Abstract
To understand how rapid changes in blood pressure can regulate Na-K-ATPase in the kidney cortex, we tested the hypothesis that a short-term (5 min) decrease in renal perfusion pressure will increase the amount of Na-K-ATPase in the plasma membranes by an angiotensin II-dependent mechanism. The abdominal aorta of anesthetized Sprague-Dawley rats was constricted with a ligature between the renal arteries, and pressure was monitored on either side during acute constriction. Left renal perfusion pressure was reduced to 70 ± 1 mmHg (n = 6), whereas right renal perfusion pressure was 112 ± 4 mmHg. In control (nonconstricted) rats (n = 5), pressure to both kidneys was similar at 119 ± 6 mmHg. After 5 min of reduced perfusion, femoral venous samples were taken for plasma renin activity (PRA) and the kidneys excised. The cortex was dissected, minced, sieved, and biotinylated. Lower perfusion left kidneys showed a 41% increase (P < 0.003) in the amount of Na-K-ATPase in the plasma membrane compared with right kidneys. In controls, there was no difference in cell surface Na-K-ATPase between left and right kidneys (P = 0.47). PRA was 57% higher in experimental animals compared with controls. To test the role of angiotensin II in mediating the increase in Na-K-ATPase, we repeated the experiments (n = 6) in rats treated with ramiprilat. When angiotensin-converting enzyme was inhibited, the cell surface Na-K-ATPase of the two kidneys was equal (P =0.46). These results confirm our hypothesis: rapid changes in blood pressure regulate trafficking of Na-K-ATPase in the kidney cortex.
Keywords: ouabain, sodium, blood pressure, renin
the proximal tubule reabsorbs approximately two-thirds of the filtered sodium, which contributes to the control of plasma blood volume and the regulation of blood pressure. The Na-K-ATPase is an active transport protein in the basolateral membrane that is widely recognized as playing a key role in sodium reabsorption. The hormone dopamine inhibits (4) and angiotensin II (ANG II) directly stimulates the short-term (≤15 min) activity of the Na-K-ATPase (1, 5, 6, 7, 21), which eventually affects blood pressure. In opossum kidney cells, a cell culture model of proximal tubules, which express the α1-subunit of the rat kidney Na-K-ATPase, the short-term stimulation of Na-K-ATPase activity by ANG II is mediated by the AT1 receptor, which stimulates PKC that in turn increases the phosphorylation of Na-K-ATPase and thereby triggers increased trafficking of Na-K-ATPase to the plasma membrane (5, 6).
Conversely, an acute increase in blood pressure reversibly inhibits Na-K-ATPase activity in the rat proximal tubule (23). This result suggests there could be a dynamic interaction between Na-K-ATPase activity and blood pressure, which is of physiological importance. If this is the case, then a rapid drop in blood pressure would be expected to stimulate Na-K-ATPase activity. Therefore, in this study we have tested the hypothesis that in vivo, a short-term (5 min) decrease in renal perfusion pressure will increase the amount of Na-K-ATPase in the plasma membranes of the kidney cortex by an ANG II-dependent mechanism.
Our experimental approach was to reduce the perfusion pressure to one kidney while maintaining the other contralateral kidney at a normal perfusion pressure. Our expectation was that reducing the perfusion pressure to one kidney would result in an increase in the amount of Na-K-ATPase in the plasma membranes of that kidney compared with the contralateral kidney without any change in the total amount of Na-K-ATPase in the cell. There should be no change in the total amount of Na-K-ATPase in the cell because the period of low pressure (5 min) is too short for there to be an increase in protein expression. We expected that the decrease in perfusion pressure would likely lead to an increase in the plasma concentrations of ANG II but also, more importantly, to a selective increase in the amount of ANG II in the low-pressure kidney compared with the contralateral kidney, as has been observed over a longer period of time (days) in a model of renovascular hypertension (19). On the basis of results in cultured cells (5), noted above, we expected this increase in ANG II would lead to an accumulation of cellular Na-K-ATPase in the plasma membrane. We anticipated that there would be an increase in the amount of Na-K-ATPase in the plasma membrane of the low-pressure kidney compared with the contralateral kidney, if there was indeed a selective increase in the amount of intrarenal ANG II in the low-pressure kidney. We expected there to be a relationship between blood pressure and the production of ANG II, because renin secretion, the rate-limiting step in the formation of angiotensin, is increased through the renal baroreceptor mechanism (20) when renal perfusion pressure is lowered near the lower limit of renal blood flow autoregulation (3). Finally, we anticipated that these studies would support the concept (23) that there is a dynamic interactive relationship between rapid changes in blood pressure and Na-K-ATPase activity in the kidney cortex.
