Abstract
Kidney ischemia-reperfusion injury (IRI) is, in part, mediated by immune and inflammatory factors. Since microbial stimuli are known to alter immune and inflammatory responses, we hypothesized that differences in perinatal microbial status would modify renal injury following IRI. We performed bilateral renal IRI on 6-wk-old germ-free and control mice and studied the effects on kidney lymphocyte trafficking, cytokines, function, and structure. Compared with control mice, normal kidneys of germ-free mice exhibited more NKT cells and lower IL-4 levels. Postischemia, more CD8 T cells trafficked into postischemic kidneys of germ-free mice compared with control mice. Renal structural injury and functional decline following IRI were more severe in germ-free mice compared with control mice. When germ-free mice were conventionalized with the addition of bacteria to their diet, the extent of renal injury after IRI became equivalent to age-matched control mice, with similar numbers and phenotypes of T cells and NKT cells, as well as cytokine expression in both normal kidneys and postischemic kidneys of conventionalized germ-free mice and age-matched control mice. Thus microbial stimuli influence the phenotype of renal lymphocytes and the expression of cytokines of normal kidneys and also modulate the outcome of IRI.
Keywords: germ-free status, immune modulation
ischemia-reperfusion injury (IRI) is a leading cause of acute kidney injury (AKI) in both native and transplanted kidneys, worsening both early and late allograft outcomes (39). IRI-induced AKI is associated with a robust inflammatory response caused by both innate and adaptive immune components in tubulointerstitial tissues (12, 23). The concept of renal IRI as an inflammatory disease mediated by components of the immune system is supported by a large body of work demonstrating the renoprotective effects of therapy targeting complements, chemokines, and adhesion molecules (13, 34, 38), and also lymphocytes (26, 48). While most work to date has focused on the different components of the immune system, there has been minimal work on elucidating the basic drivers of the immune response and whether there is a role for environmental factors such as microbial stimuli on the tissue responses in kidney IRI.
Several epidemiological studies have revealed a steep increase in the prevalence of allergies and autoimmune diseases in Western societies over the past few decades (1, 29, 37, 42). The “hygiene hypothesis” was suggested to explain the increasing prevalence of allergic disease and proposed that reduced exposure to infections in early childhood due to several factors such as improved personal hygiene, standards of living, and diminished family size may have resulted in increased risk of allergic diseases (37). Two major mechanisms, missing immune deviation and reduced immune regulation, were proposed to explain the hygiene hypothesis (21, 28, 45, 46). The increased prevalence of autoimmune diseases was also linked to an altered immune system influenced by the change in the microbial burden (4). Lymphocytes, especially T cells, came to the forefront of studies investigating the relationship between the microbial burden and the changes in the immune system involved in the increased prevalence of allergy and autoimmune disease.
The pathophysiological role of T cells has been identified in renal IRI (7, 26, 48). Direct or indirect blockade of T cells attenuated renal injury both functionally and structurally in a murine renal IRI model. CD4 and CD8 double-knockout mice were significantly protected from early renal injury in vivo, while T cells exhibited increased adherence to renal tubular epithelial cells after in vitro hypoxia reoxygenation (26). Athymic nu/nu mice, another T cell knockout mouse strain, were also protected from initial injury, and the adoptive transfer of T cells into these mice reconstituted renal injury following IRI (5). T cell-targeting medications such as FK506 and mycophenolate mofetil were found to significantly attenuate renal injury after IRI, supporting the importance of T cells in the pathogenesis of renal IRI. T cells have also been demonstrated to participate in IRI of the liver, lung, brain, and intestine (49).
Based on the data that T cells are a mediator of renal IRI and that the immune response during renal IRI could have similar features to allergy and autoimmune disease, we hypothesized that “normal nonpathogenic” germ exposure could modify renal injury following IRI. We first examined the phenotype of lymphocytes and cytokine expression in normal kidneys of control and germ-free mice. We then compared kidney lymphocyte trafficking, structure, and function in germ-free vs. control mice after renal IRI. Finally, we “conventionalized” germ-free mice with microbes and examined how this changed the kidney IRI responses in these mice. These studies open a new line of investigation into kidney IRI that will focus on germ-immune interactions on the outcome of AKI.
MATERIALS AND METHODS
Mice and germ conventionalization technique.
