Abstract
For many cell types, including pancreatic β-cells, nitric oxide is a mediator of cell death; however, it is paradoxical that for a given cell type nitric oxide can induce both necrosis and apoptosis. This report tests the hypothesis that cell death mediated by nitric oxide shifts from an early necrotic to a late apoptotic event. Central to this transition is the ability of β-cells to respond and repair nitric oxide-mediated damage. β-Cells have the ability to repair DNA that is damaged following 24-h incubation with IL-1; however, cytokine-induced DNA damage becomes irreversible following 36-h incubation. This irreversible DNA damage following 36-h incubation with IL-1 correlates with the activation of caspase-3 (cleavage and activity). The increase in caspase activity correlates with reductions in endogenous nitric oxide production, as nitric oxide is an inhibitor of caspase activity. In contrast, caspase cleavage or activation is not observed under conditions in which β-cells are capable of repairing damaged DNA (24-h incubation with cytokines). These findings provide evidence that β-cell death in response to cytokines shifts from an early necrotic process to apoptosis and that this shift is associated with irreversible DNA damage and caspase-3 activation.
Keywords: islet, insulin, death
insulin-dependent diabetes mellitus is an autoimmune disease characterized by the selective destruction of pancreatic β-cells found in islets of Langerhans (20). Cytokines, released by invading leukocytes during insulitis, are believed to play a role in β-cell destruction (32, 41). In rodent islets, the macrophage-derived cytokine interleukin (IL)-1 is sufficient to impair insulin secretion and induce islet damage (10). The destructive actions of IL-1 are augmented by IFNγ and TNF (50). In islets isolated from most mouse strains and humans, IL-1 and IFNγ are the minimal combination of cytokines required to induce damage, and this damage is enhanced by TNF (2, 13, 23, 33).
Nitric oxide plays a central role in regulating the response(s) of β-cells to cytokines. Cytokines stimulate the expression of the inducible isoform of nitric oxide synthase (iNOS) and the production of micromolar levels of nitric oxide by β-cells (9, 11, 15, 17, 48). Nitric oxide reduces β-cell viability, as determined by the neutral red assay as well as 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (49). In addition, nitric oxide inhibits insulin secretion by attenuating the oxidation of glucose to CO2 by inhibiting the activity of mitochondrial iron-sulfur center-containing enzymes such as aconitase and complexes of the electron transport system (15). This results in a fourfold decrease in cellular ATP levels (18, 30). Since glucose-stimulated insulin secretion requires the accumulation of ATP to close the ATP-sensitive K+ channels [depolarization and Ca2+-dependent exocytosis; (28, 37)], reduction in β-cell mitochondrial function (and ATP accumulation) by nitric oxide is one mechanism by which cytokines inhibit β-cell function.
The inhibitory actions of IL-1 on β-cell function are reversible (8, 40). The addition of a NOS inhibitor to islets pretreated for 18–24 h with IL-1 (without removing IL-1) results in a time-dependent recovery of insulin secretion and mitochondrial function that is complete within 8 h (12). The recovery response requires new gene expression and can be activated by nitric oxide (43). Importantly, the ability of β-cells to recover from cytokine- and nitric oxide-induced damage is temporally limited. Following a 36-h exposure to IL-1, β-cells are no longer capable of recovering metabolic and secretory function, and the islets are committed to degeneration (44).
Although we and others have shown that cytokines kill β-cells by nitric oxide-dependent necrotic mechanisms (7, 49), other reports suggest that cytokine-induced death is apoptotic (29, 31, 39). Similarly to pancreatic β-cells, nitric oxide has been implicated in both necrosis and apoptosis of multiple cell types (3, 4). Importantly, it is possible for cells to recover from a necrotic injury if energy balance is restored (57); however, apoptosis is generally considered an irreversible process that leads to the targeted degradation of cellular components. The goal of apoptosis is to rid the organism of damaged or unwanted cells in the absence of inflammation. In this report, we address the possibility that cytokines and nitric oxide stimulate an early necrotic process (24 h) that shifts to apoptosis following prolonged exposures to cytokines (36 h). In support of this hypothesis, cytokine-mediated damage is reversible following 24-h incubation; however, prolonging the incubation for periods of 36 h or longer results in an irreversible inhibition of function and commitment to degeneration (44). Consistent with these previous studies, we now show that β-cells have the ability to repair damaged DNA following a 24-h exposure to cytokines; however, DNA damage becomes irreversible following a 36-h cytokine exposure. The irreversible DNA damage correlates with caspase-3 cleavage and increased caspase-3 activity. These findings suggest that cytokine-induced β-cell death shifts from an early necrotic process (characterized by the ability of β-cells to recover metabolic function if energy balanced is restored) to a late apoptotic process that is associated with irreversible damage to DNA and caspase-3 cleavage and activation.
