Abstract
Oxalate-induced oxidative stress contributes to cell injury and promotes renal deposition of calcium oxalate crystals. However, we do not know how oxalate stimulates reactive oxygen species (ROS) in renal tubular epithelial cells. We investigated the signaling mechanism of oxalate-induced ROS formation in these cells and found that oxalate significantly increased membrane-associated protein kinase C (PKC) activity while at the same time lowering cytosolic PKC activity. Oxalate markedly translocated PKC-α and -δ from the cytosol to the cell membrane. Pretreatment of LLC-PK1 cells with specific inhibitors of PKC-α or -δ significantly blocked oxalate-induced generation of superoxide and hydrogen peroxide along with NADPH oxidase activity, LDH release, lipid hydroperoxide formation, and apoptosis. The PKC activator PMA mimicked oxalate's effect on oxidative stress in LLC-PK1 cells as well as cytosol-to-membrane translocation of PKC-α and -δ. Silencing of PKC-α expression by PKC-α-specific small interfering RNA significantly attenuated oxalate-induced cell injury by decreasing hydrogen peroxide generation and LDH release. We believe this is the first demonstration that PKC-α- and -δ-dependent activation of NADPH oxidase is one of the mechanisms responsible for oxalate-induced oxidative injury in renal tubular epithelial cells. The study suggests that the therapeutic approach might be considered toward attenuating oxalate-induced PKC signaling-mediated oxidative injury in recurrent stone formers.
Keywords: protein kinase C, oxidative stress, calcium oxalate, kidney stone, urolithiasis
hyperoxaluria is one of the major risk factors for calcium oxalate renal stone formation. Despite considerable advances in treatment of kidney stones, we know very little about the processes within the kidney that initiate stone formation or urolithiasis. Several studies support the concept that cellular injury is a predisposing factor in this process, theorizing that products of cell damage act as a nidus of calcium oxalate crystallization both in vivo and in vitro (24, 30, 63). Even without crystalluria, hyperoxaluria increases excretion of enzymes of renal tubular origin (32). Oxidative stress is considered an important contributory mechanism in cell injury and is associated with a number of disorders, including atherosclerosis, ischemia/reperfusion, arthritis, stroke, and neurodegenerative diseases. In addition, reactive oxygen species (ROS) have detrimental effects on proteins, lipids, and DNA and promote severe tissue damage and cell death. Such oxidation leads to formation of lipid hydroperoxides as an initial product of lipid peroxidation (2, 54, 66). Several studies have demonstrated that oxalate exposure increases free radical injury to the renal tubular epithelium (56, 61–64) and also promotes redistribution of phosphatidylserine to the plasma membrane surface (31, 69).
NADPH oxidase is the most important source of receptor-mediated ROS generation in nonphagocytic cells (43). Once thought to be present only in neutrophils (36), it has since been found in diverse sites including the kidney (20, 36). NADPH oxidase activity is significantly elevated in atherosclerotic lesions, leading to increased superoxide production (29). Our own studies as well as those of Umekawa et al. (51–65) have demonstrated that NADPH oxidase-mediated ROS generation plays a major role in injury of renal epithelial cells. Protein kinase C (PKC) is a critical component of intracellular signal transduction pathways and has been implicated in homeostasis, migration, proliferation, apoptosis, remodeling of the actin cytoskeleton, and modulation of ion channels (5, 25, 46). PKC is a family of serine/threonine kinases that includes at least 12 known isoenzymes, in turn grouped into 3 subfamilies based on differences in sequence homology and cofactor requirements (44). While activation of PKC has been associated with increased production of superoxide anions in phagocytes as well as vascular cells (17, 37), the cellular mechanisms that integrate these signaling events in response to oxalate toxicity in renal tubular epithelial cells are not well understood.
It is clear from our previous in vitro and in vivo studies (62, 63) that oxalate-induced free radical injury is involved in nucleation and aggregation of calcium oxalate and resultant development of kidney stones. We have now investigated the mechanisms by which oxalate induces production of ROS and leads to peroxidative injury via PKC signaling in renal epithelial cells. Our results clearly demonstrate that PKC-α and -δ play a role in oxalate-induced ROS production via activation of NADPH oxidase followed by peroxidative injury in renal epithelial cells and suggest that oxalate-mediated PKC signaling is one of the underlying molecular mechanism involved in the development of calcium oxalate kidney stones.
MATERIALS AND METHODS
Cell culture.
Serial cultures of LLC-PK1 cells of proximal tubular origin (CRL 1392, ATCC, Rockville, MD) were maintained as subconfluent monolayers in 75-cm2 Falcon T-flasks in DMEM containing 10% fetal bovine serum, streptomycin (0.20 mg/ml), and penicillin (1.0 × 102 IU/ml), pH 7.4, at 37°C in a 5% carbon dioxide-95% air atmosphere. Confluent monolayers of LLC-PK1 cells were used, and experiments were carried out with serum-free, pyruvate-free DMEM. Oxalate was prepared as we described previously (62). Briefly, a stock solution of 10 mM sodium oxalate was prepared in normal sterile PBS and diluted to 0.75 mM in defined medium.
Experiments with PKC and NADPH oxidase inhibitors.
Thirty minutes before the addition of 0.75 mM oxalate, LLC-PK1 cells were treated with the PKC inhibitor calphostin C (50 nM-1 μM), G06976 (5–20 μM), and chelerythrine chloride (1–5 μM), a PKC-α-selective inhibitor, inhibitor peptide (1–10 μg/ml), the PKC-δ selective inhibitor rottlerin (1–20 μM), or the NADPH oxidase inhibitors DPI (0.5 μM) and apocynin (0.5 mM). The cells treated with oxalate along with inhibitors for various time periods were examined as described below.
Light microscopy.
Calcium oxalate crystal formation was monitored continuously using a Leika DM IRB inverted microscope.
Transfection of LLC-PK1 cells with a PKC-α small interfering RNA.
LLC-PK1 cells were transfected with a PKC-α stealth small interfering RNA (siRNA). 5′-UGGUUCACAAGAGGUGCCAUGAGUU/AACUCAUGGCACCUCUUGUGAACCA-3′ directed toward the PKC-α mRNA target, 5′-GGUUCACAAGAGGUGCCAUGAGUU-3′ (Invitrogen Life Technology, Carlsbad, CA) using Lipofectamine 2000 according to the manufacturer's protocol (GIBCO, Gaithersburg, MD). Cells were transfected with a nonsilencing stealth siRNA duplex (control stealth siRNA, Invitrogen) (3, 38), using nontransfected cells as a second negative control (mock). Oxalate experiments were carried out 48 h after transfection. We confirmed target gene silencing by the PKC-α siRNA using Western blotting. Knockdown of PKC-α expression was determined by densitometry of the PKC-α band relative to its GAPDH loading control and comparison to cells transfected with the nonsilencing siRNA control.
Subcellular fractionation and Western blotting.
At the end of the experiments, cells were harvested and resuspended in hypotonic lysis buffer with 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin and incubated for 30 min on ice. Cytosolic and membrane particulate fractions of the lysates were isolated using a FractionPREP cell fractionation system (BioVision, Mountain View, CA) following the manufacturer's instructions. Protein content in each fraction was measured using a BCA protein assay kit (Pierce, Rockford, IL). We estimated the degree of contamination using tracking enzymes, measuring GAPDH in the cytosolic fraction and Na+-K+-ATPase in the membrane fraction. As expected, contamination was low, comprising 3–10% of total protein expression (GAPDH: membrane 10%, cytosol 90%; Na+-K+-ATPase: membrane 97%, cytosol 3%), confirming earlier studies (23, 47). Accordingly, relative amounts of PKC in the membrane fraction were corrected for cytosolic contamination using densitometric analysis of Na+-K+-ATPase and GAPDH.
