Abstract
We have previously reported that age-associated oxidative stress via protein kinase C (PKC) increases D1 receptor (D1R) phosphorylation and causes D1R-G protein uncoupling in renal proximal tubules (RPTs) of old Fischer 344 rats. This results in reduced ability of D1R agonist SKF-38393 to inhibit Na+-K+-ATPase in RPTs of old rats. Here, we studied the effect of treadmill exercise on markers of oxidative stress, PKC, D1R phosphorylation, D1R-G protein coupling, and Na+-K+-ATPase activity in RPTs of adult and old rats. We found increased levels of malondialdehyde, a marker of oxidative stress, in RPTs of old rats, which decreased during exercise. Nuclear levels of nuclear erythroid-related factor (Nrf)-2 and nuclear factor (NF)-κB in RPTs, transcription factors involved in antioxidant enzyme gene transcription, increased in exercised old rats. This was accompanied by an increase in the activity and expression of antioxidant enzymes, superoxide dismutase and heme oxygenase-1. Age-related decrease in the levels of D1R mRNAs and proteins was attenuated during exercise. Furthermore, exercise in old rats decreased PKC activity and D1R phosphorylation and increased SKF-38393-mediated [35S]guanosine 5′-O-(3-thiotriphosphate) binding (an index of D1R-G protein coupling). SKF-38393 also caused inhibition of Na+-K+-ATPase in these animals. Also, exercise caused a decrease in proteinuria and increase in phosphaturia in old rats. These results suggest beneficial effects of exercise in terms of increasing antioxidant defenses, decreasing oxidative stress, and improving kidney function in general and D1R function in particular in aging. Both Nrf-2 and NF-κB seem to play key role in this phenomenon.
Keywords: exercise, aging, D1 receptor, nuclear erythroid-related factor-2, nuclear factor-κB
renal dopamine D1 receptors play a key role in sodium homeostasis, especially during an increase in sodium intake (17, 27, 29). During salt overload, dopamine synthesis increases within the proximal tubules of the kidney, which via D1 receptor inhibits sodium transporters, Na+-K+-ATPase and Na+/H+ exchanger, resulting in an increase in sodium excretion and maintenance of sodium homeostasis (29, 45). Impaired renal D1 receptor signaling and function have been reported in aging, diabetes, and hypertension (24, 42, 47).
Aging is accompanied by a host of morphological and functional changes in several organ systems, including in the kidney (22, 39). Many of the degenerative changes observed during aging and the ensuing morbidity and mortality have been associated with an age-related increase in oxidative stress (1). Increase in oxidative stress seen in aging is associated with the age-related decline in renal dopamine D1 receptor function due, in part, to higher protein kinase C (PKC) activity and D1 receptor phosphorylation in the renal proximal tubules (RPTs) (2, 5). We have also reported that oxidative stress reduction by antioxidant tempol supplementation improves D1 receptor function in old rats (23).
Exercise is reported to reduce oxidative stress in rats and mice (4, 38). However, the mechanism as to how exercise reduces oxidative stress is not known. There are studies suggesting that moderate exercise by inducing the expression of antioxidant enzymes reduces oxidative stress (16, 25). Transcription factor nuclear erythroid-related factor (Nrf)-2 is an important mediator of the antioxidant defense mechanism that transcriptionally regulates several phase II antioxidant enzymes genes, including the glutathione S-transferase (GST), NAD(P)H-quinone reductase, γ-glutamylcysteine synthetase (GCS), superoxide dismutase (SOD), and heme oxygenase-1 (HO-1) (4, 25, 30, 33, 35, 41). Another transcription factor nuclear factor (NF)-κB, which is generally considered to be involved in the inflammatory processes (34), also is reported to mediate transcription of antioxidant enzymes such as MnSOD, GCS, and GST (25, 31, 32, 36).
In the present study, we investigated the effects of moderate exercise on oxidative stress and antioxidant enzymes in the plasma and RPTs of adult and old rats. Also, the effects of moderate exercise were studied on the activities of Nrf-2 and NF-κB in the RPTs of these animals. More importantly, we studied the effects of exercise on PKC activity, D1 receptor phosphorylation, and D1 receptor agonist SKF-38393-mediated stimulation of G proteins and inhibition of Na+-K+-ATPase in the RPTs. The effects of exercise on the above parameters were compared by including sedentary adult and old rats in the study. Furthermore, we also determined proteinuria and phosphaturia in the sedentary and exercised adult and old rats.
MATERIALS AND METHODS
Treadmill exercise.