Germ-free mice on a Swiss-Webster background were born by Caesarean section and raised in flexible plastic isolators using strict gnotobiotic techniques. Control Swiss-Webster mice were delivered by normal vaginal delivery and maintained in a conventional specific pathogen-free (SPF) environment. Male germ-free mice and male Swiss-Webster mice were purchased from Taconic Farms (Hudson, NY) and studied simultaneously. All experiment protocols were approved by the Institutional Animal Care and Use Committee. The first group of 6-wk-old germ-free (6W GF) and 6-wk-old control (6W control) mice was either killed immediately upon arrival or received bilateral IRI surgery after a 24-h acclimation period in a sterile biosafety hood. Mice that were killed were used to compare basal intrarenal-residing lymphocytes and cytokine levels (n = 7/group). Mice that received IRI surgery were maintained with autoclaved chow and water under the biosafety hood for 24 h, followed by death (n = 10/group). Functional and structural changes of 11-wk-old germ-free (11W GF) mice were also compared with the 6W GF mice at 24 h after bilateral IRI surgery. The second group of germ-free and control mice, which were the same age as the first group and also delivered at the same time, was kept in a conventional SPF environment for 5 wk. Fecal material from control mice was added to the germ-free mice with usual chow to conventionalize the germ-free mice. After 5 wk, conventionalized germ-free (11W CV-GF) mice and age-matched control (11W control) mice were either killed to analyze basal lymphocytes and cytokine changes (n = 5/group) or received bilateral IRI surgery with the same protocol as the first group (n = 10/group).
Quantification of microbial burden.
Stool from control, 6W GF, and 11W CV-GF mice was collected with rectal swabs using sterile cotton tips, weighed, and diluted with sterile saline. Stool (8 mg) was inoculated in fluid thioglycollate medium (BD Diagnostics, Sparks, MD), and 0.16 mg of each sample was placed on trypticase soy agar with 5% sheep blood (TSA II)/MacConkey II agar plate medium (BD Diagnostics). Test tubes containing the inoculated thioglycollate medium were kept in 37°C, and absorbance at 490 nm was measured at 48 h after inoculation. Plate mediums were kept at room temperature, and colony-forming units (cfu) were counted at 72 h after inoculation.
Renal ischemia-reperfusion model.
An established murine renal IRI model was used (27). Briefly, mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (75 mg/kg). Following an abdominal midline incision, both renal pedicles were bluntly dissected and clamped with a microvascular clamp (Roboz Surgical Instrument, Gaithersburg, MD) for 35 min. During the procedure, 2 ml of sterile saline at 40°C (1 ml during ischemia and 1 ml during reperfusion) was instilled into the peritoneal cavity. After the clamps were removed, the wounds were sutured and the mice were allowed to recover with free access to chow and water. Throughout the procedure, mice were placed on a heating pad (40°C).
Assessment of renal function.
Blood samples were obtained from the tail vein before and at 24 h after renal IRI. Serum creatinine levels (mg/dl) were measured with a Cobas Mira plus autoanalyzer (Roche Diagnostics, Indianapolis, IN).
Tissue histological analysis.
Mice were killed at 24 h after IRI, and kidneys were harvested after exsanguination. Tissue samples were fixed with 10% buffered formalin followed by paraffin embedding, and afterward renal sections were stained with hematoxylin and eosin (H&E). Renal tubular damage was scored in a blinded fashion by a renal pathologist.
Isolation of kidney-infiltrating mononuclear cells.
After anesthesia, mice were perfused with ∼ 30 ml of warm sterile saline, and kidneys were harvested. Kidney mononuclear cells (KMNCs) were isolated according to the previously described method (3). Briefly, decapsulated kidneys were immersed in RPMI buffer (Mediatech, Manassas, VA) containing 5% fetal bovine serum and disrupted mechanically using a Stomacher 80 Biomaster (Seward, Worthing, West Sussex, UK). Samples were strained, washed, and resuspended in 36% Percoll (Amersham Pharmacia Biotech, Piscataway, NJ) followed by gentle overlaying onto 72% Percoll. After centrifugation at 1,000 g for 30 min at room temperature, KMNCs were collected from the Percoll interface, washed twice, and counted on a hemocytometer using trypan blue exclusion.
Flow cytometric analysis of KMNCs.
Isolated KMNCs were preincubated with anti-CD16/CD32 Fc receptor-blocking antibody for 10 min to minimize nonspecific antibody binding. Cells were then incubated with various combinations of anti-mouse antibodies (all from BD Biosciences except for anti-mouse PE-conjugated anti-CD25 antibody from eBioscience) for 25 min at 4°C, washed with FACS buffer, and fixed using a 1% paraformaldehyde solution. Four-color immunofluorescence staining was acquired and analyzed using a FACSCalibur instrument (BD Biosciences, San Jose, CA) and FCS Express 3 (De Novo Software, Los Angeles, CA), respectively. Each assay included at least 10,000 gated events.
Bioplex protein array system.
A panel of cytokines was measured in whole kidney protein extracts obtained from naive control and germ-free mice with the Bioplex Protein Array system (Bio-Rad, Hercules, CA). This is a multiplexed, particle-based, flow cytometric assay that utilizes anti-cytokine monoclonal antibodies linked to microspheres incorporating distinct properties of two fluorescent dyes. Our assay was designed to detect and quantify IL-4, IL-10, and IFN-γ. Each cytokine value was normalized by dividing the raw cytokine concentration (pg/ml) with kidney protein concentration (mg/ml) measured by a Bradford assay.
