Abstract
Absorption of NaCl by the thick ascending limb (TAL) involves active transport and therefore depends on oxidative phosphorylation. Extracellular ATP has pleiotropic effects, including both stimulation and inhibition of transport and inhibition of oxidative phosphorylation. However, it is unclear whether ATP alters TAL transport and how this occurs. We hypothesized that ATP inhibits TAL Na absorption by reducing Na entry. We measured oxygen consumption in TAL suspensions. ATP reduced oxygen consumption in a concentration-dependent manner. The purinergic (P2) receptor antagonist suramin (300 μM) blocked the effect of ATP on TAL oxygen consumption (147 ± 15 vs. 146 ± 16 nmol O2·min−1·mg protein−1). In contrast, the adenosine receptor antagonist theophylline did not block the effect of ATP on oxygen consumption. When Na-K-2Cl cotransport and Na/H exchange were blocked with furosemide (100 μM) plus dimethyl amiloride (100 μM), ATP did not inhibit TAL oxygen consumption (from 78 ± 13 to 98 ± 5 nmol O2·min−1·mg protein−1). The Na ionophore nystatin (200 U/ml) increased TAL oxygen consumption to a similar extent in both ATP- and vehicle-treated samples (368 ± 41 vs. 397 ± 47 nmol O2·min−1·mg protein−1). The nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (3 mM) blocked the ATP effects on TAL oxygen consumption (157 ± 10 vs. 165 ± 15 nmol O2·min−1·mg protein−1). The P2X-selective receptor antagonist NF023 blocked the effect of ATP on oxygen consumption, whereas the P2X-selective agonist β-γ-Me-ATP reduced oxygen consumption in a concentration-dependent manner. We conclude that ATP inhibits Na transport-related oxygen consumption in TALs by reducing Na entry and P2X receptors and nitric oxide mediate this effect.
Keywords: NKCC2, purinergic signaling, NHE
the thick ascending limb of the loop of Henle reabsorbs 20–30% of the NaCl filtered by the glomerulus. NaCl reabsorbed by this segment generates the cortico-medullary osmotic gradient necessary for water reabsorption and urine concentration. Therefore, factors that affect NaCl reabsorption in this segment directly impact Na and water excretion (7, 8). NaCl reabsorption in the thick ascending limb involves an active transport mechanism in which NaCl passively enters the cell via apical Na/H exchange and Na-K-2Cl cotransport and exits via basolateral Na-K-ATPase (7).
Extracellular adenosine triphosphate (ATP) is an autocrine/paracrine factor found in the luminal fluid of the nephron (30) and interstitial space (19). Extracellular ATP may regulate thick ascending limb transport. Extracellular ATP potently activates Cl secretion in respiratory epithelia (27), while in the gastrointestinal tract it increases K and HCO3 secretion (12). In contrast, extracellular ATP inhibits HCO3 transport in the proximal tubule (1) and Na transport in the collecting ducts (21) due to reduced activity of epithelial Na channels (ENaC) (31, 32).
In the thick ascending limb, extracellular ATP could inhibit transport by at least two different mechanisms. We previously reported that extracellular ATP increases nitric oxide (NO) in a P2X receptor-mediated mechanism (22), and NO inhibits transport in the thick ascending limb (6, 20). However, extracellular ATP also inhibits oxidative phosphorylation in other cell types (9). Inhibition of oxidative phosphorylation decreases ATP production and could reduce Na transport, because thick ascending limb Na transport depends on Na-K-ATPase activity. Currently, it is unknown whether ATP inhibits transport in the thick ascending limb and the mechanism(s) involved. We hypothesized that ATP inhibits thick ascending limb transport via NO and activation of P2X receptors.
METHODS
Animals.
This study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee. All studies were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats weighing 150 to 200 g (Charles River Breeding Laboratories) were fed a diet containing 0.22% Na and 1% K (Purina) for 7–12 days before the experiments.
Rat medullary thick ascending limb suspensions.
