Abstract
C-peptide reduces diabetes-induced glomerular hyperfiltration in diabetic patients and experimental animal models. However, the mechanisms mediating the beneficial effect of C-peptide remain unclear. We investigated whether altered renal afferent-efferent arteriole tonus or alterations in tubular Na+ transport (TNa) in response to C-peptide administration mediate the reduction of diabetes-induced glomerular hyperfiltration. Glomerular filtration rate, filtration fraction, total and cortical renal blood flow, total kidney O2 consumption (Qo2), TNa, fractional Na+ and Li+ excretions, and tubular free-flow and stop-flow pressures were measured in anesthetized adult male normoglycemic and streptozotocin-diabetic Sprague-Dawley rats. The specific effect of C-peptide on transport-dependent Qo2 was investigated in vitro in freshly isolated proximal tubular cells. C-peptide reduced glomerular filtration rate (−24%), stop-flow pressure (−8%), and filtration fraction (−17%) exclusively in diabetic rats without altering renal blood flow. Diabetic rats had higher baseline TNa (+40%), which was reduced by C-peptide. Similarly, C-peptide increased fractional Na+ (+80%) and Li+ (+47%) excretions only in the diabetic rats. None of these parameters was affected by vehicle treatments in either group. Baseline Qo2 was 37% higher in proximal tubular cells from diabetic rats than controls and was normalized by C-peptide. C-peptide had no effect on ouabain-pretreated diabetic cells from diabetic rats. C-peptide reduced diabetes-induced hyperfiltration via a net dilation of the efferent arteriole and inhibition of tubular Na+ reabsorption, both potent regulators of the glomerular net filtration pressure. These findings provide new mechanistic insight into the beneficial effects of C-peptide on diabetic kidney function.
Keywords: diabetes mellitus, oxygen consumption, sodium excretion
there are several hypotheses for the mechanisms leading to the onset and progression of diabetes-induced renal dysfunction. The Diabetes Control and Complication Trial concluded that the degree of hyperglycemia is an important predictor of long-term renal complications in diabetes (7). The early augmented glomerular filtration may play a crucial role in the development of diabetic nephropathy, and inhibition of the diabetes-induced hyperfiltration has been shown to have beneficial effects, delaying the progression of kidney damage (33).
C-peptide, the 31-residue cleavage product of insulin synthesis, is secreted from the islets of Langerhans together with insulin. Since impaired insulin synthesis will affect C-peptide release to the same extent, C-peptide was initially used as a biomarker for estimation of insulin production. However, in the early 1980s, it was suggested that C-peptide may influence biological functions (59). Several recent studies have reported renoprotective effects of C-peptide when it is administered to patients with type 1 diabetes (13, 19, 20) and animal models of experimental insulinopenic diabetes (16, 36, 47–49, 51). The studies show that administration of physiologically relevant doses of C-peptide reduces diabetes-induced glomerular hyperfiltration, decreases albuminuria, reduces renal hypertrophy, and normalizes glomerular volume. Therefore, the lack of C-peptide has emerged as a possible mechanism for the development of diabetes-induced complications in diabetic patients. Peptides with the same amino acid composition as C-peptide, but in randomized sequences, have no interfering specificity (2, 35, 51).
The exact mechanism resulting in the diabetes-induced glomerular hyperfiltration is not fully understood (46). However, altered vascular tone of the afferent and efferent arterioles and increased proximal tubular Na+ reabsorption, all potential regulators of the glomerular filtration pressure and, thus, glomerular filtration rate (GFR), may contribute (46, 54). Nevertheless, glomerular hyperfiltration increases tubular electrolyte load, resulting in increased tubular Na+ reabsorption to maintain homeostasis. This will undoubtedly increase the O2 demand and, thereby, O2 consumption (Qo2) (27). A wide range of different techniques and animal models have been used to show that the increased O2 utilization eventually results in decreased tissue O2 availability in the diabetic kidney (9, 18, 23, 39, 40, 44, 45).
Na+-K+-ATPase is located in the basolateral membrane of tubular cells and creates the main driving force for tubular electrolyte reabsorption (24). Approximately 80% of total kidney Qo2 is related to tubular electrolyte transport (4). Numerous studies have reported increased renal Na+-K+-ATPase activity and increased Qo2 during the early hyperfiltration phase in streptozotocin (STZ)-induced diabetes (5, 27, 28, 58) and in other models of experimental diabetes (50). It has even been suggested that alterations in renal proximal Na+-K+-ATPase activity may regulate GFR (30). Thus regulation of GFR, tubular electrolyte handling, and renal blood flow (RBF) are interconnected via several mechanisms that must be investigated separately for a full understanding of how C-peptide might influence kidney function.
The present study aimed to provide mechanistic insights into the effect of C-peptide on GFR. We therefore tested the hypothesis that C-peptide reduces the diabetes-induced glomerular hyperfiltration via alteration of vascular tone of the afferent and/or efferent arterioles (measured as blood flow and tubular pressures) or proximal tubular Na+ reabsorption (measured as fractional urinary Na+ and Li+ excretions and inhibition of cellular Qo2 related to electrolyte transport), or a combination thereof.
MATERIALS AND METHODS
Animals and induction of diabetes.