Statistical analyses.
All data are expressed as means ± SE. Group means were compared with a Mann-Whitney test using SPSS 12.0K and ANOVA followed by a Newman-Keuls post hoc analysis using GraphPad Prism version 4. Statistical significance was determined when the P value was <0.05.
RESULTS
Baseline characteristics of mononuclear cells in kidneys from control and germ-free mice.
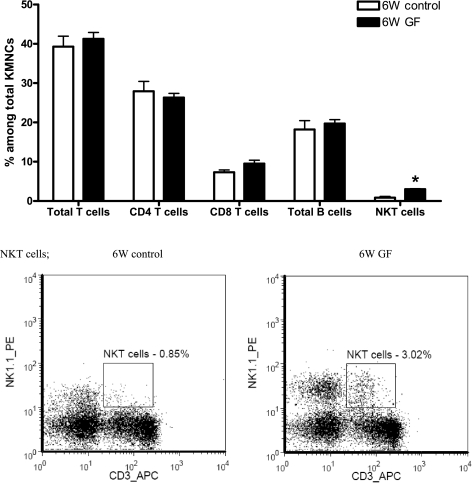
There was no difference in the total number of KMNCs among groups on both day 0 (before ischemia) and day 1 after IRI (Table 1). More NKT cells were found in the kidneys of 6W GF mice (6W control vs. 6W GF, 0.85 ± 0.34 vs. 3.02 ± 0.13% of KMNCs, P < 0.05), whereas the percentages of total T cells, CD4 T cells, CD8 T cells, and total B cells were similar between the two groups (Fig. 1). KMNCs isolated from 6W GF mice had reduced effector memory CD8 T cells (6W control vs. 6W GF, 26.94 ± 5.43 vs. 7.58 ± 1.08% of total CD8 T cells, P < 0.05), but similar percentages of effector memory CD4 T cells (control vs. germ free, 23.69 ± 2.70 vs. 20.22 ± 1.36% of total CD4 T cells, P > 0.05) compared with 6W control mice. There was no difference in the percentage of regulatory T cells expressing CD4 and CD25 among total KMNCs between the two groups (6W control vs. 6W GF, 0.84 ± 0.10 vs. 1.01 ± 0.17%).
Table 1.
Number of kidney-infiltrating mononuclear cells
| 6W Control | 6W GF | 11W Control | 11W CV-GF | |
|---|---|---|---|---|
| Day 0 | 2.36±0.29 | 2.44±0.11 | 2.73±0.39 | 2.39±0.39 |
| Day 1 | 1.95±0.25 | 1.86±0.25 | 1.80±0.15 | 1.88±0.22 |
Values are means ± SE expressed as ×106. 6W control, 6-wk-old control mice; 6W GF, 6-wk-old germ-free mice; 11W control, 11-wk-old control mice; 11W CV-GF, 11-wk-old conventionalized germ-free mice kept in a conventional specific pathogen-free environment and fed with fecal materials from control mice for 5 wk before ischemia.
Fig. 1.
Baseline phenotype of kidney lymphocytes in control and germ-free mice. Kidney mononuclear cells (KMNCs) were isolated from naive control and germ-free mice (n = 7/group) and analyzed with flow cytometry. There was no difference in the percentages of total T cells, CD4 T cells, CD8 T cells, and total B cells among KMNCs in the kidneys of control and germ-free mice before surgery. However, there were more NKT cells in the kidneys of germ-free mice. 6W control, 6-wk-old control mice; 6W GF, 6-wk-old germ-free mice. *P < 0.05 compared with 6W control.
Baseline expression of IL-4, IFN-γ, and IL-10 in the renal tissues of control and germ-free mice.
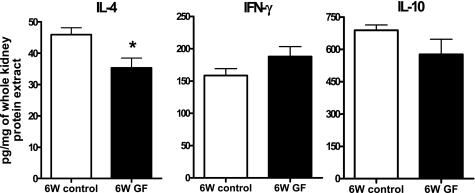
Normal kidneys of germ-free mice expressed less IL-4 compared with control mice (6W control vs. 6W GF, 45.95 ± 2.18 vs. 35.29 ± 3.15 pg/mg of total protein extracted from whole kidney, P < 0.05). IL-10 expression trended to be lower, while IFN-γ trended to be higher in normal germ-free mice kidneys (Fig. 2).
Fig. 2.
Baseline levels of IL-4, IFN-γ, and IL-10 in kidneys of control and germ-free mice. IL-4, IFN-γ, and IL-10 were measured in protein samples extracted from naive kidneys of control and germ-free mice (n = 7/group). IL-4 was significantly decreased in germ-free mice. IL-10 trended lower in germ-free mice, whereas there was a tendency for increased IFN-γ expression in renal tissues from germ-free mice. *P < 0.05 compared with 6W control.