METHODS
Animal Preparation
Male Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA) were anesthetized using thiobutabarbital (Inactin; 125 mg/kg body wt ip) and placed on a heating pad to maintain body temperature. A tracheostomy was performed using polyethylene (PE)-240 tubing to allow spontaneous breathing of room air. The femoral vein was catheterized with PE-50 tubing for maintenance infusion of 40 μl/min of 0.9% NaCl and for collection of venous blood. Femoral venous blood was sampled slowly in 250-μl volumes and then replaced with an equal amount of 6% heat-inactivated BSA. Both the left carotid artery and the right femoral artery were catheterized using heparinized PE-50 tubing attached to a three-way stopcock on a Statham pressure transducer (Viggo-Spectramed, Oxnard, CA; calibrated using a mercury manometer) and a Gould recorder (Gould Instruments, Valley View, OH) for continuous monitoring of suprarenal systemic blood pressure (BP) and renal perfusion pressure, respectively. The abdominal cavity was opened via a midventral incision. The intestines were wrapped in warm, moist gauze and tucked under the right ventral wall, and both the left and right renal arteries and veins were dissected from the surrounding tissues. To control renal perfusion pressure to the left kidney, a 1.0 silk ligature was placed around the aorta between the origins of the two renal arteries and then threaded through a plastic sleeve made from PE-140 tubing with the ends heat-annealed. This allowed the ligature to be constricted to reduce the renal perfusion pressure to only the left kidney, and left renal perfusion pressure was monitored via the femoral artery catheter while systemic perfusion of the contralateral right kidney was maintained. Control rats had an identical surgical preparation, but the ligature was not constricted, leaving both kidneys perfused with the same pressure. After surgery, the rats received a supplemental bolus of 1.0 ml of 6% heat-inactivated BSA in normal saline and stabilized for 45–60 min. At the conclusion of the protocol, rats were killed by bilateral nephrectomy and pneumothorax while still under anesthesia. All procedures using animals were approved by our Institutional Animal Care and Use Committee and adhere to the guiding principles in the care and use of experimental animals. Henry Ford Hospital's animal facility is approved by the American Association for the Accredition of Laboratory Animal Care.
Experimental Protocols
Rats were treated using one of three different experimental protocols.
Group 1.
Rats in group 1 (n = 6) were subjected to different renal perfusion pressures (ΔP) in the right and left kidney. To do this, once the rat had stabilized 45–60 min after surgery, systemic pressure was noted, and a blood sample for plasma renin activity (PRA) was obtained from the femoral venous catheter. The aortic ligature between the renal arteries was then tightened to reduce the renal perfusion pressure to the left kidney, as monitored through the femoral arterial catheter, to ∼70 mmHg. Once stabilized, this pressure was maintained for 5 min. At the conclusion of this period, the pressure to the right kidney was also determined via the carotid catheter, and a second blood sample for PRA was obtained. The left kidney was then removed, decapsulated, longitudinally transected, and placed in iced saline. The right kidney was also immediately removed, decapsulated, longitudinally transected, and placed in iced saline for processing as described below.
Group 2.
Rats in this group (n = 5) were treated identically with those in group 1, except that the aortic constrictor was not closed to reduce renal perfusion pressure to the left kidney, and both kidneys remained at normal systemic pressure throughout the 5-min experimental period. These animals served as equal pressure controls.
Group 3.
Rats in this group (n = 6) were allowed to stabilize and were then administered a 50 μg/kg body wt bolus of the ANG II-converting enzyme inhibitor ramiprilat. Rats that responded to the drug with a transient decrease of more than 10 mmHg were excluded to eliminate any errant depressor signals confounding our results. After 60 min, once blood pressure was again stable, the protocol of unilateral reduced renal perfusion pressure identical to that in group 1 was repeated, reducing the renal perfusion pressure to ∼70 mmHg over 5 min.