Age-matched male Sprague-Dawley rats (250–330 g body wt) were purchased from M & B (Ry, Denmark). Animals had free access to water and standard rat chow (0.3% Na, 0.8% K, 21% protein; R3, Ewos, Södertälje, Sweden) throughout the study. All experiments were performed in accordance with the National Institutes of Health guidelines for use and care of laboratory animals and approved by the Animal Care and Use Committee of Uppsala University. Diabetes was induced by an injection of STZ (50 mg/kg body wt; Sigma-Aldrich, St. Louis, MO) into the tail vein. Animals were considered diabetic if blood glucose concentrations increased to >18 mM within 24 h after STZ injection and remained elevated. Test reagent strips (MediSense, Bedford, MA) were used to determine blood glucose concentrations from blood samples obtained from the cut tip of the tail in all animals.
Surgical procedures.
At 2 wk after allocation to the study, the animals were anesthetized with thiobutabarbital (120 mg/kg body wt ip; Inactin, Sigma-Aldrich), placed on a thermocontrolled operating table at 37°C, and tracheotomized. Polyethylene catheters were placed in the right femoral vein for infusion of Ringer solution (5 and 10 ml·kg body wt−1·h−1 for normoglycemic controls and diabetic animals, respectively), the right femoral artery for blood pressure measurements (model P23dB, Statham Laboratories, Los Angeles, CA), and the left renal vein for blood sampling. The left ureter was catheterized for collection of urine for subsequent analysis, and the urinary bladder was catheterized to allow urinary drainage. The left kidney was exposed by a left subcostal flank incision, immobilized in a plastic cup, and embedded in pieces of saline-soaked cotton wool, and the surface was covered with paraffin oil (Apoteksbolaget, Gothenburg, Sweden).
Simultaneous measurements of GFR, total and cortical RBF, and total Qo2.
GFR was estimated by measurements of [3H]inulin clearance. 3H (American Radiolabeled, St. Louis, MO) was initially administered as a 185-kBq bolus and then continuously infused in Ringer solution (185 kBq·kg body wt−1·h−1). 3H activities in urine and plasma were measured using a standard liquid scintillation technique, and GFR was calculated as follows: GFR = U*V̇/P, where U and P denote the activity of [3H]inulin in the urine and plasma, respectively, and V̇ denotes the urine flow rate (in ml/min). After a 45-min recovery period, baseline measurements were obtained for 30 min. A single bolus dose of rat C-peptide (5 nmol/kg body wt; Sigma-Genosys, Cambridge, UK; >95% purity as measured by HPLC) was followed by a 40-min continuous infusion of C-peptide (50 pmol·kg body wt−1·min−1), which should result in plasma C-peptide concentrations within the physiological range (51). Thereafter, all parameters were measured during a second 30-min experimental period. Additional control and diabetic animals were given only saline vehicle and served as time controls, since the scrambled C-peptide has previously been shown to have no physiological effects (2, 35, 51).
Total RBF was measured using an ultrasound probe (Transonic Systems, Ithaca, NY) placed around the left renal artery, and cortical RBF was measured with laser-Doppler flowmetry (model PF 4001-2, Perimed, Stockholm, Sweden) (10). All measured parameters were continuously recorded with a MacLab instrument (AD Instruments, Hastings, UK) connected to a Macintosh Power-PC 6100. Blood gas parameters were analyzed on samples drawn from the left renal vein and femoral artery during the first and second measurement periods. Kidney weights were determined at the end of the experiment. Urine volumes were measured gravimetrically, and urinary Na+ concentrations were determined by flame spectrophotometry (model IL543, Instrumentation Lab, Milan, Italy).
Estimations of proximal tubular reabsorption using Li+ clearance.
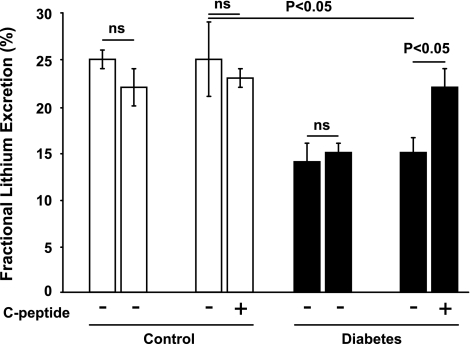
In a separate set of control and diabetic rats (n = 7–8/group), Li+ clearance was measured according to a previously published procedure (32). Briefly, the animals were subjected to the surgical protocol described above (see Surgical procedures), except only the bladder was catheterized for urine collection, and the subcostal flank incision and the immobilization of the kidney with the plastic cup were not performed. Li+ was administered as a 4-mg ip bolus of LiCl at the onset of anesthesia followed by a continuous intravenous infusion (2.1 mg·h−1·rat−1), resulting in plasma concentration of 0.2–0.4 mM. After surgery, the experimental protocol described above was performed for measurements of Li+ and inulin clearance in these animals. Since C-peptide reduces GFR and, therefore, tubular Li+ load via the same mechanism, estimations of proximal tubular reabsorption were calculated as fractional urinary Li+ excretion.
Calculations.
The filtration fraction (FF) was estimated as follows: FF = GFR/RBF*(1 − Hct). Renal vascular resistance (RVR) was calculated as mean arterial pressure (MAP) divided by RBF. In vivo renal Qo2 (μmol/min) was estimated from the arteriovenous difference in O2 content (O2ct = [Hb]*oxygen saturation*1.34 + Po2*0.22) * total RBF.
Tubular Na+ transport (TNa, μmol/min) was calculated as follows: TNa = [PNa]*GFR, where [PNa] is plasma Na+ concentration. TNa per Qo2 was calculated as TNa/Qo2. Fractional Na+ excretion was estimated from [UNa]*[Pinulin]/[PNa]*[Uinulin], and fractional Li+ excretion was calculated according to [ULi]*[Pinulin]/[PLi]*[Uinulin], where [UNa] is urinary Na+ concentration, [Pinulin] and [Uinulin] represent plasma and urinary inulin concentration, and [PLi] is plasma Li+ concentration.
