Abstract
Autosomal dominant polycystic kidney disease (ADPKD) arises following mutations of either Pkd1 or Pkd2. The proteins these genes encode, polycystin-1 (PC1) and polycystin-2 (PC2), form a signaling complex using direct intermolecular interactions. Two distinct domains in the C-terminal tail of PC2 have recently been identified, an EF-hand and a coiled-coil domain. Here, we show that the PC2 coiled-coil domain interacts with the C-terminal tail of PC1, but that the PC2 EF-hand domain does not. We measured the K0.5 of the interaction between the C-terminal tails of PC1 and PC2 and showed that the direct interaction of these proteins is abrogated by a PC1 point mutation that was identified in ADPKD patients. Finally, we showed that overexpression of the PC1 C-terminal tail in MDCK cells alters the Ca2+ response, but that overexpression of the PC1 C-terminal tail containing the disease mutation does not. These results allow a more detailed understanding of the mechanism of pathogenic mutations in the cytoplasmic regions of PC1 and PC2.
Keywords: polycystic kidney disease, calcium signaling, surface plasmon resonance, EF-hand, coiled-coil domain
autosomal dominant polycystic kidney disease (ADPKD) is a systemic hereditary disease characterized by renal and hepatic cysts. End-stage renal failure is characteristic of this disease, and ADPKD accounts for hemodialysis in ∼10% of patients in the United States (30). The consequences of mutations in Pkd1 and Pkd2 that cause ADPKD (27, 31) on the function of polycystin-1 (PC1) and PC2 are still not completely understood. However, some mutations may be linked to changes in intermolecular interactions of the components of the channel complex and consequent regulation of the channel formed by PC2.
PC2 is expressed in most adult and fetal tissues, including the kidney, heart, brain, ovaries, testis, and intestine (21, 22) and has been found in both the cilia and endoplasmic reticulum (ER) of renal tubular epithelial cells. The sequence and molecular architecture suggest that PC2 is a member of the transient receptor potential (TRP) family of channels (8, 22), consistent with its function as a nonselective cation channel that is highly permeable to Ca2+ (11, 16, 29). The role of Ca2+ in the function of the channel is not completely understood at the molecular level. However, Ca2+ regulates channel currents with a bell-shaped dependence, where currents can be measured in the presence of low concentrations and inhibited by high concentrations of Ca2+ (4). PC2 channel activity can also be modulated by intermolecular interactions with protein partners that include troponin I, α-actinin, and PC1 (17–19, 24). Alterations in the ability of PC2 to conduct Ca2+ have been seen in ADPKD, and some disease-associated point mutations result in complete loss of the ability of PC2 to function as a Ca2+ channel. This disruption of normal Ca2+ signaling by PC2 may be an important factor for development of ADPKD.
PC1 is a large protein that is expressed on the plasma membrane and primary cilia of renal cells, and it is also expressed in many other tissues including the heart, brain, muscle, and bone (10, 14). PC1 shares a region of high sequence similarity to PC2 (22), but it does not appear to be a cation channel and has less clearly understood functions. The N-terminal portion of PC1 encodes a large (∼3,000 amino acid) extracellular fragment with multiple domains and predicted functions. The C-terminal cytoplasmic portion of PC1 is much smaller (∼200 amino acids) and has been shown to translocate to the nucleus following regulated intramembrane proteolysis where it may modulate transcription (7). The PC1 C-terminal region also mediates an interaction with the PC2 C-terminal tail (13, 24, 28) and can activate channel activity of PC2 in bilayers (32).