Medullary thick ascending limb suspensions were prepared as described previously (26). Kidneys were perfused retrograde via the abdominal aorta with 40 ml of 0.1% collagenase type I (Sigma) and 100 U heparin in HEPES-buffered physiological saline containing (in mM) 130 NaCl, 4 KCl, 2.5 NaH2PO4, 1.2 MgSO4, 2 calcium dilactate, 5.5 glucose, 6 D/l-alanine, 1 trisodium citrate, and 10 HEPES. The inner stripe of the outer medulla was dissected from coronal slices of the kidneys. The tissue was minced and incubated at 37°C for 30 min in 0.1% collagenase type I while agitating the suspension and gassing it with 100% oxygen every 5 min. Tissue was centrifuged at 93 g for 2 min, resuspended in cold HEPES-buffered physiological saline, and stirred on ice for 30 min. The resulting tubule suspension was filtered through a 250-μm nylon mesh and spun again for 2 min. The pellet was washed and resuspended in 1 ml cold HEPES-buffered physiological saline. At least 93% of tubules in these suspensions are thick ascending limbs (4).
Measurement of oxygen consumption.
To examine the effect of ATP on tubular transport, we used oxygen consumption. This technique allowed us to monitor the effects of ATP on both transport and cell metabolism. Oxygen consumption can be used to measure Na transport because it is stoichiometrically related to Na transport, and 35–50% of total oxygen consumption by the thick ascending limb is associated with NaCl transport (4, 18, 23, 29). To measure oxygen consumption, thick ascending limbs were suspended in 0.1 ml of HEPES-buffered physiological saline warmed to 37°C and equilibrated with 100% oxygen. Then, they were added to a closed chamber at 37°C and oxygen consumption was recorded continuously using a Clark electrode. An initial constant slope was established for each experiment (usually within 5 min). Then, treatments were added. The effect of a treatment was measured after a new stable slope was established for at least 3 min. All experiments were completed within 20 min. At the end of the experiment, an aliquot of the suspension was used to determine protein content.
Determination of protein content.
Total protein content was determined using Coomassie Plus reagent (Pierce, Rockford, IL), based on Bradford's colorimetric method.
Statistics.
Data are reported as means ± SE. Differences in means were analyzed using either Student's t-test for paired experiments or an unpaired t-test, applying Hochberg's adjustment when appropriate to determine significance. Statistical analysis was performed by the Henry Ford Hospital Department of Biostatistics and Epidemiology.
RESULTS
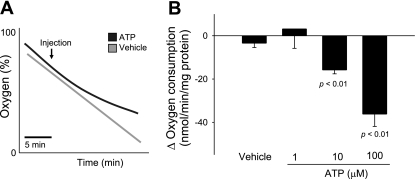
To begin to test our hypothesis, we measured the effect of ATP on basal oxygen consumption by generating a concentration-response curve. We found that increasing concentrations of ATP from 1 to 10 and 100 μM decreased oxygen consumption in a concentration-dependent manner. At the maximum concentration tested, ATP inhibited oxygen consumption by 27% (Fig. 1). In control experiments, vehicle had no effect on oxygen consumption.
Fig. 1.
Effect of extracellular ATP on thick ascending limb oxygen consumption. A: representative experiment. B: change in oxygen consumption caused by extracellular ATP (1 μM: n = 3; 10 μM: n = 4; vehicle and 100 μM: n = 6).
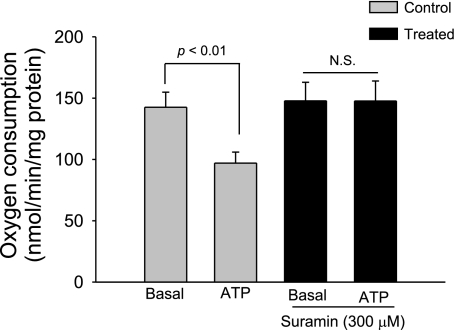
To test the possibility that the effect of ATP on thick ascending limb oxygen consumption was due to a P2 receptor-mediated mechanism, we used the P2 receptor antagonist suramin. In the absence of suramin (300 μM), basal thick ascending limb oxygen consumption was 142.3 ± 12.3 nmol O2·min−1·mg protein−1. After ATP (100 μM) was added, thick ascending limb oxygen consumption decreased to 97.6 ± 8.7 nmol O2·min−1·mg protein−1 (n = 6; P < 0.01 vs. basal). In contrast, in the presence of suramin, basal thick ascending limb oxygen consumption was 147.5 ± 15.2 nmol O2·min−1·mg protein−1. After ATP (100 μM) was added, thick ascending limb oxygen consumption remained constant at 146.6 ± 16.4 nmol O2·min−1·mg protein−1 (n = 6; Fig. 2).