Measurements of tubular pressures.
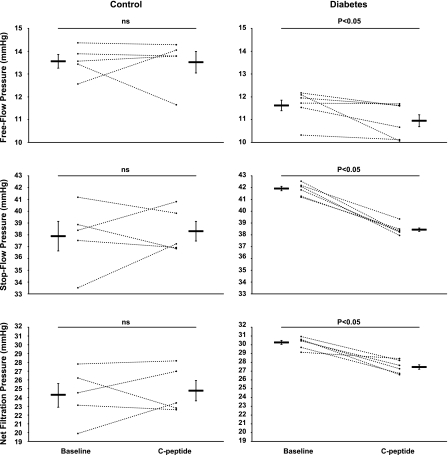
The net glomerular filtration pressure (Pnet) was estimated by stop-flow technique at baseline and after 40 min of infusion of rat C-peptide (5 nmol/kg body wt bolus + continuous infusion of 50 pmol·kg body wt−1·min−1 in 5 control and 6 diabetic animals). Early proximal tubular segments (≥5/rat) were randomly chosen on the kidney surface before and after C-peptide administration. A pipette, filled with 1 M NaCl and Lissamine green and connected to a servo-null pressure system (World Precision Instruments, New Haven, CT), was used to determine proximal tubular free-flow pressure (Pff) and stop-flow pressure (Psf), the latter after tubular flow was interrupted with a wax blockade distal to the pressure pipette. Pnet was calculated as Psf − Pff.
Measurements of Qo2 in vitro.
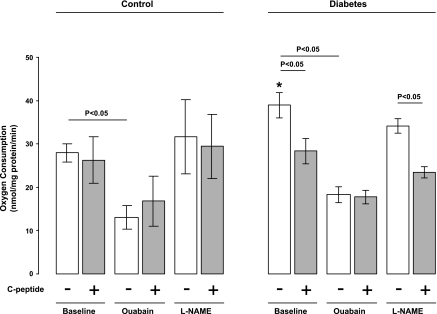
Proximal tubular cells were isolated from normoglycemic controls (n = 9) and STZ-diabetic rats (n = 12) as previously described (27, 39, 40). Briefly, the renal cortical tissue was minced through a metallic mesh strainer and immediately placed in an ice-cooled buffer solution and incubated in collagenase (0.05% wt/vol; Sigma-Aldrich) at 37°C for 60 min while the buffer was equilibrated with 95% O2-5% CO2. Thereafter, the cell suspension was cooled to 4°C and filtered through graded filters with pore sizes of 180, 75, 53, and 38 μm, respectively. The cells were pelleted by a slow centrifugation (100 g, 4 min) and resuspended in a collagenase-free buffer, a procedure that was repeated three times. The suspension was kept on ice until Qo2 was measured according to a previously described procedure (19). Briefly, a custom-made thermostatically controlled (37°C) gas-tight Plexiglas chamber with a total volume of 1.10 ml was used. The chamber was filled with buffer solution (113.0 mM NaCl, 4.0 mM KCl, 27.2 mM NaHCO3, 1.0 mM KH2PO4, 1.2 mM MgCl2, 1.0 mM CaCl2, 10.0 mM HEPES, 0.5 mM Ca lactate, 2.0 mM glutamine, and 50 U/ml streptomycin, 298 ± 2 mosM, pH 7.40) and continuously stirred with an air-driven magnetic stirrer. Glucose concentration in the medium was 5.8 mM for cells from normoglycemic controls and 23.2 mM for cells from diabetic animals. O2 content was measured by a modified O2 sensor (model 500, Unisense, Aarhus, Denmark) calibrated with the air-equilibrated buffer solution as 228 μM O2 and Na2S2O5-saturated buffer as zero. Cell suspension (100 μl) was then injected into the chamber, and the rate of O2 disappearance was measured. At the end of each experiment, a 100-μl sample was taken for determination of the protein concentration using DC protein assay (Bio-Rad Laboratories, Hercules, CA). Qo2 was calculated as the rate of O2 disappearance adjusted for protein concentration. Qo2 was measured during baseline and after incubation with rat C-peptide (1 nM), ouabain (1 mM), Nω-nitro-l-arginine methyl ester (l-NAME, 37 μM), or a combination of C-peptide and either ouabain or l-NAME.
Statistical evaluation.
All statistical analyses were performed using GraphPad Prism software (San Diego, CA). Multiple comparisons between different groups were performed using ANOVA followed by Fisher's protected least significant difference test. Multiple comparisons within the same group were performed using repeated-measures ANOVA followed by Dunnett's post hoc test for paired comparisons. For comparison before and after a treatment within the same animals, paired Student's t-test was used. Descriptive statistics are presented as means ± SE. For all comparisons, P < 0.05 was considered statistically significant.
RESULTS
All diabetic animals were hyperglycemic compared with normoglycemic controls (22.3 ± 1.4 vs. 5.8 ± 0.2 mM). Diabetic animals gained less weight than the age-matched normoglycemic controls (281 ± 5 vs. 330 ± 6 g). Left kidney weight increased in diabetic animals compared with normoglycemic controls (1.71 ± 0.03 vs. 1.24 ± 0.03 g).