The cytoplasmic C-terminal region of PC2 plays an important role in regulation of the channel, and truncation of this region results in the inability of the channel to conduct Ca2+. The molecular basis for this is not understood, but recent studies by us have begun to unravel the function of this region of PC2 by showing that there are two distinct domains in the PC2 cytoplasmic C-terminus (6). One of these domains is a Ca2+-binding EF-hand domain with predicted structural similarity to canonical EF-hand domains. The other is a coiled-coil domain (33) that may be important for channel formation. Here, we provide an investigation into the roles that these two PC2 C-terminal domains, the EF-hand domain (PC2-EF) and the coiled-coil domain (PC2-CC) have in the interaction of PC2 with PC1. These studies provide a framework to understand the functional role of the PC1 C-terminal tail in the regulation of PC2 channel activity.
MATERIALS AND METHODS
Protein expression and purification.
Briefly (see supplemental text for detailed descriptions of all experimental procedures), N-terminally glutathione S-transferase (GST)-tagged PC1 C-terminal fragments (GST-PC1-C), PC1-4183-4270, and PC1-4202-4243 and N-terminally 6× polyhistidine (His)-tagged PC2 C-terminal fragments (His-PC2-C), PC2-704-968, PC2-720-817, PC2-720-829, PC2-798-927, and PC2-821-927 fusion constructs were expressed in Escherichia. coli and purified using standard glutathione-Sepharose or nickel-affinity chromatography (all supplementary materials for this article are available on the journal web site). For mammalian expression, PC1 constructs PC1-4183-4270 and PC1-4183-4270-Q4215P were subcloned into mammalian expression vector pcDNA 3.1+. For the pull-down experiments, the PC1 proteins were bound to the glutathione-Sepharose beads. Protein concentration after elution was determined by a Bradford assay.
Pull-down assays of PC1 and PC2 protein fragments.
GST-PC1-C constructs were immobilized on glutathione-Sepharose beads and washed twice. Purified His-PC2-C was incubated with the bound GST-PC1-C. Following incubation, the beads were pelleted, washed, and analyzed using SDS-PAGE. Each experiment was conducted at least three times, and His-PC2-C binding was confirmed by Western blotting. Control pull-downs conducted for all His-PC2 fragments using both GST alone and glutathione-Sepharose beads as the bait showed no His-PC2 binding above background.
Pull down of full-length PC2 from Madin-Darby canine kidney epithelial cells by PC1-4183-4270.
PC1-4183-4270 and PC1-4183-4270-Q4215P were affinity purified as described above using glutathione-Sepharose 4B beads, separated by anion exchange, and bound to glutathione-Sepharose beads. A microsomal fraction was isolated from confluent Madin-Darby canine kidney epithelial (MDCK) cells using differential centrifugation procedures previously described (1). The MDCK microsomes were incubated with GST-PC1-C on glutathione-Sepharose beads overnight at 4°C. The beads were then pelleted, washed, analyzed using 4–20% SDS-PAGE, and visualized by Western blotting using a PC2 antibody (YCC2, provided by S. Somlo, Yale PKD Center) (5).
Surface plasmon resonance.
PC1-4183-4270 and PC2-798-927 were purified as described above and eluted in 20 mM Tris·HCl, pH 8.0, 250 mM NaCl, 500 mM imidazole. Binding studies were performed at 25°C using a Biacore 1000 optical biosensor equipped with a sensor chip coated with an anti-GST antibody (Biacore BR-1002-23). GST-PC1-C or GST alone was captured on the anti-GST antibody surface, and PC2 binding was monitored using a twofold dilution series of 0.625, 1.25, 2.5, 5.0, and 10 μM PC2 injected in duplicate. Binding responses were double-referenced against blank injections of buffer over the GST-PC1 surface and the nonspecific binding to GST alone. Steady-state responses at the end of the association phase were used to determine K0.5.
Cell culture, transfection, and imaging.