Tissue Processing
Immediately after the kidneys were removed from the rat, the cortex of each kidney was dissected, minced separately in iced phosphate-buffered saline (PBS), and then pressed through a 250-μm stainless steel sieve with a spoonula. The tissue slurry was centrifuged at 1,164 relative centrifugal force (RCF) for 10 min at 4°C, and the pellet containing the proximal-rich renal cortical sample was resuspended into a borate buffer (10 mM boric acid, 140 mM NaCl, 4 mM KCl, and 1.8 mM CaCl2, pH 8.5) containing 1.5 mg/ml NHS-SS-biotin dissolved in DMSO.
Biotinylation labeling of the membrane-bound Na-K-ATPase was carried out over a 45-min period divided into 15-min incubations in which the incubation medium was replaced with fresh biotin-containing medium. Samples were placed in capped 50-ml tubes with two small holes in the cap, one for venting and one for the gas line. The tissue was suspended in aerated medium at 4°C and overlaid with a flow of 95% O2-5% CO2 for 15 min. The tube was then centrifuged for 3 min to pellet the tissue, the incubation medium was vacuum aspirated and then replaced with new medium, the pellet was resuspended, and the next 15-min incubation period was begun. The biotinylated tissue was then washed twice by centrifugation with a lysine buffer (100 mM lysine in PBS, pH 7.8) to remove free biotin. Finally, 5 ml of chilled 3 mM ATP in PBS were added to the pellet, and the sample was placed on ice.
Next, the collected samples were vortexed, and a 1-ml aliquot of each sample was quickly removed and placed in an ice-cold microcentrifuge tube. The samples were centrifuged at 16,000 RCF for 10 min at 4°C, and the supernatant was discarded. A volume of 0.5 ml of cold RIPA buffer containing 2% SDS and protease inhibitors (22) was added to the pellet, and the tube was vortexed for 10 min at 4°C. Another 0.25 ml of the same solution was added, and the mixing continued. Ten minutes later, another 0.25 ml of the same mixture was added, and the mixing continued for another 10-min period. The sample was then centrifuged at 16,000 RCF for 10 min at 4°C. The supernatant was removed, and the protein concentration was determined using bicinchoninic acid following the manufacturer's suggestions. The final protein concentration was ∼4 mg/ml. An aliquot containing 0.3 mg of protein was removed, and the volume was increased to 0.5 ml by adding RIPA buffer supplemented with protease inhibitors (22). This sample was combined with 0.5 ml of immobilized streptavidin that had been previously washed twice with ice-cold RIPA buffer. The suspension was incubated overnight at 4°C with gentle end-over-end mixing. The next morning, the samples were briefly centrifuged to pellet the streptavidin, and the supernatant was removed to separate tubes. The streptavidin was then washed first with RIPA buffer and then with a high osmotic buffer containing 500 mM NaCl, 5 mM EDTA, 50 mM Tris, and 0.1% Triton and, finally, with a low osmotic buffer containing 50 mM Tris (8). The isolated membrane proteins were removed from the streptavidin by adding 0.5 ml of Laemmli sample buffer (9) containing excess DTT and β-mercaptoethanol.
The relative amount of biotinylated Na-K-ATPase in the right and left kidneys from each experiment was measured by immunoblotting (15) for the α-subunit of the Na-K-ATPase by using chemiluminescence as previously described (22). On each blot we ran multiple samples from the left and right kidneys from a given experiment along with known amounts of rat kidney microsomes (22). The total amount of protein added per lane was ∼5 μg or less. For instance, in each experiment, we took an ∼8-μl aliquot containing ∼5 μg of protein from the 0.5 ml of sample that was added to the streptavidin. An equal aliquot was then taken at each subsequent step in the procedure. Immunoblot signals were quantified in arbitrary units using a Fuji LAS-1000 System and Image Gauge version 3.3 software. For each blot we then constructed a standard curve of arbitrary units vs. known amounts of rat kidney microsomes and fit the data with the equation y = (a)ln x + b using least squares. This equation was then used for the samples on that blot to calculate the amount of rat kidney microsomes (in μg) that would have contained the same amount of Na-K-ATPase as the samples. Because each left kidney was paired with its own contralateral right kidney, we tested for differences in the amount of Na-K-ATPase in the plasma membranes of right and left kidneys using a paired t-test. A P value <0.05 was considered to be significant. Blots that had been probed for anti-α-subunit were stripped and probed for anti-tubulin (14).
For each experiment we also measured the total amount of Na-K-ATPase in the detergent-dissolved samples from the left and right kidneys by using the same immunoblotting procedure and found that there was no significant difference. We also confirmed that there was no Na-K-ATPase coming off the streptavidin before we added reducing agents. Controls were also run to verify that the biotin was not labeling intracellular proteins. These issues are directly addressed in the results.