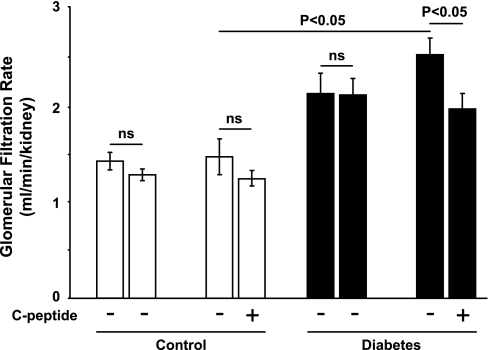
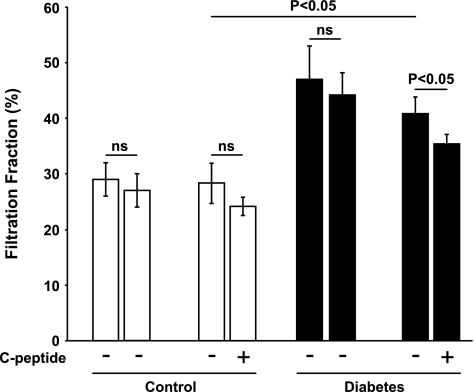
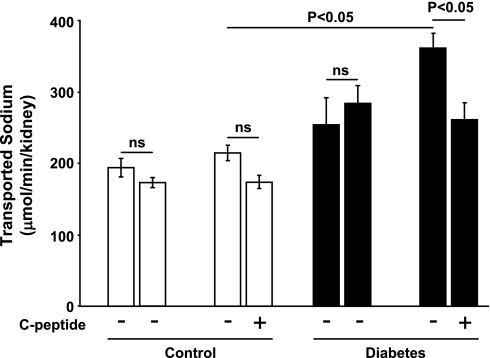
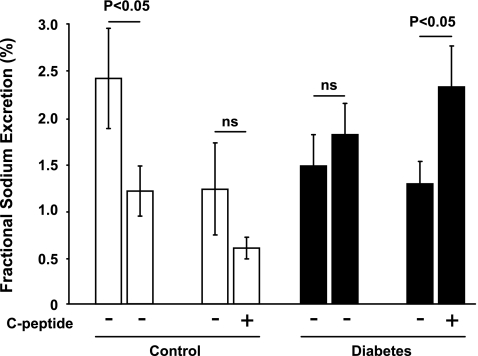
Diabetic rats displayed increased GFR (Fig. 1), which was reduced by C-peptide. FF was elevated in diabetic rats (Fig. 2) compared with controls. C-peptide selectively reduced FF by 17% in diabetic animals. MAP was similar at baseline in all groups (Table 1) and decreased in vehicle- and C-peptide-treated controls. However, MAP only decreased after C-peptide administration in the diabetic animals. Baseline TNa was 70% higher in diabetic animals than in controls (Fig. 3) and was only reduced by C-peptide in the diabetic group. Baseline fractional Na+ excretion was similar in all groups (Fig. 4), but C-peptide increased fractional Na+ excretion by 77% selectively in the diabetic group. Furthermore, fractional Li+ excretion increased only in the diabetic animals treated with C-peptide (Fig. 5).
Fig. 1.
Glomerular filtration rate in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means ± SE (n = 8–10/group). NS, not significant.
Fig. 2.
Filtration fraction in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means ± SE (n = 8–10/group).
Table 1.
MAP, total RBF, RVR, cortical RBF, in vivo renal O2 consumption, and TNa per O2 consumed in normoglycemic control and hyperglycemic diabetic rats acutely treated with vehicle or C-peptide
| n | MAP, mmHg | RBF, ml/min | RVR, mmHg·ml−1·min | Cortical RBF, LU | O2 Consumption, μmol/min | TNa/O2 Consumed | |
|---|---|---|---|---|---|---|---|
| Normoglycemic | 10 | ||||||
| Baseline | 119±4 | 9±1 | 15.0±1.3 | 425±26 | 12±1 | 16±1 | |
| Vehicle | 106±4* | 9±1 | 13.5±1.3* | 409±25 | 14±2 | 12±1* | |
| Normoglycemic | 8 | ||||||
| Baseline | 116±2 | 8±1 | 14.9±1.5 | 402±21 | 12±2 | 25±6 | |
| C-peptide | 108±2* | 9±1 | 12.4±0.9* | 430±33 | 16±3 | 16±4 | |
| Diabetic | 9 | ||||||
| Baseline | 122±4 | 9±1 | 14.3±1.3 | 370±19 | 17±2† | 17±3 | |
| Vehicle | 119±8 | 10±1 | 12.8±1.0* | 375±18 | 19±4 | 20±4 | |
| Diabetic | 9 | ||||||
| Baseline | 122±3 | 10±1 | 13.5±1.8 | 382±34 | 18±2† | 18±2 | |
| C-peptide | 113±3* | 10±1 | 12.2±1.5* | 406±33 | 20±3 | 15±3 |
Values are means ± SE. LU, laser units; MAP, mean arterial blood pressure; RBF, renal blood flow; RVR, renal vascular resistance; TNa, Na+ transport.
P < 0.05 vs. baseline in the same group.
P < 0.05 vs. normoglycemic at baseline.
Fig. 3.
Transported Na+ in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means ± SE (n = 8–10/group).
Fig. 4.
Fractional Na+ excretion in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means ± SE (n = 8–10/group).
Fig. 5.
Fractional Li+ excretion in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means ± SE (n = 7–8/group).
There was no difference in total or cortical RBF in the diabetic animals compared with the normoglycemic controls (Table 1). C-peptide did not alter RBF in any of the groups. Total in vivo Qo2 was ∼50% higher in the diabetic animals (Table 1), although Qo2 did not differ between diabetic and normoglycemic control rats during baseline conditions (Table 1). C-peptide did not affect total Qo2 or TNa/Qo2 (Table 1).
