trans geometry of the double bonds was also shown to have a noticeable effect on the ReT of the corresponding geometrical isomers (29). Thus, we analyzed the chromatographic patterns of selected ion families (as an example, ions m/z 641, 643, 645, and 647 were considered; other tested families of ions followed the trend) and found that the number of detected HPLC peaks for a given ion was proportional to the degree of unsaturation of the corresponding compound (Fig. 7): compounds with 1, 2, 3, and 4 double bonds produced, respectively, 1, 2, 3, and 4 HPLC peaks. The number of double bonds in a molecule and its cis/trans isomers ratio both influence the physiochemical properties of the compounds: the higher they are, the lower the melting point of the mixture. With an increase in the degree of unsaturation, the fragmentation pattern of the compounds also changes, indicating lesser stability of the more unsaturated compounds (Fig. 6; only two extreme cases for 2 and 4 double bonds are presented). The mechanism for the increase in the number of isoforms of the same compound remains unclear and could include peculiarities of their biosynthesis and/or postbiosynthetic isomerization.
Theoretically, the presence of one or more double bonds in their structure may make lipids susceptible to enzymatic and/or nonenzymatic (per)oxidation. One of the possible types of oxidation products reported earlier is the formation of lipid epoxides (18). Epoxides can be formed in vivo enzymatically or nonenzymatically (30). Under normal conditions, direct attachment of oxygen to the double bonds is unlikely (31). However, their enzymatic epoxidation through cytochrome P450, for example, is a possibility. CYP2B12 transcripts were found in the mouse skin and meibomian glands (32, 33). Suzuki et al. (33) studied the cytochrome P450 gene regulation by 17β-estradiol in mouse meibomian glands. Its CYP1B1 variant was localized in various human ocular structures, including cornea (34). Yet, our experiments did not produce any evidence of the epoxides of free FA and WE being a noticeable part of human meibum (Table 1 and Fig. 10). This was in contrast with earlier findings of Shine et al. (18) on the meibum epoxides of unclear origin and those of Sullivan et al. (12) who reported epoxides of DAG in human meibum. However, very little information on how the putative epoxides of DAG were identified and analyzed is presented in (12) and no actual chemical structures were proposed in either of the reports. Moreover, we have not detected noticeable amounts of regular DAG in human meibum (9, 10, 15), which makes the existence of their epoxides even less likely. Shine et al. (18) reported epoxides of C18 and C20 acids as prominent meibum lipids. However, those samples were heavily chemically manipulated and could have undergone inadvertent spontaneous postextraction epoxidation, whereas our current findings are based on the direct analyses of unmanipulated intact lipid samples, which greatly reduces the chances of their oxidation and isomerization.
Finally, an interesting but poorly characterized group of lipids, namely very long-chain OAHFA, is of special interest. Their existence was originally implied (but never claimed) by Nicolaides and Santos (19) 25 years ago. The relatively high molecular weights of OAHFA made it impossible to analyze them directly by GLC-MS or GC-MS, the earlier standard analytical techniques, without hydrolyzing the compounds first. Unfortunately, by doing so, all the information on the intact compounds is lost and their structures (i.e., the exact combinations of their detected hydrolyzed fragments) cannot be reconstructed. The demonstrated diversity of these compounds (see Scheme 4 and Fig. 11) made the earlier attempts a difficult but courageous adventure. Earlier, we reported the presence of the then-unidentified species in meibum (10). The absence of chemical standards did not allow us to positively identify the compounds. Once a related compound, (O-oleoyl)-16-hydroxypalmitic acid, had been synthesized in our laboratory, and its fragmentation pattern had been determined, OAHFA identification became possible. More than 20 related lipid species were observed in direct experiments (Fig. 11 and Table 2). However, this number could possibly be much greater if one considers the (quite possible) existence of multiple isobaric compounds (Scheme 4) of the same nature.