Germ-free mice had worse kidney function and more CD8 T cells in their postischemic kidneys at 24 h after IRI.
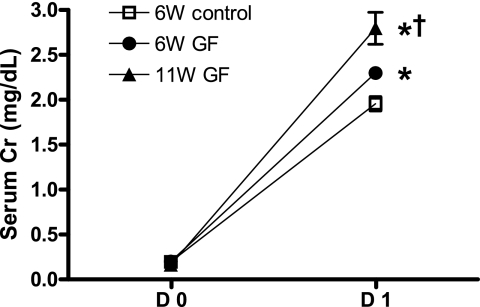
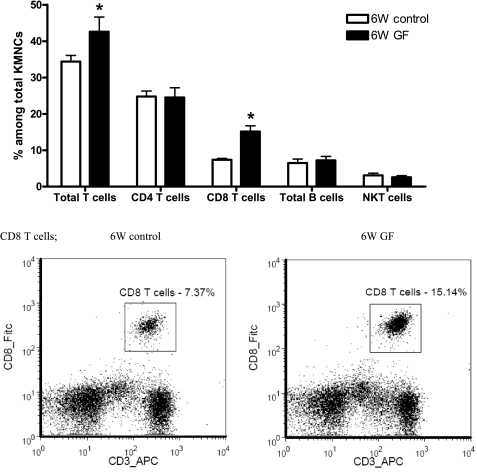
Germ-free mice had greater renal functional impairment than control mice at 24 h after bilateral renal IRI (Fig. 3). More total T cells trafficked into the postischemic kidneys of germ-free mice, and this increment of total T cells was accompanied by significantly increased trafficking of CD8 T cells (Fig. 4).
Fig. 3.
Germ-free mice show higher serum creatinine than control mice at 24 h after renal ischemia-reperfusion injury (IRI). The serum creatinine (Cr) level was measured to assess the renal function at 24 h after bilateral IRI (n = 10/group). Germ-free mice had higher serum creatinine compared with control mice. 11W GF, 11-wk-old germ free mice; D0, before bilateral IRI; D1, 24 h after bilateral IRI. *P < 0.01 compared with 6W control. †P < 0.001 compared with 6W GF.
Fig. 4.
More CD8 T cells are trafficked into the postischemic kidneys of germ-free mice. Increased trafficking of CD8 T cells into the postischemic kidneys of germ-free mice caused the increase in total T cell trafficking. There was no difference in the percentages of total CD4 T cells, total B cells, and NKT cells among KMNCs isolated from the 2 groups (n = 10/group). *P < 0.05 compared with 6W control.
Conventionalization of germ-free mice reconstituted intestinal flora.
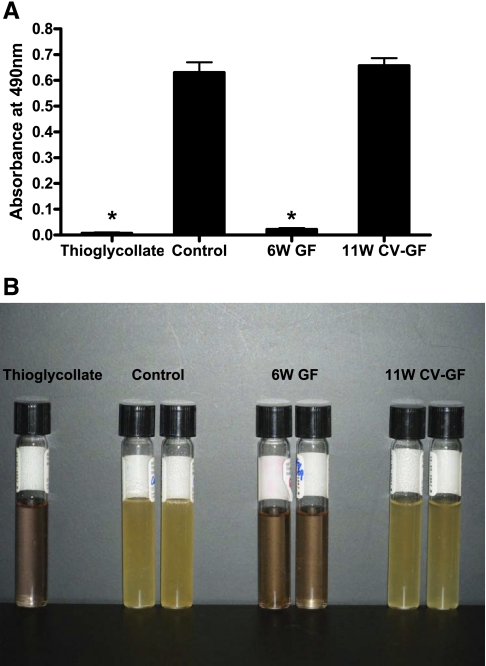
Stool culture using fluid thioglycollate medium and TSA II/MacConkey II agar plate medium showed similar amounts of microbial burden in control mice and 11W CV-GF mice (Fig. 5). There was no difference in colony counts on both TSA II and MacConkey II agar plates between control and 11W CV-GF groups (TSA II:MacConkey II, control 430 ± 98.3:427 ± 10.4, 6W GF 0:0, 11W CV-GF 404 ± 47.5:394 ± 29.5 cfu/mg stool).
Fig. 5.
Effect of conventionalization on intestinal flora of germ-free mice. A: to conventionalize, germ-free mice were kept in a conventional specific pathogen-free (SPF) environment and were fed with fecal materials from control mice for 5 wk. The absorbance of thioglycollate medium with no inoculation and that of thioglycollate medium inoculated with germ-free mice stool were similar. The absorbance of thioglycollate medium inoculated with control mice stool and 11W CV-GF mice stool was significantly higher compared with thioglycollate medium with no inoculation. B: the stool culture of 11W CV-GF mice showed a similar degree of microbial burden as control mice. Thioglycollate, thioglycollate medium with no inoculation; control, stool culture from 6W control mice; 6W GF, stool culture from 6-wk-old germ-free mice; 11W CV-GF, stool culture from 11-wk-old conventionalized germ-free mice.*P < 0.001 compared with both control and 11W CV-GF.