Fig. 2.
Effect of extracellular ATP on thick ascending limb oxygen consumption during inhibition of P2 receptors. Addition of ATP in the presence of the P2 receptor antagonist suramin (300 μM) did not inhibit thick ascending limb oxygen consumption (n = 6).
In the thick ascending limb, extracellular ATP may be hydrolyzed to adenosine, which inhibits transport in this segment (2). To test whether adenosine mediates the effects of ATP on oxygen consumption, we used the adenosine receptor antagonist theophylline. In the absence of theophylline, basal thick ascending limb oxygen consumption was 94.0 ± 7.4 nmol O2·min−1·mg protein−1. After ATP (100 μM) was added in physiological saline, thick ascending limb oxygen consumption decreased to 59.7 ± 6.1 nmol O2·min−1·mg protein−1 (n = 5; P < 0.01 vs. basal). Similarly, in the presence of theophylline (100 μM), basal thick ascending limb oxygen consumption was 103.8 ± 10.5 nmol O2·min−1·mg protein−1. After ATP was added, thick ascending limb oxygen consumption decreased to 67.6 ± 6.9 nmol O2·min−1·mg protein−1. Taken together, these data indicate that ATP inhibits thick ascending limb oxygen consumption by a P2 receptor-mediated mechanism.
To test whether ATP decreases oxygen consumption by decreasing Na transport, we first measured the effect of inhibiting Na/H exchange and Na-K-2Cl cotransport using dimethyl amiloride and furosemide on oxygen consumption (6). After the addition of both dimethyl amiloride (100 μM) and furosemide (100 μM) in physiological saline, thick ascending limb oxygen consumption decreased from 132.7 ± 9.3 to 71.9 ± 9.4 nmol O2·min−1·mg protein−1, indicating that 49% of thick ascending limb oxygen consumption was associated with transport.
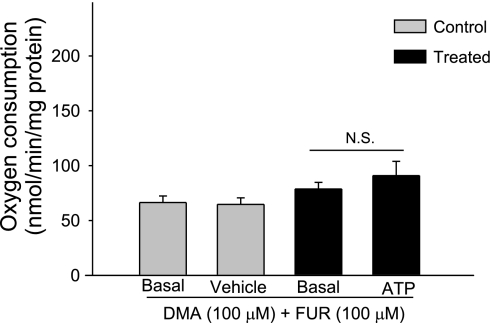
We next examined the ability of ATP to decrease oxygen consumption in the presence of the Na/H exchanger and Na-K-2Cl cotransporter inhibitors. As expected, in the presence of dimethyl amiloride (100 μM) and furosemide (100 μM), basal thick ascending limb oxygen consumption was reduced (78.8 ± 12.2 nmol O2·min−1·mg protein−1) because of decreased ATP utilization. After ATP (100 μM) was added, thick ascending limb oxygen consumption remained unchanged (98.8 ± 5.1 nmol O2·min−1·mg protein−1; n = 6; Fig. 3). In control experiments, vehicle did not alter thick ascending limb oxygen consumption (66.42 ± 5.64 vs. 64.7 ± 5.71 nmol O2·min−1·mg protein−1; n = 7). Taken together, these data indicate that the inhibitory effects of ATP on oxygen consumption are due to decreased thick ascending limb transport.
Fig. 3.
Effect of extracellular ATP on oxygen consumption in thick ascending limbs during inhibition of Na+/H+ exchanger and the Na+-K+-2Cl− cotransporter. Addition of ATP in the presence of apical transporter inhibitors Na+/H+ exchanger and the Na+-K+-2Cl− cotransporter inhibitors dimethyl amiloride (DMA) and furosemide (FUR) did not inhibit oxygen consumption, indicating that ATP inhibits oxygen consumption (n = 6). N.S., not significant.
