Abstract
To determine the relative contribution of obesity and/or insulin resistance (IR) in the development of dyslipidemia in chronic kidney disease (CKD), we investigated the transport of apolipoprotein (apo) B-100 in nonobese, nondiabetic, nonnephrotic CKD subjects and healthy controls (HC). We determined total VLDL, VLDL1, VLDL2, intermediate density lipoprotein (IDL), and LDL-apoB-100 using intravenous D3-leucine, GC-MS, and multicompartmental modeling. Plasma apoC-III and apoB-48 were immunoassayed. In this case control study, we report higher plasma triglyceride, IDL-, VLDL-, VLDL1-, and VLDL2-apoB-100 concentrations in CKD compared with HC (P < 0.05). This was associated with decreased fractional catabolic rates [FCRs (pools/day)] [IDL:CKD 3.4 (1.6) vs. HC 5.0 (3.2), P < 0.0001; VLDL:CKD 4.8 (5.2) vs. HC 7.8 (4.8), P = 0.038; VLDL1:CKD 10.1 (8.5) vs. HC 29.5 (45.1), P = 0.007; VLDL2:CKD 5.4 (4.6) vs. HC 10.4 (3.4), P = 0.001] with no difference in production rates. Plasma apoC-III and apoB-48 were significantly higher in CKD (P < 0.001) and both correlated with impaired FCRs of VLDL, VLDL1, and VLDL2 apoB-100 (P < 0.05). In CKD, apoC-III concentration was the only independent predictor of clearance defects in VLDL and its subfractions. Moderate CKD in the absence of central adiposity and IR is associated with mild hypertriglyceridemia due to delayed catabolism of triglyceride rich lipoproteins, IDL, and VLDL, without changes in production rate. Altered apoC-III metabolism may contribute to dyslipidemia in CKD, and this requires further investigation.
Keywords: lipids, kinetic analysis, insulin resistance, central adiposity
Cardiovascular (CV) morbidity and mortality is increased in patients with mild renal dysfunction (1). While the precise mechanisms for this increase in CV event are unknown, both traditional and novel CV risk factors common to both disease processes have been implicated. Renal dyslipidemia is characterized by raised triglyceride, low HDL, and normal total cholesterol concentration. However, dysregulation of lipoprotein metabolism can develop early in renal disease with alteration in apolipoprotein concentrations in spite of normal plasma lipid levels (2).
Raised plasma triglyceride concentration is now recognized as an independent risk factor for cardiovascular disease (CVD) in nonrenal subjects (3) and may reflect the direct atherogenic potential of triglyceride-rich lipoproteins (TRLs), particularly VLDLs and intermediate density lipoproteins (IDLs) (4). VLDL and IDL apolipoprotein (apo) B-100 accumulation has been reported in kinetic turnover studies of dialysis patients (5–7) and in obese chronic kidney disease (CKD) patients not yet on dialysis (8). However, the mechanism for altered apoB-100 metabolism in CKD remains uncertain with inconsistent reports of either delayed catabolism alone (7) or combination of reduced clearance and overproduction of VLDL apoB-100 particles (8). Dyslipidemia of CKD is phenotypically similar to that seen in the metabolic syndrome and obesity. Insulin resistance (IR), which is evident in early CKD (9, 10) and central adiposity, may therefore contribute to the observed dyslipidemia in these populations. Studies in hemodialysis (11) and nonrenal subjects (12) have demonstrated a role for IR and adiposity in the development of abnormal lipoproteins particles. Furthermore, several kinetic studies have shown that IR increases FFA availability, which then stimulates the release of large triglyceride-rich VLDL1 particles without a change in the small dense cholesterol-rich VLDL2 subfraction secretion (13, 14).
Inhibitors of lipolytic enzymes, such as plasma apoC-III, inflammation, oxidative modification, and carbamoylation of apolipoproteins, may also contribute to the development of dyslipidemia in CKD. The increased plasma apoC-III of CKD (15) delays the catabolism of TRL by inhibiting the action of lipolytic enzymes (i.e., LPL and HL) (16) and interfering with apoE-mediated binding to receptors, which impairs hepatic remnant clearance (17). Inflammation is common in CKD and has been associated with the development of an abnormal lipid profile (18). Inflammation upregulates apoC-III gene expression (19) and promotes protein carbamoylation, which may interfere with lipoprotein cell receptor recognition resulting in reduced clearance (20).
The kinetics of VLDL (total and subfractions), IDL-, and LDL-apoB-100 in nonobese predialysis CKD subjects controlled for the presence of IR has not previously been studied. We hypothesized that patients with moderate CKD exhibit clearance defects in TRLs, particularly, total VLDL, VLDL1, and VLDL2 and IDL apoB-100 metabolism, with relatively preserved LDL kinetics. We aimed to investigate the transport of VLDL (total and subclasses), IDL, and LDL apoB-100 in nondiabetic, nonnephrotic stage 3 CKD subjects without central adiposity and/or IR. We also explored the association between plasma apoC-III, plasma apoB-48, inflammatory markers, and lipoprotein kinetics.
METHODS AND MATERIALS
Subjects
Ten CKD [estimated glomerular filtration rate (eGFR) 30–60 ml/min by modified Modification of Diet in Renal Disease Study equation] subjects and 20 healthy controls were recruited from the community and a department of nephrology (Royal Perth Hospital, Western Australia), respectively. Healthy controls were selected to match the CKD subjects based on age, sex, and waist circumference. Total VLDL, IDL, and IDL kinetic data from 12 of the 20 control subjects were obtained from previous studies carried out in our department (21); these studies did not include kinetic data on VLDL1 and VLDL2 metabolism. We recruited eight additional healthy, control subjects in whom we measured total VLDL and VLDL subfraction kinetics. Only seven of these healthy controls could be pair-matched, based on age, sex, and waist circumference, to seven CKD subjects, in whom we also had VLDL1 and VLDL2 kinetic data. All subjects were nonobese [body mass index (BMI) <30 mkg/m2] and nondiabetic and did not have central adiposity (waist circumference of <102 cm in men and <88 cm in women as defined by the National Cholesterol Education Program Adult Treatment Panel III criteria) (22). All underwent complete physical examination and laboratory investigations. Healthy control subjects had no clinical or laboratory evidence of renal disease or any chronic disorder that required the use of regular medications. CKD subjects were excluded if they had nephrotic syndrome (or proteinuria >3g/d), significant CV disease, hypothyroidism, abnormal liver function tests, and alcohol consumption of >30g alcohol/day and presence of the apolipoprotein E2/E2 genotype. ApoE genotyping was not carried out in the control subjects, but none of these exhibited a dyslipidemic phenotype. Secondary causes of dyslipidemia were excluded in the CKD subjects. None had a family history of premature CVD in a first-degree relative. CKD subjects on lipid modifying agents, who did not have significant CV or cerebrovascular disease, underwent a 4–6 week statin wash-out prior to the study. Six of the 10 CKD subjects who were on statin underwent a 4–6 week wash-out period. Two CKD subjects were smokers. Causes of renal disease included glomerulonephritis, interstitial nephritis, previous nephrectomy, adult polycystic kidney disease, vasculitis, and systemic lupus erythematosus. In one CKD subject, the cause of renal dysfunction was unknown.