PRA Assay
PRA was determined from femoral venous blood samples as previously described (2). PRA was assayed for the generation of angiotensin I using a Gamma Coat RIA kit (DiaSorin, Stillwater, MN). Units are expressed in nanograms of ANG I per hour.
Materials
We purchased acrylamide from Bio-Rad Laboratories (Hercules, CA); PVDF from Millipore (Billerica, MA); BCA, NHS-SS-biotin, and immobilized streptavidin from Pierce Biotechnology (Rockford, I:); goat horseradish peroxidase-conjugated secondary anti-mouse antibodies from Jackson ImmunoResearch (West Grove, PA); KPL chemiluminescence reagents from Insight Biotechnology (Wembley, UK); and ramiprilat from Astra Zeneca (Newark, DE). Mouse anti-tubulin antibody was obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). All other reagents, including protease inhibitor cocktail and the anti-α-subunit antibody (M8-P1-A3), were purchased from Sigma-Aldrich (St. Louis, MO).
RESULTS
Group 1
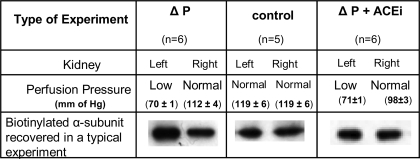
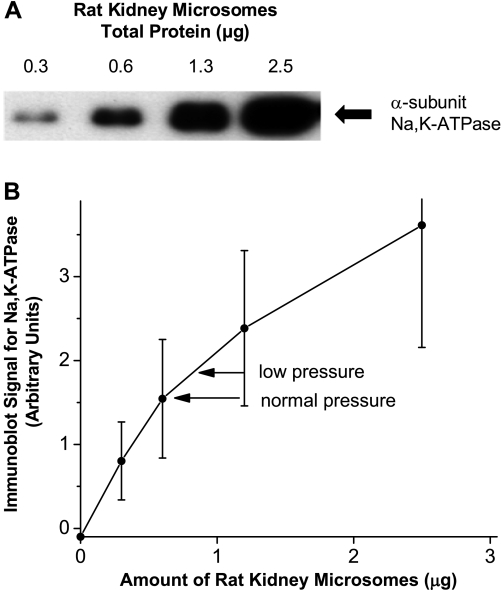
We first tested whether a brief reduction in renal perfusion pressure would increase the incorporation of Na-K-ATPase into the plasma membranes of the kidney cortex. In these experiments the perfusion pressure to the left kidney was reduced to 70 ± 1 mmHg, a change of ∼40 mmHg from that to the nonconstricted right kidney (Fig. 1). We found more biotinylated Na-K-ATPase recovered from the left, low-pressure kidney than from the right kidney maintained at a normal perfusion pressure (Fig. 1). In these experiments, the amount of protein analyzed by means of immunoblotting was well within the range in which the intensity of the response was sensitive to the amount of Na-K-ATPase present (Fig. 2). The immunoblot signal from biotinylated α-subunit from the left kidneys (mean ± SE) was the equivalent of 0.87 ± 0.20 μg of rat kidney microsomes compared with 0.69 ± 0.20 μg in the right kidneys maintained at normal pressure, which were significantly different from each other (P = 0.003) as shown by a paired t-test.
Fig. 1.
Perfusion pressure of the left and right kidneys and an example of the amount of biotinylated Na-K-ATPase recovered from left and right kidneys in the 3 types of experiments performed in this study: pressure change (ΔP, group 1), control (group 2), and pressure change in the presence of ANG II-converting enzyme inhibitor (ΔP + ACEi, group 3). Values of perfusion pressures are means ± SE for the number (n) of experiments shown. Immunoblots are shown for the α-subunit of Na-K-ATPase.
Fig. 2.
Response of the antibody to the α-subunit of Na-K-ATPase to known amounts of rat kidney microsomes. A: example of a typical immunoblot showing the response of the anti-α antibody as a function of known amounts of rat kidney microsomes. B: standard curve showing the average response of the anti-α antibody as a function of known amounts of rat kidney microsomes in the 6 experiments in group 1. Arrows indicate the mean value for the samples from the low-pressure and normal pressure kidney for the rats in group 1. Values are means ± SE.
