Abstract
Assessing insulin resistance in rodent models gives insight into mechanisms that cause type 2 diabetes and the metabolic syndrome. The hyperinsulinemic euglycemic glucose clamp, the reference standard for measuring insulin sensitivity in humans and animals, is labor intensive and technically demanding. A number of simple surrogate indexes of insulin sensitivity/resistance have been developed and validated primarily for use in large human studies. These same surrogates are also frequently used in rodent studies. However, in general, these indexes have not been rigorously evaluated in animals. In a recent validation study in mice, we demonstrated that surrogates have a weaker correlation with glucose clamp estimates of insulin sensitivity/resistance than in humans. This may be due to increased technical difficulties in mice and/or intrinsic differences between human and rodent physiology. To help distinguish among these possibilities, in the present study, using data from rats substantially larger than mice, we compared the clamp glucose infusion rate (GIR) with surrogate indexes, including QUICKI, HOMA, 1/HOMA, log (HOMA), and 1/fasting insulin. All surrogates were modestly correlated with GIR (r = 0.34–0.40). Calibration analyses of surrogates adjusted for body weight demonstrated similar predictive accuracy for GIR among all surrogates. We conclude that linear correlations of surrogate indexes with clamp estimates and predictive accuracy of surrogate indexes in rats are similar to those in mice (but not as substantial as in humans). This additional rat study (taken with the previous mouse study) suggests that application of surrogate insulin sensitivity indexes developed for humans may not be appropriate for determining primary outcomes in rodent studies due to intrinsic differences in metabolic physiology. However, use of surrogates may be appropriate in rodents, where feasibility of clamps is an obstacle and measurement of insulin sensitivity is a secondary outcome.
Keywords: glucose clamp, calibration model, quantitative insulin-sensitivity check index
insulin resistance is typically defined as decreased sensitivity and/or responsiveness to metabolic actions of insulin (19). This cardinal feature of diabetes, obesity, and dyslipidemias is also a prominent component of cardiovascular diseases, including hypertension, coronary heart disease, and atherosclerosis (6, 26). Rodent models of metabolic diseases and their cardiovascular complications are useful for elucidating underlying pathophysiology and for evaluating novel therapeutic approaches (5, 12, 22). The ability to efficiently and accurately quantify insulin sensitivity/resistance is an essential aspect of ascertaining the metabolic phenotype of rodent models of human diseases and their response to therapeutic interventions. In both humans and animals, the reference standard method for directly quantifying insulin sensitivity/resistance is the hyperinsulinemic euglycemic glucose clamp (19). This is a technically demanding, time-consuming procedure that is not feasible to apply in large studies. Therefore, more tractable surrogate indexes of insulin sensitivity/resistance have been developed and validated in humans (3, 13, 17–19, 23). In rodents, performing adequate glucose clamps is even more challenging than in humans. This is due to small body mass, total blood volumes (∼2 ml in mice) that limit blood sampling, substantial fasting-induced loss in body weight prior to the clamp study, stress from anesthesia and/or instrumentation, and a variety of other technical issues. A number of animal studies have adopted the use of surrogate indexes developed in humans to determine insulin sensitivity/resistance (11, 15, 24, 25, 28). However, little is known about the validity of these indexes in animal studies. Therefore, it is important to directly compare glucose clamp estimates of insulin sensitivity with surrogate indexes of insulin sensitivity/resistance in animals to determine whether it is appropriate to use these surrogates in the context of animal studies. In the only published study that directly compares the glucose clamp with surrogate indexes of insulin sensitivity/resistance in mice, we observed a weaker correlation between clamp estimates and surrogate indexes than in comparable human studies (14). One potential reason for poorer performance of surrogate indexes in mice may be the associated technical difficulties caused by small body size. This raises the possibility that comparisons in rats with larger body size and where studies are less technically demanding will give results more in line with comparisons in human studies. Alternatively, inherent differences between human and rodent physiology may be the overriding reason that surrogate indexes developed and validated in humans do not perform as well in rodents. To help evaluate these possibilities, we used data derived from rats with a wide range of insulin sensitivities to compare glucose clamp estimates of insulin sensitivity with a variety of surrogate indexes, including quantitative insulin sensitivity check index (QUICKI), homeostasis model assessment (HOMA), 1/HOMA, log (HOMA), and 1/fasting insulin. In addition, we formally assessed the predictive accuracy of these surrogates using a calibration model analysis.