The study was approved by the Royal Perth Hospital Ethics Committee and was performed in accordance with the ethical standards set by the Helsinki Declaration of 1975 (as revised in 1983). Written informed consent was obtained from all participants. This trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12605000726651).
Study design and clinical protocols
All subjects presented to the metabolic ward in the morning of the scheduled kinetic study after a 12 h fast. Body weight and height were measured, and arterial blood pressure was obtained using a Dinamap1846 SX/P monitor (Critikon, Tampa, FL). Dietary intake was assessed using a 24 h dietary diary and analyzed using DIET 4 Nutrient Calculation Software (Xyris Software, Queensland, Australia).
A Teflon cannula was inserted into an antecubital vein, and venous blood samples were collected into Vacutainer tubes for biochemical analysis. Participants received a single bolus of D3-leucine (5 mg/kg) intravenously over 2 min and allowed water only for the first 10 h of the study. Venous blood samples were taken at baseline and at 5, 10, 20, 30, and 40 min and 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, and 10 h after the isotope injection. Additional blood samples were obtained after a 12 h fast in the morning at 24, 48, 72, and 96 h. All samples were immediately centrifuged at 4°C for 15 min at 3500 rpm to obtain plasma and then stored at 4°C until processed.
Isotopic enrichment of apoB
Total VLDL, VLDL1, VLDL2, IDL, and LDL concentrations were measured using a modification of the method as described by Beghin et al. (23) In brief, total VLDL, IDL, and LDL were first isolated from 2 ml of plasma by ultracentrifugation (42,000 rpm, 23°C, 16 h; Beckman 50.4Ti fixed angle rotor; Beckman Instruments, Palo Alto, CA). VLDL1 and VLDL2 were isolated from total VLDL in a six-step discontinuous salt gradient of densities 1.0988–1.0588 g/ml (24). ApoB was precipitated and delipidated by isopropanol, hydrolyzed with 6 M HCl at 110°C for 16 h, and dried and derivatizated to an oxazolinone derivative (25). The free amino acid in plasma was isolated by cation-exchange chromatography (AG 50W-X8 resin; Bio-Rad, Richmond, CA) following removal of protein with 60% perchloric acid. Isotopic enrichment was determined using negative chemical ionization GC-MS with selected ion monitoring at m/z of 212 and 209. Tracer-to-tracee ratios were derived from isotopic ratios of each sample.
Biochemical analyses
All biochemical analyses were measured in samples obtained at baseline. Plasma cholesterol and triglyceride concentrations were determined by standard enzymatic methods (Hitachi, Tokyo, Japan; Roche Diagnostic, Mannheim, Germany). HDL-cholesterol was measured by an enzymatic colorimetric method using a commercial kit (Boehringer Mannheim, Mannheim, Germany), while nonHDL-cholesterol was calculated by subtracting HDL-cholesterol from total cholesterol. LDL-cholesterol was calculated by the Friedewald calculation. Plasma total apoA-I, apoA-II, and apoB concentrations were determined by immunonephelometry (Dade Behring, Illinois). Plasma apoC-III, apoC-II, and apoE were measured by the turbidimetric immunoassay kit (Wako Pure Chemicals Industries, Osaka, Japan). Plasma apoB-48 concentration was measured in a 12 h fasting plasma sample by sandwich enzyme-linked immunoassay using a validated commercially available anti-human apoB-48 monoclonal antibodies (interassay coefficient of variation 5%) (26). LPL mass was measured in preheparin plasma using sandwich enzyme immunoassay (LPL Elisa Daiichi; Daiichi Pure Chemicals, Tokyo, Japan) (coefficient of variation <10%). Plasma adiponectin was determined using an enzyme immunoassay kit (interassay coefficient of variation <7%, Quantikine; R and D Systems, Minneapolis, MN). Plasma insulin was measured by solid-phase two-site sequential chemiluminescent immunometric assay (Diagnostic Products, Los Angeles, CA) and glucose by hexokinase method (Hitachi, Tokyo, Japan). Insulin resistance was estimated by the homeostasis model assessment (HOMA) score [fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5]. High-sensitivity C-reactive protein was assayed using a high-sensitivity immunonephelometric method (Dade Behring Marburg, Marburg, Germany). Tumor necrosis factor-α and interleukin 6 were measured using enzyme immunoassay technique (Quantikine HS; R and D Systems). Genomic DNA was extracted from whole blood, and apoE genotypes were determined using the TaqMan assay on an ABI PRISM® 7000 sequence detection analyzer.
Kinetic analyses
Total VLDL, IDL, and LDL apoB-100 kinetics.
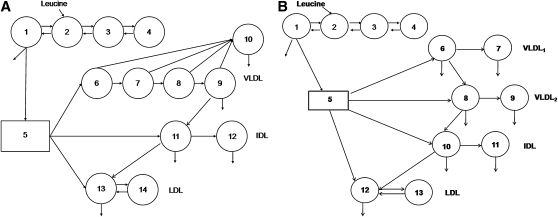
The SAAM II program (SAAM Institute, Seattle WA) was used for modeling the data. The details and assumptions of the model for the VLDL-, IDL-, and LDL-apoB were described previously (21). In brief, part of the model consists of a four-compartment subsystem that describes plasma leucine kinetics (compartments 1–4) (see Fig. 1, model 1a). This subsystem is connected to an intrahepatic delay compartment (compartment 5) that accounts for the time required for the assembly, synthesis, and secretion of apoB-100 into plasma. Five compartments (compartments 6–10) are used to describe the kinetics of apoB-100 in VLDL, which also takes into account the delipidation cascade (compartments 6–9) and a slowly turning over VLDL apoB-100 pool (compartment 10). The kinetics of plasma IDL was described by compartments 11 and 12. Compartment 11 represents the conversion of IDL to LDL (compartment 13) or its removal directly from plasma. Compartment 12 represents a slowly turning-over pool of IDL particles. LDL apoB is described by compartment 13 and an extravascular compartment, compartment 14. The fractional catabolic rates (FCRs) of VLDL, IDL, and LDL-apoB were derived from the model parameters giving the best fit. The corresponding production rates were calculated as the product of FCRs and pool size, which equals the plasma concentration multiplied by plasma volume. Plasma volume was estimated as 4.5% of body weight.