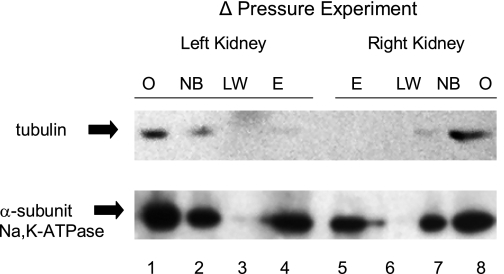
The amount of biotinylated Na-K-ATPase recovered in these experiments varied between 20 and 40% of the total amount of Na-K-ATPase in the cell (data not shown). The biotinylated Na-K-ATPase that was eluted from the streptavidin is from the plasma membranes of the kidney cortex and not from intracellular vesicles, because intracellular proteins were not eluted from the streptavidin (Fig. 3). For example, both the Na-K-ATPase, which can be in either the plasma membrane or intracellular vesicles, and tubulin, which is only found intracellularly, were present in the mixture of cellular proteins from left and right kidneys that were combined with the streptavidin (Fig. 3, lanes 1 and 8); both were present in the mixture of proteins that did not bind to the streptavidin (Fig. 3, lanes 2 and 7), but only Na-K-ATPase was present in the sample that was released from the streptavidin on the addition of reducing agent (Fig. 3, lanes 4 and 5).
Fig. 3.
Relative amount of tubulin and Na-K-ATPase in the samples at critical points during the processing of samples from a ΔP experiment. The results show that some of the cellular Na-K-ATPase, but none of the tubulin, which is known to be present only inside cells, was recovered from streptavidin. Lanes 1 and 8 were loaded with 5 μg of total protein in 8 μl from the detergent-dissolved cellular lysate (O) of which 0.5 ml containing 0.3 mg of protein was added to and incubated with the streptavidin. Lanes 2 and 7 contain an equal volume of the supernatant after the streptavidin was cleared from the sample following the overnight incubation. Proteins present in this sample were not biotinylated (NB) and therefore did not bind to the streptavidin. Lanes 3 and 6 contain an equal volume of a subsequent supernatant sample after the last wash (LW) of the streptavidin. Lanes 4 and 5 contain an equal volume of the supernatant after the streptavidin was exposed to reducing agents that eluted (E) biotinylated proteins. The E sample contains only Na-K-ATPase, despite tubulin being present in samples O and NB.
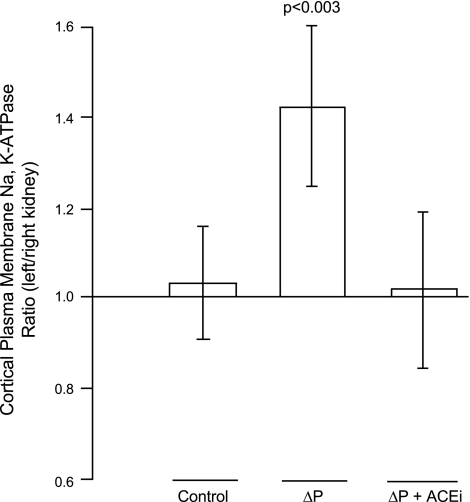
To compensate for differences between experiments, we also calculated the ratio of biotinylated Na-K-ATPase in the left renal cortex compared with the right renal cortex in each experiment. This ratio will most accurately reflect the differences in biotinylated Na-K-ATPase between the two kidneys. We found a significant increase in the amount of biotinylated Na-K-ATPase in the low-pressure kidney of >40% (Fig. 4). These data suggest that decreasing the perfusion pressure for a brief 5-min period significantly increased the amount of Na-K-ATPase in the plasma membranes of cells that were in the kidney cortex.
Fig. 4.
Ratio of the relative amount of biotinylated α-subunit of Na-K-ATPase between the left and right kidneys of the 3 types of experiments performed in this study. Values are the means ± SE of the amount of biotinylated α-subunit recovered from the left kidney divided by the amount recovered from the right kidney. In experiments where the left renal perfusion pressure was reduced (ΔP), the amount recovered in the left kidney was significantly different from that in the right (P < 0.003), whereas there was no difference in the amount recovered between kidneys in the control experiments or when pressure was changed in the presence of ramiprilat (ΔP + ACEi).
To determine whether these acute changes in renal perfusion pressure would result in stimulating the renin-angiotensin system, we measured PRA as an index of angiotensin formation. PRA increased 57% from 9.7 ± 2.5 to 15.2 ± 2.5 ng ANG I·ml−1·h−1 over the 5-min period in which the perfusion pressure was reduced.