Equal protein aliquots of cytosol and membrane fractions were subjected to SDS-PAGE, and the separated proteins were transferred to a nitrocellulose membrane. Blots were probed with antibodies specific for PKC isoenzymes (Cell Signaling Technology, Boston, MA; Santa Cruz Biotechnology, Santa Cruz, CA). Specific reactive bands were detected using goat anti-rabbit or goat anti-mouse secondary antibodies conjugated with horseradish peroxides. The immunoreactive bands were visualized using an enhanced chemiluminescence Western blot detection kit (GE Healthcare Bio-Sciences, Piscataway, NJ) and analyzed by densitometric scanning using Kodak imaging software. After stripping, the membrane was reprobed with antibodies to GAPDH (cytosolic marker, Biodesign International, Saco, ME) or Na+-K+-ATPase (membrane marker, Sigma-Aldrich, St. Louis, MO) to ensure equal protein loading.
Determination of PKC activity.
PKC activity in the cytosolic and membrane fractions was measured using a nonradioactive ELISA that utilizes a synthetic PKC pseudosubstrate and a monoclonal antibody that recognizes the phosphorylated form of the peptide, according to the manufacturer's instructions (Calbiochem, EMB Biosciences, San Diego, CA). Protein concentration was determined with a BCA assay kit. PKC activity was expressed as optical density (OD) per milligram protein, and the data were normalized to control.
Determination of NADPH oxidase activity.
NADPH oxidase activity was determined using an assay based on the chemiluminescence of lucigenin (bis-N-methylacridinium nitrate; CL) as described previously (22). Briefly, control cultures or cultures exposed to oxalate with or without inhibitors were washed with 5 ml ice-cold PBS and scraped from the plate into 5 ml of the same solution. Samples were transferred to a 50-ml tube and centrifuged at 750 g for 10 min at 4°C. The pellet was resuspended (0.5–1.0 ml/dish) in lysis buffer containing protease inhibitors (20 mM monobasic potassium phosphate, pH 7.0, 1 mM EGTA, 10 μg/ml aprotinin, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, and 0.5 mM phenylmethylsulfonyl fluoride). The cell suspension was then disrupted using a dounce homogenizer on ice, and the homogenate was stored on ice until use. Protein content was measured in a homogenate aliquot by Lowry's method (39), and NADPH oxidase activity was assessed by luminescence assay in 50 mM phosphate buffer (pH 7.0) containing 1 mM EGTA, 150 mM sucrose, 500 μM lucigenin as the electron acceptor, and 100 μM NADPH as the substrate. Enzyme activity was expressed as nanomoles superoxide produced per minute per milligram protein, and the data were normalized to control. To confirm the validity of the CL method, specific NADPH oxidase activity was also measured by SOD-inhibitable cytochrome c reduction using NADPH as a substrate and expressed as nanomoles superoxide produced per minute per milligram protein (58).
Determination of apoptosis.
Apoptosis was detected using an ELISA Plus cell death detection kit (Roche Applied Science, Indianapolis, IN). This technique is based on a quantitative sandwich enzyme immunoassay that allows specific determination of mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates, using mouse monoclonal antibodies directed against DNA and histones. Nucleosome enrichment was quantified based on absorbance at 405 nm. ODs in the treated samples were normalized to control.
Determination of superoxide anions.
At the end of the experiments, intracellular superoxide anions were measured by a nitroblue tetrazolium (NBT) reduction assay as we described previously (60). The amount of reduced NBT was determined based on absorbance at 630 nm. Values were expressed as OD at 630 nm, and ODs in the treated samples were normalized to control.
Determination of H2O2 release.
Hydrogen peroxide in the medium was measured with an assay kit according to the manufacturer's instructions (Assay Designs, Ann Arbor, MI). This assay is based on the reaction of xylenol orange with sorbitol and ammonium iron sulfate in an acidic solution, producing a purple color proportional to the concentration of H2O2 in the medium. The reaction product was quantified at 550 nm and expressed as micromolar H2O2 released. H2O2 production in treated cells was normalized to control.
Determination of LDH release.
Cellular injury was assessed by release of lactic dehydrogenase (LDH). The medium from control and the experiment was centrifuged to remove crystals and cellular debris. LDH activity was determined using a commercial kit (Roche Diagnostics). All determinations were made against appropriate reagent blanks. The reaction product was read at 490 nm and expressed as percent release. Values in treated samples were normalized to control.
Determination of lipid hydroperoxide.
Cells were harvested in HPLC-grade water. Lipid hydroperoxide (LHP) was assayed immediately after sonication, according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI). Briefly, cells were extracted with 0.5 ml Extract-R-saturated methanol and vortexed for 15 s; 1 ml deoxygenated chloroform was added to each test tube, vortexed for 15 s, and the mixture was centrifuged at 1,500 g for 5 min at 0°C. The lower chloroform phase was transferred to clean tubes and stored on ice. LHP was prepared, and the chromogenic reaction was assayed according to the manufacturer's protocol. After color development, the samples were pipetted into a 96-well glass plate and absorbance was measured at 492 nm. Values were expressed as nanomoles LHP formed per milligram protein, and the experimental data were normalized to control.
Statistical analysis.
All data are expressed as means ± SE. Data were analyzed by ANOVA followed by Tukey's multiple comparisons test. Student's t-test was used for comparison between two groups. A P value of < 0.05 was considered significant.
RESULTS
Inhibition of PKC activation attenuates oxalate-induced ROS production and cell injury.
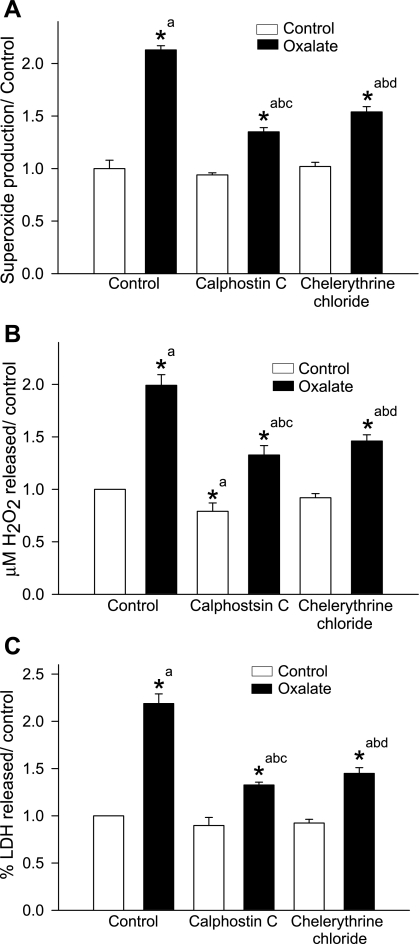
We determined the effects of PKC inhibitors on oxalate-induced ROS production and LDH release by exposing LLC-PK1 cells to 0.75 mM oxalate for 3 h in the presence or absence of a PKC inhibitor, specifically, calphostin C (100 nM) or chelerythrine chloride (5 μM). Following 3-h exposure to oxalate, there was a significant increase in superoxide (Fig. 1A) and hydrogen peroxide (Fig. 1B) compared with control. Both calphostin C and chelerythrine chloride significantly attenuated this increase, indicating that PKC activation is required for oxalate-mediated ROS production.
Fig. 1.
Effect of PKC inhibitors on oxalate-induced reactive oxygen species (ROS) production and cell injury. LLC-PK1 cells were pretreated with a PKC inhibitor, either calphostin C (100 nM) or chelerythrine chloride (5 μM), for 30 min and then treated with 0.75 mM oxalate along with inhibitors for 3 h. Superoxide production (A), H2O2 production (B), and LDH release (C) as a marker of cell injury were determined. DMSO was used as a vehicle. Comparisons shown: a, significant compared with control; b, significant compared with oxalate; c, significant compared with calphostin C-treated control; d, significant compared with chelerythrine chloride-treated control. Data are normalized to control, and values are expressed as means ± SE. *P < 0.05; n = 6.