Fig. 1.
Multicompartmental models employed for analysis of total VLDL, IDL, and LDL apoB-100 kinetics (a) and VLDL1 and VLDL2 apoB-100 kinetics (b).
Total VLDL1, VLDL2, IDL, and LDL apoB-100 kinetics.
The SAAM II program (SAAM Institute, Seattle WA) was also used for modeling the data. This model consisted of 13 compartments (27), which includes a four-compartment subsystem that describes plasma leucine kinetics (compartments 1–4) (see Fig. 1, model 1b) This subsystem is connected to an intrahepatic delay compartment (compartment 5) that accounts for the synthesis and secretion of apoB-100 into plasma. Compartments 6 and 7 describe the kinetics of apoB-100 in the VLDL1, corresponding to fast and slowly turning-over VLDL1 apoB-100 pools, respectively. The kinetics of apoB-100 in VLDL2 are described by compartments 8 and 9, which also takes into account the fast and slowly turning over VLDL2 apoB-100 pools. IDL apoB-100 kinetics is described by compartments 10 and 11. IDL apoB-100 is either cleared from plasma or converted to LDL (compartment 12). LDL-apoB-100 is cleared from this compartment or exchanges with an extravascular LDL pool (compartment 13). VLDL1, VLDL2, IDL, and LDL apoB-100 FCRs were derived from the model parameters giving the best fit. The corresponding production rates were calculated as the product of FCR and pool size, as described above.
Statistical analyses
Statistical analyses were performed using SPSS version 15.0 (SPSS Software, Chicago IL). Data are presented as median and interquartile range (IQR) to account for the skewed nature of the parameters given the small sample size. Group comparisons were performed using the Mann-Whitney test. Statistical associations were examined using simple, stepwise, and multiple linear regression methods. Statistical significance was defined as P < 0.05.
RESULTS
Subject characteristics
The clinical and biochemical characteristics of the CKD subjects and healthy controls are shown in Table 1. The two groups were well matched for age, body weight, BMI, and waist circumference. Blood pressure, plasma glucose, insulin, HOMA score, adiponectin, and alanine transaminase did not differ significantly between groups (see Table 1). There was no significant between group difference in markers of inflammation, including high-sensitivity C-reactive protein, interleukin-6, and tumor necrosis factor-α (all P > 0.05). Of the 10 CKD subjects, six did not have the apoE2/E2 allele and four were heterozygous for the apoE2/E3. Average daily energy intake and the proportion of energy from protein, fat, carbohydrates, and alcohol were not different between groups. [CKD: total energy median 8,333 (IQR 3270) kJ, protein 19 (8)%, fat 35 (13)%, carbohydrates 39 (10)%; alcohol 2 (11)% vs. controls: total energy 7,215 (5,102) kJ, protein 20 (11)%, fat 32 (7)%, carbohydrates 50 (12)%, and alcohol 0 (4)%; all P > 0.05)].
TABLE 1.
Clinical and biochemical characteristics of CKD and control subjects
| CKD (n = 10) | Controls (n = 20) | P | |
|---|---|---|---|
| Age (years) | 57 (21) | 58 (10) | 0.98 |
| Male/female (n) | 7/3 | 14/6 | 0.90 |
| Body weight (kg) | 73.5 (25.9) | 73.9 (16.6) | 0.91 |
| Waist circumference (cm) | 93.5 (19.2) | 89.8 (16.1) | 0.59 |
| BMI (kg/m2) | 24.4 (5.5) | 23.6 (7.0) | 0.98 |
| Systolic blood pressure (mm Hg) | 126 (10) | 124 (19) | 0.85 |
| Diastolic blood pressure (mm Hg) | 72 (3) | 74 (11) | 0.71 |
| Serum creatinine (μmol/L) | 130 (56) | 78 (24) | <0.001 |
| Estimated glomerular filtration rate (ml/min) | 51 (17) | 89 (23) | <0.001 |
| Urine protein:creatinine ratio (mg/mmol) | 17.0 (12.5) | – | – |
| Glucose (mg/dl) | 90.0 (3.6) | 90.0 (16.2) | 1.0 |
| Insulin (μU/ml) | 6.7 (4.2) | 7.0 (4.4) | 0.85 |
| HOMA Score | 1.5 (1.1) | 1.5 (1.1) | 0.85 |
| Alanine transaminase (U/L) | 14.5 (8.5) | 18.5 (7.0) | 0.15 |
| Adiponectin (mg/L) | 5.6 (2.4) | 4.3 (2.8) | 0.53 |
| High sensitivity C-reactive protein | 2.7 (6.3) | 1.4 (1.3) | 0.27 |
| Interleukin-6 (pg/ml) | 1.0 (3.2) | 1.0 (1.2) | 0.85 |
| Tumor necrosis factor-α (pg/ml) | 3.0 (9.1) | 3.1 (4.2) | 0.56 |
Data are presented as median (IQR). To convert estimated glomerular filtration rate in ml/min to ml/s, multiply by 0.01667; glucose in mg/dl to mmol/L, multiply by 0.05551; insulin in μU/ml to pmol/L, multiply by 7.175.
As expected, the CKD group had significantly higher serum creatinine (sCr) and lower eGFR (P < 0.001) (see Table 1). CKD subjects had significantly higher serum triglyceride, apoB, apoC-III, and apoB-48 concentrations compared with healthy controls (P < 0.05). Since plasma apoC-II concentration was not different between groups, the ratio of apoC-II/apoC-III was significantly reduced in the CKD subjects (P < 0.05). Total cholesterol, LDL-cholesterol, HDL-cholesterol, apoA-I, apoA-II, apoE concentrations, and LPL mass were not significantly different between CKD and healthy control subjects (see Table 2).