Male Fischer (F344/NNiaHsd) rats 3 (adult) and 21 (old) mo of age, supplied by Harlan Sprague-Dawley (Indianapolis, IN), were purchased from the National Institute on Aging (Bethesda, MD). These animals were housed in the University Animal Care Facility, and the present study was approved by the Institutional Animal Care and Use Committee. The rats were acclimatized for 7 days before any experimental maneuver. Adult and old rats were subdivided into sedentary and exercised groups. The exercised groups of adult and old rats were subjected to a treadmill exercise for 12 wk (12 meter·min−1·60 min−1, 15 degree grade, 5 days/wk) according to a published protocol (4). The sedentary groups of adult and old rats were not exercised. All four groups of rats had free access to standard rodent chow and water.
Animal surgery.
The rats were anesthetized with pentobarbital sodium (50 mg/kg body wt) 48 h after the last exercise session along with the respective age-matched sedentary control rats. A tracheotomy was performed to assist ventilation, and a midline abdominal incision was made. The bladder urine was aspirated by a syringe and kept frozen at −80°C for further analysis. The abdominal aorta was cannulated with PE-50 tubing below the renal artery bifurcations, and blood was collected in EDTA-coated tubes. The kidneys were perfused with Krebs-Henseleit buffer (KHB) without calcium [KHB-B (in mM): 118 NaCl, 27.2 NaHCO3, 4 KCl, 0.12 MgCl2, 1 KH2PO4, 5 glucose, and 10 HEPES, pH 7.4]. In situ kidney digestion was performed with KHB-B buffer containing 230 U/ml g collagenase and 250 U/ml hyalurodinase. The kidneys were excised, and RPTs were isolated by a method routinely used in the laboratory (18). Plasma was isolated by centrifuging blood at 3,000 rpm for 15 min at 4°C.
PKC activity.
PKC activity was determined using a nonradioactive assay kit according to the manufacturer's protocol (Promega, Madison, WI).
SOD activity.
SOD activity was measured in the RPT homogenates using the Superoxide Dismutase Assay kit from Cayman Chemical (Ann Arbor, MI).
Measurement of malondialdehyde.
The levels of malondialdehyde (MDA) in the RPT homogenates were measured as thiobarbituric acid-reactive substances by a previously published method (23). Molar extinction coefficient, 1.56 X 105 M/cm, was used to quantify MDA.
Measurement of HO-1.
The levels of HO-1 in plasma and RPT homogenates were determined using rat HO-1 enzyme-linked immunosorbent assay kit from Assay Designs (Ann Arbor, MI).
Measurement of urinary protein.
Urinary protein levels were measured using the bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Rockford, IL) and BSA as standard.
Measurement of urinary creatinine.
The levels of creatinine in the urine were measured by Jaffe reaction using the method of Taussky (43). Briefly, 50 μl of 10% NaOH was added to 500 μl of diluted (1:100) urine samples. Saturated picric acid solution (200 μl) was added, and the mixture was incubated at room temperature for 20 min. The samples were then read at 520 nm, and the concentration was determined using a standard curve generated from known concentrations of creatinine.
Measurement of urinary phosphate.
The levels of inorganic phosphate in the urine were measured using the method of Taussky and Shorr (44).
[35S]5′-O-(3-thiotriphosphate) binding assay.
[35S]5′-O-(3-thiotriphosphate) (GTPγS) binding was performed as previously described (26). In brief, 5 μg renal proximal tubular membranes were incubated with vehicle or various concentration of SKF-38393 (10 pmol/l to 10 μmol/l) in the presence of [35S]GTPγS (final concn 6 nM) for 1 h at 30°C. Nonspecific [35S]GTPγS binding was determined in the presence of 100 μM GTP.
Na+-K+-ATPase activity.
Freshly isolated RPTs were used to determine Na+-K+-ATPase activity, as we have reported previously (10–12, 37). Briefly, RPTs (1 mg/ml) were incubated with vehicle (0.1% sodium metabisulfite) or SKF-38393 (final concn 10−9 M to 10−5 M) for 15 min at 37°C. The reaction was terminated by rapidly freezing the RPTs in liquid nitrogen. RPT suspension (1 mg protein/ml) was used to assay ouabain (1 mmol/l)-sensitive Na+-K+-ATPase activity using end-point phosphate hydrolysis of ATP (4 mmol/l) as an index. The inorganic phosphate that is released was colorimetrically measured.
Protein measurement.
Proteins were measured using the BCA protein assay kit and BSA as standards.
Isolation of nuclear and cytosolic proteins.
Nuclear and cytosolic fractions of RPTs were isolated as per the manufacturer's instructions using NE-PER nuclear and cytosolic extraction reagents (Thermo Scientific, Rockford, IL).