The effect of PKC inhibitors on oxalate-induced cell injury was examined by measuring LDH release into the medium (Fig. 1C). Following exposure to oxalate for 3 h, there was a significant increase in LDH release by oxalate-treated cells compared with control (2.18 ± 0.10-fold increase; n = 6; P < 0.05). In the presence of a PKC inhibitor, LDH release was significantly lower compared with cells exposed to oxalate for 3 h (calphostin C: 1.32 ± 0.03-fold increase; chelerythrine chloride: 1.45 ± 0.06-fold increase; n = 6; P < 0.05). Thus oxalate toxicity is dependent on PKC activation.
Effect of oxalate on subcellular distribution of PKC isoenzymes.
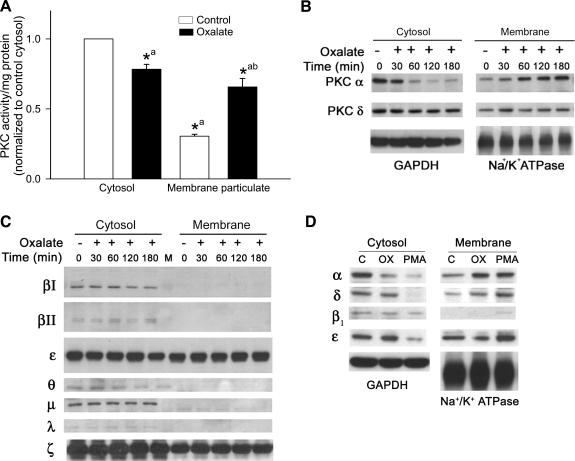
We tested whether oxalate exposure activates PKC in LLC-PK1 cells by determining PKC activity using ELISA. Under control conditions, PKC activity was found mainly in the cytosolic compartment and translocated to the membrane compartment upon activation, but total PKC activity in the homogenate remained unchanged. This shows that the increase in the ratio of membrane to cytosol activity accurately reflects activation of PKC (11). PKC activity from cytosolic and particulate membrane fractions was quantified after oxalate treatment to LLC-PK1 cells for 3 h. Oxalate exposure significantly increased PKC activity in the particulate fraction (control: 0.305 ± 0.1; oxalate: 0.657 ± 0.06; n = 6; P < 0.05), whereas activity was decreased in the cytosol (control: 1.0 ± 0.1; oxalate: 0.78 ± 0.03; n = 6; P <0.05) (Fig. 2A).
Fig. 2.
Effect of oxalate on PKC activity and PKC isoenzyme translocation in LLC-PK1 cells. A: LLC-PK1 cells were treated with or without 0.75 mM oxalate for 3 h, and PKC activity in the cytosol and membrane particulate fraction was determined. Data are normalized to cytosolic control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with cytosolic control; b, significant compared with membrane control. *P < 0.05; n = 6. B: effect of oxalate on translocation of PKC-α and -δ. LLC-PK1 cells were treated with oxalate for different time periods. Lysates of cytosolic and membrane fractions were analyzed for PKC-α and -δ expression by Western blotting. A typical Western blot from 1 of 4 experiments is shown. GAPDH was used as a cytosolic loading control and Na+-K+-ATPase as a membrane loading control. C: effect of oxalate on translocation of endogenous conventional PKCs, novel PKCs, and atypical PKCs. LLC-PK1 cells were treated with oxalate for different time periods. Lysates of cytosolic and membrane fractions were analyzed for PKC isoenzyme expression by Western blotting. A typical blot from 1 of 4 experiments is shown. D: effect of PMA or oxalate on PKC isoenzyme translocation from cytosol to membrane fraction. LLC-PK1 cells were treated with PMA (1 μM) or oxalate (0.75 mM) for 3 h. Lysates of cytosolic and membrane fractions were analyzed for PKC isoenzyme expression by Western blotting. GAPDH was used as a cytosolic loading control and Na+-K+-ATPase as a membrane loading control. A typical blot from 1 of 4 experiments is shown.
Since PKC activity was increased in the membrane fraction, we further quantified the subcellular distribution of the PKC isoenzymes in control cells and cells exposed to oxalate. PKC distribution in the particulate fraction was examined in oxalate-treated cells using Western blotting. We observed significant translocation of PKC-α and -δ to the membrane fraction in oxalate-treated cells (Fig. 2B); however, the membrane distribution of other PKC isoenzymes remained unaltered (Fig. 2C). PMA, a known activator of PKC isoenzymes that translocates PKC from the cytosol to the membrane, was used as a positive control for PKC activation. Figure 2D suggests that PMA and oxalate differ markedly in their ability to translocate PKC. While both PMA and oxalate induced significant redistribution of PKC-α and -δ to the plasma membrane, PMA translocated PKC-ε and -β1 to the membrane whereas oxalate did not. Thus oxalate has a unique profile of PKC isoenzyme activation, inducing free radical-mediated injury in LLC-PK1 cells (Fig. 2D).
Effect of PKC-α- and -δ-specific inhibitors on oxalate-induced ROS generation and cell injury.
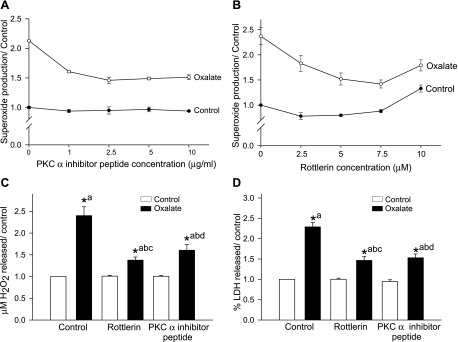
Since oxalate activated PKC-α and -δ in LLC-PK1 cells, we next studied whether activation of PKC-α or -δ plays a role in oxalate-induced ROS production using isoenzyme-specific inhibitors. To determine the optimum concentration of inhibitors of PKC-α (“inhibitor peptide” and G06976) and PKC-δ (rottlerin), LLC-PK1 cells were exposed to increasing concentrations of the inhibitor for 30 min and then coincubated with 0.75 mM oxalate for 3 h, measuring superoxide generation. Inhibitor peptide at 1–10 μg/ml significantly decreased superoxide generation in a concentration-dependent manner (P < 0.05; n = 6) (Fig. 3A). Similar effects were observed with another PKC-α inhibitor, G06976 (data not shown). Similarly, the PKC-δ inhibitor rottlerin (2.5–10 μM) had a concentration-dependent effect on cells exposed to oxalate for 3 h, resulting in a significant decrease in superoxide production (P < 0.05; n = 6) at 7.5 μM (∼50%). At higher concentrations, rottlerin (10 and 20 μM) induced superoxide production both at baseline and following oxalate treatment (Fig. 3B).
Fig. 3.
Effect of PKC-α- or -δ-specific inhibitors on oxalate-induced ROS production and cell injury. A and B: both the PKC-α inhibitor inhibitor peptide (A) and the PKC-δ inhibitor rottlerin (B) attenuated oxalate-induced superoxide production. LLC-PK1 cells were pretreated with different concentrations of inhibitor peptide (1–10 μg/ml) or rottlerin (2–5-10 μM) for 30 min and then treated with 0.75 mM oxalate along with inhibitor for 3 h, after which superoxide production was determined. C and D: effect of PKC-α and -δ inhibitors on oxalate-induced H2O2 production (C) and LDH release (D). LLC-PK1 cells were treated with PKC-α (2.5 μg/ml) or -δ inhibitor (7.5 μM) for 30 min and then exposed to 0.75 mm oxalate along with inhibitors for 3 h, after which H2O2 and LDH were determined. DMSO was used as a vehicle. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with vehicle-treated control; b, significant compared with oxalate; c, significant compared with rottlerin-treated control; d, significant compared with inhibitor peptide-treated control. *P < 0.05; n = 6.