EXPERIMENTAL PROCEDURES
Materials and animals.
Male Sprague-Dawley rats (250–300 g) were purchased from Harlan (Indianapolis, IN). RINm5F cells were obtained from Washington University Tissue Culture Support Center (St. Louis, MO). Human islet preparations were obtained from the Islet Cell Resource Centers at Joslin Diabetes Center (Boston, MA), City of Hope (Duarte, CA), and Washington University. RPMI 1640, CMRL-1066 tissue culture medium, l-glutamine, streptomycin, and penicillin were from GIBCO-BRL (Grand Island, NY). Fetal calf serum was from Hyclone Laboratories (Logan, UT). Human recombinant IL-1 and IFNγ were purchased from PeproTech (Rocky Hill, NJ). Tunicamycin, staurosporine, and MTT were from Sigma (St. Louis, MO). BCA protein assay was purchased from Pierce (Rockford, IL). Caspase-3 fluorometric assay was obtained from R & D Systems (Minneapolis, MN). NG-monomethyl-l-arginine (l-NMMA) and (Z)-1(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA-NO) were purchased from Axxora (San Diego, CA). pRC-CMV bcl2 and pRC-CMV/bcl2.CB5 [restricted localization to endoplasmic reticulum (ER)] constructs were a generous gift of Dr. David Andrews (McMaster University, Hamilton, ON, Canada) and have been described previously (56). Rabbit anti-active caspase-3 and rabbit anti-caspase-3 were from Cell Signaling Technology (Beverly, MA). Guinea pig anti-insulin was from DakoCytomation (Carpinteria, CA). Horseradish peroxidase-conjugated donkey anti-rabbit antiserum and Cy3-conjugated donkey anti-rabbit antiserum were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Goat anti-guinea pig Alexa fluor 488 was from Molecular Probes (Carlsbad, CA). All other reagents were obtained from commercially available sources. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Islet isolation and cell culture.
Islets were isolated from male Sprague-Dawley rats (250–300 g) by collagenase digestion, as described previously (25). Islets were cultured overnight in CMRL-1066 (containing 2 mM l-glutamine, 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin) at 37°C under an atmosphere of 95% air and 5% CO2 prior to experimentation. RINm5F cells were removed from growth flasks by treatment with 0.05% trypsin and 0.02% EDTA for 5 min at 37°C, washed twice with RPMI, and plated at the indicated cell densities. Human islets were cultured for 48 h at 37°C in complete CMRL-1066 tissue culture medium at an atmosphere of 95% air and 5% CO2 prior to experimentation.
Nitrite determination.
Nitrite production was determined by adding 50 μl of the Greiss reagent to 50 μl of culture supernatant (21). The absorbance was measured at 540 nm, and nitrite concentrations were calculated using a sodium nitrite standard curve.
Cell viability.
Cell viability was determined using the MTT assay as described previously (38, 49). Rat islets (150 islets/400 μl complete CMRL-1066) were washed with CMRL, and MTT was added (0.5 mg/ml). Following 1 h incubation, the islets were harvested by centrifugation. The islets were lysed with 200 μl of isopropanol and incubated for 30 min at room temperature. The optical density was determined at a wavelength of 562 nm. Results are from three independent experiments and are presented as the percent of dead cells ± SE compared with unstimulated controls.
Comet assay.