Measurement of transcription factors Nrf-2 and NF-κb.
Nuclear levels of Nrf-2 and NF-κB in RPTs were determined by Western blotting using specific Nrf-2 and NF-κB antibodies (Santa Cruz, Santa Cruz, CA) and horseradish peroxidase (HRP)-conjugated goat-anti-rabbit secondary antibodies.
Immunoprecipitation of D1 receptor.
Renal proximal tubular membranes were used to immunoprecipitate D1 receptor using specific D1 receptor antibody as published previously (5). Briefly, RPTs were homogenized in sucrose buffer [in mM: 250 sucrose, 10 Tris, 1 phenylmethylsulfonyl fluoride (PMSF), 1 orthovanadate, 2.5 sodium pyrophosphate, 1 β-glycerophosphate, and protease inhibitor cocktail, pH 7.4], and the membranes were isolated by differential centrifugation. The membranes (1.5 mg/ml) were suspended in immunoprecipitation (IP) buffer (in mM: 140 NaCl, 3 KCl, 10 Na2HPO4, 2 KH2PO4, 1 sodium orthovanadate, 1 PMSF, 1% Nonidet P-40, 0.5% sodium cholate, 0.1% SDS, and protease inhibitor cocktail, pH 7.4), incubated with protein A/G agarose beads for 30 min at 4°C, and centrifuged at 14,000 g. The supernatant (devoid of nonspecific proteins bound to agarose beads) was incubated with 2.5 μg of D1 receptor antibody (Millipore, Billerica, MA) for 2 h at 4°C followed by incubation with 50 μl agarose beads overnight at 4°C. The antigen-antibody-A/G agarose beads complex was settled down by centrifugation and washed two times with IP buffer and one time with 50 mM Tris·HCl, pH 8.0. The antigen-antibody complex was dissociated with Laemmli buffer (125 mM Tris·HCl, 4% SDS, 5% β-mercaptoethanol, and 20% glycerol) at 37°C for 1 h. The samples were vortexed and centrifuged at room temperature, and the supernatant was used for electrophoresis.
Detection of D1 receptor serine phosphorylation.
The immunoprecipitated D1 receptor samples (5 μl) were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% BSA in PBS with 0.05% Tween 20 for 2 h at room temperature and incubated with a specific phosphoserine antibody (clone 16B4; Calbiochem, Gibbstown, NJ) 1:400 in 2.5% BSA overnight at 4°C. HRP-conjugated secondary antibody was used to probe phosphoserine antibody, and an enhanced chemiluminescence reagent kit was used to visualize the bands (Alpha Diagnostics, San Antonio, TX). The membranes were then stripped using antibody stripping solution (Alpha Diagnostics) and reprobed for D1 receptor protein using specific D1 receptor antibody (Millipore).
D1 RT-PCR.
RPTs were stored in RNA preservation reagent at −80°C, and total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA) as per the manufacturer's instructions. RNA was then used to synthesize cDNA using the Advantage RT for PCR kit from Clontech (Mountain View, CA). Dopamine D1 receptor message was amplified using published gene specific primers (forward 5′-AGATCTCTTGGTGGCTGTC-3′ and reverse 5′-ATAATGGCTACGGGGATGT-3′) corresponding to nucleotide sequences 263–281 and 688–706, respectively (40). The amplified samples were run on 0.8% agarose gel containing ethidium bromide, and the bands were visualized using an ultraviolet transilluminator and quantified using Alpha Innotech software.
RESULTS
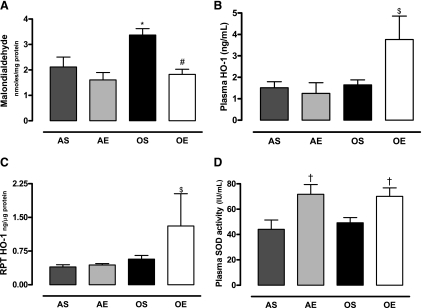
The levels of oxidative stress marker, MDA, were higher in the RPTs of sedentary old compared with adult rats. Exercise in old rats decreased the levels of MDA in the RPTs to the levels seen in adult rats (Fig. 1A). Antioxidant enzyme HO-1 levels increased in the plasma (Fig. 1B) and RPTs (Fig. 1C) of exercised old rats. Total SOD activity, another marker of antioxidant capacity, increased in the plasma of exercised adult and old rats (Fig. 1D).
Fig. 1.