Exposure to germs abolished the difference in basal NKT cell trafficking and cytokine expression in normal kidneys.
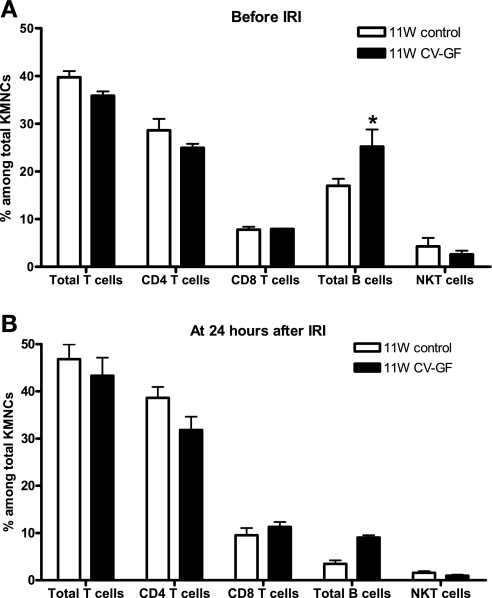
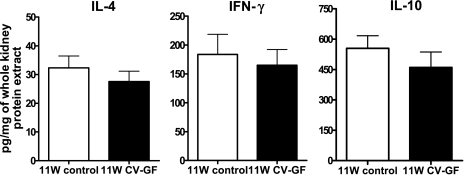
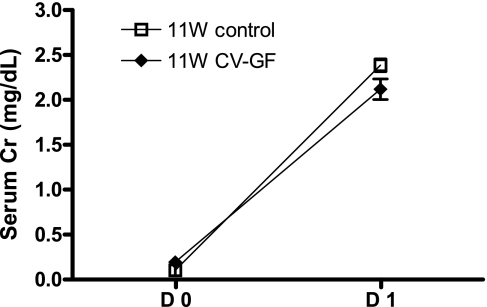
KMNCs isolated from 11W control mice and 11W CV-GF mice were similar in the total number and the percentages of T cells, CD4 T cells, CD8 T cells, and NKT cells. However, there were more B cells in the normal kidneys of 11W CV-GF mice (Fig. 6). There was no difference in IL-4, IFN-γ, and IL-10 levels measured in normal kidneys between the two groups (Fig. 7). Both 11W CV-GF mice and 11W control mice showed similar renal functional impairment (Fig. 8) and lymphocyte trafficking into the postischemic kidneys (Fig. 6).
Fig. 6.
Conventionalized germ-free mice show similar phenotypes of kidney-resident lymphocytes as age-matched control mice. A: KMNCs isolated from 11W CV-GF mice were analyzed and compared with KMNCs from age-matched 11W control mice (n = 5/group). There was no difference in the percentages of total T cells, CD4 T cells, CD8 T cells, and NKT cells. Total B cells were increased in the naive kidneys of 11W CV-GF mice. B: KMNCs were analyzed again at 24 h after bilateral renal IRI (n = 10/group). The percentages of total T cells, CD4 T cells, CD8 T cells, total B cells, and NKT cells were similar between groups. 11W control; 11-wk-old control mice; 11W CV-GF; 11-wk-old conventionalized germ-free mice kept in a conventional SPF environment and fed fecal materials from control mice for 5 wk before ischemia. *P < 0.05 compared with 11W control.
Fig. 7.
Baseline levels of IL-4, IFN-γ, and IL-10 in kidneys of conventionalized germ-free mice and age-matched control mice. IL-4, IFN-γ, and IL-10 were measured in protein samples extracted from naive kidneys of 11W control mice and 11W CV-GF mice (n = 5/group). There was no difference in the expression level of the three cytokines. Groups are defined as in Fig. 6.
Fig. 8.
Introduction of microorganisms in germ-free mice leads to the development of equivalent kidney dysfunction after IRI to age-matched control mice. There was no difference in serum creatinine (Cr) levels measured at 24 h after bilateral renal IRI between the 2 groups. Groups are defined as in Fig. 6.
Effects of conventionalization of bacteria on tubular injury following IRI.