Infusion of vehicle did not alter any of the parameters in controls or diabetic animals, except for RVR, which decreased in all groups as a function of time, and TNa/Qo2, which decreased in the normoglycemic controls (Table 1).
C-peptide reduced Pff by 5.2% (11.6 ± 0.2 vs. 11.0 ± 0.2 mmHg), Psf by 8.4% (41.9 ± 0.3 vs. 38.4 ± 0.3 mmHg), and calculated Pnet by 9.6% (30.3 ± 0.4 vs. 27.4 ± 0.3 mmHg) in the diabetic animals, whereas it had no effect in controls (Fig. 6).
Fig. 6.
Tubular free-flow pressure (top), stop-flow pressure (middle), and calculated glomerular net filtration pressure (bottom) in control (n = 5) and diabetic (n = 6) rats before and after C-peptide. Values are means ± SE. P < 0.05 vs. control at baseline.
Baseline Qo2 was increased in isolated proximal tubular cells from diabetic animals compared with controls (39 ± 3 vs. 28 ± 2 nmol·mg protein−1·min−1; Fig. 7). C-peptide reduced Qo2 in diabetic cells (28 ± 3 nmol·mg protein−1·min−1) but had no effect on cells from normoglycemic controls (26 ± 5 nmol·mg protein−1·min−1). C-peptide had no effect on ouabain-pretreated cells (17 ± 6 vs. 18 ± 2 nmol·mg protein−1·min−1; Fig. 7). Pretreatment with l-NAME had no effect in any of the groups and did not alter the effect of C-peptide in the diabetic cells (Fig. 7).
Fig. 7.
O2 consumption in isolated proximal tubular cells from normoglycemic control (n = 9) and hyperglycemic diabetic (n = 12) rats at baseline and after incubation with C-peptide, ouabain, Nω-nitro-l-arginine methyl ester (l-NAME), or a combination of C-peptide and ouabain or l-NAME. Values are means ± SE. *P < 0.05 vs. untreated control at baseline.
DISCUSSION
The main new finding from the present study was that acute administration of C-peptide reduced GFR in diabetic rats via two seemingly different mechanisms. 1) C-peptide caused a net dilation of the efferent arteriole in the diabetic rats, as evident from decreased FF and Psf in the absence of altered RBF. 2) C-peptide inhibited tubular Na+ reabsorption in diabetic rats, as evident from increased fractional Na+ and Li+ excretions and reduced transport-dependent Qo2 in isolated proximal tubule cells from diabetic rats.
Acute alterations in GFR are determined by the glomerular capillary hydrostatic pressure (Pcap), the hydrostatic pressure in Bowman's space, and the oncotic pressure (ΔΠonc). Changes in Pcap are a result of alterations in the interplay of afferent and efferent arteriolar diameter, which alter the pressure transmitted to the glomerular capillaries (41, 43). Constriction of the afferent arteriole or dilation of the efferent arteriole will reduce Pcap and lower Pnet, which reduces GFR. Intratubular hydrostatic pressure is mainly determined by early proximal tubular reabsorption and, under certain conditions, by the hydraulic resistance in the distal nephron segments, e.g., obstruction of the tubules (22). ΔΠonc is determined by differences in the protein concentration between the blood in the glomerular capillaries and the fluid in Bowman's space. The main intrarenal factor influencing acute changes in ΔΠonc is the FF (3, 38), which reflects the balance between the afferent and efferent arterioles during normal conditions. Reduced FF will decrease the concentration of proteins in the blood that leaves the glomerulus through the afferent arteriole and, thus, reduce the driving force for reabsorption along the proximal part of the nephron into the peritubular capillaries, commonly referred to as the glomerulotubular balance.
Diabetes-induced glomerular hyperfiltration is reduced by C-peptide in patients with type 1 diabetes (19, 20) and in animal models of insulinopenic diabetes (16, 36, 48, 49, 51), with concomitant reduction of diabetes-induced renal damage (14, 16, 48). The glomerular arterioles exert critical actions to control Pcap, thereby influencing the GFR. We previously reported that C-peptide constricts isolated afferent glomerular arterioles from diabetic mice in vitro (35). A similarly pronounced constriction of the afferent arterioles in vivo with no effect on efferent arterioles would undoubtedly reduce GFR and RBF and increase RVR. However, C-peptide reduced the elevated GFR and FF in the diabetic rats in the present study, but did not affect RBF, which is consistent with previous reports (20, 49). None of these parameters were altered in the animals treated with vehicle only. Furthermore, RVR was not increased in the diabetic animals treated with C-peptide but, rather, decreased as a function of time in all groups. Taken together with our finding of reduced Psf after C-peptide administration, this can only be attributed to a balancing dilation of the efferent arteriole. Similar dual actions have been reported for nitric oxide (NO) synthase inhibitors, Ca2+ channel blockers, atrial natriuretic peptide, and adenosine (1, 11, 15, 31). However, given the lowering of Pnet, this mechanism could have a significant effect on the outcome for diabetic patients, since it lowers GFR and, thus, the tubular Na+ load while maintaining RBF.