Group 2
In these control experiments, the perfusion pressure to both kidneys was maintained at equal pressures for 5 min (Fig. 1). The amount of Na-K-ATPase in the plasma membranes of the left and right kidneys had equal amounts of biotinylated Na-K-ATPase (Fig. 1). When the amount of α-subunit was quantified, the values (means ± SE) were 0.68 ± 0.11 μg for the left kidney and 0.67 ± 0.09 μg for the right kidney, which were not different from each other (P = 0.47) as determined by a paired t-test. The mean ratio of biotinylated α-subunit in the left kidney compared with the right kidney was not different from 1 (Fig. 4), meaning that the cortices from both the left and right kidneys contained the same amount of Na-K-ATPase in their plasma membranes. These data confirm that when the perfusion pressure is the same in both kidneys, the amount of Na-K-ATPase in the plasma membranes of both kidneys is the same. Likewise, there was no difference between the amount of Na-K-ATPase in the plasma membranes of the right kidneys in group 1 compared with group 2 as analyzed using a normal t-test (P = 0.47).
Group 3
In this group with angiotensin-converting enzyme inhibited (ACEi), reducing renal perfusion pressure to the left kidney failed to change the amount of Na-K-ATPase in the plasma membranes from the renal cortex compared with the amount in the right kidney maintained at the basal renal perfusion pressure (Fig. 1). The α-subunit in the left, reduced-pressure kidneys was 0.81 ± 0.22 μg compared with 0.79 ± 0.14 μg in the right kidneys. These values were not different from each other (P = 0.46) as analyzed using a paired t-test, and the ratio of Na-K-ATPase in the left and right kidneys was not different from 1 (Fig. 4). These data suggest the increase in the incorporation of Na-K-ATPase in the plasma membranes of cells in the cortex of the kidney in response to a decrease in perfusion pressure is mediated, at least in part, by a local increase in ANG II. We could detect no difference in the amount of Na-K-ATPase in the plasma membranes of the right (normal pressure) kidney of rats in group 1 (ΔP) and group 3 (ΔP + ACEi), as determined using a normal t-test (P = 0.35). The latter result shows that there is no evidence that the administration of ramiprilat significantly changed the amount of Na-K-ATPase in the plasma membrane under our experimental conditions.
DISCUSSION
Our results directly support the hypothesis that, in vivo, a short-term (5 min) decrease in renal perfusion pressure will increase the amount of Na-K-ATPase in the plasma membranes of the kidney cortex by an ANG II-dependent mechanism without a change in the total amount of cellular Na-K-ATPase. This rapid increase in the amount of Na-K-ATPase in the plasma membrane in response to a decrease in perfusion pressure supports the concept that there is a dynamic interaction between blood pressure and Na-K-ATPase activity in the kidney cortex. It was previously known that an acute short-term increase in blood pressure rapidly inhibits Na-K-ATPase activity (23). Now we also know that a rapid decrease in pressure will increase the amount of Na-K-ATPase in the plasma membrane, which would be predicted to increase Na-K-ATPase activity. An increase in activity would be expected to initiate an increase in sodium reabsorption, which could help restore blood volume and normalize blood pressure.
Our results also support the conclusion that this rapid increase in the amount of Na-K-ATPase in the plasma membrane is mediated by ANG II. ANG II is implicated because ramiprilat, an ANG II-converting enzyme inhibitor, blocked the increase in the amount of Na-K-ATPase in the plasma membrane of the low-pressure kidney relative to the contralateral kidney maintained at a normal perfusion pressure in group 1. The increased amounts of Na-K-ATPase in the plasma membranes of the low-pressure kidney are likely due to a local increase in intrarenal ANG II in the low-pressure kidney compared with the contralateral kidney. Although it is true that we observed an increase in PRA in the rats in group 1, it is difficult to see how an increase in PRA and circulating ANG II can explain the preferential increase in Na-K-ATPase in the plasma membranes of the low-pressure kidney. There is no evidence that this increase in PRA had an effect on the amount of Na-K-ATPase in the plasma membranes of both kidneys, because we detected no increase in the amount of Na-K-ATPase in the plasma membranes of the right kidney in group 1 compared with group 2. Likewise, there was not a significant difference in the absolute amount of Na-K-ATPase that was in the right kidneys in groups 1 and 3 that had been treated with ramiprilat. It is, of course, possible that differences in the amount of biotinylated Na-K-ATPase between experiments obscured our ability to detect a difference in these unpaired comparisons. Nevertheless, it is difficult to see how an increase in the plasma levels of ANG II can explain the increased amounts of Na-K-ATPase in the low-pressure kidney relative to its matched control.