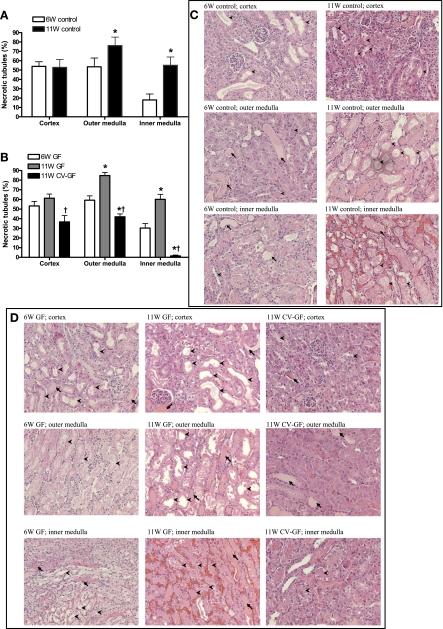
When GF mice are conventionalized to standard bacterial flora, age increases during the conventionalization process. Prior reports have shown that aging increases susceptibility to kidney IRI (25, 40). We found that 11W control mice showed significantly increased tubular injury in the outer medulla and inner medulla after the same duration of kidney ischemia compared with 6W control mice (Fig. 9, A and C). 11W GF mice also showed significantly increased tubular injury in the outer medulla and inner medulla compared with 6W GF mice (Fig. 9B). After conventionalization, 11W CV-GF mice had less tubular injury in all three areas of the postischemic kidney compared with 11W GF mice. Unexpectedly, the 11W CV-GF mice also had less tubular injury compared with 6W GF mice in the outer medulla and inner medulla (Fig. 9, B and D).
Fig. 9.
Introduction of microorganisms in germ-free mice attenuates tubular injury. A: tubular injury was scored with the percentage of necrotic tubules among total tubules. Old control (11-wk-old) mice showed significantly increased tubular injury in both the outer medulla and inner medulla compared with control (6-wk-old) mice. *P < 0.05 compared with 6W control. B: 11W CV-GF mice showed decreased tubular injury compared with germ-free mice. *P < 0.05 compared with 6W GF. †P < 0.05 compared with 11W GF. C: both the outer medulla and inner medulla of old control (11-wk-old) mice exhibited higher proportions of necrotic tubules than control (6-wk-old) mice. D: postischemic kidneys of 11W CV-GF mice showed mitigated tubular injury compared with both 6W GF and 11W GF mice. Arrowheads indicate necrotic tubules, and arrows indicate tubular casts. Groups are defined as in Figs. 1, 3, and 6.
DISCUSSION
These data demonstrate that germ-free status alters NKT cells and IL-4 content of normal mouse kidneys and worsens kidney histological and functional responses to IRI, with enhanced CD8 T cell trafficking. Furthermore, conventionalizing germ-free mice by feeding intestinal microbiota normalized the kidney IRI functional response, making germ-free mice similar to control mice.
Previous reports demonstrated that germ-free mice were protected from intestinal IRI due to increased production of IL-10 (35, 36). In contrast, we found that the germ-free mice had an enhanced kidney dysfunction and worse structural injury after IRI. At baseline, kidneys of germ-free mice expressed less IL-4 but showed a tendency toward increased IFN-γ compared with control mice. These cytokine profiles suggest immune deviation in the germ-free mice, similar to Th1-skewed autoimmune disease. According to the hypothesis of missing immune deviation, the reduced microbial burden during childhood caused by a Westernized lifestyle induces an imbalance of Th1 and Th2 responses, resulting in an exaggerated Th2 response in allergic disease and an exaggerated Th1 response in autoimmune disease (4, 8). Thus our results are more similar to that seen in autoimmune disease rather than allergic disease. Our results are consistent with previous reports on liver and kidney IRI showing that CD4 T cells of the Th1 phenotype are pathogenic and those of the Th2 phenotype are protective (33, 47). A number of studies reported that preceding or concomitant activation of the immune system with whole bacteria or bacterial-derived products led to protection from experimental allergic disease (10, 14, 19, 41) and autoimmune disease (6, 9, 17, 24, 30, 31).
Regulatory T cells and IL-10 have been implemented in the pathogenesis of autoimmune disease and allergic disease as major mediators in the hypothesis of reduced immune regulation. This hypothesis proposes that the increased prevalence of allergic disease is a consequence of reduced stimulation of regulatory T cells caused by a reduced microbial burden during childhood. Despite ongoing debates for the underlying pathogenic mechanisms, several studies have reported the protective role of regulatory T cells in autoimmune disease (15, 22, 32) and allergic disease (11, 20, 50). One recent study reported that there is a small percentage of natural regulatory T cells expressing CD4 and CD25 in naive mice kidneys (2). In the current study, we also found a small number of natural regulatory T cells in normal kidneys of control and germ-free mice, but there was no difference between the two groups, suggesting that the difference in microbial burden does not affect the population of intrarenal regulatory T cells. There was also no significant difference in the level of renal IL-10 expression, implying that reduced immune suppression may not occur in the kidneys of germ-free mice.