DNA damage was assessed using the comet assay (single-cell gel electrophoresis) as described previously (47). Briefly, cells were harvested and embedded in 0.6% low-melting agarose on slides precoated with 1.0% agar. Samples were then incubated in lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Trizma base, 1% Triton X-100) overnight. The next day, the slides were incubated in an alkaline electrophoresis buffer (0.3 M NaOH, 1 mM EDTA, pH >13) for 40 min followed by electrophoresis at 25 V for 20 min. Slides were washed three times in 0.4 M Tris, pH 7.5, and stained with ethidium bromide (2 μg/ml). Comet images were captured using a Nikon eclipse 90i, and the CASP program (27) was used to quantify the mean tail moment from 30–50 cells/condition.
Caspase-3 activity.
Following treatment, RINm5F cells, isolated rat islets, or human islets were washed and then lysed in 55 μl of lysis buffer. Caspase-3 activity was determined using 50 μl of the lysate following the manufacturer's protocol (Caspase-3 Fluorometric Assay Kit; R & D Systems). The relative fluorescence units were normalized to total protein content of the sample as determined by the BCA assay (Pierce). Treated samples are compared with unstimulated controls, and activity is presented as fold increase over control.
Immunocytochemistry.
Islets were dispersed into single cells, as described previously (11), and plated at a density of 5.0 × 104 cells/400 μl CMRL. Following the indicated treatments, islet cells were centrifuged onto microscope slides. Immunohistochemistry was performed as described previously (1). Briefly, the cells were fixed in 3% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100 and 0.1% sodium citrate for 2 min on ice, and then blocked with 10% goat serum (1× PBS) for 30 min. Cleaved caspase-3 and insulin were identified using rabbit anti-active caspase-3 (1:200 dilution) and guinea pig anti-insulin (1:100 dilution). Cy3-conjugated donkey anti-rabbit and goat anti-guinea pig Alexa fluor 488 were used as secondary antibodies (1:200 dilutions). Immunocytochemistry images were generated using a Nikon Diaphot 200 inverted microscope fitted with a fluorescence upgrade (human islets) and a Nikon 90i upright microscope (rat islets) and were taken at ×20 magnification.
Transfection.
RINm5F cells were transiently transfected using the Amaxa Nucleofect electroporator (Amaxa Biosystems, Gaithersburg, MD). RINm5F cells were removed from growth flasks by treatment with 0.05% trypsin and 0.02% EDTA for 5 min at 37°C, washed two times with RPMI 1640, and incubated for 1 h at 37°C in a 50-ml conical tube. The cells (2.0 × 106/ml) were harvested by centrifugation and resuspended in 100 μl of Amaxa buffer V. Two micrograms of pRC-CMV (vector) or pRC-CMV.bcl2 ± 2 μg of pEGFP were added to the cells, and electroporation was performed using program G-016. Immediately following electroporation, cells were placed in medium and aliquoted into a 24-well tissue culture to give a final volume of 2.0 × 105 cells/400 μl RPMI. Cells were incubated overnight at 37°C and washed three times with RPMI the next day. Cells were cultured in 400 μl of fresh RPMI 1640 for an additional 24 h prior to experimentation. Using this method, we routinely achieve transfection efficiencies of ∼50–70% (determined by enhanced green fluorescent protein expression).
Real-time PCR.
RNA was isolated using the RNeasy kit (Qiagen). cDNA synthesis was performed using oligo(dT) and reverse transcriptase Superscript Preamplification System according to the manufacturer's instructions (Invitrogen). Real-time PCR was performed using the Light Cycler 280 (Roche Applied Biosciences) to detect SYBR Green incorporation according to the manufacturer's instructions. The fold increase of p53 upregulated mediator of apoptosis (PUMA) mRNA accumulation was normalized to the housekeeping gene GAPDH. Primer sequences were as follows: PUMA primers: forward 5′-GCACTGATGGAGATACGGACTTG-3′, reverse 5′-ATGAAGGTGAGGCAGGCATTGC-3′; and GAPDH: forward 5′-GCTGGGGCTCACCTGAAGGG-3′, reverse 5′-GGATGACCTTGCCCACAGCC-3′.
Statistics.
Statistical comparisons were made between groups using one-way ANOVA. Significant differences between groups, cytokine-treated samples compared with cytokine-treated samples plus l-NMMA (P < 0.05), were determined by Newman-Keuls post hoc analysis.
RESULTS
IL-1 induces rat islet degradation and rat islet cell death.