METHODS
Rat models used.
For the present study, we used glucose clamp data from a total of 80 rats with a wide range of insulin sensitivity. The phenotypes of these rats have been described previously. These include 1) 6-mo-old female Sprague-Dawley rats [nonpregnant sham-operated rats (n = 3), pregnant sham-operated rats (n = 8), and pregnant rats with visceral fat surgically removed 1 mo before mating (n = 4) (7)], 2) 2- and 20-mo-old male F1 hybrids of Brown Norway × Fischer 344 rats fed ad libitum (AL) or assigned to caloric-restricted diet [55% of the calories consumed by AL (n = 21) (9, 10)], 3) 20-mo-old sham-operated rats fed AL or rats with visceral fat surgically removed 5 mo before the clamp study (n = 12) (8, 9), and 4) 3-mo-old male Sprague-Dawley rats (n = 32) (20, 21).
Hyperinsulinemic euglycemic clamp.
Glucose clamp procedures were performed in awake, precatheterized, unrestrained, unanesthetized rats as described (7, 9, 10). Briefly, rats were fasted for 6 h, and a 2-h hyperinsulinemic euglycemic clamp procedure was conducted. A primed continuous infusion of insulin (3 mU·kg−1·min−1) was initiated to acutely increase and then maintain high, but physiological, plasma insulin levels. Somatostatin (1.5 μg·kg−1·min−1) was infused to suppress endogenous insulin secretion. A variable infusion of 25% dextrose was adjusted periodically to clamp the plasma glucose concentration at the basal level during the hyperinsulinemic phase of the clamp study. A continuous infusion of [3-3H]glucose was given for 2 h prior to and throughout the clamps to allow estimation of basal and insulin-stimulated whole body glucose turnover. The glucose infusion rate (GIR) adjusted for body weight was used as a measure of insulin sensitivity. When plasma glucose is at steady state during the clamp, the rate of glucose disappearance (Rd) is equal to the rate of glucose appearance (Ra). Ra was determined as described (7, 9, 10). Hepatic glucose production (HGP) during the clamp was calculated as the difference between Ra and GIR. Animal study protocols were reviewed and approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine.
Surrogate indexes of insulin sensitivity/resistance.
Fasting samples for glucose and insulin were obtained from chronically placed intravenous lines at the beginning of the clamp studies. Surrogate indexes were calculated from fasting blood glucose and plasma insulin concentrations as follows: QUICKI = 1/[log(I0) + log(G0)], where I0 is fasting insulin (μU/ml) and G0 is fasting glucose (mg/dl); and HOMA = (G0 × I0)/22.5, with glucose expressed as mmol/l and insulin expressed as μU/ml. Other surrogate indexes examined were 1/HOMA, log (HOMA), and 1/fasting insulin.
Calibration model analysis of surrogate indexes adjusted for body weight.
Calibration model analysis was performed as described previously (3, 14). Calibration is inverse regression. For the model y = f (x; θ) + ε, x is the explanatory variable, y is the response variable, θ is an unknown parameter, and ε is the random error. Using an estimated model y = f (x; θ̂) to predict a new y* for a given x* is regression. Conversely, predicting a new x* for a given y* is calibration. If the value of x is prespecified as part of an experimental design, this is called classical or controlled calibration. Since QUICKI (or other surrogates), GIR, and weight are measured with error from an experimental population, random calibration is the more appropriate method to use. In random calibration, there is no difficulty in specifying the conditional distribution of x given y, so that random calibration is similar to regression in prediction. Here, we fitted a calibration model xi = α + β1Si + β2Wi + β3 Si·Wi + εi, where xi is GIR, Si is the surrogate index, Wi is the body weight, Si·Wi is the product of surrogate index and body weight, and εi is the random error for the ith subject. It was assumed that the random error has Gaussian distribution with mean of 0 and a constant variance. Although GIR was measured with error, it was assumed for our model that the measurement error of GIR (determined from a robust, direct, and data-intensive protocol) was very small relative to measurement error of simple surrogates adjusted for body weight determined from single fasting measurements (e.g., QUICKI). Therefore, to simplify the analysis, we neglected measurement error for GIR in our calibration model.