Exercise decreases elevated malondialdehyde (MDA) levels in old rats, increases heme oxygenase (HO) levels in plasma and renal proximal tubules (RPT) of old rats, and increases total superoxide dismutase (SOD) activity in adult and old rats. AS, adult sedentary; AE, adult exercised; OS, old sedentary; OE, old exercised. A: MDA was measured in RPT homogenate as a marker of oxidative stress as described in materials and methods. B and C: HO-1 was measured in plasma (B) and RPT homogenate (C) using a HO-1 enzyme-linked immunosorbent assay kit. D: total SOD activity was determined using a SOD assay kit. Results are means ± SD; n = 4 or 5 animals in A–D. P < 0.05 vs. AS (*), vs. OS (#), vs. AS, AE, and OE ($), and vs. AS and OS (†).
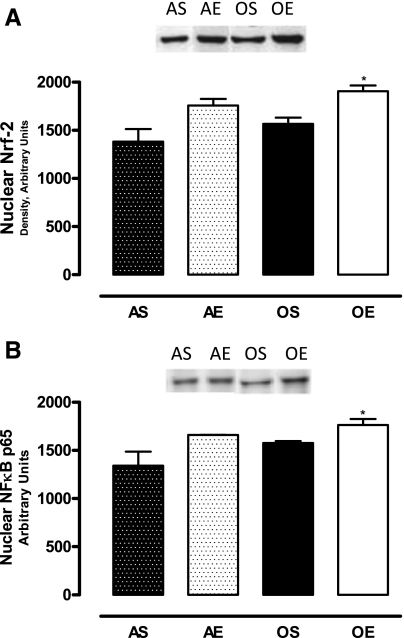
The nuclear levels of transcription factors Nrf-2 and NF-κB in the RPTs increased in exercised old rats (Fig. 2, A and B).
Fig. 2.
Exercise increases transcription factors nuclear erythroid-related factor (Nrf)-2 and nuclear factor (NF)-κB p65 in the nuclear fraction of RPTs of old rats. A: nuclear Nrf-2 levels in RPTs. B: nuclear NF-κB p65 levels in RPTs. Nuclear proteins (15 μg) (A and B) were resolved by SDS-PAGE and immunoblotted for Nrf-2 and NF-κB p65 using specific antibodies. Results are means ± SD; n = 4 animals/group. *P < 0.05 vs. AS.
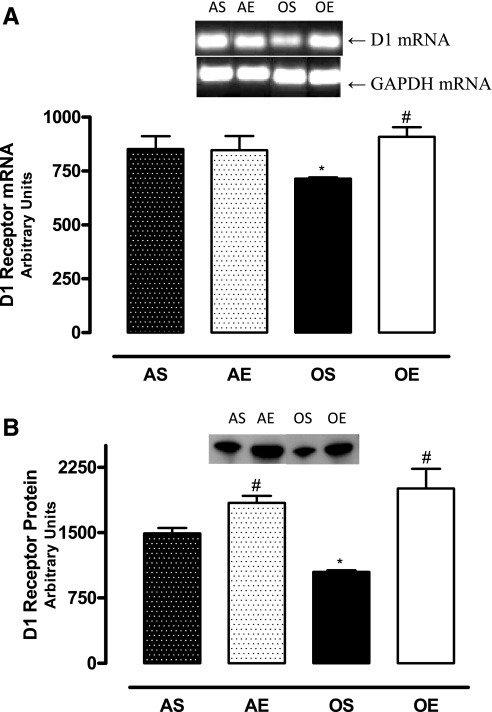
The D1 receptor mRNA levels were lower in the RPTs of sedentary old rats. Exercise increased the D1 receptor mRNA levels in old rats (Fig. 3A). Also, there were decreased levels of D1 receptor proteins in the RPT membranes of sedentary old rats, which increased with exercise in these animals (Fig. 3B).
Fig. 3.
Exercise increases the levels of D1 receptor mRNAs and proteins. A: D1 receptor mRNA was amplified using gene specific primers and resolved on 0.8% agarose gel. The bands were normalized by glyceraldehydes-3-phosphate dehydrogenase. B: 20 μg RPT membrane protein was resolved by SDS-PAGE, and D1 receptor proteins were determined by immunoblotting with specific antibody; n = 4 animals/group. Results are means ± SD. P < 0.05 vs. AS (*) and vs. OS (#).