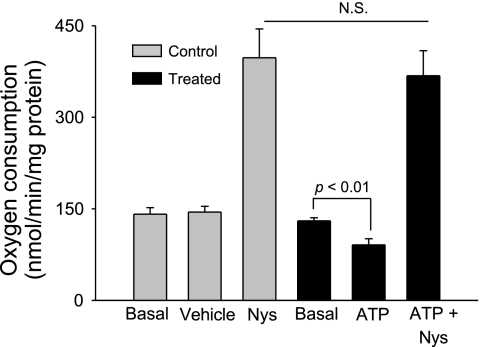
To distinguish between an effect of ATP on Na entry and exit, we used the Na ionophore nystatin (3). Nystatin increases intracellular Na by forming cation-selective channels in the plasma membrane, and therefore enhances Na-K-ATPase activity. Basal oxygen consumption was 130.3 ± 4.9 nmol O2·min−1·mg protein−1. After ATP (100 μM) was added, thick ascending limb oxygen consumption decreased to 91.1 ± 10.4 nmol O2·min−1·mg protein−1 (n = 7; P < 0.01 vs. basal). Addition of nystatin in dimethyl sulfoxide (200 U/ml) increased thick ascending limb oxygen consumption to 368.2 ± 41.5 nmol O2·min−1·mg protein−1. In control experiments, vehicle did not alter thick ascending limb oxygen consumption (141.2 ± 10.7 vs. 144.7 ± 9.3 nmol O2·min−1·mg protein−1; n = 7). Addition of nystatin increased thick ascending limb oxygen consumption to 397.4 ± 47.4 nmol O2·min−1·mg protein−1, similar to the suspensions treated with ATP (n = 7; not significant vs. tubules treated with ATP; Fig. 4). These data indicate that the inhibitory effects of ATP on oxygen consumption are not due to a decrease in maximum pump activity.
Fig. 4.
Effect of extracellular ATP on thick ascending limb oxygen consumption. Effect of extracellular ATP on Na exit. Nystatin (Nys; 200 U/ml) increased thick ascending limb oxygen consumption in the same extent in vehicle- and ATP-treated samples, indicating that Na exit is not inhibited by extracellular ATP (n = 6).
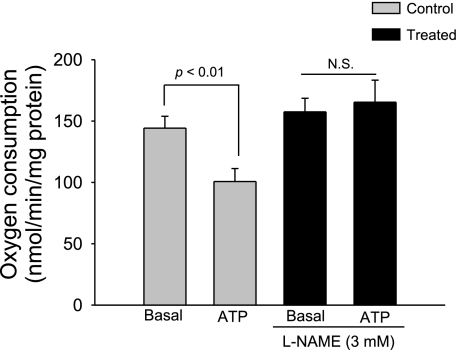
To study whether the inhibitory effects of ATP on thick ascending limb transport-related oxygen consumption are mediated by NO, we used the NO synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME). In the absence of l-NAME, basal thick ascending limb oxygen consumption was 144.2 ± 9.5 nmol O2·min−1·mg protein−1. After ATP (100 μM) was added, thick ascending limb oxygen consumption decreased to 100.7 ± 10.6 nmol O2·min−1·mg protein−1 (n = 6; P < 0.01 vs. basal). In contrast, in the presence of l-NAME (3 mM), basal thick ascending limb oxygen consumption was 157.5 ± 10.9 nmol O2·min−1·mg protein−1. After ATP was added, thick ascending limb oxygen consumption remained constant at 165.3 ± 15.5 nmol O2·min−1·mg protein−1 (n = 6; not significant vs. basal; Fig. 5). These data indicate that the inhibitory effects of ATP on oxygen consumption in the thick ascending limb are mediated by NO.
Fig. 5.
Effect of extracellular ATP on thick ascending limb oxygen consumption during inhibition of nitric oxide synthase (NOS). Addition of ATP in the presence of the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 3 mM) did not inhibit thick ascending limb oxygen consumption (n = 6).