TABLE 2.
Plasma lipid, lipoprotein, and apolipoprotein concentrations in CKD and control subjects
| Lipid, Lipoprotein, Apolipoprotein | CKD (n = 10) | Controls (n = 20) | P |
|---|---|---|---|
| Total cholesterol (mg/dl) | 218 (86) | 203 (70) | 0.18 |
| LDL cholesterol (mg/dl) | 133 (66) | 117 (43) | 0.91 |
| Triglycerides (mg/dl) | 124 (142) | 71 (53) | 0.004 |
| HDL cholesterol (mg/dl) | 59 (35) | 59 (35) | 0.95 |
| apoB (mg/dl) | 120 (40) | 92 (30) | 0.035 |
| apoA-I (mg/dl) | 160 (70) | 160 (50) | 0.62 |
| apoA-II (mg/dl) | 30 (7) | 30 (10) | 0.65 |
| apoC-II (mg/l) | 34.2 (26.7) | 32.4 (13.0) | 0. 71 |
| apoC-III (mg/l) | 200 (74) | 98 (34) | <0.0001 |
| apoC-II/C-III ratio | 0.21 (0.12) | 0.32 (0.2) | 0.001 |
| apoB-48 (mg/l) | 4.5 (2.2) | 2.2 (2.6) | <0.0001 |
| apoE (mg/l) | 62.2 (51.5) | 43.6 (52.8) | 0.21 |
| Lipoprotein lipase mass (ng/ml) | 51.0 (31.6) | 42.7 (41.0) | 0.81 |
Data are presented as median (IQR). To convert cholesterol, HDL-cholesterol. and LDL-cholesterol in mg/dl to mmol/L, multiply by 0.02586; triglycerides in mg/dl to mmol/L, multiply by 0.01129; apoC-III in mg/dl to mg/L, multiply by 10.
Table 3 shows plasma VLDL-, IDL-, and LDL-apoB-100 concentrations and corresponding kinetic parameters in the CKD subjects and healthy controls (as derived from model 1a in Fig. 1). Table 4 shows the plasma VLDL1 and VLDL2 apoB-100 concentrations and kinetic parameters in a subgroup of CKD subjects and pair-matched healthy controls (as derived from model 1b of Fig. 1). Serum triglyceride levels were significantly different between the subgroup of CKD (n = 7) and healthy control (n = 7) subjects with VLDL subfraction data [CKD: 150 (257) vs. HC 62 (35), P = 0.004]. Compared with controls, CKD subjects had significantly higher total IDL, VLDL, VLDL1, and VLDL2 apoB-100 concentrations. These kinetic changes were related to lower FCRs (P < 0.05) without a change in corresponding production rates. LDL concentration and kinetics were not significantly different between groups. There were no differences in the percentage of conversion rates of VLDL to IDL apoB, IDL to LDL apoB, VLDL1 to IDL apoB, VLDL1 to VLDL2 apoB, and VLDL2 to IDL apoB (Tables 3 and 4).
TABLE 3.
Kinetic estimates of total VLDL, IDL, and LDL apoB-100 in the CKD and control subjects
| CKD (n = 10) | Controls (n = 20) | P | |
|---|---|---|---|
| Plasma concentrations (mg/l) | |||
| VLDL apoB | 73.1 [17.2, 371.9] | 46.4 [23.7, 85.7] | 0.039 |
| IDL apoB | 86.5 [39.6, 224.0] | 55.2 [11.7, 135.2] | 0.006 |
| LDL apoB | 814.2 [581.6, 1070.7] | 839.9 [595.0, 1042.7] | 0.98 |
| FCR (pools/day) | |||
| VLDL apoB | 4.3 [0.63, 17.99] | 6.8 [4.3, 14.4] | 0.008 |
| IDL apoB | 3.4 [1.0, 4.3] | 5.0 [2.2, 21.6] | <0.0001 |
| LDL apoB | 0.39 [0.16, 0.49] | 0.45 [0.24, 0.76] | 0.31 |
| Production rate (mg/kg/day) | |||
| VLDL apoB | 14.4 [10.0, 31.0] | 17.0 [11.0, 25.5] | 0.18 |
| IDL apoB | 13.6 [7.2, 18.2] | 13.6 [9.6, 25.3] | 0.53 |
| LDL apoB | 12.7 [5.8, 22.0] | 14.9 [7.5, 24.4] | 0.13 |
| Conversion rate (%) | |||
| VLDL to IDL apoB | 71.7 [39.8, 96.5] | 70.4 [44.9, 99.9] | 0.71 |
| IDL to LDL apoB | 92.2 [60.6, 100.0] | 99.1 [64.4, 100.0] | 0.14 |
Kinetic data presented as median [minimum, maximum].
TABLE 4.
Kinetic estimates of VLDL1 and VLDL2 apoB-100 in the CKD and control subjects as derived from model 1b
| CKD (n = 7) | Controls (n = 7) | P | |
|---|---|---|---|
| Plasma concentrations (mg/l) | |||
| Triglyceride | 150 (257) | 62 (35) | 0.004 |
| VLDL1 apoB | 17.0 [8.2, 94.4] | 8.4 [5.4, 18.1] | 0.035 |
| VLDL2 apoB | 65.3 [35.3, 277.6] | 34.5 [18.3, 57.6] | 0.013 |
| IDL apoB | 105.2 [39.6, 224.0] | 59.3 [32.5, 80.1] | 0.013 |
| LDL apoB | 811.7 [585.1, 1050.8] | 857.1 [669.4, 1029.6] | 0.34 |
| FCR (pools/day) | |||
| VLDL1 apoB | 10.8 [2.0, 25.7] | 29.5 [13.3, 70.3] | 0.009 |
| VLDL2 apoB | 5.4 [1.1, 8.5] | 10.4 [7.5, 14.4] | 0.003 |
| IDL apoB | 3.3 [1.9, 5.1] | 6.4 [4.5, 9.0] | 0.003 |
| LDL apoB | 0.38 [0.20, 0.58] | 0.47 [0.36, 0.56] | 0.3 |
| Production rate (mg/kg/day) | |||
| VLDL1 apoB | 9.5 [5.6, 17.5] | 13.0 [9.0, 19.7] | 0.18 |
| VLDL2 apoB | 15.4 (7.4) | 15.0 (3.4) | 0.95 |
| IDL apoB | 16.1 [11.3, 22.0] | 16.8 [12.2, 18.7] | 0.85 |
| LDL apoB | 14.1 [7.3, 19.8] | 17.8 [12.3, 21.5] | 0.08 |
| Conversion rate (%) | |||
| IDL to LDL apoB | 83.6 [59.1, 100.0] | 96.6 [83.0, 99.3] | 0.18 |
| VLDL1 to IDL apoB | 96.3 [55.1, 97.8] | 68.9 [7.8, 98.6] | 0.085 |
| VLDL1 to VLDL2 apoB | 96.7 [55.1, 97.8] | 68.9 [7.8, 98.6] | 0.085 |
| VLDL2 to IDL apoB | 100 [95.9, 100.0] | 100 [ - ] | 0.32 |
Data are presented as median (IQR). Kinetic data presented as median [minimum, maximum].