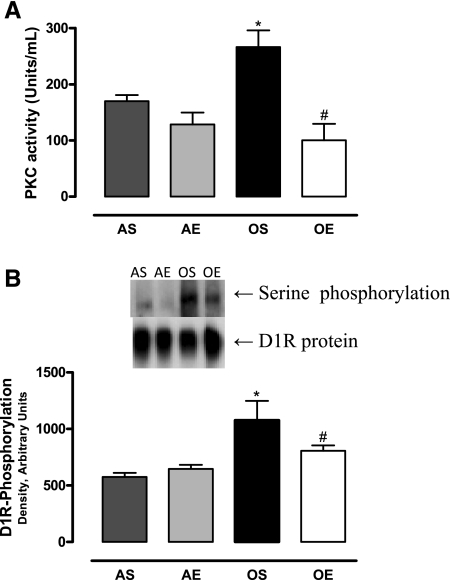
The basal PKC activity was higher in the RPTs of sedentary old compared with adult rats. The PKC activity in the RPTs decreased in exercised old rats (Fig. 4A). The levels of D1 receptor phosphorylation in the RPT membranes were higher in sedentary old rats, which decreased with exercise in these animals (Fig. 4B).
Fig. 4.
Exercise reduces the elevated protein kinase C (PKC) activity and D1 receptor phosphorylation in old sedentary rats. A: PKC activity was measured using the PepTag assay kit from Promega. B: D1 receptor protein was immunoprecipitated as described in materials and methods, and receptor phosphorylation was determined using a specific phosphoserine antibody; n = 4 animals/group. Results are means ± SD. P < 0.05 vs. AS (*) and vs. OS (#).
D1 receptor agonist SKF-38393 increased the [35S]GTPγS binding (Fig. 5A) and inhibited Na+-K+-ATPase activity (Fig. 5B) in the RPTs of adult rats. The SKF-38393-mediated increase in [35S]GTPγS binding (Fig. 5A) and inhibition of Na+-K+-ATPase (Fig. 5B) were reduced in sedentary old rats. The stimulation of [35S]GTPγS (Fig. 5A) and inhibition of Na+-K+-ATPase (Fig. 5B) by SKF-38393 were restored in exercised old rats.
Fig. 5.
Exercise increases [35S]5′-O-(3-thiotriphosphate) (GTPγS) binding and Na+-K+-ATPase inhibition in response to D1 receptor agonist (SKF-38393) in old exercised rats. A: [35S]GTPγS binding, an index of G protein activation, was measured in 5 μg RPT membranes. B: Na+-K+-ATPase measured in the RPTs as described in materials and methods; n = 5 animals/group. Results are means ± SD. P < 0.05 vs. AS (*), vs. OE (#), and vs. OS ($).
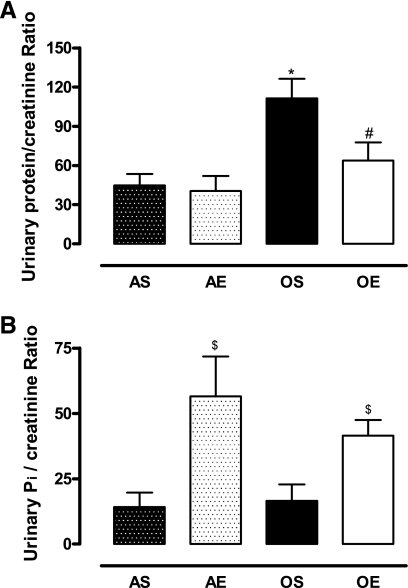
The levels of urinary proteins were higher in sedentary old rats, which decreased with exercise in these rats (Fig. 6A). Exercise increased the levels of urinary phosphate in adult and old rats (Fig. 6B).
Fig. 6.
Exercise decreases proteinuria and increases phosphaturia in Fischer 344 rats. A: urinary proteins levels normalized to urinary creatinine. B: urinary phosphate levels normalized to urinary creatinine. Total urinary proteins were measured using the bicinchoninic acid method. Urinary phosphate was measured using the Taussky-Shorr (44) method. Urinary creatinine was measured using the Jaffe method (43) as described in materials and methods. Results are means ± SD; n = 5 animals/group. P < 0.05 vs. AS (*), vs. OS (#), and vs. AS and OS ($).
DISCUSSION
Our study clearly demonstrates that exercise decreased oxidative stress, stimulated transcription factors (Nrf-2 and NF-κB), and increased antioxidant enzymes such as HO-1 and SOD. It also increased the levels of D1 receptor mRNAs and proteins in the RPTs of old rats. Furthermore, exercise decreased the age-related increase in PKC activity and D1 receptor phosphorylation and restored D1 receptor function in the RPTs of old rats. Moreover, exercise improved aspects of kidney function in terms of reducing proteinuria and phosphate retention.