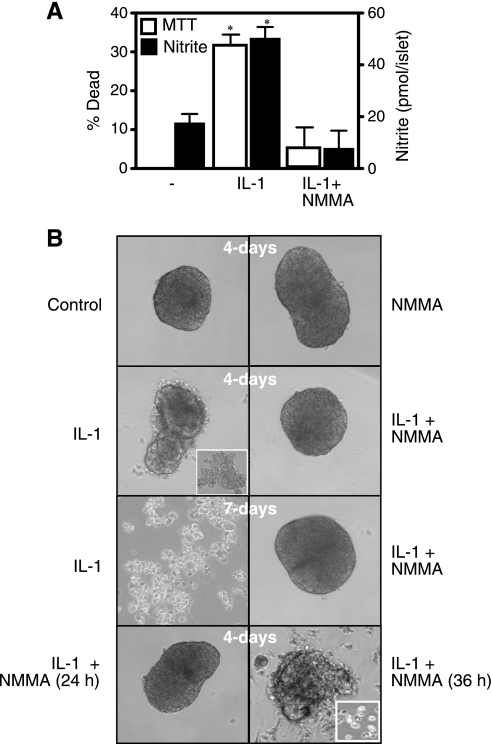
IL-1 induces the death of 33% of islet cells following 48-h incubation, as determined by the MTT assay (Fig. 1A). IL-1-induced death correlates with a threefold increase in nitrite production (following the 48-h incubation), and both islet cell death and nitric oxide production are attenuated by l-NMMA (Fig. 1A). The biochemical measurements of islet cell death (MTT) correlate with the morphological degeneration of islets. IL-1 induces islet cell sloughing (first apparent following a 48-h incubation; data not shown) and islet degeneration into single cells or clusters of cells following 4-day incubation (day 4 of IL-1 treatment shows islet degeneration into fragments or clusters of cells; Fig. 1B, upper middle, inset). There are a limited number of intact islets (<10%; data not shown) (12) that appear opaque and display significant sloughing of cells following 4-day incubation (Fig. 1B). Following 7-day incubation, IL-1 causes the islets to completely degenerate into single cells or clusters of cells. Importantly, when the NOS inhibitor l-NMMA is cocultured for the entire 4–7 days with IL-1, islet degeneration is completely prevented.
Fig. 1.
IL-1 induces β-cell death and rat islet degeneration in a nitric oxide-dependent manner. A: rat islets (150 islets/400 μl CMRL) were incubated with IL-1 (10 U/ml) ± ng-monomethyl-l-arginine (l-NMMA) (2 mM) for 48 h. Cell viability was determined using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The culture supernatants were harvested for nitrite determination. B: rat islets (150 islets/400 μl CMRL) were treated with IL-1 (10 U/ml) ± l-NMMA (2 mM) for 4 or 7 days as indicated. B, lower middle and bottom: effects of the addition of l-NMMA 24 (bottom left) or 36 h (bottom right) after IL-1, followed by continued culture with IL-1 and l-NMMA for a total of 96 h. Islet morphology was examined by phase contrast microscopy. Note that the islet shown in B, bottom right (IL-1 + l-NMMA added at 36 h), is representative of the few healthy islets observed at this time point. The majority of the islets have degenerated into clusters of 100–200 cells (IL-1 at 4 days; upper middle left, inset). Results are the average ± SE of 3 independent experiments (A) or representative of 3 experiments (B). Statistically significant increases in cell death and nitrite production are indicated (*P < 0.05).
In previous studies, we have demonstrated that β-cells have a limited ability to recover metabolic and secretory function following cytokine-mediated damage (44). Consistent with the recovery of oxidative metabolism, islets maintain a normal morphology and do not degenerate when l-NMMA is added 24 h after the addition of IL-1 and continued culture for an additional 72 h in the presence of both IL-1 and l-NMMA (Fig. 1B). In contrast, the addition of l-NMMA to islets treated for 36 h with IL-1 and the incubation continued for a total time of 96 h results in islet degeneration (degeneration into single cells and small clusters of cells; Fig. 1B, bottom right, inset). These findings suggest that multiple pathways are responsible for cytokine-induced β-cell death. There is an early induction of a necrotic-like process that is reversible if energy balance is restored, in this case, by the removal of nitric oxide from the system using l-NMMA. This is followed by a later, second event that is irreversible and seems to commit β-cells to death. This second pathway occurs following a 36-h incubation with IL-1 (Fig. 1B, bottom).