MDCK cells were cotransfected with pcDNA3.1+ vector containing either wild-type or Q4125P PC1 constructs (PC1-4183-4270 or PC1-4183-4270-Q4215P) and pIRES2-DsRed2 (Clontech, Mountain View, CA). Controls were cotransfected with pcDNA3.1+ and pIRES2-DsRed2. Cells were loaded with 10 μM fura 2-AM (Molecular Probes-Invitrogen, Carlsbad, CA). Ratiometric Ca2+ imaging was performed with a Zeiss Axiovert 100 microscope. Intracellular Ca2+ concentrations ([Ca2+]i) were derived from background-subtracted F340/F380 fluorescent ratios (R) after in situ calibration (12, 15) using [Ca2+]i (nM) = Kd × β × (R − Rmin)/(Rmax − R), where Kd is the dissociation constant of fura 2 for Ca2+ at 37°C (225 nM), Rmin and Rmax were experimentally determined, and β was the fluorescence ratio of the emission intensity excited by 380 nm for Ca2+-free compared with Ca2+-saturating imaging buffer. Nonviable cells were excluded from the evaluation.
Statistical analysis.
Released Ca2+ (peak and total) was calculated by subtracting baseline Ca2+ of each individual cell and plotted over time. Total Ca2+ release (area under the curve) was calculated using SigmaPlot and resting Ca2+ by averaging the baseline Ca2+ of each individual cell over a period of at least 30 s with Microsoft Excel. Statistical analysis was performed using one-way ANOVA (Student-Newman-Keuls method) for multiple group comparisons, and a P value of <0.05 was considered statistically significant.
RESULTS
PC2 interaction with PC1 is mediated by C-terminal coiled coils.
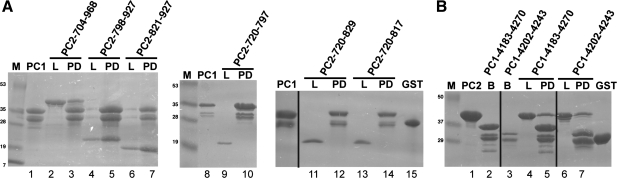
The C-terminus of PC2 is critical for channel activity (16). We recently showed that there are two domains in this cytoplasmic region, an EF-hand domain and a coiled-coil domain (6) and that these domains are separated by an ∼30-residue linker. We also demonstrated that the presumed function of these two domains is distinct, with biophysical analyses showing that the EF-hand domain binds Ca2+ with micromolar affinity and the coiled-coil domain oligomerizes (6). To investigate the role of these two domains for association with the PC1 C-terminal tail construct (PC1-4183-4270), we conducted pull-down experiments utilizing three constructs: PC2-704-968, PC2-821-927, and PC2-720-797. These constructs encoded the full cytoplasmic region of PC2, the newly identified coiled-coil domain, and the EF-hand domain, respectively (6) (Fig. 1). Our results showed that the C-terminal tails of PC1 and PC2 interact in this system, an observation that correlates well with results reported by others (24, 28, 32). More significantly, these results show that this interaction is mediated by the newly identified PC2 coiled-coil domain and not by the PC2 EF-hand domain (Fig. 2A).
Fig. 1.
Polycystin-1 (PC1) and PC2 sequence and domain structure. A: schematic representation of PC1 and PC2 domain structure and topology. PC1 is predicted to have a >3,000-amino acid extracellular region and 11 transmembrane spans. PC2 is predicted to have 6 transmembrane spans and intracellular N- and C-termini. PC1 and PC2 are predicted to interact via their C-terminal tails. B: sequences of the PC1 and PC2 C-terminal tails showing constructs, regions of predicted secondary structure (SS-Pred) (26) and predicted coiled-coil domains (CC-Pred). Human Q4224 and mouse Q4215 are shaded in the PC1 alignment. C: schematic illustrating constructs used in this study. The locations of EF-hand and coiled-coil domains are indicated (schematics not to scale). Numbering for human PC2 and mouse PC1 are shown.
Fig. 2.