To determine whether activation of PKC-α or -δ can modulate production of H2O2 and injury in LLC-PK1 cells following oxalate challenge, studies were carried out using PKC-α- and -δ-selective inhibitors. Inhibitor peptide and rottlerin pretreatment of LLC-PK1 cells significantly inhibited H2O2 generation following oxalate challenge. Inhibitor peptide at 2.5 μg/ml significantly decreased hydrogen peroxide generation (−35%), while rottlerin at 7.5 μM significantly decreased H2O2 generation (−45%) (Fig. 3C). LDH release was also significantly attenuated by inhibitor peptide and rottlerin at concentrations of 2.5 μg/ml and 7.5 μM, respectively (oxalate: 2.29 ± 0.10; rottlerin+oxalate: 1.46 ± 0.09; inhibitor peptide+oxalate: 1.53 ± 0.09: n = 6; P <0.05) (Fig. 3D). The results suggest that oxalate-induced oxidative cell injury is dependent on both PKC-α and -δ.
Effect of PKC-α and -δ inhibitors on oxalate-induced peroxidative injury.
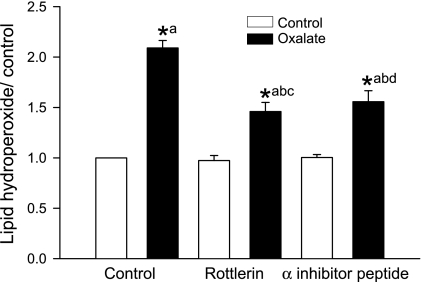
Since oxidative stress has been linked to oxalate-induced cell injury, we measured lipid hydroperoxide (LHP), an end product of oxidative injury, in renal epithelial cells (Fig. 4). To determine whether PKC-α and -δ are involved in LHP formation in LLC-PK1 cells following oxalate exposure, studies were carried out using PKC-α- and δ-selective inhibitors. Treatment with 0.75 mM oxalate significantly increased LHP whereas rottlerin or inhibitor peptide pretreatment significantly inhibited LHP formation following oxalate exposure.
Fig. 4.
Effect of PKC isoenzyme-specific inhibitors on levels of oxalate-induced cellular lipid hydroperoxide (LHP). LLC-PK1 cells were pretreated with inhibitor peptide (2.5 μg/ml) or rottlerin (7.5 μM) for 30 min and then exposed to 0.75 mM oxalate along with inhibitors for 3 h, after which LHP was measured. DMSO was used as a vehicle. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with control; b, significant compared with oxalate; c, significant compared with rottlerin-treated control; d, significant compared with inhibitor peptide-treated control. *P < 0.05; n = 6.
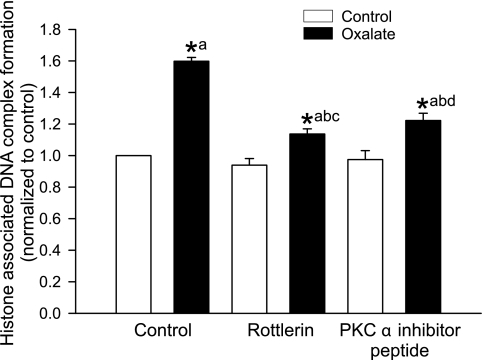
Effect of PKC-α and -δ inhibitors on induction of apoptosis and necrosis following oxalate treatment.
Apoptosis was quantified after exposure of LLC-PK1 cells to oxalate, using an ELISA that measures histone-associated DNA fragmentation (Fig. 5). In the early stages of cell death, endogenous endonucleases cleave double-stranded DNA at the most accessible internucleosome-linked regions, generating mono- and oligonucleosomes. We found that apoptosis of LLC-PK1 cells increased following oxalate exposure, as evidenced by accumulation of nucleosomal DNA. In the presence of a PKC-α or -δ inhibitor, enrichment of nucleosomal DNA was significantly lower compared with oxalate exposure for 3 h. Oxalate also induced necrosis as indicated by increased release of LDH (Fig. 1C). Thus our results demonstrated that renal epithelial cells can undergo both apoptosis and necrosis following oxalate exposure.
Fig. 5.
Effect of PKC-α and -δ inhibitors on oxalate-induced apoptosis in LLC-PK1 cells. LLC-PK1 cells were pretreated with 7.5 μM rottlerin or 2.5 μg/ml inhibitor peptide for 30 min and then exposed to 0.75 mM oxalate along with inhibitors for 3 h, after which histone-associated DNA complex formation was measured. DMSO was used as a vehicle. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with control; b, significant compared with oxalate-treated cells; c, significant compared with rottlerin-treated control; d, significant compared with inhibitor peptide-treated control. *P < 0.05; n = 4.
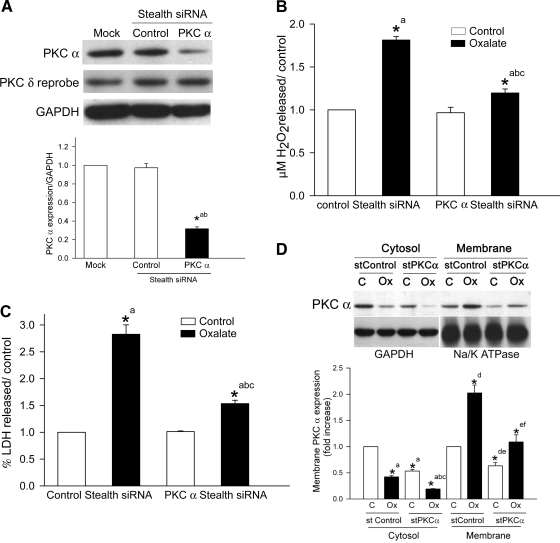
Effects of downregulating PKC-α expression on oxalate-induced ROS production and cell injury.
The effectiveness of siRNA transfection in reducing PKC-α expression was determined using semiquantitative Western blotting. We found that by 48 h after transfection, the siRNA had significantly reduced PKC-α protein expression (∼70%) compared with cells transfected with a nonsilencing siRNA or mock (Fig. 6A; n = 4; P < 0.05). To determine the possible off-target effect of the PKC-α siRNA sequences, we reprobed the same blot with a PKC-δ antibody but found that reduction of PKC-α protein expression by siRNA transfection did not reduce PKC-δ protein expression (Fig. 6A).
Fig. 6.
Effect of knockdown of PKC-α by a PKC-α siRNA on ROS production, LDH release, and translocation. A: LLC-PK1 cells were transiently transfected with a chemically modified PKC-α siRNA for 48 h. Mock and nonsilencing siRNAs (control stealth siRNA) were used as controls. Expression of PKC-α was analyzed by Western blotting with GAPDH as a loading control. To check possible off-target effects of PKC-α siRNA in LLC-PK1 cells, the same membrane was reprobed with PKC-δ antibody with no change in protein concentration between control and PKC-α siRNA-transfected cells, establishing the isoenzyme specificity of the PKC-α siRNA. A typical blot from 1 of 4 experiments is shown. The graph represents the ratio of densitometric analysis of PKC-α protein expression to GAPDH. Comparisons shown: a, significant compared with mock; b, significant compared with cells transfected with the control stealth siRNA. B and C: effect of sequence-specific siRNA inhibition of PKC-α protein expression on oxalate-induced H2O2 production (B) and LDH release (C) in LLC-PK1 cells. After 48-h transfection of LLC-PK1 cells with a nonsilencing siRNA (control stealth siRNA) or PKC-α siRNA, they were treated with or without 0.75 mM oxalate for 3 h and H2O2 production and LDH release were determined. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with respective control of siRNA-transfected cells; b, significant compared with oxalate-treated nonsilencing siRNA-transfected cells; c, significant compared with PKC-α siRNA-transfected cells. *P < 0.05; n = 6. D: effect of PKC-α siRNA on oxalate-induced PKC-α translocation from cytosol to membrane in LLC-PK1 cells. LLC-PK1 cells were transfected with a siRNA specific for PKC-α. Cells transfected with a nonsilencing siRNA served as a control. LLC-PK1 cells transfected with the nonsilencing siRNA (control stealth siRNA) or PKC-α siRNA (stealth PKC-α siRNA) were exposed to oxalate for 3 h. Membrane and cytosol fractions were isolated and analyzed by Western blotting with an anti-PKC-α antibody. Top: typical blot from 1 of 3 experiments. Bottom: densitometric analysis of the blots. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with nonsilencing siRNA cytosol control; b, significant compared with oxalate-treated nonsilencing siRNA cytosol; c, significant compared with PKC siRNA-transfected cytosol control; d, significant compared with nonsilencing siRNA membrane control; e, significant compared with oxalate-treated nonsilencing siRNA membrane; f, significant compared with PKC siRNA-treated membrane. *P < 0.05; n = 3.