Repair of cytokine-mediated DNA damage.
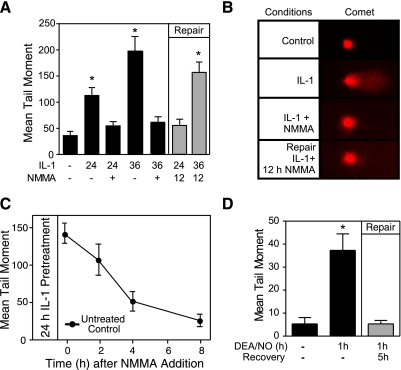
The comet assay was used to explore the integrity of islet DNA in response to IL-1 and nitric oxide. Although previous studies have shown that cytokines induce DNA damage in rat, mouse, and human islets, it is unclear whether β-cells have the capacity to repair this damage (16). Treatment of insulinoma INS 832/13 cells for 24 or 36 h with IL-1 results in a 2.5- and fourfold increase in DNA damage, respectively (Fig. 2A). When cocultured with IL-1, l-NMMA completely prevents this DNA damage, indicating that it is mediated by nitric oxide. To examine whether DNA damage can be repaired, l-NMMA was added to INS 832/13 cells treated for 24 or 36 h with IL-1, and the cells were cultured for an additional 12 h with both the cytokine and NOS inhibitor. As evidenced by a mean tail moment similar to those in the untreated control, INS 832/13 cells completely repair damaged DNA when l-NMMA is added 24 h after the addition of IL-1 (Fig. 2A and representative images of comets in Fig. 2B, right). The repair of damaged DNA is time dependent with half-maximal repair after 3 h and complete repair after 8-h incubation with l-NMMA (Fig. 2C). Following 36-h incubation with IL-1, the addition of l-NMMA does not restore the mean tail moment to that of the control, indicating that the damaged DNA is not repaired (Fig. 2A). This temporally correlates the inability to repair damaged DNA with the irreversible inhibition of oxidative metabolism and secretory function (44) and a commitment of islet degeneration (Fig. 1B).
Fig. 2.
Repair of DNA damage in cytokine-treated insulinoma cells. INS 832/13 cells (2.0 × 105 cells/400 μl RPMI) were treated with IL-1 (10 U/ml) for 24 or 36 h ± l-NMMA (2 mM) (black bars) or incubated with IL-1 for the indicated times followed by the addition of l-NMMA and continued culture for 12 h (without removing IL-1, DNA repair conditions; gray bars). A: DNA damage, quantified using the comet assay, is expressed as the mean tail moment. B: representative comets are shown. C: the rate of DNA repair was quantified by comet assay using INS 832/13 cells pretreated with IL-1 for 24 h followed by the addition of l-NMMA and continued culture (with l-NMMA and IL-1) for 1, 2, 4, and 8 h. INS 832/13 cells were treated with (Z)-1(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA-NO; 500 μM) for 1 h. DEA-NO was removed by washing, and the cells were allowed to recover for an additional 5 h. D: the comet assay was used to quantify DNA damage following the 1-h exposure to DEA-NO and after the 5-h recovery (gray bar). Results are the average ± SE of 3 independent experiments. Statistically significant increases in mean tail moment vs. control are indicated (*P < 0.05).
To determine whether the repair of damaged DNA requires the presence of IL-1, INS 832/13 cells were treated for 1 h with the nitric oxide donor DEA-NO. The cells were washed to remove the nitric oxide and cultured for an additional 5 h in the absence of nitric oxide. DNA damage was determined following the 1-h incubation with DEA-NO and compared with the 5-h recovery period using the comet assay. As shown in Fig. 2D, DEA-NO stimulates DNA damage, as indicated by aprroximately sevenfold increase in the mean tail moment. Removal of nitric oxide by washing and continued culture for 5 h results in the complete repair of INS 832/13 cell DNA. These findings indicate that nitric oxide can directly induce islet cell DNA damage and that nitric oxide, or the DNA damage induced by nitric oxide, is sufficient to activate the biological pathways responsible for DNA repair.