We found that NKT cells were increased in the normal kidneys of germ-free mice compared with control mice. NKT cells have been found in normal mice kidneys (2) and postischemic kidneys (3). Recently, two studies reported that NKT cells play an important role in early renal injury following IRI (16, 18). NKT cells were suggested to contribute to renal injury by mediating neutrophil infiltration and production of IFN-γ (18). Our findings of an increased number of NKT cells and an increased tendency of IFN-γ expression in normal kidneys of germ-free mice are consistent with an enhanced injury response.
Regarding effector memory T cells, the percentage of effector memory CD8 T cells in naive germ-free mice kidney was less than that of naive control mice, whereas there was no difference in the percentage of effector memory CD4 T cells between the two groups. Memory T cells are antigen-generated, generally long-lived, and quiescent cells that respond very rapidly and effectively to a subsequent challenge with the same antigen. Normal mice kidneys are known to contain more effector memory CD4 and CD8 T cells which express high CD44 and low CD62L than the spleen or blood (2). Our results imply that the number of kidney-infiltrating effector memory CD8 T cells is influenced by the previous microbial burden but that those cells are not involved in the pathogenesis of ischemic AKI because the kidneys of germ-free mice, with less effector memory CD8 T cells, had more severe injury after renal IRI.
At 24 h after renal IRI, more CD8 T cells trafficked into the postischemic kidneys of germ-free mice, suggesting the important role of CD8 T cells in the initial injury phase of renal IRI. CD4 T cells have been identified as a crucial mediator of renal IRI. CD4-deficient mice were significantly protected from renal IRI (5), and less CD4 T cell infiltration mediated by IL-16 deficiency also showed a protective effect (43). However, the role of CD8 T cells in renal IRI is not known. Our results suggest that naive CD8 T cells, having less chance of microbial stimuli, could infiltrate postischemic kidneys and play more roles in establishing renal injury following IRI. CD8 T cells isolated from postischemic kidneys were recently reported to produce more IFN-γ compared with normal and sham-operated kidneys (2).
Normal kidneys of conventionalized germ-free mice revealed similar expression of IL-4, IL-10, and IFN-γ compared with age-matched control mice. Thus expression of these cytokines in normal kidneys is influenced by microbial stimuli. There was also no difference in the number of NKT cells between conventionalized germ-free mice and age-matched control mice. Conventionalized germ-free mice showed equivalent renal functional injury to age-matched (11-wk-old) control, whereas germ-free mice showed significantly more renal functional injury than control mice. The degree of tubular injury with the percentage of necrotic tubules in the sets of 6W control vs. 11W control and 6W GF vs. 11W GF vs. 11W CV-GF mice was compared. Older control (11W control) mice showed more tubular injury compared with 6W control mice. There was more tubular injury in 11W GF mice compared with 6W GF mice. Conventionalized germ-free (11W CV-GF) mice showed less tubular injury in all three areas of the postischemic kidney compared with 11W GF mice and in the medulla of postischemic kidney compared with 6W GF mice, suggesting that exposure to “nonpathogenic” microbes has some protective effect on initial renal injury following renal IRI.
Commensal microbes are also reported to have a protective role in the pathogenesis of type I diabetes, a debilitating autoimmune disease caused by T cell-mediated destruction of the pancreas (44). In this study, germ-free, MyD88 (an adaptor for multiple innate immune receptors that recognize microbial stimuli)-negative NOD mice developed robust type I diabetes, while specific pathogen-free NOD mice lacking MyD88 did not. Conventionalization of germ-free, MyD88-negative NOD mice attenuated the development of diabetes.
Our study demonstrates, for the first time, the important role of microbial stimuli in kidney lymphocytes, cytokine expression, and the response to kidney IRI. Future studies can be geared to identify the details of the microbiota that modulate these effects in the kidney and alter microbiota therapeutically to improve outcomes from kidney IRI.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 to H. Rabb. H. R. Jang was supported by the Korea Research Foundation.
ACKNOWLEDGMENTS
We thank Chaitali Sarkar and Priya Kesari for technical assistance.