Pull downs of PC1 and PC2 C-terminal tails. A: PC2-C, PC2-EF, and PC2-CC pull downs with PC1-C. Constructs that include the coiled-coil domain of PC2 pull down with PC1 (lanes 2–7), while constructs encoding only the EF-hand domain do not (lanes 9 and 10, 12–15). Example pull downs are shown for PC2 constructs with glutathione S-transferase (GST)-PC1-4183-4270 on beads. M, marker; PC1, bound PC1 on beads; L, loaded PC2; PD, pull down on beads following 3× wash; GST, GST marker; n = ≥3. Lane numbers are used in the text. B: predicted coiled-coil domain of PC1 is sufficient to pull down PC2-C. Example pull downs are shown for 93 μM PC2-704-968 with GST-PC1-constructs on beads. M, marker; PC2 PC2 sample; B, bound PC1; L, loaded PC2; PD, pull down on beads following 3× wash; GST, GST marker; n = ≥3. Black lines indicate the intervening lanes have been spliced out. Lane numbers are used in the text.
PC2 linker is not sufficient to mediate interaction with PC1.
We next investigated the role of the linker between the EF-hand and coiled-coil domains of PC2. The role of this linker region in PC2 channel activity is not fully described; however, posttranslational modification in this region (S812) alters PC2 channel activity and localization (4). To investigate whether the linker between the PC2 EF-hand and coiled-coil domains was sufficient for PC2 association with PC1, we made two sets of constructs. One set contained PC2 coiled-coil domain constructs with different N-terminal start residues, whose constructs began at R798 and G821 (Fig. 1). These constructs were likely to bind PC1-C due to the presence of the coiled-coil domain of PC2. We found that both constructs bind GST-PC1-C (Fig. 2A, lanes 2–7). The second set of constructs encoded the PC2-EF-hand domain, which does not bind to PC1, and portions of the PC2 linker. These constructs (PC2-720-817 and PC2-720-829) terminated either immediately following the phosphorylation site (S812) (4) or at the N terminus of the coiled-coil domain (6). These constructs were not expected to bind PC1-C because they lacked the coiled-coil domain of PC2. We found that the inclusion of the linker region was not sufficient to enable the association of PC2 EF-hand with GST-PC1-C (Fig. 2A, lanes 9 and 10 and 12–14). These results suggest that the interaction of PC2 with PC1 is mediated by the PC2 C-terminal coiled-coil domain and that the linker region between the EF-hand and coiled-coil domains is not sufficient to mediate the association by itself.
In similar studies, using shortened constructs of the PC2 coiled-coil domain that included variable lengths of the linker region, we observed a trend toward weaker binding for constructs that did not include the linker. We were, however, unable to obtain quantitative measurements of these interactions because of the inherent variability due to low overall binding. Nonetheless, the results suggest that the presence of the N-terminal linker to the PC2 coiled-coil domain allows a tighter association.
The PC1 coiled-coil domain is necessary and sufficient for association with PC2.
We designed a PC1 construct to encompass a minimal coiled-coil domain for mouse PC1, residues P4202 to G4243. This construct pulls down PC2, suggesting that the PC1-predicted coiled-coil domain is sufficient for association of PC1 and PC2 C-terminal tails (Fig. 2B, lanes 6 and 7).
Affinity of his-PC2-C for GST-PC1-C.
The affinity of the C-terminal tails of PC1 and PC2 for one another has never been measured. In our pull-down analyses above, we showed that the EF-hand domain did not contribute to the binding of these two proteins. Therefore, we investigated the affinity of this interaction using the longer PC1-C construct and a PC2 construct that included the linker and coiled-coil domain (PC1-4183-4270 and PC2-798-927). To obtain an approximate value for K0.5 between the C-terminal tails of PC1 and PC2, we conducted pull-down and surface plasmon resonance (SPR). Densitometric analysis of Coomassie-stained SDS-PAGE pull downs yielded a K0.5 in the low-micromolar range (data not shown). To obtain a quantitative estimate of PC1-PC2 C-terminal tail interaction, we then conducted SPR (Fig. 3). In the SPR system, oligomerization of the analyte can result in avidity effects which preclude fitting the kinetics to a simple 1:1 binding model (23). These effects were present in our SPR analysis and indicate that the coiled-coil domain of PC2 is indeed multivalent for PC1, a result that suggests the presence of multiple PC2-PC1 interactions in the polycystin ion channel complex. For complicated systems such as this, a steady-state analysis can, however, provide a reasonable estimate of K0.5. The steady-state analysis (Fig. 3, bottom) yields a K0.5 of 2.9 ± 0.9 μM.