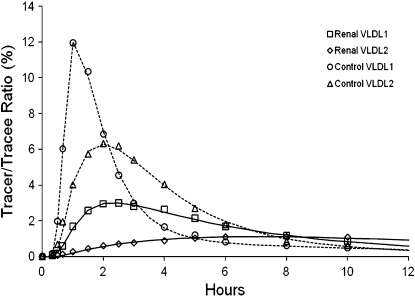
To confirm the validity of our kinetic analysis, Fig. 2 shows representative VLDL1 and VLDL2 apoB-100 tracer curves in a CKD subject with high apoC-III and a control subject with low apoC-III. This shows good agreement between the model fit and the VLDL1 and VLDL2 apoB-100 tracer data over a 12 h period. The average coefficient of variation [percentage ± SD (range)] for estimates of VLDL1 and VLDL2 FCRs in the CKD and control subjects are as follows: CKD: VLDL1, apoB-100 [4.39 ± 1.17% (2.98–6.12%)], VLDL2 apoB-100 [6.20 ± 1.91% (3.84–9.16%)]; controls: VLDL1 apoB-100 [2.93 ± 0.88% (2.08–4.41%)], VLDL2 apoB-100 [3.69 ± 0.94% (2.92–5.66%)].
Fig. 2.
Representative VLDL1 and VLDL2 apoB-100 tracer to trace ratios for CKD subject with high apoC-III and control subject with low apoC-III concentration.
There was a significant positive correlation between sCr and triglyceride concentrations in the CKD subjects (r = 0.71, P = 0.022). sCr and eGFR in the CKD subjects correlated with total VLDL apoB-100 FCR (sCr: r = −0.75, P = 0.013; eGFR r = 0.62, P = 0.056), but this did not reach statistical significance for the FCRs of VLDL1 apoB-100 (sCr: r = −0.63, P = 0.13; eGFR r = 0.48, P = 0.28) and VLDL2 apoB-100 (sCr r = −0.72, P = 0.06; eGFR r = 0.63, P = 0.13).
Plasma apoC-III and apoB-48 concentrations were significantly and inversely correlated with the total VLDL apoB-100 (apoC-III: r = −0.79, P = 0.007; apoB-48: r = −0.80, P = 0.005), FCR of VLDL1 apoB-100 (apoC-III: r = −0.76, P = 0.049, apoB-48: r = −0.75, P = 0.05), and FCR of VLDL2 apoB-100 (apoC-III: r = −0.80, P = 0.029, apoB-48: r = −0.86, P = 0.012) in the CKD subjects. Furthermore, there was positive correlation between plasma apoC-III and plasma apoB-48 concentration (r = 0.72, P = 0.02) in the CKD subjects. In a stepwise regression model that included apoB-48 and creatinine, apoC-III was the only significant independent predictor of the FCRs of total VLDL (adjusted R2 = 52%, P = 0.01), VLDL1 (adjusted R2 = 62%, P = 0.038), and VLDL2 (adjusted R2 = 65%, P = 0.029) apoB-100. ApoC-II/apoC-III ratio was only correlated with total VLDL apoB-100 FCR (r = 0.64, P = 0.045) but not with VLDL1 apoB-100 (r = 0.41, P = 0.36) nor VLDL2 apoB-100 (r = 0.612, P = 0.14) FCRs.
DISCUSSION
We have demonstrated that nonobese subjects with moderate CKD have mild hypertriglyceridemia that relates to higher plasma concentrations of total VLDL, VLDL1, VLDL2, and IDL apoB-100. This could be a consequence of impaired clearance of IDL apoB-100, and of total VLDL apoB-100, including its major subclasses, with no significant difference in corresponding production rates. We also demonstrated that elevated plasma apoC-III levels in CKD independently predicted clearance defects in total VLDL, VLDL1, and VLDL2 apoB-100 particles. Our kinetic inferences are based on multicompartmental models of VLDL (total and subfractions) apoB-100 that accords well with previous studies (28, 29).
IR contributes to changes in lipoprotein metabolism and development of dyslipidemia in subjects with diabetes mellitus and the metabolic syndrome. A previous kinetic study by Batista et al. (8) in predialysis obese CKD subjects reported higher VLDL and IDL apoB-100 concentrations, which related to a change in clearance but not a change in production rate. The presence of the metabolic syndrome and obesity could have contributed to uremic dyslipidemia in that study, but it was also confounded by a very small sample size of three predialysis CKD subjects. Hence, the relative contributions of visceral adiposity or IR could not be determined. Other radio-iodine tracer studies by Chan et al. (5) in 12 dialysis patients reported increase in VLDL apoB-100 concentration due to decreased VLDL apo-B100 fractional catabolic rate and increased production rate. A stable isotope study in 12 Japanese hemodialysis patients reported impaired catabolism of IDL apoB, delayed clearance and decreased production of LDL apoB, and no difference in the metabolism of VLDL apoB (6). In a study of peritoneal dialysis patients, Prinsen et al. (7) reported increased VLDL1 apoB-100 pool size due to increased synthesis and decreased clearance, while increased VLDL2 apoB-100 pool size was a consequence only of delayed catabolism. The increase in VLDL1 apoB-100 synthesis was partially explained by IR and increased FFA availability. Our data extends these kinetic findings in a larger group of nonobese, nondialyzed CKD subjects using a nonradioactive labeled technique. We observed clearance defects in total VLDL, VLDL1, and VLDL2 apoB-100 without changes in production rates, suggesting that VLDL (total and subclass) apoB-100 overproduction in CKD subjects is possibly driven by visceral adiposity and IR. Thus, the presence of IR may further exacerbate the lipid abnormalities associated with CKD.