C-peptide may also decrease diabetic hyperfiltration through inhibition of Na+-K+-ATPase activity, as evident from the increase in fractional urinary Na+ and Li+ excretions and decreased transport-independent Qo2 in isolated proximal tubular cells from diabetic rats. It has previously been postulated that diabetic hyperfiltration occurs as a result of primary hyperreabsorption upstream of the macula densa and the normal physiology of tubuloglomerular feedback (53, 54). However, it has been suggested that diabetes-induced glomerular hyperfiltration is caused by TGF-independent mechanisms (10, 46), although conflicting results have been reported (55). These studies utilize adenosine A1 receptor-deficient mice, which have been shown to lack the TGF mechanism, but additional TGF-independent effects of adenosine A1 receptors on afferent arteriolar tone complicate the interpretation of these findings. Also, the degree of hyperglycemia must be considered in a discussion of the potential role of TGF in diabetes-induced glomerular hyperfiltration (55). The diabetic rats displayed reduced proximal tubular Na+ reabsorption after C-peptide administration, and it is possible that the reduced GFR in these animals is caused by a TGF activation. Furthermore, earlier work demonstrated that tubular reabsorption directly influences hydraulic resistance in the proximal tubule and, therefore, affects GFR (30). It has been shown that sustained hyperglycemia in patients (56), as well as experimental animal models (27, 54), results in increased proximal tubular Na+ reabsorption secondary to increased Na+-linked glucose reabsorption. It has also been shown that early distal tubular Na+ concentrations are decreased during hyperglycemia, together with increased cortical Na+-K+-ATPase activity (27, 50). Conversely, decreased Na+ reabsorption in the early proximal tubule will increase hydraulic resistance, which in turn lowers Pnet and, therefore, reduces GFR. This hypothesis is consistent with reports of a linear correlation between GFR and Na+-K+-ATPase activity in the kidney cortex during experimental diabetes (27, 50). Therefore, a reduced or normalized Na+ reabsorption in the early proximal tubule is likely to reduce GFR in the diabetic kidney. The differences in the reduction of Psf (−8.4%) and Pff (−5.2%) in this study are not definite proof but indicate that proximal tubular reabsorption is involved in the decrease in GFR. No reduction was seen in total Na+ excretion after C-peptide, a finding that is consistent with previous studies (42). However, the reduction in tubular Na+ load, due to reduced GFR after C-peptide, will conceal the inhibitory effect on total urinary Na+ excretion. The calculated fractional Na+ and Li+ excretions increased in the diabetic animals after C-peptide administration, which shows that C-peptide directly inhibits Na+ reabsorption exclusively in the diabetic rats.
Electrolyte transport via Na+-K+-ATPase activity accounts for ∼80% of total Qo2 in the kidney (52). Therefore, diabetes-induced increase in Na+-K+-ATPase activity is likely to influence total Qo2 and, therefore, possibly limit renal O2 availability. The finding of increased cellular Qo2 in diabetic rats in the present study is consistent with previous reports (27, 39). However, the present study shows that C-peptide directly inhibits Qo2 in isolated proximal tubular cells but that the in vivo total kidney Qo2 was not altered by C-peptide administration, even though TNa decreased by ∼17%. A possible explanation can be a shift of the Na+ reabsorption site along the nephron after inhibition of proximal tubular Na+-K+-ATPase activity. Qo2/TNa is significantly less in the proximal tubule than in more distal parts of the nephron, since a notable part of the Na reabsorption in the proximal tubule is achieved via paracellular transport (29). This explanation would account for lack of reduced Qo2 in the diabetic rats in vivo after C-peptide administration.
Na+-K+-ATPase is inhibited by ouabain, although complete inhibition cannot be achieved in rats (12, 34). Ouabain treatment reduced cellular Qo2 in both groups. C-peptide had no further effect on ouabain-treated cells, which indicates that C-peptide reduces Na+-K+-ATPase activity.
C-peptide reduced blood pressure in the present study, similar to previously reported findings (36), although most studies show no such effect. However, it is predicted that increased NaCl load to the macula densa will decrease renin release and, therefore, angiotensin II production (25), and it is possible that reduced tubular Na+-K+-ATPase activity may reduce long-term arterial blood pressure, which should further add to the renoprotective effects of C-peptide. However, the effect of long-term C-peptide administration on arterial blood pressure control in diabetic patients is beyond the scope of this study.
The beneficial effects of C-peptide on diabetes mellitus have been observed in various tissues (14, 17). However, on the molecular level, the data may seem contradictory. In the present study, C-peptide decreased the ouabain-sensitive Qo2 of cells from diabetic rats but did not elicit an effect in cells isolated from normoglycemic rats. The inhibitory effect of C-peptide on isolated diabetic proximal tubular cells persisted also when the cells where pretreated with a nonselective NO synthase blocker, indicating that the mechanism does not involve a stimulated NO release with concomitant inhibition of mitochondrial respiration. Several previous studies report stimulatory effects of C-peptide on Na+-K+-ATPase, but these studies were performed under normoglycemic conditions (37, 60). Furthermore, Na+-K+-ATPase activities in neurons and erythrocytes are reduced during diabetes, and C-peptide normalizes these impairments (8, 13, 17). However, Na+-K+-ATPase activity in the kidney is elevated during the early onset of diabetes, probably due to the increased tubular electrolyte load resulting from the elevated GFR (6, 27, 57, 58). We report increased fractional urinary Na+ excretion and decreased transport-related cellular Qo2 after C-peptide administration, which demonstrates an inhibitory effect of C-peptide on Na+-K+-ATPase in the kidney. Indeed, the effects of C-peptide on endothelial NO synthase under normoglycemic conditions are different from those under hyperglycemic conditions (21, 26).
In conclusion, these results indicate that C-peptide reduces the diabetes-induced hyperfiltration via a net dilation of the efferent arteriole and inhibition of tubular Na+ reabsorption, both potent regulators of Pnet. These findings provide new mechanistic insight into the beneficial effects of C-peptide on diabetic kidney function.