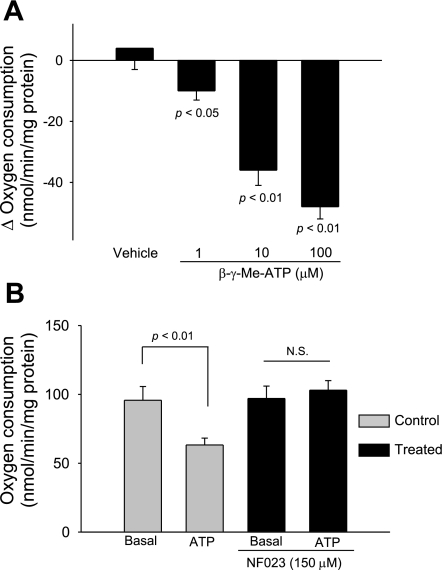
Because we previously reported that ATP increases NO production in the thick ascending limb via P2X receptor activation (22), we investigated the role of P2X receptors in ATP-induced inhibition of oxygen consumption. First, we examined the efficacy of the P2X-selective agonist β-γ-Me-ATP. We found that increasing concentrations of β-γ-Me-ATP from 1 to 10 and 100 μM decreased oxygen consumption in a concentration-dependent manner. At the maximum concentration tested, β-γ-Me-ATP inhibited oxygen consumption by 38% (Fig. 6A). Then, we tested the effect of ATP on oxygen consumption in the presence and absence of the P2X-selective antagonist NF023. In the absence of NF023, basal thick ascending limb oxygen consumption was 95.7 ± 9.3 nmol O2·min−1·mg protein−1. After ATP (100 μM) was added, thick ascending limb oxygen consumption decreased to 63.3 ± 5.0 nmol O2·min−1·mg protein−1 (n = 5; P < 0.01 vs. basal). In contrast, in the presence of NF023 (150 μM), thick ascending limb oxygen consumption was 97.4 ± 8.6 nmol O2·min−1·mg protein−1. After ATP (100 μM) was added, thick ascending limb oxygen consumption remained constant at 100.5 ± 7.2 nmol O2·min−1·mg protein−1 (n = 5; Fig. 6B). Because P2Y receptors are also expressed in this segment, we tested the ability of the P2Y-selective agonist uridine-triphosphate (UTP) to inhibit thick ascending limb oxygen consumption. We found that in thick ascending limb suspensions, basal oxygen consumption was 111.8 ± 3.2 nmol O2·min−1·mg protein−1. After UTP (100 μM) was added, thick ascending limb oxygen consumption only decreased to 101.3 ± 4.2 nmol O2·min−1·mg protein−1 (n = 5; P < 0.05 vs. basal), only a 9% inhibition. In control experiments, vehicle had no effect on oxygen consumption. Taken together, these data indicate that ATP inhibits thick ascending limb oxygen consumption primarily via activation of P2X receptors.
Fig. 6.
A: change in thick ascending limb oxygen consumption caused by β-γ-Me-ATP. B: effect of extracellular ATP on thick ascending limb oxygen consumption during inhibition of P2X receptors. Addition of ATP in the presence of the P2X receptor NF023 (150 μM) did not inhibit thick ascending limb oxygen consumption (n = 5).
DISCUSSION
To study the effect of extracellular ATP on the thick ascending limb, we measured its effects on oxygen consumption (18). In thick ascending limb suspensions, ATP decreased oxygen consumption by 27%. Most of the cellular effects of ATP are mediated by purinergic receptors (16). To determine whether P2 receptors were involved in ATP-induced inhibition of oxygen consumption in the thick ascending limb, we used the P2 receptor antagonist suramin. We found that it blocked the effects of ATP on oxygen consumption by 90%. These data indicate that most of the inhibition of oxygen consumption by ATP is primarily receptor mediated.
We showed that ATP is hydrolyzed in the extracellular space of the thick ascending limb (22), forming adenosine (13, 28) which should inhibit transport (2). To determine whether the adenosine that could be generated by ATP hydrolysis was involved in the ATP-induced inhibition of oxygen consumption in the thick ascending limb, we used the adenosine receptor antagonist theophylline. We found that it did not block the effects of ATP on oxygen consumption. However, the P2 receptor antagonist suramin blocked ATP-induced inhibition of transport. The inability to show an effect of adenosine resulting from ATP hydrolysis is likely due to the fact that the adenosine generation is insufficient to have an effect, under our experimental conditions.
About 50% of oxygen consumption by the thick ascending limb is the result of active Na transport. Thus, we tested whether inhibition of oxygen consumption by extracellular ATP was related to Na transport. To do this, we measured the effects of ATP in tubules preincubated with both furosemide and dimethyl amiloride at concentrations previously shown to abolish Na entry (6, 20). We found that in the presence of these transport inhibitors, ATP no longer inhibits oxygen consumption. These data indicate that the inhibitory effects of extracellular ATP on thick ascending limb oxygen consumption at least at the concentrations we tested are limited to transport. However, these experiments did not address the issue of how Na transport is inhibited by ATP.