Accumulation of intact and/or partially metabolized triglyceride-rich apoB-containing lipoproteins (TRLs) in CKD has been related to defective lipolytic degradation (30). Elevated total plasma apoC-III concentration and accumulation of apoC-III in VLDL particles can occur in early CKD (31). Compositional changes in apoB-100-contaning lipoproteins may render them less suitable substrates for lipolysis and result in reduced receptor mediated uptake (32). In this study, we noted that higher apoC-III concentration was associated with slower VLDL, VLDL1, and VLDL2 apoB-100 catabolism. This could mean that delayed catabolism of TRLs resulted in increased plasma apoC-III concentration or that elevated apoC-III led to reduced clearance of these TRLs.
There are several potential mechanisms for the increase apoC-III in CKD. Renal impairment is associated with excess sialylation of apoCIII, which may render these triglyceride-rich particles less suitable substrate for LPL (33). The kidney is partly involved in clearing apoC-III from plasma (33), so elevated apoC-III could be secondary to early CKD. In advanced CKD, where there is greater degree of inflammation (34), inflammatory mediators have been shown to increase the gene expression of apoC-III (35). However, markers of inflammation were not different in our CKD subjects who had early uncomplicated disease and were not insulin resistant. Thus, inflammation is an unlikely mechanism for the raised plasma apoC-III and delayed catabolism of VLDL apoB-100 and its subclasses in subjects with early CKD.
We did not observe a reduction in LPL mass that could account for the slower VLDL apoB-100 catabolism in the CKD subjects. While the value of measuring LPL mass may be questioned, LPL mass is highly correlated with postheparin lipolytic activity (r = 0.777, P < 0.001) (36). Collectively, our findings suggest that in early CKD subjects without IR, change in LPL mass is an unlikely contributing factor to defective catabolism of apoB-100-containing particles.
Protein carbamoylation (37) and accumulation of advanced glycation end products (38) in the setting of CKD can lead to alteration in protein structure, enzymatic activity, and binding to cell surface receptors. Covalent modification of LPL in the setting of uremia could decrease LPL activity. This may also involve modification of hepatic VLDL apoB-100 and remnant receptors and their ligands. Measurement of LPL activity in postheparin plasma may better clarify changes in in vivo LPL activity in CKD.
Dysregulation of intestinal lipoprotein metabolism with accumulation of intestinally derived apoB-48-containing lipoproteins is known to occur in diabetes, obesity, and insulin-resistant states (39, 40). Although raised plasma apoB-48 concentration has been reported in end-stage renal disease in nondiabetic patients (40), we report for the first time that plasma apoB-48 concentration is elevated in nondiabetic predialysis subjects and that this is inversely associated with VLDL apoB-100 FCR. This suggests that early CKD impairs the metabolism of remnant lipoproteins, which may involve competing with apoB-100-containing lipoproteins for common clearance pathways (21, 41). ApoB-48 concentration after a 12 h fast is found mainly in IDL and chylomicron remnants and is predictive of intestinally derived TRLs in the postprandial state. Further studies should investigate postprandial lipoprotein metabolism in early CKD.
Our study has several limitations. The observational design limits inferring causal associations. The small sample size could have confounded an adequate test of the null hypothesis, particularly with the VLDL1 and VLDL2 kinetics. There is also the potential for selection bias, given that control subjects were selected for having normal lipid levels, while there were no lipid criteria for inclusion in the CKD subjects. However, the cases were not selected for having dyslipidemia and secondary causes for dyslipidemia beyond CKD were excluded. Another potential limitation is the technical difficulty in isolating pure apoB-100 VLDL particles. However, it has previously been reported that in the fasted state, apoB-48 contributes to only ∼1% of total plasma VLDL apoB fraction (42). In our study, the contribution of apoB-48 to total VLDL mass was at most 6%, which is consistent with other reports (42). Additional studies of apoB-48 in lipoprotein fractions in the postprandial state could be of interest. The kinetics of VLDL triglyceride was not studied, and further isotopic investigations would be required to increase our understanding of lipoprotein metabolism in CKD. A strength of our study, however, is the exclusion of obese subjects with diabetes and proteinuria. This provided us with an opportunity to dissect the contribution of CKD alone to the dysregulation of lipoprotein metabolism.
We conclude that moderate CKD per se, in the absence of central adiposity, may lead to mild hypertriglyceridemia with accumulation of TRLs, particularly VLDL and IDL, and that this is related to defective catabolism. In CKD, this relationship may partly relate to abnormal metabolism of apoC-III or a hitherto unidentified uraemic toxin. The association between apoC-III and apoB-48 in the CKD subjects suggest that apoC-III may contribute to postprandial hypertriglyceridemia and accumulation of chylomicron remnants (43, 44). This needs further investigation. To clarify the relationship between apoC-III and VLDL kinetics, future ex vivo studies should examine the ability of VLDL isolated from CKD to act as a substrate for LPL.
Increased plasma triglyceride is increasingly recognized as an important risk factor for CVD in the general population (45) and may be an important target in CKD subjects who have the characteristic dyslipidemia. Increased VLDL1, a source of plasma triglyceride, promotes the formation of atherogenic small dense LDL particles and may contribute to the accumulation of remnant particles and to changes in HDL composition (29, 46). Modification of VLDL catabolism may be a new therapeutic target for reducing CV risk in early CKD.
Acknowledgments
The authors thank J. Ji and T.W.K. Ng for their laboratory assistance and Mary Anne Powell for nursing support. We thank all Royal Perth Hospital Nephrologists for their clinical support.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- BMI
- body mass index
- CKD
- chronic kidney disease
- CV
- cardiovascular
- CVD
- cardiovascular disease
- eGFR
- estimated glomerular filtration rate
- FCR
- fractional catabolic rate
- IDL
- intermediate density lipoprotein
- IQR
- interquartile range
- IR
- insulin resistance
- sCr
- serum creatinine
- TRL
- triglyceride-rich lipoprotein
This work was supported by a Pfizer Cardiovascular Lipid Research Grant. D.T.C. is in receipt of a National Health and Medical Research Council (NHMRC) Medical and Dental Postgraduate Research Scholarship. E.M.O. is a research fellow of the National Heart Foundation of Australia (PF 07P 3263). P.H.B. is a senior research fellow of the NHMRC. D.C.C. is supported by an NHMRC Career Development Award.