A role for ANG II in regulating the amount of Na-K-ATPase in the plasma membrane in response to a change in blood pressure is consistent with the observation that a drop in plasma ANG II concentration mediates inhibition of sodium reabsorption (11) and a step in the redistribution of Na/H exchanger 3 (NHE3) in the apical membrane of the proximal tubule (10) in response to an acute increase in blood pressure. Zhou and colleagues have suggested that in proximal tubules, ANG II acting through AT1 receptors in either the plasma membrane or internalized vesicles (12) stimulates a series of cellular signaling events including increased intracellular calcium (25), activation of NF-κB (24), redistribution of NHE3, and regulation of proximal sodium transport (13). The involvement of ANG II in mediating Na-K-ATPase in the plasma membrane of proximal tubular cells would complement the proposed ANG II mediation of NHE3 (10, 13) in local regulation of proximal sodium reabsorption.
It has been known for some time that ANG II-induced changes in the phosphorylation of Na-K-ATPase directly stimulate Na-K-ATPase activity in the rat proximal tubule (1, 7) and that this effect could occur in as little as 2 min (21). However, it was first suggested that the stimulation was due to an increase in the affinity of the Na-K-ATPase for intracellular sodium (1), its rate-limiting substrate. It was later shown in opossum kidney cells that express the rat kidney Na-K-ATPase that ANG II directly stimulates Na-K-ATPase activity in ∼15 min or less via the AT1 receptor (5). Activation of the AT1 receptor stimulates PKC, which in turn phosphorylates the rat Na-K-ATPase at Ser11 and Ser18, which triggers a rapid accumulation of Na-K-ATPase in the plasma membrane (5). Our results are the first to show that this trafficking-type mechanism may also be relevant to how ANG II directly simulates Na-K-ATPase activity in intact animals and to relate this effect to changes in blood pressure.
The concept that the amount of Na-K-ATPase in the plasma membrane quickly (≤5 min) responds to physiological changes of renal perfusion pressure and baroreceptor-mediated angiotensin formation near the lower limit of renal autoregulation (3) may represent an underappreciated mechanism with a fast response to preserve sodium. Although this may reflect the response to rapid changes and normal fluctuations in blood pressure, it also may be part of a complex mechanism involved in renal pathophysiology, such as adaptation of the stenotic kidney in the development of renovascular hypertension (17, 18), or more rigorous compromises to renal function such as the response to hypovolemic shock. Greater reductions in perfusion pressure than what we used in our experiments would be expected to compromise renal blood flow, leading to ATP depletion and associated cellular changes (16). Changes in the trafficking of Na-K-ATPase to the plasma membrane in response to changes in pressure could be a major factor in pathophysiology, such as adaptation of the stenotic kidney in the development of renovascular hypertension (17, 18), or more rigorous compromises to renal function, such as the response to hypovolemic shock. None of these has yet been studied in the context of sodium transport controlled by local ANG II production, nor has the pathway between AT1-mediated signaling and translocation and incorporation of the Na-K-ATPase in the plasma membrane, but these may reveal a new understanding of local, filtered, or reabsorbed ANG II as a key factor in controlling sodium reabsorption. The current study purposefully does not address these more aggressive and complex responses.
In conclusion, we found that acute reductions in renal perfusion pressure in vivo lead to a significant increase in Na-K-ATPase incorporation into the plasma membrane of cells in the renal cortex. Furthermore, we observed that the unilateral renal baroreceptor stimulation of renin, and presumably local tissue generation of ANG II, is the signal that mediates this increased trafficking and incorporation of Na-K-ATPase into the basolateral membrane. This response is consistent with an acute increase in the ability to reabsorb sodium and help maintain blood pressure in the face of a perceived decline in renal perfusion pressure.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-60752 (to D. Yingst).
ACKNOWLEDGMENTS
We thank Haiping Chen for the preparation of rat kidney microsomes, D'Anna Potter for expertise in the in vivo preparations, and Albert Chow for immunoblotting.