For analysis of each surrogate index, two types of predicted residuals were considered. The first type of residual is the difference between measured GIR (xi for the ith subject) and fitted GIR (x^i = α^ + β^1Si + β^2Wi + β^3Si·Wi). That is, the residual, ei = xi − x^i, is derived from the calibration model, with all subjects included in the estimation of model parameters α, β1, β2, and β3. The second type of residual considered is a cross-validation type predicted residual, e(i) = xi − x^(i), where xi is still measured GIR, but x^(i) is predicted GIR from the calibration model that excludes the ith subject. The subscript (i) means “with the ith subject deleted”. From these two types of residuals, criterion functions were used to evaluate prediction accuracy: square root of the mean squared error of prediction, RMSE = , and leave-one-out cross-validation-type RMSE of prediction, CVPE = . Smaller values of RMSE and CVPE indicate better predictive power. RMSE is likely to underestimate prediction errors, whereas CVPE is more robust.
Statistical analysis.
Pearson correlation coefficients (r) and corresponding P values were calculated to assess the statistical significance of the model using a linear least-squares fit method to obtain the linear regression. To compare predictive accuracy of QUICKI (adjusted for weight) and other surrogates (adjusted for weight) in terms of CVPE and RMSE, we performed hypothesis testing with the one-sided alternative hypothesis that weight-adjusted QUICKI had a smaller RMSE or CVPE than another weight-adjusted surrogate, using a Bootstrap percentile method with 60,000 replications performed for each comparison. The bootstrap method is appropriate because the RMSEs (or CVPEs) corresponding to QUICKI and other surrogates were derived from the same group of subjects and thus correlated. The P values calculated from comparison of RMSE and CVPE were for pair-wise comparisons. For example, when weight-adjusted QUICKI and weight-adjusted HOMA were compared with respect to CVPE based on 80 rats, a bootstrap percentile method with 60,000 replications was used to get a sample of 60,000 differences in CVPE [CVPE (HOMA) − CVPE (QUICKI)], and then a P value for one-sided superiority testing was estimated as the proportion of the bootstrap replications less than zero. One-sided hypothesis testing was used because multiple previous studies in humans have demonstrated the superiority of weight-adjusted QUICKI as a surrogate index of insulin sensitivity from a variety of perspectives supporting an a priori expectation. P < 0.05 was considered to indicate statistical significance. The software used for statistical analysis and the random calibration model was SAS System V9.
RESULTS
Rat models of insulin sensitivity/resistance.
Metabolic characteristics of the 80 rats analyzed in our study under fasting and steady-state glucose clamp conditions are shown in Table 1. Detailed phenotyping of these rats has been described previously (7–10, 21). In the present study, we included data from rats with normal insulin sensitivity (n = 41) as well as models of pregnancy- and aging-associated insulin resistance (n = 12 and 27, respectively). These data include rat studies that examined effects of visceral fat removal and chronic calorie restriction on insulin sensitivity (n = 8 and 11, respectively). GIR (mg·kg−1·min−1) was highest in nonpregnant (19.9 ± 1.1), lowest in pregnant (11.6 ± 1.0), and intermediate in pregnant rats in which visceral fat was surgically removed (14.6 ± 2.2). In studies related to age-associated insulin resistance, GIR was higher in calorie-restricted rats compared with rats fed AL (18.9 ± 1.7 vs. 7.0 ± 1.0). Thus, we have analyzed data from a variety of rat models with a wide range of insulin sensitivity, directly determined using the reference standard glucose clamp technique.
Table 1.
Metabolic characteristics of rats included in this study (n = 80)
| Parameters | (n = 80) |
|---|---|
| Body weight, g | 316 (296–385) |
| Fasting glucose, mg/dl | 133 (119–151) |
| Fasting insulin, μU/ml | 52 (33–73) |
| GIR, mg·kg−1·min−1 | 14.5 (8.9–18.6) |
| Rd clamp | 20 (15–23) |
| Clamp HGP, mg·kg−1·min−1 | 5.5 (4.1–7.5) |
| QUICKI | 0.263 (0.248–0.278) |
| Log (HOMA) | 1.190 (0.973–1.407) |
| HOMA | 15.51 (9.40–25.53) |
| 1/HOMA | 0.064 (0.039–0.106) |
| 1/Fasting insulin, ml/μU | 0.019 (0.013–0.030) |
Data shown are median (interquartile range) from glucose clamp studies at baseline (fasting) or under steady-state clamp conditions. GIR, glucose infusion rate; Rd, rate of glucose disappearance; HGP, hepatic glucose production; QUICKI, quantitative insulin sensitivity check index; HOMA, homeostasis model assessment.