REFERENCES
- 1.Go A. S., Chertow G. M., Fan D., McCulloch C. E., Hsu C. Y. 2004. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson O., Attman P. O., Knight-Gibson C., Kron B., Larsson R., Mulec H., Weiss L., Alaupovic P. 1994. Lipoprotein abnormalities without hyperlipidaemia in moderate renal insufficiency. Nephrol. Dial. Transplant. 9: 1580–1585 [PubMed] [Google Scholar]
- 3.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S. M., Khaw K. T., Gudnason V. 2007. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 115: 450–458 [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg H. N.2002. New perspectives on atherogenesis: role of abnormal triglyceride-rich lipoprotein metabolism. Circulation. 106: 2137–2142 [DOI] [PubMed] [Google Scholar]
- 5.Chan P. C., Persaud J., Varghese Z., Kingstone D., Baillod R. A., Moorhead J. F. 1989. Apolipoprotein B turnover in dialysis patients: its relationship to pathogenesis of hyperlipidemia. Clin. Nephrol. 31: 88–95 [PubMed] [Google Scholar]
- 6.Ikewaki K., Schaefer J. R., Frischmann M. E., Okubo K., Hosoya T., Mochizuki S., Dieplinger B., Trenkwalder E., Schweer H., Kronenberg F., et al. 2005. Delayed in vivo catabolism of intermediate-density lipoprotein and low-density lipoprotein in hemodialysis patients as potential cause of premature atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25: 2615–2622 [DOI] [PubMed] [Google Scholar]
- 7.Prinsen B. H. C. M., Rabelink T. J., Romijn J. A., Bisschop P. H., De Barse M. M. J., De Boer J., van Haeften T. W., Barrett P. H. R., Berger R., de Sain-van der Velden M. 2004. A broad-based metabolic approach to study VLDL apoB100 metabolism in patients with ESRD and patients treated with peritoneal dialysis. Kidney Int. 65: 1064–1075 [DOI] [PubMed] [Google Scholar]
- 8.Batista M. C., Welty F. K., Diffenderfer M. R., Sarnak M. J., Schaefer E. J., Lamon-Fava S., Asztalos B. F., Dolnikowski G. G., Brousseau M. E., Marsh J. B. 2004. Apolipoprotein A-I, B-100, and B-48 metabolism in subjects with chronic kidney disease, obesity, and the metabolic syndrome. Metabolism. 53: 1255–1261 [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Muntner P., Hamm L. L., Fonseca V., Batuman V., Whelton P. K., He J. 2003. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J. Am. Soc. Nephrol. 14: 469–477 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S., Maesato K., Moriya H., Ohtake T., Ikeda T. 2005. Insulin resistance in patients with chronic kidney disease. Am. J. Kidney Dis. 45: 275–280 [DOI] [PubMed] [Google Scholar]
- 11.Lee P., O'Neal D., Murphy B., Best J. 1997. The role of abdominal adiposity and insulin resistance in dyslipidemia of chronic renal failure. Am. J. Kidney Dis. 29: 54–65 [DOI] [PubMed] [Google Scholar]
- 12.Goff D. C., Jr., D'Agostino R. B., Jr., Haffner S. M., Otvos J. D. 2005. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism. 54: 264–270 [DOI] [PubMed] [Google Scholar]
- 13.Adiels M., Boren J., Caslake M. J., Stewart P., Soro A., Westerbacka J., Wennberg B., Olofsson S. O., Packard C., Taskinen M. R. 2005. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 25: 1697–1703 [DOI] [PubMed] [Google Scholar]
- 14.Gill J. M. R., Brown J. C., Bedford D., Wright D. M., Cooney J., Hughes D. A., Packard C. J., Caslake M. J. 2004. Hepatic production of VLDL1 but not VLDL2 is related to insulin resistance in normoglycaemic middle-aged subjects. Atherosclerosis. 176: 49–56 [DOI] [PubMed] [Google Scholar]
- 15.Vaziri N. D.2006. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am. J. Physiol. Renal Physiol. 290: F262–F272 [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg H. N., Le N. A., Goldberg I. J., Gibson J. C., Rubinstein A., Wang-Iverson P., Norum R., Brown W. V. 1986. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J. Clin. Invest. 78: 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnon R., Sehayek E., Vogel T., Eisenberg S. 1991. Effects of exogenous apo E-3 and of cholesterol-enriched meals on the cellular metabolism of human chylomicrons and their remnants. Biochim. Biophys. Acta. 1085: 336–342 [DOI] [PubMed] [Google Scholar]
- 18.Irish A.1998. Cardiovascular disease, fibrinogen and the acute phase response: Associations with lipids and blood pressure in patients with chronic renal disease. Atherosclerosis. 137: 133–139 [DOI] [PubMed] [Google Scholar]
- 19.Gruber P. J., Torres-Rosado A., Wolak M. L., Leff T. 1994. Apo CIII gene transcription is regulated by a cytokine inducible NF-kappa B element. Nucleic Acids Res. 22: 2417–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z., Nicholls S. J., Rodriguez E. R., Kummu O., Horkko S., Barnard J., Reynolds W. F., Topol E. J., DiDonato J. A., Hazen S. L. 2007. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 13: 1176–1184 [DOI] [PubMed] [Google Scholar]
- 21.Chan D. C., Watts G. F., Redgrave T. G., Mori T. A., Barrett P. H. 2002. Apolipoprotein B-100 kinetics in visceral obesity: associations with plasma apolipoprotein C-III concentration. Metabolism. 51: 1041–1046 [DOI] [PubMed] [Google Scholar]
- 22.Stone N. J., Bilek S., Rosenbaum S. 2005. Recent national cholesterol education program adult treatment panel III update: adjustments and options. Am. J. Cardiol. 96: 53E–59E [DOI] [PubMed] [Google Scholar]