ATP-induced inhibition of transport-related oxygen consumption could be due to inhibition of either 1) Na entry and/or exit or 2) oxidative phosphorylation. To determine which was the case, we used the Na ionophore nystatin. Addition of nystatin increases Na entry, and thus stimulates Na exit via Na-K-ATPase. Because of the enhanced pump activity, nystatin also increases oxidative phosphorylation, ATP production, and therefore oxygen consumption. If extracellular ATP inhibits Na entry, we would expect nystatin to increase oxygen consumption equally in both vehicle- and ATP-treated cells. Conversely, if extracellular ATP inhibits Na exit or oxidative phosphorylation, we would expect nystatin not to stimulate oxygen consumption in ATP-treated cells as much as in vehicle-treated cells. We found that nystatin increased oxygen consumption equally in ATP- and vehicle-treated samples. These data indicate that the inhibitory effects of ATP on oxygen consumption are due to inhibition of Na entry rather than Na exit or oxidative phosphorylation.
Our data indicating that ATP inhibits Na transport by reducing Na entry are similar to other data reported in the literature. In Madin-Darby canine kidney cells, extracellular ATP inhibits Na entry via Na-K-2Cl cotransport (5). ATP inhibits Na reabsorption in collecting ducts, when rats are fed a low-Na diet. This maneuver increases ENaC activity, which is also an apical ion channel (21). Moreover, ATP reportedly blocks Na currents in M1 collecting duct cells (15). In addition, ATP also reduces activity of apical small-conductance K channels by activating the P2 receptor (17).
Our data indicate that ATP does not inhibit oxidative phosphorylation or Na-K-ATPase activity. These data contrast with those reported for the proximal tubule in which ATP inhibits Na exit via Na-K-ATPase (11). Our report also varies with data showing that extracellular ATP reduces oxidative phosphorylation (9). These differences may be related to the type of cell studied and/or the concentration of ATP. They also raise the question of how ATP inhibits Na entry into the thick ascending limb.
Extracellular ATP stimulates NO production in the thick ascending limb (22), and we showed that NO inhibits Na-K-2Cl cotransport (20) and Na/H exchange (6) in this segment. Therefore, we investigated the role of NO in ATP-induced inhibition of transport. In the presence of the NOS inhibitor l-NAME, ATP-induced inhibition of oxygen consumption was abolished. These data indicate that the inhibitory effects of extracellular ATP on thick ascending limb transport are primarily mediated by NO.
Our observation that NO mediates the effect of extracellular ATP is similar to other published reports. We previously found that the inhibitory effect of NO on thick ascending limb transport is due to a reduction in Na/H exchange and Na-K-2Cl cotransport rather than decreased Na-K-ATPase (6, 20). ATP-induced inhibition of K conductance in the cortical collecting duct is reproduced by adding cGMP and inhibited by adding l-NAME (17). In medullary collecting ducts, ATP inhibits Na currents in a mechanism dependent on PI3 kinase (33), an enzyme which mediates the ATP-induced NO production in the thick ascending limb (25).
P2X receptor activation in the thick ascending limb stimulates NO production (22). Therefore, we tested whether ATP-induced inhibition of oxygen consumption was mediated by P2X receptors. We found that the P2X-selective agonist β-γ-Me-ATP decreased oxygen consumption in a concentration-dependent manner and the P2X-selective antagonist NF023 blocked the effects of ATP on thick ascending limb oxygen consumption. These data indicate that P2X receptors mediate the effects of ATP on thick ascending limb oxygen consumption.
P2X receptor activation has been shown to inhibit transport in other cell types. Activation of P2X receptors reportedly blocks Na currents in ENaC expressing Xenopus laevis oocytes by decreasing ENaC abundance in the apical membrane (32). Similarly, electrophysiological experiments with inner medullary collecting duct cells indicate that P2X receptor activation inhibits ENaC activity (33). As we mention before, this mechanism is sensitive to inhibition of PI3 kinase, an enzyme involved in NOS activation by ATP (25). Therefore, it is probable that in these cells P2X receptor activation inhibits ENaC in a mechanism mediated by NO.