To further examine the ability of PKC-α-downregulated cells to generate hydrogen peroxide upon oxalate challenge, we exposed cells to oxalate for 3 h and measured hydrogen peroxide in the medium. Cells transfected with the nonsilencing siRNA (control stealth siRNA) showed a significant increase in hydrogen peroxide generation (Fig. 6B) and LDH release (Fig. 6C) following oxalate exposure. When cells transfected with the PKC-α stealth siRNA were exposed to oxalate for 3 h, both hydrogen peroxide and LDH were significantly decreased compared with cells transfected with the nonsilencing siRNA.
Since PKC-δ siRNA transcripts of porcine origin (LLC-PK1 cells) were not available in GenBank, we used a PKC-δ siRNA which is specific to the human PKC-δ transcript. Although an antibody specific to the human PKC-δ transcript recognized PKC-δ of porcine origin (LLC-PK1 cells), the siRNA targeted to human PKC-δ did not reduce PKC-δ protein expression in LLC-PK1 cells (data not shown). Consistent with our findings, other studies have shown that a siRNA targeted to mammalian PKC-δ does not suppress PKC-δ protein expression in cells derived from rats (27). Therefore, we were unable to perform oxalate experiments with PKC-δ siRNA. While the data described here confirm the role of PKC-α in oxalate-induced oxidative cell injury, the fact that the PKC-δ inhibitor attenuated oxalate-induced ROS and cell injury suggests that the role of PKC-δ in oxalate toxicity cannot be disregarded.
In addition, LLC-PK1 cells transfected with a PKC-α siRNA or a nonsilencing siRNA were treated with oxalate for 3 h, fractionated, and protein expression was determined by Western blotting. Significant accumulation of PKC-α was observed in the membrane fraction of the cells transfected with the nonsilencing siRNA. PKC-α siRNA transfection significantly decreased PKC-α protein expression in the cytosol. Although oxalate translocated PKC-α to the membrane fraction in the cells transfected with the PKC-α siRNA (Fig. 6D), the level of expression in the membrane fraction was not sufficient to induce free radical production or LDH release compared with oxalate treatment (Fig. 6, B and C).
Effect of inhibitors on oxalate-induced membrane translocation of PKC isoenzymes.
Given that PKC-α or -δ inhibitors attenuated oxalate-induced ROS and cell injury, we questioned whether this effect was due to inhibition of PKC-α or -δ translocation from the cytosol to the membrane, which is necessary for PKC function. This supposition proved to be correct (Figs. 7, A and B), as oxalate treatment significantly increased PKC-α and -δ protein expression in the membrane fraction. Inhibitor peptide and rottlerin significantly reduced PKC-α and -δ protein expression, respectively, in the cells exposed to oxalate.
Fig. 7.
A and B: effect of PKC isoenzyme-specific inhibitors on oxalate-induced membrane translocation of PKC-α (A) and PKC-δ (B) protein expression in LLC-PK1 cells. LLC-PK1 cells were pretreated with inhibitor peptide (2.5 μg/ml) or rottlerin (7.5 μM) for 30 min and then exposed to 0.75 mM oxalate along with inhibitors for 3 h. Lysates of the membrane fractions were analyzed for PKC-α (A) and PKC-δ (B) expression by Western blotting. DMSO was used as a vehicle. Data are normalized to control, and values are expressed as means ± SE. A typical blot from 1 of 3 experiments is shown. The graph represents the ratio of densitometric analysis of PKC-α or PKC-δ protein expression to Na+-K+-ATPase. Comparisons shown: a, significant compared with vehicle-treated control; b, significant compared with oxalate-treated cells; c, significant compared with inhibitor peptide- or rottlerin-treated cells. *P < 0.05; n = 3.
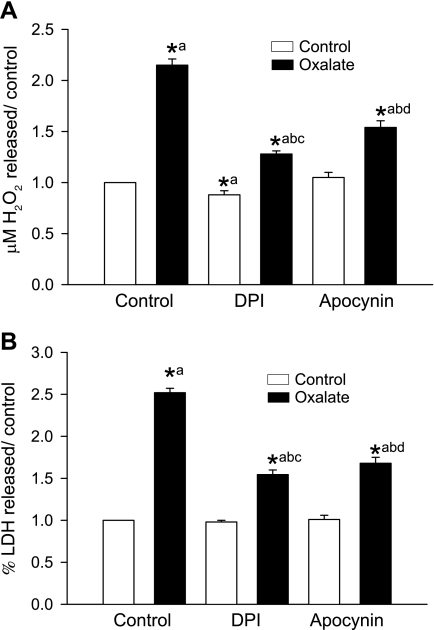
Oxalate-induced ROS formation and cell injury are dependent on NADPH oxidase activity.
Exposure of LLC-PK1 cells to oxalate for 3 h significantly increased production of hydrogen peroxide (Fig. 8A) and LDH release (Fig. 8B). Treatment with a NADPH oxidase inhibitor, either 0.5 μM DPI or 0.5 mM apocynin, significantly blocked oxalate-induced hydrogen peroxide formation or LDH release, suggesting that NADPH oxidase is involved in oxalate-mediated ROS generation.
Fig. 8.
A and B: effect of DPI and apocynin on oxalate-induced H2O2 generation (A) and LDH release (B). LLC-PK1 cells were pretreated with 0.5 μM DPI or 0.5 mM apocynin for 30 min and then exposed to oxalate along with inhibitors for 3 h, after which H2O2 production and LDH release were determined. DMSO was used as a vehicle. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with control; b, significant compared with oxalate; c, significant compared with DPI-treated control; d, significant compared with apocynin-treated control. *P < 0.05; n = 6.
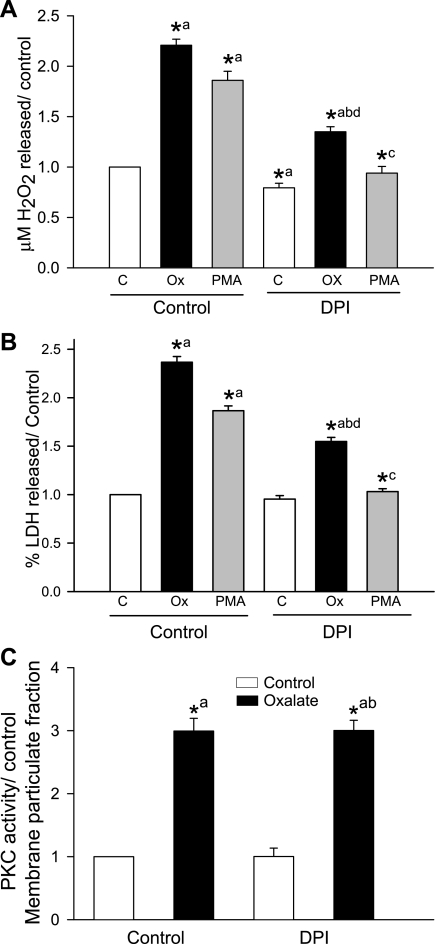
Effect of PKC-α- and -δ-selective inhibitors on oxalate-induced NADPH oxidase activity.