What physiological significance may these compounds have? In skin, they are related to a family of very long-chain (O-acyl)-Cers (35). Recently, the latter were linked to a vital function of skin permeability (35, 36). However, neither (O-acyl)-Cer nor regular Cer were found in our studies as the major meibum components. Thus, OAHFA should play some role in meibum and tear film lipid layer on their own. A striking difference between OAHFAs and the meibum lipids of regular type (WEs, CEs, and TAGs) is that OAHFAs are carrying an overall negative charge due to their free carboxyl groups whereas the other lipids are electroneutral and very hydrophobic. Indeed, the carboxyl group of a typical aliphatic compound has a pKa value of around 4.6, which may slightly change one way or the other, depending on the chain length and the solvent. However, under the conditions of physiological pH [∼7.5 for tears (37)] a large part of these groups is ionized, thus, providing them amphiphilic and surfactant properties. With the absence of appreciable amounts of phospholipids, Cers, monoacylglycerols, and DAG in meibum (9–11, 15), OAHFAs are perfect candidates for fulfilling the role of a somewhat elusive amphiphilic barrier between the postulated very thick TF nonpolar lipid sub-layer comprised of WE, CE, TAG, etc., and the underlaying aqueous layer, which is in contact with the cornea (38). Thus, a finely tuned balance between OAHFA and other less polar components of the human tear film lipid layer could play a role in its stabilization. Future work will address this hypothesis.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- Cer
- ceramide
- DAG
- diacylglycerol
- FAl
- fatty alcohol
- MGS
- meibomian gland secretion
- NP HPLC
- normal-phase HPLC
- OAHFA
- (O-acyl)-ω-hydroxy fatty acid
- RP HPLC
- reverse-phase HPLC
- ReT
- retention time
- SIM
- selected ion monitoring
- TAG
- triacylglycerol
- TF
- tear film
- TIC
- total ion chromatogram
- WE
- wax ester
REFERENCES
- 1.Tiffany J. M.2008. The normal tear film. Dev. Ophthalmol. 41: 1–20 [DOI] [PubMed] [Google Scholar]
- 2.Bron A. J., Tiffany J. M., Gouveia S. M., Yokoi N., Voon L. W. 2004. Functional aspects of the tear film lipid layer. Exp. Eye Res. 78: 347–360 [DOI] [PubMed] [Google Scholar]
- 3.Bron A. J., Tiffany J. M. 1998. The meibomian glands and tear film lipids-structure, function, and control. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2 438: 281–295 [DOI] [PubMed] [Google Scholar]
- 4.Sullivan D. A., Yamagami H., Liu M., Steagall R. J., Schirra F., Suzuki T., Krenzer K. L., Cermak J. M., Sullivan R. M., Richards S. M., et al. 2002. Sex steroids, the meibomian gland and evaporative dry eye. Adv. Exp. Med. Biol. 506: 389–399 [DOI] [PubMed] [Google Scholar]
- 5.Ohashi Y., Dogru M., Tsubota K. 2006. Laboratory findings in tear fluid analysis. Clin. Chim. Acta. 369: 17–28 [DOI] [PubMed] [Google Scholar]
- 6.Foulks G. N.2007. The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 52: 369–374 [DOI] [PubMed] [Google Scholar]
- 7.McCulley J. P., Shine W. E. 2001. The lipid layer: the outer surface of the ocular surface tear film. Biosci. Rep. 21: 407–418 [DOI] [PubMed] [Google Scholar]
- 8.Mathers W. D., Lane J. A. 1998. Meibomian gland lipids, evaporation, and tear film stability. Adv. Exp. Med. Biol. 438: 349–360 [DOI] [PubMed] [Google Scholar]
- 9.Butovich I. A., Uchiyama E., Di Pascuale M. A., McCulley J. P. 2007. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 42: 765–776 [DOI] [PubMed] [Google Scholar]
- 10.Butovich I. A., Uchiyama E., McCulley J. P. 2007. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J. Lipid Res. 48: 2220–2235 [DOI] [PubMed] [Google Scholar]
- 11.Butovich I. A.2009. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J. Lipid Res. 50: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan B. D., Evans J. E., Krenzer K. L., Reza Dana M., Sullivan D. A. 2000. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J. Clin. Endocrinol. Metab. 85: 4866–4873 [DOI] [PubMed] [Google Scholar]
- 13.Krenzer K. L., Dana M. R., Ullman M. D., Cermak J. M., Tolls D. B., Evans J. E., Sullivan D. A. 2000. Effect of androgen deficiency on the human meibomian gland and ocular surface. J. Clin. Endocrinol. Metab. 85: 4874–4882 [DOI] [PubMed] [Google Scholar]
- 14.Borchman D., Foulks G. N., Yappert M. C., Tang D., Ho D. V. 2007. Spectroscopic evaluation of human tear lipids. Chem. Phys. Lipids. 147: 87–102 [DOI] [PubMed] [Google Scholar]
- 15.Butovich I. A.2008. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest. Ophthalmol. Vis. Sci. 49: 3779–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith K. R., Thiboutot D. M. 2008. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J. Lipid Res. 49: 271–281 [DOI] [PubMed] [Google Scholar]