Correlations between clamp-derived measurement of insulin sensitivity and surrogate indexes of insulin sensitivity/resistance.
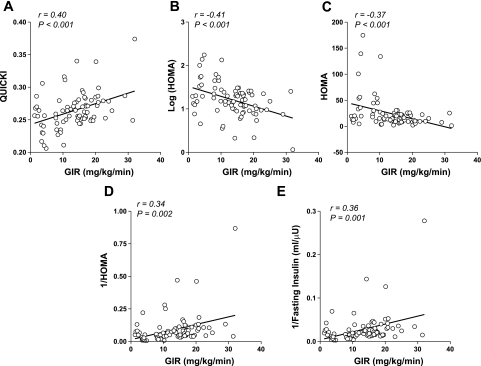
When we compared data from each of the surrogate indexes and clamp-derived GIR for all study rats, modest linear correlations (r = 0.34–0.40) were observed (Fig. 1). From this analysis the surrogate indexes [QUICKI, HOMA, 1/HOMA, log (HOMA)] appeared comparable, with no particular advantage evident among any of the surrogates. We also examined the relationships between various surrogate indexes and a measurement of hepatic insulin sensitivity, clamp HGP (CHGP), in our rat cohort (n = 80). Fasting insulin levels (r = 0.36, P = 0.001), HOMA (r = 0.35, P = 0.001), and log (HOMA) (r = 0.25, P = 0.02) but not 1/fasting insulin, fasting glucose levels, QUICKI, or 1/HOMA were significantly related to CHGP. Thus, surrogate indexes correlate modestly with both clamp hepatic glucose output (r = 0.21–0.35) and GIR (r = 0.34–0.40), but CGHP does not outperform GIR in rats when compared with surrogate indexes.
Fig. 1.
Linear correlations between surrogate indexes of insulin sensitivity/resistance and glucose infusion rate (GIR) derived from glucose clamp studies (n = 80). The solid line represents the linear least-squares fit between each calculated surrogate index of insulin sensitivity/resistance and measured GIR, using data from the entire cohort. Correlation coefficients (r) and corresponding P values are shown in each part. A: results derived from quantitative insulin sensitivity check index (QUICKI). B: results derived from log [homeostasis model assessment (HOMA)]. C: results derived from HOMA. D: results derived from 1/HOMA. E: results derived from 1/fasting insulin.
Correlations between GIR and body weight in rats.
In our previous study in mice, body weight was strongly correlated with insulin sensitivity determined by either glucose clamp or surrogate measures (14). Similarly, in the present study, the body weight of rats was significantly related to GIR (r = −0.55, P < 0.0001) and all surrogate indexes of insulin sensitivity/resistance examined [QUICKI: r = −0.49, P < 0.0001; log (HOMA): r = 0.55, P < 0.0001; HOMA: r = 0.62, P < 0.0001; 1/HOMA: r = −0.32, P < 0.003; and 1/fasting insulin: r = −0.32, P < 0.002]. Therefore, for our calibration model analyses we used surrogate indexes of insulin sensitivity/resistance adjusted for body weight.
Calibration model analysis of surrogate indexes adjusted for body weight.
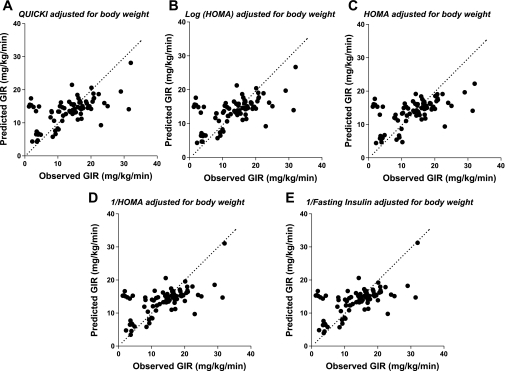
We tested the absolute accuracy of various surrogate indexes adjusted for body weight using calibration model analysis. The calibration model predicts GIR based on data from each surrogate index. We regressed experimentally determined GIR on each surrogate index and fitted these data to a calibration model, as described in methods. We determined model parameters α and β for each surrogate index for the entire cohort. We then used the fitted calibration model (using leave-one-out cross-validation analysis) to generate plots of the values for predicted GIR by each surrogate index as a function of the measured GIR in our entire cohort (Fig. 2). If a surrogate index adjusted for body weight perfectly predicted GIR, results for each rat would fall on a straight line with a slope of 1 and a y-intercept of 0. By inspection, all surrogates gave comparable predictions of GIR, with no one particular surrogate index emerging as a superior predictor of GIR. To quantitatively assess predictive accuracy for each surrogate index, residuals (measured GIR − predicted GIR) generated from random calibration analysis were used to calculate CVPE and RMSE (Table 2). No significant differences were noted among CVPE or RMSE from any of the surrogate indexes when compared with QUICKI.