Although we used DPI and apocynin to demonstrate that oxalate-induced activation of NADPH oxidase is required for ROS generation, a recent review indicates these are not specific inhibitors (1). Therefore, as an alternate approach, we used PKC-α- and -δ-specific inhibitors to demonstrate the crucial role of NADPH oxidase in oxalate-induced oxidative injury by measuring enzyme activity, as adding exogenous NADPH to assess NADPH oxidase activity has been performed by several groups. We also assessed NADPH oxidase activity in cell homogenates by SOD-inhibitable cytochrome c reduction assay. Using this method, NADPH oxidase-dependent superoxide production in LLC-PK1 cells exposed to oxalate was still significantly increased compared with control (data not shown). Comparison of lucigenin-amplified CL and cytochrome c reduction showed excellent correlation. NADPH oxidase activity was significantly increased in LLC-PK1 cells treated with oxalate for 3 h (Fig. 9). To investigate the potential role of PKC-α and -δ in regulating NADPH oxidase activity, we examined the effects of selective inhibitors of PKC-α and -δ. As shown in Fig. 9, inhibitor peptide and rottlerin significantly blocked the oxalate-induced increase in NADPH oxidase activity, indicating that PKC-α and -δ are the isoenzymes involved in activation of oxalate-induced NADPH oxidase. NADPH oxidase activity was also significantly abolished by DPI or apocynin in cells treated with or without oxalate.
Fig. 9.
Effect of PKC isoenzyme-specific inhibitors and NADPH oxidase inhibitors on oxalate-induced NADPH oxidase activity in LLC-PK1 cells. LLC-PK1 cells were pretreated with 0.5 μM DPI, 0.5 mM apocynin, 7.5 μM rottlerin or 2.5 μg/ml inhibitor peptide for 30 min and then exposed to 0.75 mM oxalate along with inhibitors for 3 h, after which NADPH oxidase activity was determined. DMSO was used as a vehicle. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with control; b, significant compared with oxalate-treated; c, significant compared with inhibitor peptide-treated. *P < 0.05; n = 4.
Oxalate-induced PKC activation leading to NADPH oxidase-mediated ROS production and cell injury.
Since activation of PKC by oxalate induced ROS production in LLC-PK1 cells, we also tested whether ROS formation is mediated via PKC-dependent NADPH oxidase activation, using PMA, a known activator of PKC, as a positive control. Adding PMA increased ROS production (Fig. 10A) or LDH release (Fig. 10B) in the absence of DPI. However, ROS levels and LDH release remained unchanged when cells were first treated with DPI and then exposed to PMA, indicating that activation of PKC is required for NADPH oxidase-mediated ROS generation or LDH release. Similarly, when NADPH oxidase was inhibited with DPI and oxalate was added, ROS and LDH were reduced while PKC activity increased (Fig. 10C). These results suggest that oxalate-induced ROS production and cell injury involve PKC-mediated activation of NADPH oxidase.
Fig. 10.
Effect of the NADPH oxidase inhibitor DPI on oxalate- or PMA-induced H2O2 production, LDH release, and PKC activity. A and B: LLC-PK1 cells were pretreated with 0.5 μM DPI for 30 min and then exposed to 0.75 mM oxalate or 1 μM PMA along with DPI for 3 h, after which H2O2 production (A) and LDH release (B) were determined. DMSO was used as a vehicle. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with control; b, significant compared with oxalate-treated; c, significant compared with PMA-treated; d, significant compared with DPI-treated control.*P < 0.05; n = 6. C: effect of DPI on oxalate-induced PKC activity in the membrane fraction. LLC-PK1 cells were pretreated with 0.5 μM DPI for 30 min and then exposed to 0.75 mM oxalate along with DPI for 3 h, after which PKC activity was measured. DMSO was used as a vehicle. Data are normalized to control, and values are expressed as means ± SE. Comparisons shown: a, significant compared with control; b, significant compared with DPI-treated control. *P < 0.05; n = 3.
Next, we examined whether NADPH oxidase activity is required for oxalate-induced PKC activation. As shown in Fig. 10C, PKC activity in the membrane fraction increased significantly when the cells were treated with oxalate, but when we inhibited NADPH oxidase with DPI and exposed the cells to oxalate PKC activity remained increased, indicating that PKC activation was upstream from NADPH oxidase. Even though PKC activity was increased by oxalate in the DPI-treated cells, ROS levels and LDH release remained low (Fig. 10, A and B). Moreover, inhibitor peptide (PKC-α inhibitor) and rottlerin (PKC-δ inhibitor) suppressed oxalate-induced NADPH oxidase activity (Fig. 9), ROS levels, and cell injury (Fig. 3, A–D). Taken together, these data indicate that oxalate-induced PKC activation is required for NADPH oxidase activity.
DISCUSSION
The new findings of this study address the signaling mechanism upstream from NADPH oxidase-mediated oxidative injury and demonstrate that oxalate increases ROS production and peroxidative injury in renal epithelial cells via PKC-α- and -δ-mediated activation of NADPH oxidase. We found that 1) oxalate increases PKC activity, translocates PKC-α and -δ to the membrane particulate fraction, and increases NADPH oxidase activity, superoxide, and H2O2 generation, apoptosis, and necrosis, and peroxidative injury in renal epithelial cells; 2) inhibition of PKC-α or -δ decreases oxalate-induced NADPH oxidase activity, superoxide, H2O2, apoptosis, and necrosis, and peroxidative injury; and 3) treatment with DPI or apocynin prevented oxalate-induced ROS generation and cell injury, indicating that oxalate-induced activation of PKC-α and -δ plays a significant role in NADPH oxidase-mediated peroxidative injury in renal epithelial cells. Peroxidative injury stemming from the oxalate-induced activation of PKC signaling might result in calcium oxalate nucleation and aggregation and lead to development of kidney stones.
Oxalate may cause precipitation of calcium oxalate, especially when inhibitors of calcium oxalate crystallization are low. When we treated LLC-PK1 cells with oxalate and monitored them for 30 min, we did not find calcium oxalate crystals under high-power magnification; however, superoxide production (data not shown), translocation of PKC-α, and PKC-δ were increased. After 1 h, we detected a few calcium oxalate crystals, while superoxide continued to increase. Finally, by 2–3 h we observed significant crystal accumulation along with further increases in superoxide production and cell injury. Clearly, when oxalate-induced free radical generation overwhelms cellular defense mechanisms, cells become damaged, as we reported previously (61). Our study reveals that oxalate itself can initiate ROS generation and peroxidative injury to renal tubular cells.
Concentration-dependent oxalate studies involving renal cell cultures have shown that many birefringent calcium oxalate crystals are formed in 1 h at oxalate concentrations of 1.0 mM or greater (4). It has been shown that both oxalate and calcium oxalate crystals independently increase free radical generation in a time- and concentration-dependent manner (8, 21, 56) and that oxalate alone increases oxidative cell injury while calcium oxalate crystals potentiate injury (60). There is evidence that exposure to elevated levels of oxalate leads to progressive as well as dose- and time-dependent changes in the morphology and viability of LLC-PK1 and HK2 cells. Morphological changes accompanying oxalate treatment of renal epithelial cells led to an increase in both cytoplasmic vacuolization and pyknotic nuclei as well as the appearance of dysmorphic cells and condensation and disintegration of the nuclei (41, 55).
Intracellular reactive oxidants are generated by both enzymatic and nonenzymatic sources, including the mitochondrial electron transport system (9), NADPH oxidase (35), 5-lipoxygenase (52), several oxidases located in subcellular compartments such as peroxidases and mono- and dioxygenases, and isoenzymes of the cytochrome P-450 superfamily, including nitric oxide synthase (67), xanthine oxidase (50), and cyclooxygenase (52). Because PKC is a critical component of intracellular signal transduction pathways, the studies presented here were designed to extend our earlier work on oxalate-induced NADPH oxidase activation (51). We still need to evaluate the effect of oxalate exposure on other free radical sources.
A number of specific pharmacological PKC inhibitors have been synthesized and used to study the role of PKC in signaling. In the present study, oxalate-induced ROS generation in renal epithelial cells was effectively inhibited by the PKC inhibitors calphostin C and chelerythrine chloride, suggesting that oxalate-induced ROS generation is PKC dependent. Many renal diseases are known to be associated with activation of the PKC pathway, and consistent with our findings PMA-, ANG-, and glucose-stimulated ROS generation in BAE cells were all reportedly inhibited by calphostin C and chelerythrine chloride (40). Increased production of ROS by PKC activation has also been demonstrated in mononuclear cells in patients with multiple sclerosis (68), endothelial cells treated with cyclic strain (13), and mesangial cells treated with high glucose (34).