- 17.Feingold K. R.2007. The importance of lipids in cutaneous function. J. Lipid Res. 48: 2529–2530 [DOI] [PubMed] [Google Scholar]
- 18.Shine W. E., McCulley J. P. 1993. Role of wax ester fatty alcohols in chronic blepharitis. Invest. Ophthalmol. Vis. Sci. 34: 3515–3521 [PubMed] [Google Scholar]
- 19.Nicolaides N., Santos E. C. 1985. The di- and triesters of the lipids of steer and human meibomian glands. Lipids. 20: 454–467 [DOI] [PubMed] [Google Scholar]
- 20.Nicolaides N., Kaitaranta J. K., Rawdah T. N., Macy J. I., Boswell F. M., 3rd, Smith R. E. 1981. Meibomian gland studies: comparison of steer and human lipids. Invest. Ophthalmol. Vis. Sci. 20: 522–536 [PubMed] [Google Scholar]
- 21.Shine W. E., McCulley J. P. 1991. The role of cholesterol in chronic blepharitis. Invest. Ophthalmol. Vis. Sci. 32: 2272–2280 [PubMed] [Google Scholar]
- 22.Shine W. E., McCulley J. P. 2000. Association of meibum oleic acid with meibomian seborrhea. Cornea. 19: 72–74 [DOI] [PubMed] [Google Scholar]
- 23.Joffre C., Souchier M., Gregoire S., Viau S., Bretillon L., Acar N., Bron A. M., Creuzot-Garcher C. 2008. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br. J. Ophthalmol. 92: 116–119 [DOI] [PubMed] [Google Scholar]
- 24.Nichols K. K., Ham B. M., Nichols J. J., Ziegler C., Green-Church K. B. 2007. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest. Ophthalmol. Vis. Sci. 48: 34–39 [DOI] [PubMed] [Google Scholar]
- 25.Shine W. E., McCulley J. P. 2003. Polar lipids in human meibomian gland secretions. Curr. Eye Res. 26: 89–94 [DOI] [PubMed] [Google Scholar]
- 26.Shine W. E., McCulley J. P. 2004. Meibomianitis: polar lipid abnormalities. Cornea. 23: 781–783 [DOI] [PubMed] [Google Scholar]
- 27.Thomas M. C., Mitchell T. W., Harman D. G., Deeley J. M., Nealon J. R., Blanksby S. J. 2008. Ozone-induced dissociation: elucidation of double bond position within mass-selected lipid ions. Anal. Chem. 80: 303–311 [DOI] [PubMed] [Google Scholar]
- 28.Butovich I. A., Hamberg M., Radmark O. 2005. Novel oxylipins formed from docosahexaenoic acid by potato lipoxygenase–10(S)-hydroxydocosahexaenoic acid and 10,20-dihydroxydocosahexaenoic acid. Lipids. 40: 249–257 [DOI] [PubMed] [Google Scholar]
- 29.Butovich I. A., Reddy C. C. 2001. Enzyme-catalyzed and enzyme-triggered pathways in dioxygenation of 1-monolinoleoyl-rac-glycerol by potato tuber lipoxygenase. Biochim. Biophys. Acta. 1546: 379–398 [DOI] [PubMed] [Google Scholar]
- 30.Schneider C., Boeglin W. E., Yin H., Porter N. A., Brash A. R. 2008. Intermolecular peroxyl radical reactions during autoxidation of hydroxy and hydroperoxy arachidonic acids generate a novel series of epoxidized products. Chem. Res. Toxicol. 21: 895–903 [DOI] [PubMed] [Google Scholar]
- 31.Yamada T., Takai T., Rhode O., Mukaiyama T. 1991. Direct epoxidation of olefins catalyzed by nickel(Ii) complexes with molecular-oxygen and aldehydes. Bull. Chem. Soc. Jpn. 64: 2109–2117 [Google Scholar]
- 32.Keeney D. S., Skinner C., Wei S., Friedberg T., Waterman M. R. 1998. A keratinocyte-specific epoxygenase, CYP2B12, metabolizes arachidonic acid with unusual selectivity, producing a single major epoxyeicosatrienoic acid. J. Biol. Chem. 273: 9279–9284 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T., Schirra F., Richards S. M., Jensen R. V., Sullivan D. A. 2008. Estrogen and progesterone control of gene expression in the mouse meibomian gland. Invest. Ophthalmol. Vis. Sci. 49: 1797–1808 [DOI] [PubMed] [Google Scholar]
- 34.Doshi M., Marcus C., Bejjani B. A., Edward D. P. 2006. Immunolocalization of CYP1B1 in normal, human, fetal and adult eyes. Exp. Eye Res. 82: 24–32 [DOI] [PubMed] [Google Scholar]
- 35.McMahon A., Butovich I. A., Mata N. L., Klein M., Ritter R., 3rd, Richardson J., Birch D. G., Edwards A. O., Kedzierski W. 2007. Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Mol. Vis. 13: 258–272 [PMC free article] [PubMed] [Google Scholar]
- 36.Zuo Y., Zhuang D. Z., Han R., Isaac G., Tobin J. J., McKee M., Welti R., Brissette J. L., Fitzgerald M. L., Freeman M. W. 2008. ABCA12 maintains the epidermal lipid permeability barrier by facilitating formation of ceramide linoleic esters. J. Biol. Chem. 283: 36624–36635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer F. H., Wiederholt M. 1982. Human precorneal tear film pH measured by microelectrodes. Graefes Arch. Clin. Exp. Ophthalmol. 218: 168–170 [DOI] [PubMed] [Google Scholar]
- 38.McCulley J. P., Shine W. 1997. A compositional based model for the tear film lipid layer. Trans. Am. Ophthalmol. Soc. 95: 79–88 (discussion 88–93) [PMC free article] [PubMed] [Google Scholar]