- 23.Beghin L., Duhal N., Poulain P., Hauw P., Lacroix B., Lecerf J. M., Bonte J. P., Fruchart J. C., Luc G. 2000. Measurement of apolipoprotein B concentration in plasma lipoproteins by combining selective precipitation and mass spectrometry. J. Lipid Res. 41: 1172–1176 [PubMed] [Google Scholar]
- 24.Lindgren F. T. JLHF. 1972. The isolation and quantitative analysis of serum lipoproteins. Blood Lipids and Lipoproteins. Nelson G. J., editor John Wiley & Sons, New York: 181–274 [Google Scholar]
- 25.Dwyer K. P., Barrett P. H. R., Chan D., Foo J. I., Watts G. F., Croft K. D. 2002. Oxazolinone derivative of leucine for GC-MS: a sensitive and robust method for stable isotope kinetic studies of lipoproteins. J. Lipid Res. 43: 344–349 [PubMed] [Google Scholar]
- 26.Uchida Y., Kurano Y., Ito S. 1998. Establishment of monoclonal antibody against human Apo B-48 and measurement of Apo B-48 in serum by ELISA method. J. Clin. Lab. Anal. 12: 289–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millar J. S., Maugeais C., Ikewaki K., Kolansky D. M., Barrett P. H. R., Budreck E. C., Boston R. C., Tada N., Mochizuki S., Defesche J. C., et al. 2005. Complete deficiency of the low-density lipoprotein receptor is associated with increased apolipoprotein B-100 production. Arterioscler. Thromb. Vasc. Biol. 25: 560–565 [DOI] [PubMed] [Google Scholar]
- 28.Chan D. C., Watts G. F., Barrett P. H. R., Beilin L. J., Redgrave T. G., Mori T. A. 2002. Regulatory effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B-100 kinetics in insulin-resistant obese male subjects with dyslipidemia. Diabetes. 51: 2377–2386 [DOI] [PubMed] [Google Scholar]
- 29.Packard C. J., Demant T., Stewart J. P., Bedford D., Caslake M. J., Schwertfeger G., Bedynek A., Shepherd J., Seidel D. 2000. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J. Lipid Res. 41: 305–318 [PubMed] [Google Scholar]
- 30.Lee D. M., Knight-Gibson C., Samuelsson O., Attman P. O., Wang C. S., Alaupovic P. 2002. Lipoprotein particle abnormalities and the impaired lipolysis in renal insufficiency. Kidney Int. 61: 209–218 [DOI] [PubMed] [Google Scholar]
- 31.Kimak E., Solski J. 2002. apoA- and apoB-containing lipoproteins and Lp(a) concentration in non-dialyzed patients with chronic renal failure. Ren. Fail. 24: 485–492 [DOI] [PubMed] [Google Scholar]
- 32.Arnadottir M., Thysell H., Dallongeville J., Fruchart J. C., Nilsson-Ehle P. 1995. Evidence that reduced lipoprotein lipase activity is not a primary pathogenetic factor for hypertriglyceridemia in renal failure. Kidney Int. 48: 779–784 [DOI] [PubMed] [Google Scholar]
- 33.Holdsworth G., Stocks J., Dodson P., Galton D. J. 1982. An abnormal triglyceride-rich lipoprotein containing excess sialylated apolipoprotein C-III. J. Clin. Invest. 69: 932–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenvinkel P., Alvestrand A. 2002. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin. Dial. 15: 329–337 [DOI] [PubMed] [Google Scholar]
- 35.Navarro M. A., Carpintero R., Acin S., Arbones-Mainar J. M., Calleja L., Carnicer R., Surra J.C., Guzman-Garcia M. A., Gonzalez-Ramon N., Iturralde M., et al. 2005. Immune-regulation of the apolipoprotein A-I/C-III/A-IV gene cluster in experimental inflammation. Cytokine. 31: 52–63 [DOI] [PubMed] [Google Scholar]
- 36.Tornvall P., Olivecrona G., Karpe F., Hamsten A., Olivecrona T. 1995. Lipoprotein lipase mass and activity in plasma and their increase after heparin are separate parameters with different relations to plasma lipoproteins. Arterioscler. Thromb. Vasc. Biol. 15: 1086–1093 [DOI] [PubMed] [Google Scholar]
- 37.Kraus L. M., Kraus A. P. 2001. Carbamoylation of amino acids and proteins in uremia. Kidney Int. 78 (Suppl): S102–S107 [DOI] [PubMed] [Google Scholar]
- 38.Miyata T., Sugiyama S., Saito A., Kurokawa K. 2001. Reactive carbonyl compounds related uremic toxicity (“carbonyl stress”). Kidney Int. 78 (Suppl.): S25–S31 [DOI] [PubMed] [Google Scholar]
- 39.Adeli K., Lewis G. F. 2008. Intestinal lipoprotein overproduction in insulin-resistant states. Curr. Opin. Lipidol. 19: 221–228 [DOI] [PubMed] [Google Scholar]
- 40.Hayashi T., Hirano T., Taira T., Tokuno A., Mori Y., Koba S., Adachi M. Remarkable increase of apolipoprotein B48 level in diabetic patients with end-stage renal disease. Atherosclerosis. 197: 154–158 [DOI] [PubMed] [Google Scholar]
- 41.Brunzell J. D., Hazzard W. R., Porte D., Jr., Bierman E. L. 1973. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J. Clin. Invest. 52: 1578–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campos H., Khoo C., Sacks F. M. 2005. Diurnal and acute patterns of postprandial apolipoprotein B-48 in VLDL, IDL, and LDL from normolipidemic humans. Atherosclerosis. 181: 345–351 [DOI] [PubMed] [Google Scholar]
- 43.Charlesworth J. A., Kriketos A. D., Jones J. E., Erlich J. H., Campbell L. V., Peake P. W. 2005. Insulin resistance and postprandial triglyceride levels in primary renal disease. Metabolism. 54: 821–828 [DOI] [PubMed] [Google Scholar]
- 44.Weintraub M., Burstein A., Rassin T., Liron M., Ringel Y., Cabili S., Blum M., Peer G., Iaina A. 1992. Severe defect in clearing postprandial chylomicron remnants in dialysis patients. Kidney Int. 42: 1247–1252 [DOI] [PubMed] [Google Scholar]
- 45.Hokanson J. E., Austin M. A. 1996. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J. Cardiovasc. Risk. 3: 213–219 [PubMed] [Google Scholar]
- 46.Taskinen M. R.2003. Diabetic dyslipidemia: from basic research to clinical practice. Diabetologia. 46: 733–749 [DOI] [PubMed] [Google Scholar]