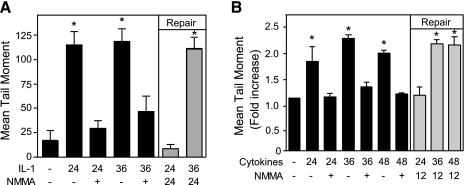
Similarly to the DNA repair observed in INS 832/13 cells, rat (Fig. 3A) and human islets (Fig. 3B) are also capable of repairing DNA damaged by nitric oxide. Treatment of rat islets with IL-1 or human islets with IL-1 and IFNγ for 24, 36, or 48 h results in nitric oxide-dependent DNA damage since it is prevented by coincubation with l-NMMA. Human islets require multiple cytokines for the induction of iNOS. Alone, IL-1 fails to stimulate iNOS expression, whereas IL-1 plus IFN-γ is the minimal combination of cytokine required to stimulate iNOS expression and nitric oxide production by human islets (13). Under repair conditions (gray bars), l-NMMA is added after a 24-, 36-, or 48-h incubation with cytokines, and the islets are cultured for an additional 12 or 24 h with the NOS inhibitor and cytokines. Rat and human islets maintain the ability to repair damaged DNA if l-NMMA is added 24 h after the addition of cytokine; however, DNA is damaged irreversibly following 36-h incubation with cytokines because l-NMMA addition does not stimulate rat and human islet DNA repair (Fig. 3). These findings correlate the inhibition of DNA repair (Figs. 2 and 3) with the irreversible inhibition of oxidative metabolism and secretory function (44) and a commitment of islets to morphological degeneration (Fig. 1B) following a 36-h incubation with cytokines. In contrast, islets have the ability to overcome the damaging actions of 24-h incubation with cytokines on insulin secretion and metabolic function (14, 43, 44) and to repair damaged DNA (Fig. 2A). These findings suggest that there is a shift in the response of islets to cytokine-induced nitric oxide production from an early (24 h) recovery/repair response that is followed by irreversible damage (36 h).
Fig. 3.
Repair of rat and human islet DNA. Rat (A) and human islets (B) were treated with IL-1 (10 U/ml) or a combination of cytokines (75 U/ml IL-1 and 750 U/ml IFNγ), respectively, for 24, 36, or 48 h in the presence (+) or absence of l-NMMA (black bars). DNA damage was quantified by comet assay (black bars). The repair of DNA damage (repair; gray bars) was examined in islets treated for 24 or 36 h with IL-1 (rat; A) or the cytokine mixture (human; B), and l-NMMA was added and the islets were cultured for an additional 12 (A) or 24 h (B) with both the cytokines and l-NMMA. DNA damage was determined using the comet assay. Results are the average ± SE of 3 independent experiments. The induction of DNA damage (vs. control) and the repair of this damage at 24 h are statistically significant (*P < 0.05).
Time-dependent effects of IL-1 on nitric oxide production by RINm5F cells.
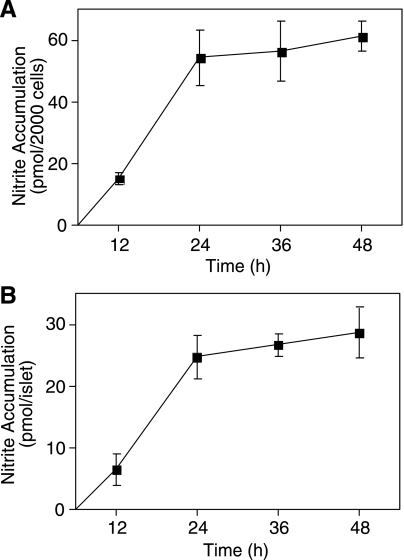
As shown in Figs. 1 and 2 and in previous publications (10, 16, 52), nitric oxide mediates the inhibitory actions of cytokines on oxidative metabolism and insulin secretion and induces DNA damage. Nitric oxide is also an endogenous inhibitor of caspase activity (26, 42), and we have shown that exogenously supplied nitric oxide inhibits ER stress-induced caspase-3 activity in insulinoma cells (5). To examine whether the shift to irreversible islet damage following prolonged cytokine treatment correlates with changes in nitric oxide production, the time-dependent accumulation of nitrite was examined using insulinoma RINm5F cells (Fig. 4A) and rat islets (Fig. 4B). IL-1 stimulates the time-dependent accumulation of nitrite that is linear for the first 24 h (Fig. 4). After 36- to 48-h incubation, a minimal amount of additional nitrite accumulates above the levels observed at 24 h. To determine whether nitrite accumulation reflects the rates of nitric oxide production, islets were treated for 24 and 36 h with IL-1, and the islets were washed two times and then incubated for an additional 2 h in fresh prewarmed medium. Nitrite accumulation during the 2-h exposure was used to quantify rates of nitrite production. At 24 h, the rate of IL-1-induced nitrite formation was 3 ± 0.4 pmol nitrite·islet−1·h−1, whereas at 36 h nitrite was produced at a rate of 0.5 ± 0.07 pmol·islet−1·h−1 (results are the average ± SE of 3 independent experiments; P < 0.05). These findings correlate a reduction in the rate of cytokine-induced nitric oxide production at 36 h with the irreversible inhibition of β-cell function and a commitment of islets to degeneration.