Fig. 3.
Affinity of the cytoplasmic interaction of PC1 and PC2 C-terminal fragments. The interaction of PC2-798-927 and GST-PC1-4183-4270 produced a concentration-dependent and PC1-specific surface plasmon resonance (SPR) response [the traces presented (top) are corrected for responses generated by PC2 interacting with the chip surface that contained GST alone]. Concentrations of PC2 tested over the immobilized PC1 were 0.625, 1.25, 2.5, 5.0, and 10 μM, and the regions of the sensograms used for the steady-state analysis are marked by a horizontal bar; the errors shown (bottom) represent an average noise of ± 2 RU observed in the sensograms during data collection. The steady-state analysis (bottom) yields a K0.5 of 2.9 ± 0.9 μM. Data points are averages from duplicates, with results from 1 experiment shown (n = 3).
Helix-breaking disease mutant results in abrogation of interaction between PC1 and PC2.
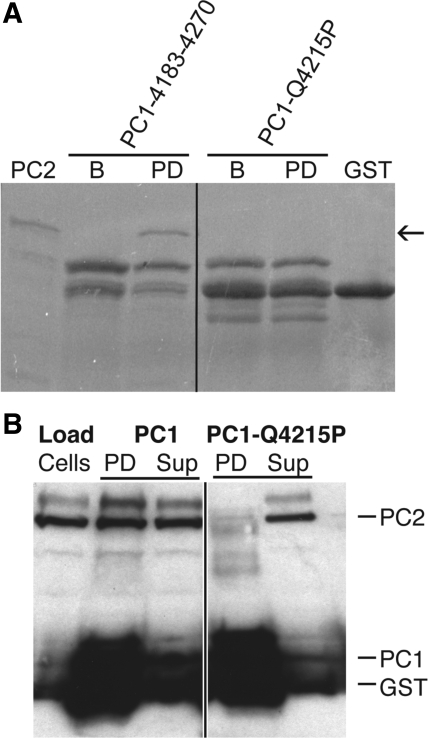
One of the point mutations in PC1 that has been documented to cause ADPKD in humans is Q4224P (3). The effect of this point mutation on the direct interaction of PC1 and PC2 has not been shown but may arise from the helix-breaking proline residue, preventing formation of an intact PC1 binding site for PC2. In the mouse, the equivalent mutation is Q4215P (see alignment in Fig. 1A). This mutation was incorporated into construct PC1-4183-4270 to investigate whether it does indeed abrogate the binding of PC1 and PC2 C-terminal tails. No interaction was detected with the mutated PC1. Densitometric analysis of the pull-down experiments showed virtually no association above background for PC1-4183-4270-Q4215P and PC2-704-968 (Fig. 4A).
Fig. 4.
Polycystin-1 Q4215P mutant does not bind PC2. A: pull downs show that introduction of the Q4215P mutation into PC1-4183-4270 interrupts association with PC2-798-927. Arrow indicates PC2-798-927. PC2, PC2-704-968 loaded; B, bound PC1 on beads; PD, pull down on beads following 3× wash; GST, GST marker; n = ≥3. B: GST-PC1-C pulls down full-length PC2 from Madin-Darby canine kidney epithelial cell (MDCK) microsomes; however, introduction of the disease-related mutation Q4215P into PC1 abrogates this pull down. PC1, PC1-4183-4270; “cells,” loaded MDCK microsomes; PD, pull down on beads; Sup, supernatant following pull down. Black lines indicate the intervening lanes have been spliced out. PC2 in the supernatant was in excess of PC1 on the beads to maximize any possible interaction. Blot is representative of >3 experiments.