Fig. 2.
Comparisons between predicted GIR derived from surrogate indexes of insulin sensitivity/resistance adjusted for body weight (leave-one-out cross-validation analysis) and actual GIR measured during glucose clamp studies. Calibration model analysis of data from surrogate indexes was used to generate predictions of GIR, as described in methods. The dashed line indicates ideal predictive accuracy with a slope of 1 and intercept of 0. A: results derived from QUICKI adjusted for body weight. B: results derived from log (HOMA) adjusted for body weight. C: results derived from HOMA adjusted for body weight. D: results derived from 1/HOMA adjusted for body weight. E: results derived from 1/fasting insulin adjusted for body weight.
Table 2.
CVPE and RMSE estimates of error calculated from calibration analysis of surrogate indexes of insulin sensitivity/resistance adjusted for weight
| CVPE | P Value | RMSE | P Value | |
|---|---|---|---|---|
| QUICKI | 5.86 | 5.80 | ||
| Log (HOMA) | 5.88 | 0.39 | 5.81 | 0.44 |
| HOMA | 5.96 | 0.29 | 5.88 | 0.29 |
| 1/HOMA | 5.80 | 0.50 | 5.79 | 0.44 |
| 1/Fasting insulin | 5.80 | 0.44 | 5.79 | 0.45 |
CVPE, leave-one-out cross-validation-type root mean squared error of prediction; RMSE, square root of the mean squared error of prediction. These error estimates are derived from comparing observed GIR from glucose clamp studies with predicted GIR derived from calibration analysis of surrogate indexes, as described in methods (n = 80). P values are for statistical comparisons between CVPE or RMSE for QUICKI adjusted for weight and respective error estimates from alternative surrogate indexes adjusted for weight.
DISCUSSION
Insulin resistance plays a major pathophysiological role in type 2 diabetes and is associated with obesity, hypertension, coronary artery disease, dyslipidemias, and a cluster of metabolic and cardiovascular abnormalities that define the metabolic syndrome (6, 26). An epidemic of obesity is driving increased incidence and prevalence of type 2 diabetes and its cardiovascular complications. Thus, it is essential to develop and validate accurate tools for measuring insulin sensitivity/resistance in both humans and animals. Among animal studies, validation and explicit comparisons between surrogate indexes of insulin sensitivity/resistance and glucose clamp estimates are sparse. In our previous study of mice with a wide spectrum of insulin sensitivity/resistance, we demonstrated that linear correlations of surrogate indexes with clamp estimates and predictive accuracy of surrogate indexes in mice are not as substantial as in humans. Smaller body size with accompanying technical difficulties may contribute to diminished precision of clamp estimates in mice. Therefore, in this study, we formally examined the relationship of surrogate measurements with clamp estimates of insulin sensitivity/resistance in rats, which are less technically demanding to work with than mice.
Comparisons between glucose clamp estimates and surrogate indexes of insulin sensitivity/resistance.
A total of 80 rats were studied in this report; in addition to normal rats, pregnancy and aging-induced insulin resistance in rats were included. Furthermore, data from interventions that ameliorate insulin resistance in rats such as calorie restriction and visceral fat removal were included. All of the surrogate indexes evaluated [QUICKI, log (HOMA), HOMA, 1/HOMA, and 1/fasting insulin] had modest linear correlations with GIR (r = 0.34–0.40), with no clear advantage among QUICKI, log (HOMA), HOMA, 1/HOMA, or 1/fasting insulin. The strength of these correlations was comparable with that observed in our previous study in mice. By contrast, in humans, both QUICKI and log (HOMA) have much more substantial and stronger linear correlations with glucose clamp estimates in multiple independent studies (r ≈ 0.70–0.90) (3, 4, 13, 16, 27, 29, 30). The use of the human-specific normalizing factor 22.5 in the calculation of HOMA, rather than a rat-specific linear normalizing factor, does not affect our results in terms of the magnitude or significance of linear correlation between surrogates and GIR, our calibration analysis, or the conclusions drawn from our data.