Fig. 4.
Time-dependent effects of IL-1 on RINm5F cell and rat islet nitrite formation. RINm5F cells (2.0 × 105/400 μl RPMI; A) or rat islets (150 islets/400 μl CMRL; B) were treated with IL-1 (1 U/ml) for 12, 24, 36, or 48 h. Nitrite formation was determined from the culture supernatants. Results are presented as the average ± SE of 3 independent experiments. Nitrite production is statistically significant compared with control at 12, 24, 36, and 48 h (P < 0.05).
Cytokine-induced caspase-3 cleavage in human islets.
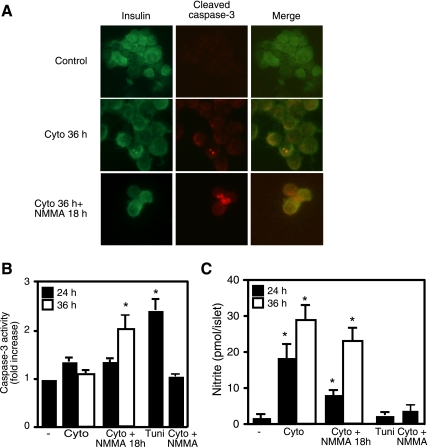
To examine whether the irreversible inhibition of β-cell function correlates with a shift in the type of death from necrosis to apoptosis, we examined the effects of cytokines and NOS inhibitors on caspase-3 cleavage and activity in dispersed human islets. Treatment of human islets for 36 h with IL-1 plus IFNγ results in caspase-3 cleavage (red immunofluorescence staining; Fig. 5A) in insulin-containing cells (green immunofluorescence). The cleavage of caspase-3 was not observed following a 24-h incubation with cytokines or in human islets cocultured with l-NMMA and the cytokine combination for 24, 36, or 48 h (49). Although we observe caspase-3 cleavage in human islets treated for 36 h with cytokines, the enzymatic activity of this executioner of apoptosis is similar to the levels detected in untreated control islets. This appears to reflect the ability of nitric oxide to inhibit caspase-3 activity, because the addition of l-NMMA to the islets treated for 36 h with cytokines and continued culture for an additional 18 h results in a twofold increase in measurable caspase-3 activity (Fig. 5B). In contrast, caspase-3 activity was not detectable in islets cocultured with cytokines and l-NMMA (24 or 36 h), islets incubated for 24 h with cytokines, and islets cultured for 24 h with cytokines and an additional 18 h with cytokines and l-NMMA (Fig. 5C and data not shown). As a positive control for caspase activation, the effects of a 24-h incubation with the ER stress inducer tunicamycin on caspase-3 activity in human islets are shown (Fig. 5B).
Fig. 5.