In summary, extracellular ATP inhibits thick ascending limb Na transport via activation of P2X receptors by reducing Na entry by stimulating NO production which, in turn, blocks Na/H exchange and Na-K-2Cl cotransport. The effects of ATP on the thick ascending limb may be important when ATP release is stimulated, which occurs with increased luminal flow (10), changes in interstitial osmolality (24), or increased Na delivery to the distal nephron (14).
GRANTS
This work was supported in part by National Institutes of Health Grants HL-090550-01 and HL-028982-27 to J. L. Garvin and from the American Heart Association-Greater Midwest Grant 0615718Z to G. B. Silva.
REFERENCES
- 1.Bailey MA. Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. Am J Physiol Renal Physiol 287: F789–F796, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Beach RE, Good DW. Effects of adenosine on ion transport in rat medullary thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 263: F482–F487, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Brezis M, Rosen S, Silva P, Spokes K, Epstein FH. Polyene toxicity in renal medulla: injury mediated by transport activity. Science 224: 66–68, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Chamberlin ME, LeFurgey A, Mandel LJ. Suspension of medullary thick ascending limb tubules from the rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol 247: F955–F964, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Gagnon F, Dulin NO, Tremblay J, Hamet P, Orlov SN. ATP-induced inhibition of Na+, K+, Cl− cotransport in Madin-Darby canine kidney cells: lack of involvement of known purinoceptor-coupled signaling pathways. J Membr Biol 167: 193–204, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Garvin JL, Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. Am J Physiol Renal Physiol 277: F377–F382, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev 65: 760–797, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Greger R. Physiology of renal sodium transport. Am J Med Sci 319: 51–62, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Ichai C, El Mir MY, Nogueira V, Piquet MA, Chauvin C, Fontaine E, Leverve XM. Exogenous Mg-ATP induces a large inhibition of pyruvate kinase in intact rat hepatocytes. J Biol Chem 276: 6398–6403, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Jin W, Hopfer U. Purinergic-mediated inhibition of Na+-K+-ATPase in proximal tubule cells: elevated cytosolic Ca2+ is not required. Am J Physiol Cell Physiol 272: C1169–C1177, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Kerstan D, Gordjani N, Nitschke R, Greger R, Leipziger J. Luminal ATP induces K+ secretion via a P2Y2 receptor in rat distal colonic mucosa. Pflügers Arch 436: 712–716, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmermann H, Sevigny J, Robson SC. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288: F1032–F1043, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol 286: F1054–F1058, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J 19: 142–143, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lu M, MacGregor GG, Wang W, Giebisch G. Extracellular ATP inhibits the small-conductance K channel on the apical membrane of the cortical collecting duct from mouse kidney. J Gen Physiol 116: 299–310, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandel LJ. Primary active sodium transport, oxygen consumption, and ATP: coupling and regulation. Kidney Int 29: 3–9, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama A, Majid DS, Walker M III, Miyatake A, Navar LG. Renal interstitial ATP responses to changes in arterial pressure during alterations in tubuloglomerular feedback activity. Hypertension 37: 753–759, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Shirley DG, Bailey MA, Unwin RJ. In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol Renal Physiol 288: F1243–F1248, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Silva GB, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension 47: 563–567, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Silva GB, Garvin JL. Angiotensin II-dependent hypertension increases Na transport-related oxygen consumption by the thick ascending limb. Hypertension 52: 1091–1098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol 295: F1090–F1095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva GB, Garvin JL. Akt1 mediates purinergic-dependent NOS3 activation in thick ascending limbs. Am J Physiol Renal Physiol 297: F646–F652, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Stutts MJ, Lazarowski ER, Paradiso AM, Boucher RC. Activation of CFTR Cl− conductance in polarized T84 cells by luminal extracellular ATP. Am J Physiol Cell Physiol 268: C425–C433, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Varela M, Garvin JL. Acute and chronic regulation of thick ascending limb endothelial nitric oxide synthase by statins. J Am Soc Nephrol 15: 269–275, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Wildman SS, King BF. P2X receptors: epithelial ion channels and regulators of salt and water transport. Nephron Physiol 108: 60–67, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Wildman SS, Marks J, Churchill LJ, Peppiatt CM, Chraibi A, Shirley DG, Horisberger JD, King BF, Unwin RJ. Regulatory interdependence of cloned epithelial Na+ channels and P2X receptors. J Am Soc Nephrol 16: 2586–2597, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]