GRANTS
This study was supported by the Swedish Medical Research Council, the Swedish Society for Medical Research, the Fredrik and Ingrid Thuring Foundation, the Marcus and Amalia Wallenberg Foundation, the Magnus Bergvall Foundation, and National Institute of Diabetes and Digestive and Kidney Diseases Grant K99/R00 DK-077858.
REFERENCES
- 1.Al-Mashhadi RH, Skott O, Vanhoutte PM, Hansen PB. Activation of A2 adenosine receptors dilates cortical efferent arterioles in mouse. Kidney Int 75: 793–799, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signalling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia 47: 987–997, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Troy JL. Postglomerular vascular protein concentration: evidence for a causal role in governing fluid reabsorption and glomerulotublar balance by the renal proximal tubule. J Clin Invest 50: 336–349, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodwall EK, Laake H. The relation between oxygen consumption and transport of sodium in the human kidney. Scand J Clin Lab Invest 16: 281–286, 1964 [PubMed] [Google Scholar]
- 5.Chen CC. Differentiation of renal Na+-K+-ATPase in control and streptozotocin-induced diabetic mice by G-protein acting toxins and phorbol esters. Eur J Pharmacol 225: 275–279, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Cohen RA, MacGregor LC, Spokes KC, Silva P, Epstein FH. Effect of myo-inositol on renal Na-K-ATPase in experimental diabetes. Metabolism 39: 1026–1032, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Djemli-Shipkolye A, Gallice P, Coste T, Jannot MF, Tsimaratos M, Raccah D, Vague P. The effects ex vivo and in vitro of insulin and C-peptide on Na/K adenosine triphosphatase activity in red blood cell membranes of type 1 diabetic patients. Metabolism 49: 868–872, 2000 [DOI] [PubMed] [Google Scholar]
- 9.dos Santos EA, Li LP, Ji L, Prasad PV. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol 42: 157–162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol 19: 722–730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng MG, Navar LG. Nitric oxide synthase inhibition activates L- and T-type Ca2+ channels in afferent and efferent arterioles. Am J Physiol Renal Physiol 290: F873–F879, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Feraille E, Barlet-Bas C, Cheval L, Rousselot M, Carranza ML, Dreher D, Arystarkhova E, Doucet A, Favre H. Presence of two isoforms of Na,K-ATPase with different pharmacological and immunological properties in the rat kidney. Pflügers Arch 430: 205–212, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Forst T, De La Tour DD, Kunt T, Pfutzner A, Goitom K, Pohlmann T, Schneider S, Johansson BL, Wahren J, Lobig M, Engelbach M, Beyer J, Vague P. Effects of proinsulin C-peptide on nitric oxide, microvascular blood flow and erythrocyte Na+,K+-ATPase activity in diabetes mellitus type I. Clin Sci (Lond) 98: 283–290, 2000 [PubMed] [Google Scholar]
- 14.Forst T, Kunt T, Pfutzner A, Beyer J, Wahren J. New aspects on biological activity of C-peptide in IDDM patients. Exp Clin Endocrinol Diabetes 106: 270–276, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T. Ca2+ channel subtypes and pharmacology in the kidney. Circ Res 100: 342–353, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Huang DY, Richter K, Breidenbach A, Vallon V. Human C-peptide acutely lowers glomerular hyperfiltration and proteinuria in diabetic rats: a dose-response study. Naunyn Schmiedebergs Arch Pharmacol 365: 67–73, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Ido Y, Vindigni A, Chang K, Stramm L, Chance R, Heath WF, DiMarchi RD, Di Cera E, Williamson JR. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science 277: 563–566, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Izuhara Y, Nangaku M, Inagi R, Tominaga N, Aizawa T, Kurokawa K, van Ypersele de Strihou C, Miyata T. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J Am Soc Nephrol 16: 3631–3641, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with type 1 diabetes mellitus. Diabet Med 17: 181–189, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Johansson BL, Sjoberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia 35: 121–128, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Kamikawa A, Ishii T, Shimada K, Makondo K, Inanami O, Sakane N, Yoshida T, Saito M, Kimura K. Proinsulin C-peptide abrogates type-1 diabetes-induced increase of renal endothelial nitric oxide synthase in rats. Diabetes Metab Res Rev 24: 331–338, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Karlsen FM, Holstein-Rathlou NH, Leyssac PP. A re-evaluation of the determinants of glomerular filtration rate. Acta Physiol Scand 155: 335–350, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Katavetin P, Miyata T, Inagi R, Tanaka T, Sassa R, Ingelfinger JR, Fujita T, Nangaku M. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J Am Soc Nephrol 17: 1405–1413, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Katz AI, Epstein FH. The role of sodium-potassium-activated adenosine triphosphatase in the reabsorption of sodium by the kidney. J Clin Invest 46: 1999–2011, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol 290: F1016–F1023, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kitamura T, Kimura K, Makondo K, Furuya DT, Suzuki M, Yoshida T, Saito M. Proinsulin C-peptide increases nitric oxide production by enhancing mitogen-activated protein-kinase-dependent transcription of endothelial nitric oxide synthase in aortic endothelial cells of Wistar rats. Diabetologia 46: 1698–1705, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 43: 629–633, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Ku DD, Sellers BM, Meezan E. Development of renal hypertrophy and increased renal Na,K-ATPase in streptozotocin-diabetic rats. Endocrinology 119: 672–679, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Larsen EH, Mobjerg N, Sorensen JN. Fluid transport and ion fluxes in mammalian kidney proximal tubule: a model analysis of isotonic transport. Acta Physiol (Oxf) 187: 177–189, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Leyssac PP. Dependence of glomerular filtration rate on proximal tubular reabsorption of salt. Acta Physiol Scand 58: 236–242, 1963 [DOI] [PubMed] [Google Scholar]