Native full-length PC2 is pulled down by GST-PC1-C but not the disease mutant.
To investigate whether full-length PC2 from native tissue was able to associate with the C-terminal tail of PC1 and whether the ADPKD mutation, Q4224P, abrogates this interaction, we conducted pull downs using native PC2 and beads coated with PC1-4183-4270 or PC1-4183-4270-Q4215P, the mouse ortholog of the disease mutant (Fig. 1A). Full-length PC2 is expressed in the ER, which is included in the cytoplasmic fraction of MDCK cells. The ER was isolated from MDCK cells as the microsomal fraction of the cytoplasm and used in pull-down experiments. We found that full-length PC2 is pulled down by PC1-4183-4270 but not by the construct containing the disease mutant (Fig. 4B). These results suggest that a major effect of the Q4224P ADPKD mutation is to interfere with the association of PC1 and PC2.
Role of C-terminal region of PC1 on intracellular PC2 Ca2+ signaling.
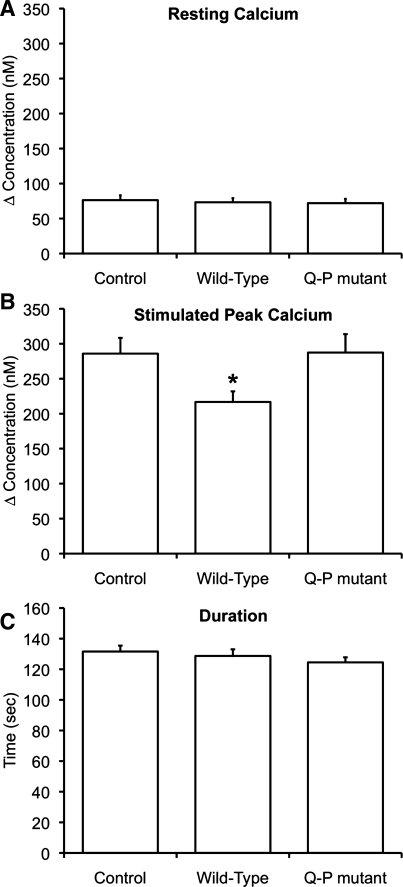
The functional role of the C-terminal tail of PC1 in maintaining normal Ca2+ signaling within a cell has not been well described. We hypothesized that overexpression of the C-terminal portion of PC1 would alter Ca2+ signaling by interfering with normal PC1-PC2 interaction and that overexpression of the disease mutant C-terminal portion of PC1 would not. To investigate this, we overexpressed the PC1-C constructs PC1-4183-4270 and PC1-C-4183-4270-Q4215P in MDCK cells (Supplementary Fig. S2). We found that expression of these constructs in MDCK cells did not alter the resting Ca2+ (Fig. 5A). Also, the ER Ca2+ load was not altered by expression of these constructs, as shown by the magnitude of the release of Ca2+ stored in the ER after addition of the inhibitor thapsigargin (data not shown); the released Ca2+ was similar in all groups monitored.
Fig. 5.
Expression of PC1-C, but not PC1-C-Q4215P, alters intracellular Ca2+ signaling. MDCK cells were loaded with fura 2-AM, and changes in intracellular Ca2+ levels were monitored upon addition of extracellular ATP in the absence of extracellular Ca2+. In all cases, the values plotted represent the average of the response measured in 92-254 cells from experiments performed on at least 5 different days. Control, experiments where cells were mock transfected with empty vector. A: resting Ca2+ levels were unchanged by the expression of PC1-4183-4270 (wild-type) or PC1-4183-4270-Q4215P (Q-P mutant). B: peak Ca2+ release was attenuated after addition of 3 μM ATP in cells expressing PC1-C wild-type, but not the Q-P mutant, presumably because expression of PC1-C disrupts the normal interaction between full-length PC1 and PC2 in MDCK cells. C: duration of the response to 3 μM ATP was similar in cells expressing PC1-C wild-type and the Q-P mutant. *Statistically significant (P <0.05).