Exercise is known to reduce morbidity and mortality, improve physiological outcomes, mitigate functional impairments, and prevent the progression of chronic disease in the elderly (14). Exercise is also known to increase antioxidant enzymes, reduce oxidative stress, and improve endothelial function and reduce coronary artery disease risk (20). Previously, we reported an age-related increase in oxidative stress in F344/NNiaHsd rats that was associated with renal D1 receptor dysfunction in old rats (23). In this study, we found that exercise decreased the levels of oxidative stress in old (24-mo) rats. However, there is study demonstrating that beneficial effects of exercise in terms of decreasing oxidative stress were apparent in 52-wk (13-mo)-old mice but not in 78-wk (19.5-mo)-old mice (37). The failure of exercise training to reduce oxidative stress in 78-wk mice is not known. However, we found that exercise training in 24-mo-old rats reduces oxidative stress. The possible explanation for this discrepancy could be because of a different animal species and exercise protocol used in our studies. Navarro et al. (38) in their studies used an exercise protocol (treadmill speed of 10, 15, and 20 cm/s for 5 min/wk for 78 wk) in mice starting at 28 wk. We used a different exercise protocol (treadmill speed of 12 meter·min−1·60 min−1, 15 degree grade, 5 days/wk for 3 mo) in adult and old Fischer 344 rats starting at 3 and 21 mo, respectively.
HO-1, an inducible antioxidant enzyme capable of catalyzing the conversion of heme to bilirubin (32), was upregulated with exercise in old rats. Bilirubin is also a potent antioxidant capable of combating oxidant species (32). An increase in HO-1 with exercise in old rats might represent a compensatory mechanism to fight the elevated oxidative stress in aging. Exercise increased HO-1 in the plasma and RPTs of old rats but not in the adult rats. The SOD activity increased similarly in the plasma of adult and old rats. Greater reduction in oxidative stress observed in old exercised rats might be mediated by the upregulation of two antioxidant enzymes, namely HO-1 and SOD, compared with SOD alone in adult exercised rats. Although the reason for this is not known, the greater response of exercise on antioxidant enzyme regulation in old rats may be to combat a higher degree of oxidative stress in these animals (22). It should be noted that higher oxidative stress in old rats is not the result of impaired antioxidant mechanism in these animals. Rather, an age-related increase in one of the subunits of renal NADPH oxidase seems to contribute to higher oxidative stress in old rats (3).
Both HO-1 and SOD are regulated by the transcription factors Nrf-2 and NF-κB (25, 28, 32). These transcriptions factors were upregulated with exercise in both adult and old rats, albeit significantly only in old rats. Nrf-2 is known to bind to antioxidant response elements on genes of a myriad of enzymes such as HO-1, SOD, GST, GCS, and catalase (4, 25, 30, 33, 35, 41). On the other hand, the role of NF-κB as a transcription factor capable of upregulating antioxidant enzymes such as HO-1, MnSOD, GCS, and GST has only been recognized recently (25, 31, 32, 36). There are reports indicating that exercise upregulates NF-κB and is beneficial in antioxidant enzyme induction (13, 25). We found that exercise mediated activation of Nrf-2 and NF-κB by 29 and 25%, respectively, in the RPTs of old rats. These increases in the levels of Nrf-2 and NF-κB together, as opposed to alone, may be sufficient to upregulate antioxidant defenses, such as HO-1 (Fig. 1C), resulting in the reduction of oxidative stress and restoration of D1 receptor function in the RPTs of exercised old rats. It should be mentioned that the nuclear fractions used in the present study were enriched with nuclear proteins. This was confirmed by detecting histone deacetylase (HDAC), a nuclear marker, in cytosolic and nuclear fractions by Western blotting. The levels of HDAC were higher in nuclear than in cytosolic fractions (data not shown). However, we failed to use HDAC as loading control for the reason that the amounts of nuclear proteins (15 μg) used to detect Nrf-2 and NF-kB resulted in a higher background signal for HDAC posing difficulty for its quantification.
Our previous studies had shown that the age-related decrease in D1 receptor and G protein coupling contributed to diminished Na+-K+-ATPase inhibition and natriuretic response to dopamine (15). Earlier, we have shown that exercise restores the natriuretic response to SKF-38393 in old rats (4). However, the mechanisms involved in the restoration of this response following exercise were not studied. Therefore, the present study was undertaken to examine the effects of exercise on D1 receptor function in RPTs in both adult and old rats. Here we show that exercise restores D1 receptor-G protein coupling and Na+-K+-ATPase inhibition in response to D1 receptor agonist SKF-38393 in old animals. D1 receptor-G protein coupling was not affected by exercise in adult animals. Exercise also resulted in increased D1 receptor protein in the RPT membranes of adult and old rats and normalized D1 receptor mRNA in old exercised rats. Also, there was a trend of exercise-induced increase in D1 receptor protein in the RPT homogenates (data not shown).