- 31.Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 324: 473–476, 1986 [DOI] [PubMed] [Google Scholar]
- 32.Miracle CM, Rieg T, Blantz RC, Vallon V, Thomson SC. Combined effects of carbonic anhydrase inhibitor and adenosine A1 receptor antagonist on hemodynamic and tubular function in the kidney. Kidney Blood Press Res 30: 388–399, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nath KA, Kren SM, Hostetter TH. Dietary protein restriction in established renal injury in the rat. Selective role of glomerular capillary pressure in progressive glomerular dysfunction. J Clin Invest 78: 1199–1205, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noel F, Fagoo M, Godfraind T. A comparison of the affinities of rat (Na+ + K+)-ATPase isozymes for cardioactive steroids, role of lactone ring, sugar moiety and KCl concentration. Biochem Pharmacol 40: 2611–2616, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Nordquist L, Lai EY, Sjoquist M, Patzak A, Persson AE. Proinsulin C-peptide constricts glomerular afferent arterioles in diabetic mice. A potential renoprotective mechanism. Am J Physiol Regul Integr Comp Physiol 294: R836–R841, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Nordquist L, Moe E, Sjoquist M. The C-peptide fragment EVARQ reduces glomerular hyperfiltration in streptozotocin-induced diabetic rats. Diabetes Metab Res Rev 23: 400–405, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Ohtomo Y, Aperia A, Sahlgren B, Johansson BL, Wahren J. C-peptide stimulates rat renal tubular Na+,K+-ATPase activity in synergism with neuropeptide Y. Diabetologia 39: 199–205, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Ott CE, Marchand GR, Diaz-Buxo JA, Knox FG. Determinants of glomerular filtration rate in the dog. Am J Physiol 231: 235–239, 1976 [DOI] [PubMed] [Google Scholar]
- 39.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 46: 1153–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Palm F, Hansell P, Ronquist G, Waldenstrom A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia 47: 1223–1231, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Persson AE, Gushwa LC, Blantz RC. Feedback pressure-flow responses in normal and angiotensin-prostaglandin-blocked rats. Am J Physiol Renal Fluid Electrolyte Physiol 247: F925–F931, 1984 [DOI] [PubMed] [Google Scholar]
- 42.Rebsomen L, Pitel S, Boubred F, Buffat C, Feuerstein JM, Raccah D, Vague P, Tsimaratos M. C-peptide replacement improves weight gain and renal function in diabetic rats. Diabetes Metab 32: 223–228, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Ren YL, Garvin JL, Ito S, Carretero OA. Role of neuronal nitric oxide synthase in the macula densa. Kidney Int 60: 1676–1683, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Ries M, Basseau F, Tyndal B, Jones R, Deminiere C, Catargi B, Combe C, Moonen CW, Grenier N. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging 17: 104–113, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Rosenberger C, Khamaisi M, Abassi Z, Shilo V, Weksler-Zangen S, Goldfarb M, Shina A, Zibertrest F, Eckardt KU, Rosen S, Heyman SN. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int 73: 34–42, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Sallstrom J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F. Diabetes-induced hyperfiltration in adenosine A1-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 190: 253–259, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Ekberg K, Isaksson B, Eriksson L, Wahren J, Sjoquist M. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrol Dial Transplant 20: 532–538, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Sjoquist M. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int 60: 1258–1265, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Samnegard B, Jacobson SH, Johansson BL, Ekberg K, Isaksson B, Wahren J, Sjoquist M. C-peptide and captopril are equally effective in lowering glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant 19: 1385–1391, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Scherzer P, Nachliel I, Bar-On H, Popovtzer MM, Ziv E. Renal Na-K-ATPase hyperactivity in diabetic Psammomys obesus is related to glomerular hyperfiltration but is insulin-independent. J Endocrinol 167: 347–354, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Sjoquist M, Huang W, Johansson BL. Effects of C-peptide on renal function at the early stage of experimental diabetes. Kidney Int 54: 758–764, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Thaysen JH, Lassen NA, Munck O. Sodium transport and oxygen consumption in the mammalian kidney. Nature 190: 919–921, 1961 [DOI] [PubMed] [Google Scholar]
- 53.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 286: F8–F15, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A1 receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol 111: p30–p38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vervoort G, Veldman B, Berden JH, Smits P, Wetzels JF. Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur J Clin Invest 35: 330–336, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Wald H, Scherzer P, Popovtzer MM. Enhanced renal tubular ouabain-sensitive ATPase in streptozotocin diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 251: F164–F170, 1986 [DOI] [PubMed] [Google Scholar]
- 58.Wald H, Scherzer P, Rasch R, Popovtzer MM. Renal tubular Na+-K+-ATPase in diabetes mellitus: relationship to metabolic abnormality. Am J Physiol Endocrinol Metab 265: E96–E101, 1993 [DOI] [PubMed] [Google Scholar]
- 59.Wojcikowski C, Maier V, Dominiak K, Fussganger R, Pfeiffer EF. Effects of synthetic rat C-peptide in normal and diabetic rats. Diabetologia 25: 288–290, 1983 [DOI] [PubMed] [Google Scholar]
- 60.Zhong Z, Kotova O, Davidescu A, Ehren I, Ekberg K, Jornvall H, Wahren J, Chibalin AV. C-peptide stimulates Na+,K+-ATPase via activation of ERK1/2 MAP kinases in human renal tubular cells. Cell Mol Life Sci 61: 2782–2790, 2004 [DOI] [PubMed] [Google Scholar]