When cells were stimulated with a saturating concentration of agonist (15 μM ATP), the response was maximal for all conditions and it was not possible to separate out differences among the treatment groups (data not shown). However, when cells were stimulated with a lower concentration of agonist (3 μM ATP), we found that the peak Ca2+ level achieved was reduced when PC1-C was overexpressed (Fig. 5B), a situation where the normal interaction between full-length PC1 and PC2 would be lost; note that the duration of response was unchanged (Fig. 5C). Expression of the mutated version of PC1-C (PC1-C-4183-4270-Q4215P) did not alter the peak Ca2+ levels (Fig. 5B), as expected for a construct that does not interfere with the normal interaction between PC1 and PC2. These results show that the interaction between PC1-C and PC2-C can influence intracellular Ca2+ signals and that the modulation does not occur when the interaction between the two proteins is lost. The disease-related point mutation alters this functional interaction.
DISCUSSION
In this study, we show that the newly identified coiled-coil domain of PC2 (6) is critical for maintenance of the PC1-PC2 interaction and that disruption of the interaction of the C-terminal tails of PC1 and PC2 can alter normal cellular Ca2+ signaling. This result helps explain the pathological effect of extreme C-terminal truncations observed in PC2 in ADPKD patients (25); normal channel regulation by PC1 cannot be maintained in these patients. Other studies have shown that this interaction is important for PC2 channel regulation (32) but divergent expression patterns suggest that PC1 and PC2 are not always associated (9). Here, we provide the first quantitation of the affinity of this interaction and show that K0.5 for C-terminal tail-mediated interaction between PC1 and PC2 is in the low-micromolar range. This is an interesting result in the context of the recent structural study showing crystallization of only the PC2 coiled-coil domain under crystallization conditions that contained both PC1 and PC2 (33). Additionally, our observation that the linker may be important for binding provides a molecular basis for the finding that phosphorylation of S812 regulates PC2 channel activity (4).
Intracellular Ca2+ changes have been shown to control an increasingly large number of cellular events, and modulation of intracellular Ca2+ signaling is necessary to maintain cellular integrity. Recently, it has become clear that PC2 plays a role in maintaining Ca2+ signaling in a number of cell types. For example, there is synergy with both the inositol 1,4,5-trisphosphate receptor (20) and the ryanodine receptor (2), and there are profound changes in the intracellular Ca2+ transients when regulation by full-length PC2 is deleted (2) or overexpressed (16). Similarly, when pathogenic mutations are expressed intracellular signaling is altered (16). Here, we also show that mutation of a portion of PC1, the C-terminal region, disrupts its ability to bind to its cellular partner PC2 and that this change leads to alterations in Ca2+ signaling.
Multiple proteins have been suggested to interact with the C-terminal fragments of both PC1 and PC2. Many of these protein-binding partners can modulate channel activity (13, 24, 28, 32), and some of these interactions with PC2 have been suggested to take place in what is now described as the C-terminal coiled-coil domain (18, 19), overlapping with the region that we describe here as critical for PC1-PC2 association. It is not currently clear whether the multiple protein-binding partners of PC2 (13, 24, 28, 32) are exclusive of or cooperative with PC1, and how these proteins interact with PC2; however, the diversity in modulation of channel activity and the similarity in binding sites suggest that active protein-mediated regulation of PC2 Ca2+ signaling may occur in normal channel function.