In the present study, glucose clamps were performed in unrestrained, conscious, and chronically catheterized rats. Consequently, these animals are less stressed, blood sampling for glucose and insulin measurements are relatively easier than in mice, and glucose metabolism is not affected by coadministration of anesthetics. However, it is possible that animals are less stressed and acclimatized at the later part of the clamp study than the beginning, thus affecting surrogate indexes obtained from basal blood samples (1). Despite the larger size of rats with larger blood volumes and less demanding technical issues, our results with rats were similar to those we reported previously for mice (14). In addition to technical issues in rodents that present more difficulties than in humans, inherent differences between rodent and human metabolic physiology include the absence of a true physiological fasting state in rodents. This is an important consideration because all of the simple surrogate indexes examined in this study depend on fasting plasma insulin and/or glucose levels that primarily reflect hepatic insulin sensitivity/resistance (19). GIR determined under hyperinsulinemic glucose clamp conditions reflects primarily insulin-mediated glucose disposal in skeletal muscle. In humans, hepatic insulin sensitivity/resistance is normally quite tightly coupled to peripheral insulin sensitivity and overall insulin-stimulated glucose disposal. This accounts for the excellent performance of surrogates, including QUICKI and log (HOMA) in humans. In rodents that do not have a physiological fasting state, it is possible that the coupling between hepatic and skeletal muscle insulin sensitivity may be different than in humans. In fact, in our study, CGHP, a measurement of hepatic insulin sensitivity, does not outperform GIR in rats when compared with surrogate indexes. These results suggest that, because rodents do not have a physiological fasting state, surrogate indexes based on experimentally imposed fasting glucose and insulin may not correlate as robustly with either hepatic or whole body insulin sensitivity/resistance as in humans.
In contrast with our current study, a recent study in nonpregnant and pregnant rats from two rat strains (Wistar and Sprague-Dawley) reported that the relationship between surrogate indexes and GIR was similar in magnitude (r = ∼0.70) to that observed in humans (2). Incidentally, comparison of GIR with various surrogate indexes in the Sprague-Dawley and female pregnant rat subgroup (n = 40) in our study demonstrated much weaker correlations compared with the overall cohort (data not shown). This is most likely because the range of insulin sensitivity in this subgroup of more homogenous rats is much narrower than the overall cohort. It is not clear why results of this previous rat study are different from our current study. Some possible explanations for these differences may be that the previous study used isoglycemic rather than euglycemic clamps. In addition, the previous study had other technical differences from our study, including blood sampling from tail vein instead of indwelling catheter and the use of anesthesia instead of unrestrained conscious animals that may affect hepatic and/or peripheral insulin sensitivity. Moreover, in our more heterogenous cohort, various interventions, including age-associated insulin resistance, caloric restriction, and visceral fat removal, may have differentially affected hepatic and muscle insulin sensitivity contributing to weaker overall correlations between surrogate indexes and glucose clamp estimates in our study. Unfortunately, because of the small numbers in each of these intervention subgroups, subgroup analysis would not be informative.
In this study, we included data from highly heterogeneous rat cohorts ranging from very insulin sensitive to very insulin resistant. That is, clamp GIR, a direct measurement of insulin sensitivity, ranged between 1.25 and 32 mg·kg−1·min−1. The purpose of including this wide range of insulin sensitivities was to attempt to maximize the linear correlation between surrogate indexes and clamp-derived measurements of insulin sensitivity. This effect is seen in human studies (13, 16) and simply reflects the statistical property that larger dynamic ranges in the variable of interest have a tendency to improve linear correlations with surrogate indexes (although this does not affect absolute accuracy as measured by calibration analysis). Despite inclusion in our study of rats with a wide range of insulin sensitivity, surrogate indexes had modest linear correlations with clamp estimates of insulin sensitivity that would likely be worse in homogeneous rats with a narrower range of insulin sensitivities.
Calibration model analysis of surrogate indexes adjusted for body weight.
Evaluating the absolute accuracy of various surrogate indexes of insulin sensitivity/resistance is crucial in formally assessing the validity of these indexes. To that end, we used calibration model analysis to assess the predictive accuracy of each surrogate index adjusted for body weight. Using calibration model analysis, weight-adjusted QUICKI (or other weight-adjusted surrogate indexes) was reasonable at predicting GIR. This finding was similar to that observed in our previous study in mice (14). However, in humans, QUICKI and log (HOMA) are both excellent at predicting insulin sensitivity derived from glucose clamp studies, with a much smaller CVPE and RMSE compared with rodents (3). In our calibration model analysis of mice or rats, there were no significant differences in predictive accuracy among any of the surrogates tested. Taken together with our previously published mice study, our results suggests that intrinsic differences between human and rodent physiology may be the overriding reason that surrogate indexes developed and validated in humans do not perform as well in rodents.