REFERENCES
- 1.Aperia A, Holtbäck U, Syrén ML, Svensson LB, Fryckstedt J, Greengard P. Activation/deactivation of renal Na+,K+-ATPase: a final common pathway for regulation of natriuresis. FASEB J 8: 436–439, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Beierwaltes WH. cGMP stimulates renin secretion in vivo by inhibiting phosphodiesterase-3. Am J Physiol Renal Physiol 290: F1376–F1381, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Beierwaltes WH, Sigmon DH, Carretero OA. Endothelium modulates renal blood flow but not autoregulation. Am J Physiol Renal Fluid Electrolyte Physiol 262: F943–F949, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren PO, Bertorello AM. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and Is responsible for the decreased activity in epithelial cells. J Biol Chem 274: 1920–1927, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem 278: 28719–28726, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Efendiev R, Pedemonte CH. Contrary to rat-type, human-type Na,K-ATPase is phosphorylated at the same amino acid by hormones that produce opposite effects on enzyme activity. J Am Soc Nephrol 17: 31–38, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Garvin JL. Angiotensin stimulates bicarbonate transport and Na+/K+ ATPase in at proximal straight tubules. J Am Soc Nephrol 1: 1146–1152, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Gottardi CJ, Dunbar LA, Caplan MJ. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol Renal Fluid Electrolyte Physiol 268: F285–F295, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 10.Leong PK, Yang LE, Holstein-Rathlou NH, McDonough AA. Angiotensin II clamp prevents the second step in renal apical NHE3 internalization during acute hypertension. Am J Physiol Renal Physiol 283: F1142–F1150, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Leong PK, Zhang Y, Yang LE, Holstein-Rathlou NH, McDonough AA. Diuretic response to acute hypertension is blunted during angiotensin II clamp. Am J Physiol Regul Integr Comp Physiol 283: R837–R842, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Li XC, Carretero OA, Navar LG, Zhou JL. AT1 receptor-mediated accumulation of extracellular angiotensin II in proximal tubule cells: role of cytoskeleton microtubules and tyrosine phosphatases. Am J Physiol Renal Physiol 291: F375–F383, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XC, Zhou JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-ANG II endocytosis and NHE-3 expression in immortalized rabbit proximal tubular cells. Am J Physiol Cell Physiol 293: C367–C378, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Mullins SR, Sloane BF, Mattingly RR. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia 10: 314–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattingly RR, Milstein ML, Mirkin BL. Down-regulation of growth factor-stimulated MAP kinase signaling in cytotoxic drug-resistant human neuroblastoma cells. Cell Signal 13: 499–505, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Molitoris BA, Wagner MC. Surface membrane polarity of proximal tubular cells: alterations as a basis for malfunction. Kidney Int 49: 1592–1597 1996 [DOI] [PubMed] [Google Scholar]
- 17.Sigmon DH, Beierwaltes WH. Degree of renal artery stenosis alters nitric oxide regulation of renal hemodynamics. J Am Soc Nephrol 5: 1369–1377, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Sigmon DH, Beierwaltes WH. Renal nitric oxide and angiotensin II interaction in renovascular hypertension. Hypertension 22: 237–242, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension 33: 1237–1242, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Tobian L, Tomboulian A, Janecek J. Effect of high perfusion pressure on the granulation of juxtaglomerular cells in an isolated kidney. J Clin Invest 38: 605–610, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yingst DR, Massey KJ, Rossi NF, Mohanty MJ, Mattingly RR. Angiotensin II directly stimulates activity and alters the phosphorylation of Na-K-ATPase in rat proximal tubule with a rapid time course. Am J Physiol Renal Physiol 287: F713–F721, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Yingst DR, Doci TM, Massey KJ, Rossi NF, Rucker E, Mattingly RR. Angiotensin II stimulates elution of Na-K-ATPase from a digoxin-affinity column by increasing the kinetic response to ligands that trigger the decay of E2-P. Am J Physiol Renal Physiol 294: F990–F1000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Magyar CE, Norian JM, Holstein-Rathlou NH, Mircheff AK, McDonough AA. Reversible effects of acute hypertension on proximal tubule sodium transporters. Am J Physiol Cell Physiol 274: C1090–C1100, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Zhou JL, Carretero OA, Li XC. Effects of AT1 receptor-mediated endocytosis of extracellular Ang II on activation of nuclear factor-kappa B in proximal tubule cells. Ann NY Acad Sci 1091: 336–345, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol 290: F1383–F1390, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]