Previous work from our laboratory has shown that oxidative stress increases higher basal serine phosphorylation via the PKC-G protein-coupled receptor kinase (GRK2)-dependent pathway and leads to D1 receptor dysfunction in old animals (5, 8, 9). Antioxidant supplementation to old rats reduced PKC and GRK2 activities, decreased D1 receptor phosphorylation, and restored D1 receptor function in RPTs of old rats (2, 5). In the present study, although we did not measure GRK2 activity, we found that exercise reduced PKC activity and the D1 receptor phosphorylation in old rats. The exercise-induced reduction of oxidative stress may cause the mitigation in PKC activity and subsequently a decrease in D1 receptor phosphorylation in old rats. The reduction in basal D1 receptor phosphorylation may be responsible for the restoration of D1 receptor function as measured by increased [35S]GTPγS binding and Na+-K+-ATPase inhibition in response to D1 receptor agonist in the RPTs of old rats.
Another finding to note is that exercise decreased proteinuria in old animals and improved phosphate excretion in adult and old rats. Proteinuria and phosphate retention are complications of chronic kidney disease that worsen the quality of life and increase the disease burden (21, 30b). Phosphate retention in patients with advanced kidney disease is treated with dietary phosphate restriction and phosphate binders to prevent complications such as calcium × phosphate product deposition in vascular beds and soft tissues, secondary hyperparathyroidism, and renal osteodystrophy (19, 30a). Proteinuria in chronic kidney disease is a prognostic indicator and signals the rate of disease progression (21). The mechanism of reduced proteinuria during exercise in 24-mo-old Fischer 344 rats is not known. It is likely that exercise has improved the glomerular basement membrane (GBM) filtration mechanism leading to reduced proteinuria in old rats. In this regard, it should be noted that nephrin, an integral protein of podocyte, a key cell that supports and maintains the GBM filtration mechanism, is reduced in 24-mo-old Fischer 344 rats. The reduced levels of nephrin have been implicated to cause proteinuria in these rats (46). It is likely that exercise induces an increase in the levels of nephrin, which may be the mechanism of reduced proteinuria in exercised old rats that needs to be determined. Nevertheless, moderate exercise as a treatment modality that could potentially decrease phosphate retention and proteinuria in kidney disease is a promising finding in the present study.
In conclusion, exercise-mediated upregulation of antioxidant enzymes and subsequent reduction of oxidative stress lead to normalization of PKC activity and D1 receptor phosphorylation, which results in restoration of D1 receptor function in old rats. Redox-sensitive transcription factors, Nrf-2 and NF-κB, could be the pivotal players in reducing the age-related increase in oxidative stress, since they can signal the seminal event in the cascade of antioxidant enzyme upregulation. Further experiments are needed to elucidate the precise role these transcription factors play in reducing oxidative stress and restoring renal D1 receptor function.
GRANTS
This work was supported by National Institute on Aging Grants AG-25056 (to M. F. Lokhandwala) and AG-029904 (to M. Asghar).
REFERENCES
- 1.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90: 7915–7922, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asghar M, Banday AA, Fardoun RZ, Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G-protein-coupled receptor kinase 2. Free Radic Biol Med 40: 13–20, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Asghar M, Chillar A, Lokhandwala MF. Renal proximal tubules from old Fischer 344 rats grow into epithelial cells in cultures and exhibit increased oxidative stress and reduced D1 receptor function. Am J Physiol Cell Physiol 295: C1326–C1331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol 293: F914–F919, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Asghar M, Hussain T, Lokhandwala MF. Higher basal serine phosphorylation of D1A receptors in proximal tubules of old Fischer 344 rats. Am J Physiol Renal Physiol 283: F350–F355, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Asghar M, Lokhandwala MF. Antioxidant supplementation normalizes elevated protein kinase C activity in the proximal tubules of old rats. Exp Biol Med (Maywood) 229: 270–275, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Asghar M, Lokhandwala MF. Antioxidant tempol lowers age-related increases in insulin resistance in Fischer 344 rats. Clin Exp Hypertens 28: 533–541, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopamine-mediated inhibition of renal Na,K-ATPase is reduced by insulin. Hypertension 41: 1353–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Banday AA, Hussain T, Lokhandwala MF. Renal dopamine D1 receptor dysfunction is acquired and not inherited in obese Zucker rats. Am J Physiol Renal Physiol 287: F109–F116, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension 51: 367–375, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bar-Shai M, Carmeli E, Ljubuncic P, Reznick AZ. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-kappaB activation. Free Radic Biol Med 44: 202–214, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil 85: S31–S34, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Beheray S, Kansra V, Hussain T, Lokhandwala MF. Diminished natriuretic response to dopamine in old rats is due to an impaired D1-like receptor-signaling pathway. Kidney Int 58: 712–720, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol 43: 175–197, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Carey RM. Theodore Cooper Lecture: renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension 38: 297–302, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Beach RE, Lokhandwala MF. Dopamine fails to inhibit renal tubular sodium pump in hypertensive rats. Hypertension 21: 364–372, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Dillon M, Burke SK, Steg M, Bleyer AJ, Garrett BN, Domoto DT, Wilkes BM, Wombolt DG, Slatopolsky E. A randomized trial of sevelamer hydrochloride (RenaGel) with and without supplemental calcium. Strategies for the control of hyperphosphatemia and hyperparathyroidism in hemodialysis patients. Clin Nephrol 51: 18–26, 1999 [PubMed] [Google Scholar]
- 20.Edwards DG, Schofield RS, Lennon SL, Pierce GL, Nichols WW, Braith RW. Effect of exercise training on endothelial function in men with coronary artery disease. Am J Cardiol 93: 617–620, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, Steffes MW, Toto R. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis 42: 617–622, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Epstein M. Aging and the kidney. J Am Soc Nephrol 7: 1106–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Fardoun RZ, Asghar M, Lokhandwala M. Role of oxidative stress in defective renal dopamine D1 receptor-G protein coupling and function in old Fischer 344 rats. Am J Physiol Renal Physiol 291: F945–F951, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA 99: 3872–3877, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44: 126–131, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Hussain T, Lokhandwala MF. Renal dopamine DA1 receptor coupling with G(S) and G(q/11) proteins in spontaneously hypertensive rats. Am J Physiol Renal Physiol 272: F339–F346, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 228: 134–142, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Johnson J, Maher P, Hanneken A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Invest Ophthalmol Vis Sci 50: 2398–2406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jose PA, Eisner GM, Felder RA. Renal dopamine and sodium homeostasis. Curr Hypertens Rep 2: 174–183, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal 7: 1664–1673, 2005 [DOI] [PubMed] [Google Scholar]
- 30a.K/DOQI Clinical practice guidelines for bone metabolism, and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003 [PubMed] [Google Scholar]
- 30b.K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 31.Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Korthuis RJ. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol 68: 2337–2343, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Lavrovsky Y, Song CS, Chatterjee B, Roy AK. Age-dependent increase of heme oxygenase-1 gene expression in the liver mediated by NFkappaB. Mech Ageing Dev 114: 49–60, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O'Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J 20: 2624–2626, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol 30: 285–296, 2002 [DOI] [PubMed] [Google Scholar]
- 35.McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res 61: 3299–3307, 2001 [PubMed] [Google Scholar]
- 36.Morceau F, Duvoix A, Delhalle S, Schnekenburger M, Dicato M, Diederich M. Regulation of glutathione S-transferase P1-1 gene expression by NF-kappaB in tumor necrosis factor alpha-treated K562 leukemia cells. Biochem Pharmacol 67: 1227–1238, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Narkar V, Hussain T, Lokhandwala M. Role of tyrosine kinase and p44/42 MAPK in D2-like receptor-mediated stimulation of Na+,K+-ATPase in kidney. Am J Physiol Renal Physiol 282: F697–F702, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol 286: R505–R511, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Niedermuller H, Hofecker G, Skalicky M. The change in the supply with reduction equivalents in different organs of the rat during aging. Z Gerontol 24: 61–65, 1991 [PubMed] [Google Scholar]
- 40.O'Connell DP, Aherne AM, Lane E, Felder RA, Carey RM. Detection of dopamine receptor D1A subtype-specific mRNA in rat kidney by in situ amplification. Am J Physiol Renal Physiol 274: F232–F241, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol 62: 606–612, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension 33: 1036–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Taussky HH. A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem 208: 853–861, 1954 [PubMed] [Google Scholar]
- 44.Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem 202: 675–685, 1953 [PubMed] [Google Scholar]
- 45.Vieira-Coelho MA, Hussain T, Kansra V, Serrao MP, Guimaraes JT, Pestana M, Soares-Da-Silva P, Lokhandwala MF. Aging, high salt intake, and renal dopaminergic activity in Fischer 344 rats. Hypertension 34: 666–672, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics 19: 233–246, 2004 [DOI] [PubMed] [Google Scholar]