We found that oxalate, a potent inducer of free radicals, also triggers cytosol-to-membrane translocation of PKC-α and -δ but not other PKC isoenzymes (δ, ε, θ, η, βI, βII, λ, ζ, and, μ). Chang and Beezhold (10) have shown that cytosol-to-membrane translocation of isoenzymes reflects their enzymic activity and that PMA-triggered cytosol-to-membrane translocation of PKC-α is accompanied by a shift of PKC enzymatic activity from the cytosol to the membrane. As a positive control, we confirmed our findings using PMA, which also induces free radical formation and triggers translocation of PKC isoenzymes. Different PKC isoenzymes have been shown to be involved in diverse responses of various types of cells (59). For example, in LLC-PK1 cells PKC involvement has been demonstrated in tight junction barrier function, H2O2-induced cell injury, regulation of sodium metabolism, and cell growth, although each function is regulated by a different PKC isoenzyme(s) (18, 45, 49, 53). Blockade of activation and translocation of PKC by PKC inhibitors reduces 4-hydroxy-2-nonenal-induced ROS generation in airway smooth muscle cells (57). In addition to the classic PKCs and PKC-δ, researchers have reported that PKC-ε and PKC-ζ also play a role in oxidative stress in different types of cells (28, 59). However, in our study neither PKC-ε nor PKC-ζ was altered by oxalate treatment.
Although PKC inhibitors such as calphostin C and chelerythrine chloride have been shown to inhibit oxalate-induced ROS generation and cell injury, they are not specific for PKC isoenzymes. To determine PKC isoenzyme specificity in oxalate-induced ROS generation, we used the PKC-α- and -δ-specific blockers, inhibitor peptide and rottlerin, respectively. PKC-δ is reportedly a key signaling molecule in the ROS-induced apoptotic pathway through generation of active catalytic fragments by proteolytic cleavage (19), and consistent with our findings rottlerin also inhibited agonist-induced translocation of PKC-δ in rat skeletal muscle and glutathione-depleted neuroblastoma cells (6, 14). PKC-δ has also been shown to be important for a number of other cell functions such as stimulation of the Na+/K+ antiporter in C8 glioma cells (12).
G06976 is a potent inhibitor of the Ca2+-dependent PKC isoenzymes α, βI, βII, and γ (42). Consistent with our findings, reduction of ROS generation by G06976 has been correlated with KCN-induced LDH release in PC12 cells (26). When we used specific inhibitors of PKC-α and a PKC-α siRNA to evaluate the role of PKC-α in oxalate-induced oxidative stress, we found that both inhibitor peptide and PKC-α siRNA transfection significantly reduced oxalate-induced ROS production and cell injury. Adenoviral overexpression of a dominant negative mutant PKC-α has also been shown to modulate oxidative stress-mediated signaling pathways in cardiac myocytes by decreasing superoxide production (16). Furthermore, inhibition of PKC-α activity by either siRNA transfection or overexpression of a dominant negative mutant decreased nitric oxide production in primary aortic endothelial cells (48). Our results convincingly demonstrate the essential role of PKC-α and -δ in oxalate-induced ROS production and cell injury.
Not only oxalate-induced PKC activation but also ROS-mediated cell injury was mimicked by PMA, a PKC activator, in renal epithelial cells. The present findings also demonstrate that PMA-induced ROS production and cell injury were prevented by the NADPH oxidase inhibitors DPI and apocynin, providing further evidence that NADPH oxidase-dependent ROS generation requires PKC activation in LLC-PK1 cells. NADPH oxidase is accepted as the most important mechanism of receptor-stimulated ROS generation in nonphagocytic cells (43). Furthermore, recent studies have suggested that PKCs activate NADPH oxidase by phosphorylating the NADPH oxidase subunit p47phox, and PKC-β was shown to increase NADPH oxidase activity in diabetic glomeruli and HL60 cells (15, 33). PKC-δ has also been implicated as a regulator of NADPH oxidase and appears to be required for assembly of this complex enzyme's components (7).
In conclusion, we believe this is the first demonstration that the mechanism of oxalate-induced free radical production involves activation of NADPH oxidase in renal tubular cells and is mediated by PKC signaling. We also provide evidence that among the PKC isoenzymes studied, PKC-α and -δ play a particularly important role in oxalate-induced free radical production. These findings strongly suggest that PKC-dependent activation of NADPH oxidase may be one of the essential mechanisms responsible for peroxidative cell injury in hyperoxaluria. As a result of oxalate exposure, the injured renal tubular membrane plays a significant role in calcium oxalate adhesion, aggregation, and growth of kidney stones. These novel findings support PKC signaling as a significant therapeutic target in the prevention of renal tubular calcium oxalate adhesion and retention in recurrent stone formers.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK56249.
ACKNOWLEDGMENTS
This work was presented in part at the 29th Congress of the Société Internationale d'Urologie, September 2–6, 2007, Paris, France, and published in abstract form (Urology 70, Suppl 1: 53–54, 2007) and the Annual Meeting of the American Urological Association, May 17–22, 2008, Orlando, FL (J Urol 179: 507–508, 2008).
REFERENCES
- 1.Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9: 686–696, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Aliev G, Smith MA, Seyidov D, Neal ML, Lamb BT, Nunomura A, Gasimov EK, Vinters HV, Perry G, LaManna JC, Friedland RP. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer's disease. Brain Pathol 12: 21–35, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner M, Sillman AL, Blackwood EM, Srivastava J, Madson N, Schilling JW, Wright JH, Barber DL. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci USA 103: 13391–13396, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhandari A, Koul S, Sekhon A, Pramanik SK, Chaturvedi LS, Huang M, Menon M, Koul HK. Effects of oxalate on HK-2 cells, a line of proximal tubular epithelial cells from normal human kidney. J Urol 168: 253–259, 2002 [PubMed] [Google Scholar]
- 5.Bokhari SM, Zhou L, Karasek MA, Paturi SG, Chaudhuri V. Regulation of skin microvasculature angiogenesis, cell migration, and permeability by a specific inhibitor of PKC alpha. J Invest Dermatol 126: 460–467, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Protein kinase C delta mediates insulin-induced glucose transport in primary cultures of rat skeletal muscle. Mol Endocrinol 13: 2002–2012, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Brown GE, Stewart MQ, Liu H, Ha VL, Yaffe MB. A novel assay system implicates PtdIns(3,4)P(2), PtdIns(3)P, and PKC delta in intracellular production of reactive oxygen species by the NADPH oxidase. Mol Cell 11: 35–47, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Byer K, Khan SR. Citrate provides protection against oxalate and calcium oxalate crystal induced oxidative damage to renal epithelium. J Urol 173: 640–646, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979 [DOI] [PubMed] [Google Scholar]
- 10.Chang ZL, Beezhold DH. Protein kinase C activation in human monocytes: regulation of PKC isoforms. Immunology 80: 360–366, 1993 [PMC free article] [PubMed] [Google Scholar]
- 11.Chanson M, Bruzzone R, Spray DC, Regazzi R, Meda P. Cell uncoupling and protein kinase C: correlation in a cell line but not in a differentiated tissue. Am J Physiol Cell Physiol 255: C699–C704, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Chen CC, Wu ML. Protein kinase C isoform delta is involved in the stimulation of the Na+-H+ exchanger in C6 glioma cells. Mol Pharmacol 48: 995–1003, 1995 [PubMed] [Google Scholar]