GRANTS
This work was supported, in whole or in part, by National Institutes of Health Grants P50 DK057328 (pilot grant to T. J. Boggon, project to B. E. Ehrlich.), DK57328 (to B. E. Ehrlich), CA009085 (training grant to E. T. Petri), and N01-HV-28186 contract (partial support for E. F. Folta-Stogniew), and two German National Merit Foundation scholarships (to J. Casuscelli and S. Schmidt). This work was also supported in part by a grant from the Polycystic Kidney Disease Foundation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank David Calderwood, Amit Kumar, Xiaofeng Li, Nilda Alicea-Velázquez, and Rong Zhang. The Somlo (Yale) and Germino (Johns Hopkins) laboratories kindly supplied antibodies.
Present address of J. Casuscelli: Witten/Herdecke University, Alfred-Herrhausen-Str. 50, 58448 Witten, Germany.
REFERENCES
- 1.Anyatonwu GI, Ehrlich BE. Organic cation permeation through the channel formed by polycystin-2. J Biol Chem 280: 29488–29493, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Anyatonwu GI, Estrada M, Tian X, Somlo S, Ehrlich BE. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci USA 104: 6454–6459, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badenas C, Torra R, San Millan JL, Lucero L, Mila M, Estivill X, Darnell A. Mutational analysis within the 3′ region of the PKD1 gene. Kidney Int 55: 1225–1233, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE, Somlo S. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem 279: 19987–19995, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 274: 28557–28565, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Ćelić A, Petri ET, Demeler B, Ehrlich BE, Boggon TJ. Domain mapping of the polycystin-2 C-terminal tail using de novo molecular modeling and biophysical analysis. J Biol Chem 283: 28305–28312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S, Caplan MJ. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest 114: 1433–1443, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol 11: 814–827, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Geng L, Segal Y, Pavlova A, Barros EJ, Lohning C, Lu W, Nigam SK, Frischauf AM, Reeders ST, Zhou J. Distribution and developmentally regulated expression of murine polycystin. Am J Physiol Renal Physiol 272: F451–F459, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 13.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151–160, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Kao JP. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol 40: 155–181, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Liu Y, Shen PY, Dai XQ, Wang S, Smillie LB, Sandford R, Chen XZ. Troponin I binds polycystin-L and inhibits its calcium-induced channel activation. Biochemistry 42: 7618–7625, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Montalbetti N, Shen PY, Dai XQ, Cheeseman CI, Karpinski E, Wu G, Cantiello HF, Chen XZ. Alpha-actinin associates with polycystin-2 and regulates its channel activity. Hum Mol Genet 14: 1587–1603, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Shen PY, Wu G, Chen XZ. Polycystin-2 interacts with troponin I, an angiogenesis inhibitor. Biochemistry 42: 450–457, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wright JM, Qian F, Germino GG, Guggino WB. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem 280: 41298–41306, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23: 2600–2607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Myszka DG. Improving biosensor analysis. J Mol Recognit 12: 279–284, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Reynolds DM, Hayashi T, Cai Y, Veldhuisen B, Watnick TJ, Lens XM, Mochizuki T, Qian F, Maeda Y, Li L, Fossdal R, Coto E, Wu G, Breuning MH, Germino GG, Peters DJ, Somlo S. Aberrant splicing in the PKD2 gene as a cause of polycystic kidney disease. J Am Soc Nephrol 10: 2342–2351, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res 32: W321–W326, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somlo S, Ehrlich B. Human disease: calcium signaling in polycystic kidney disease. Curr Biol 11: R356–360, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94: 6965–6970, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye C, Brown EM, Hediger MA, Zhou J. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282: 341–350, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Wilson PD. Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Xu GM, Gonzalez-Perrett S, Essafi M, Timpanaro GA, Montalbetti N, Arnaout MA, Cantiello HF. Polycystin-1 activates and stabilizes the polycystin-2 channel. J Biol Chem 278: 1457–1462, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, Tong L, Isacoff EY, Yang J. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci USA 106: 11558–11563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.