REFERENCES
- 1.Anonymous. Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet 355: 873–876, 2000 [PubMed] [Google Scholar]
- 2.Ascon DB, Ascon M, Satpute S, Lopez-Briones S, Racusen L, Colvin RB, Soloski MJ, Rabb H. Normal mouse kidneys contain activated and CD3+CD4-CD8- double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol 84: 1400–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380–3387, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347: 911–920, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, Rabb H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calcinaro F, Gambelunghe G, Lafferty KJ. Protection from autoimmune diabetes by adjuvant therapy in the non-obese diabetic mouse: the role of interleukin-4 and interleukin-10. Immunol Cell Biol 75: 467–471, 1997 [DOI] [PubMed] [Google Scholar]
- 7.De Greef KE, Ysebaert DK, Dauwe S, Persy V, Vercauteren SR, Mey D, De Broe ME. Anti-B7–1 blocks mononuclear cell adherence in vasa recta after ischemia. Kidney Int 60: 1415–1427, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Garn H, Renz H. Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiology 212: 441–452, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Greenwood BM, Herrick EM, Voller A. Suppression of autoimmune disease in NZB and (NZB × NZW) F1 hybrid mice by infection with malaria. Nature 226: 266–267, 1970 [DOI] [PubMed] [Google Scholar]
- 10.Han X, Fan Y, Wang S, Yang J, Bilenki L, Qiu H, Jiao L, Yang X. Dendritic cells from Chlamydia-infected mice show altered Toll-like receptor expression and play a crucial role in inhibition of allergic responses to ovalbumin. Eur J Immunol 34: 981–989, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol 172: 3842–3849, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol 130: 41–50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YS, Kwon KS, Kim DK, Choi IW, Lee HK. Inhibition of murine allergic airway disease by Bordetella pertussis. Immunology 112: 624–630, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochetkova I, Trunkle T, Callis G, Pascual DW. Vaccination without autoantigen protects against collagen II-induced arthritis via immune deviation and regulatory T cells. J Immunol 181: 2741–2752, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HT, Kim M, Kim M, Kim N, Billings FT IV, D'Agati VD, Emala CW., Sr Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol 293: F713–F722, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Lehmann D, Ben-Nun A. Bacterial agents protect against autoimmune disease. I. Mice pre-exposed to Bordetella pertussis or Mycobacterium tuberculosis are highly refractory to induction of experimental autoimmune encephalomyelitis. J Autoimmun 5: 675–690, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol 170: 3289–3295, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet 363: 608–615, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Martinez FD. The coming-of-age of the hygiene hypothesis. Respir Res 2: 129–132, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochoa-Reparaz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol 178: 1791–1799, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron 90: 133–138, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Oldstone MB, Dixon FJ. Inhibition of antibodies to nuclear antigen and to DNA in New Zealand mice infected with lactate dehydrogenase virus. Science 175: 784–786, 1972 [DOI] [PubMed] [Google Scholar]
- 25.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, Shi S, Li J, Xie Y, Lu Y, Wang Z. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci 60: 830–839, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Rabb H, Daniels F, O'Donnell M, Haq M, Saba SR, Keane W, Tang WW. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 279: F525–F531, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Rabb H, Ramirez G, Saba SR, Reynolds D, Xu J, Flavell R, Antonia S. Renal ischemic-reperfusion injury in l-selectin-deficient mice. Am J Physiol Renal Fluid Electrolyte Physiol 271: F408–F413, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Romagnani S. Regulation of the development of type 2 T-helper cells in allergy. Curr Opin Immunol 6: 838–846, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Rosati G, Aiello I, Mannu L, Pirastru MI, Agnetti V, Sau G, Garau M, Gioia R, Sanna G. Incidence of multiple sclerosis in the town of Sassari, Sardinia, 1965 to 1985: evidence for increasing occurrence of the disease. Neurology 38: 384–388, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Sadelain MW, Qin HY, Lauzon J, Singh B. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes 39: 583–589, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J Immunol 166: 1352–1359, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Sharabi A, Mozes E. The suppression of murine lupus by a tolerogenic peptide involves foxp3-expressing CD8 cells that are required for the optimal induction and function of foxp3-expressing CD4 cells. J Immunol 181: 3243–3251, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, Busuttil RW, Kupiec-Weglinski JW. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology 37: 296–303, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Singbartl K, Ley K. Protection from ischemia-reperfusion induced severe acute renal failure by blocking E-selectin. Crit Care Med 28: 2507–2514, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM, Teixeira MM. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol 179: 8533–8543, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol 173: 4137–4146, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Strachan DP. Hay fever, hygiene, and household size. BMJ 299: 1259–1260, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest 99: 2682–2690, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Thomas SE, Anderson S, Gordon KL, Oyama TT, Shankland SJ, Johnson RJ. Tubulointerstitial disease in aging: evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol 9: 231–242, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Tukenmez F, Bahceciler NN, Barlan IB, Basaran MM. Effect of pre-immunization by killed Mycobacterium bovis and vaccae on immunoglobulin E response in ovalbumin-sensitized newborn mice. Pediatr Allergy Immunol 10: 107–111, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Upton MN, McConnachie A, McSharry C, Hart CL, Smith GD, Gillis CR, Watt GC. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspring. BMJ 321: 88–92, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Diao H, Guan Q, Cruikshank WW, Delovitch TL, Jevnikar AM, Du C. Decreased renal ischemia-reperfusion injury by IL-16 inactivation. Kidney Int 73: 318–326, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455: 1109–1113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol 1: 69–75, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science 296: 490–494, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Yokota N, Burne-Taney M, Racusen L, Rabb H. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 285: F319–F325, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Yokota N, Daniels F, Crosson J, Rabb H. Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation 74: 759–763, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, De Broe ME. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int 66: 491–496, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med 8: 625–629, 2002 [DOI] [PubMed] [Google Scholar]