In summary, in rats, as in mice, simple surrogate indexes of insulin sensitivity/resistance originally developed and extensively validated for use in humans modestly correlate with and predict GIR from the reference standard glucose clamp. In rodent studies where determination of insulin sensitivity is a primary study outcome, the glucose clamp remains the method of choice. However, if feasibility is an issue due to large numbers of animals or other issues, and determination of insulin sensitivity/resistance is a secondary outcome, it may still be useful to employ a surrogate index in rats to determine and follow up changes in insulin sensitivity/resistance in response to therapeutic interventions.
GRANTS
This work was supported by the Intramural Research Program, the National Center for Complementary and Alternative Medicine, and grants from the National Institutes of Health (K08-AG-027462 and P60-DK-20541 to R. H. Muzumdar; R01-AG-18381, T32-AG-23475, and P01-AG-021654 to N. Barzilai) and the Core Laboratories of the Albert Einstein Diabetes Research and Training Center (P60-DK-20541).
REFERENCES
- 1.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43: 42–51, 2004 [PubMed] [Google Scholar]
- 2.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab 295: E1269–E1276, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes 54: 1914–1925, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Sullivan G, Yue LQ, Katz A, Quon MJ. QUICKI is a useful index of insulin sensitivity in subjects with hypertension. Am J Physiol Endocrinol Metab 284: E804–E812, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocr Rev 28: 48–83, 2007 [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88: 787–835, ix, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Einstein FH, Fishman S, Muzumdar RH, Yang XM, Atzmon G, Barzilai N. Accretion of visceral fat and hepatic insulin resistance in pregnant rats. Am J Physiol Endocrinol Metab 294: E451–E455, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 51: 2951–2958, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gupta G, Cases JA, She L, Ma XH, Yang XM, Hu M, Wu J, Rossetti L, Barzilai N. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Am J Physiol Endocrinol Metab 278: E985–E991, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Gupta G, She L, Ma XH, Yang XM, Hu M, Cases JA, Vuguin P, Rossetti L, Barzilai N. Aging does not contribute to the decline in insulin action on storage of muscle glycogen in rats. Am J Physiol Regul Integr Comp Physiol 278: R111–R117, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, de Angelis MH, Halban PA, Wolf E, Aigner B, Wanke R. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes 56: 1268–1276, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, Rabadan-Diehl C, Goldberg IJ. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res 100: 1415–1427, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, Kim JK, Quon MJ. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 294: E261–E270, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Mather KJ, Hunt AE, Steinberg HO, Paradisi G, Hook G, Katz A, Quon MJ, Baron AD. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab 86: 5457–5464, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294: E15–E26, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS One 4: e6334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muzumdar RH, Ma X, Fishman S, Yang X, Atzmon G, Vuguin P, Einstein FH, Hwang D, Cohen P, Barzilai N. Central and opposing effects of IGF-I and IGF-binding protein-3 on systemic insulin action. Diabetes 55: 2788–2796, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Nandi A, Kitamura Y, Kahn CR, Accili D. Mouse models of insulin resistance. Physiol Rev 84: 623–647, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab 17: 305–322, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M. Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes 55: 3594–3603, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 292: E1378–E1387, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res 59: 207–223, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Skrha J, Haas T, Sindelka G, Prazny M, Widimsky J, Cibula D, Svacina S. Comparison of the insulin action parameters from hyperinsulinemic clamps with homeostasis model assessment and QUICKI indexes in subjects with different endocrine disorders. J Clin Endocrinol Metab 89: 135–141, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, Xu JY, Chen B, Chow WS, Tso AW, Lam KS. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA 102: 6086–6091, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, Tahara H, Koyama H, Shoji T, Inaba M, Nishizawa Y. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment are useful indexes of insulin resistance in type 2 diabetic patients with wide range of fasting plasma glucose. J Clin Endocrinol Metab 89: 1481–1484, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, Tahara H, Shoji T, Okuno Y, Nishizawa Y. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care 26: 2426–2432, 2003 [DOI] [PubMed] [Google Scholar]