- 13.Cheng JJ, Chao YJ, Wang DL. Cyclic strain activates redox-sensitive proline-rich tyrosine kinase 2 (PYK2) in endothelial cells. J Biol Chem 277: 48152–48157, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Domenicotti C, Marengo B, Verzola D, Garibotto G, Traverso N, Patriarca S, Maloberti G, Cottalasso D, Poli G, Passalacqua M, Melloni E, Pronzato MA, Marinari UM. Role of PKC-delta activity in glutathione-depleted neuroblastoma cells. Free Radic Biol Med 35: 504–516, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41: 7743–7750, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Frazier DP, Wilson A, Dougherty CJ, Li H, Bishopric NH, Webster KA. PKC-α and TAK-1 are intermediates in the activation of c-Jun NH2-terminal kinase by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 292: H1675–H1684, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKC zeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res 90: 1012–1019, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Ocana A, De Miguel F, Penaranda C, Albar JP, Sarasa JL, Esbrit P. Parathyroid hormone-related protein is an autocrine modulator of rabbit proximal tubule cell growth. J Bone Miner Res 10: 1875–1884, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med 184: 2399–2404, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Greene EL, Farell G, Yu S, Matthews T, Kumar V, Lieske JC. Renal cell adaptation to oxalate. Urol Res 33: 340–348, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Grummer B, Beer M, Liebler-Tenorio E, Greiser-Wilke I. Localization of viral proteins in cells infected with bovine viral diarrhoea virus. J Gen Virol 82: 2597–2605, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hackett RL, Shevock PN, Khan SR. Cell injury associated calcium oxalate crystalluria. J Urol 144: 1535–1538, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Hryciw DH, Pollock CA, Poronnik P. PKC-α-mediated remodeling of the actin cytoskeleton is involved in constitutive albumin uptake by proximal tubule cells. Am J Physiol Renal Physiol 288: F1227–F1235, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Huang HM, Weng CH, Ou SC, Hwang T. Selective subcellular redistributions of protein kinase C isoforms by chemical hypoxia. J Neurosci Res 56: 668–678, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Irie N, Sakai N, Ueyama T, Kajimoto T, Shirai Y, Saito N. Subtype- and species-specific knockdown of PKC using short interfering RNA. Biochem Biophys Res Commun 298: 738–743, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Jung YS, Ryu BR, Lee BK, Mook-Jung I, Kim SU, Lee SH, Baik EJ, Moon CH. Role for PKC-epsilon in neuronal death induced by oxidative stress. Biochem Biophys Res Commun 320: 789–794, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Kalinina N, Agrotis A, Tararak E, Antropova Y, Kanellakis P, Ilyinskaya O, Quinn MT, Smirnov V, Bobik A. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler Thromb Vasc Biol 22: 2037–2043, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Khan SR. Heterogeneous nucleation of calcium oxalate crystals in mammalian urine. Scanning Microsc 9: 597–614, 1995 [PubMed] [Google Scholar]
- 31.Khan SR, Byer KJ, Thamilselvan S, Hackett RL, McCormack WT, Benson NA, Vaughn KL, Erdös GW. Crystal-cell interaction and apoptosis in oxalate-associated injury of renal epithelial cells. J Am Soc Nephrol 10, Suppl 14: S457–S463, 1999 [PubMed] [Google Scholar]
- 32.Khan SR, Hackett RL. Hyperoxaluria, enzymuria and nephrolithiasis. Contrib Nephrol 101: 190–193, 1993 [PubMed] [Google Scholar]
- 33.Korchak HM, Kilpatrick LE. Roles for beta II-protein kinase C and RACK1 in positive and negative signaling for superoxide anion generation in differentiated HL60 cells. J Biol Chem 276: 8910–8917, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Kwan J, Wang H, Munk S, Xia L, Goldberg HJ, Whiteside CI. In high glucose protein kinase C-zeta activation is required for mesangial cell generation of reactive oxygen species. Kidney Int 68: 2526–2541, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 43: 332–347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassègue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Subbulakshmi V, Fields AP, Murray NR, Cathcart MK. Protein kinase c alpha regulates human monocyte O2− production and low density lipoprotein lipid oxidation. J Biol Chem 274: 3764–3771, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Li S, Crothers J, Haqq CM, Blackburn EH. Cellular and gene expression responses involved in the rapid growth inhibition of human cancer cells by RNA interference-mediated depletion of telomerase RNA. J Biol Chem 280: 23709–23717, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 40.Mahrouf M, Ouslimani N, Peynet J, Djelidi R, Couturier M, Therond P, Legrand A, Beaudeux JL. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem Pharmacol 72: 176–183, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Maroni PD, Koul S, Chandhoke PS, Meacham RB, Koul HK. Oxalate toxicity in cultured mouse inner medullary collecting duct cells. J Urol 174: 757–760, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993 [PubMed] [Google Scholar]
- 43.Meier B. Superoxide generation of phagocytes and nonphagocytic cells. Protoplasma 217: 117–124, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J 332: 281–292, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowicki S, Kruse MS, Brismar H, Aperia A. Dopamine-induced translocation of protein kinase C isoforms visualized in renal epithelial cells. Am J Physiol Cell Physiol 279: C1812–C1818, 2000 [DOI] [PubMed] [Google Scholar]
- 46.O'Brian CA, Ward NE. Biology of the protein kinase C family. Cancer Metastasis Rev 8: 199–214, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Pal-Bhowmick I, Vora HK, Jarori GK. Sub-cellular localization and post-translational modifications of the Plasmodium yoelii enolase suggest moonlighting functions. Malar J 6: 45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Partovian C, Zhuang Z, Moodie K, Lin M, Ouchi N, Sessa WC, Walsh K, Simons M. PKC alpha activates eNOS and increases arterial blood flow in vivo. Circ Res 97: 482–487, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Polosukhina D, Singaravelu K, Padanilam BJ. Activation of protein kinase C isozymes protects LLC-PK1 cells from H2O2 induced necrotic cell death. Am J Nephrol 23: 380–389, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Pritsos CA. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem Biol Interact 129: 195–208, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Rashed T, Menon M, Thamilselvan S. Molecular mechanism of oxalate-induced free radical production and glutathione redox imbalance in renal epithelial cells: effect of antioxidants. Am J Nephrol 24: 557–568, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Romano M, Claria J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. FASEB J 17: 1986–1995, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Rosson D, O'Brien TG, Kampherstein JA, Szallasi Z, Bogi K, Blumberg PM, Mullin JM. Protein kinase C-alpha activity modulates transepithelial permeability and cell junctions in the LLC-PK1 epithelial cell line. J Biol Chem 272: 14950–14953, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9: 49–89, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Scheid C, Koul H, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Kennington L, Kohli R, Hodapp J, Ayvazian P, Menon M. Oxalate toxicity in LLC-PK1 cells, a line of renal epithelial cells. J Urol 155: 1112–1116, 1996 [PubMed] [Google Scholar]
- 56.Scheid C, Koul H, Hill WA, Luber-Narod J, Kennington L, Honeyman T, Jonassen J, Menon M. Oxalate toxicity in LLC-PK1 cells: role of free radicals. Kidney Int 49: 413–419, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Shao MX, Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha-converting enzyme. J Immunol 175: 4009–4016, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Shi Y, Niculescu R, Wang D, Patel S, Davenpeck KL, Zalewski A. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol 21: 739–745, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Takeishi Y, Jalili T, Ball NA, Walsh RA. Responses of cardiac protein kinase C isoforms to distinct pathological stimuli are differentially regulated. Circ Res 85: 264–271, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol 164: 224–229, 2000 [PubMed] [Google Scholar]
- 61.Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol 157: 1059–1063, 1997 [PubMed] [Google Scholar]
- 62.Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31: 3–9, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Thamilselvan S, Menon M. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int 96: 117–126, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res 33: 65–69, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Umekawa T, Tsuji H, Uemura H, Khan SR. Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vladimirova O, Lu FM, Shawver L, Kalman B. The activation of protein kinase C induces higher production of reactive oxygen species by mononuclear cells in patients with multiple sclerosis than in controls. Inflamm Res 48: 412–416, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Wiessner JH, Hasegawa AT, Hung LY, Mandel NS. Oxalate-induced exposure of phosphatidylserine on the surface of renal epithelial cells in culture. J Am Soc Nephrol 10, Suppl 14: S441–S445, 1999 [PubMed] [Google Scholar]