Cytokines induce caspase-3 cleavage and activity in human islets. Dispersed human islet cells were pretreated with IL-1 (75 U/ml) and IFNγ (500 U/ml) for 36 h (cyto 36-h condition) or pretreated with the cytokines for 36 h, followed by the addition of l-NMMA and continued culture for an additional 18 h (cytokines were not removed). A: the human islet cells were centrifuged onto slides, and cleaved caspase-3 (red) and insulin (green) were detected by immunocytochemistry. B and C: human islets (100 islets/400 μl CMRL) were pretreated with the cytokines (cyto) IL-1 (75 U/ml) + IFNγ (500 U/ml) for 24 (black bars) or 36 h (open bars) or treated with the cytokine mix for 24 or 36 h, followed by the addition of l-NMMA (2 mM) and continued culture for 18 h [in the presence of the cytokines and nitric oxide synthase (NOS) inhibitor; cyto + l-NMMA 18 h]. The islets were harvested, and caspase-3 activity was determined on the cell lysate (B), and nitrite formation was determined from the culture supernatants (C). As controls, caspase-3 activity was examined on human islets treated with tunicamycin (2 μg/ml) for 24 h (positive control), and human islets were cocultured with cytokines + l-NMMA. Results are representative of 2 independent experiments (A) or the average ± SE of 3 independent experiments (B and C). Statistically significant increases in caspase-3 activity and nitrite production compared with untreated controls are as indicated (*P < 0.05).
Cytokine stimulation of caspase-3 activity in rodent islets.
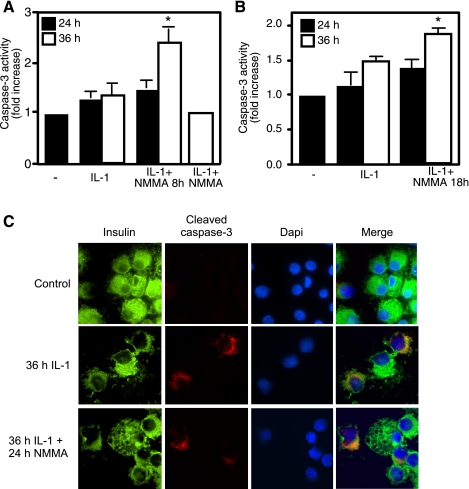
Since reports have suggested that there is a species difference in the response of islets to cytokines, we examined whether IL-1 stimulates caspase-3 activity in rat islets and RINm5F cells in a temporal fashion that correlates with the irreversible inhibition of β-cell function following a 36-h treatment. Consistent with the response of human islets to a combination of cytokines, increased activity of caspase-3 was not observed in RINm5F cells treated for 24 or 36 h with IL-1. However, the addition of l-NMMA to islets treated for 36 h with IL-1 followed by an additional 8 h of culture results in a twofold increase in caspase-3 activity (Fig. 6A). Caspase-3 activity is not increased in RINm5F cells treated for 24 h with IL-1 followed by an additional 8 h of culture in the presence of l-NMMA. In addition, we fail to observe cytokine-induced caspase-3 activity in RINm5F cells cocultured with IL-1 and l-NMMA for 24 or 48 h (Fig. 6A) (49).
Fig. 6.
Cytokines induce caspase-3 activation in RINm5F cells and rat islets. RINm5F cells (2.0 × 105 cells/400 μl RPMI; A) or rat islets (150 islets/400 μl CMRL; B and C) were treated with IL-1 (10 U/ml) for 24 (black bars) or 36 h (open bars), followed by the addition of l-NMMA and continued culture for an additional 8 or 18 h in the presence of both the NOS inhibitor and cytokine, as indicated. A and B: the cells and islets were harvested, and caspase-3 activity was determined. A also provides a control in which RINm5F cells were coincubated with IL-1 and l-NMMA for 36 h. C: following a 36-h IL-1 treatment or 36 h with IL-1 followed by the addition of l-NMMA and continued incubation for 24 h, the islets were dispersed and the cells centrifuged onto slides. The presence of active caspase-3 was examined by immunohistochemistry using antibodies specific for cleaved caspase-3 (red), insulin (green), and 4′,6-diamidino-2-phenylindole (DAPI; blue). Results are the average ± SE of at least 3 independent experiments. Statistically significant increases in caspase-3 activity compared with untreated controls are as indicated (*P < 0.05).
Like human islets and RINm5F cells, treatment of rat islets for 36 h with IL-1 and then an additional 8 h with l-NMMA in addition to IL-1 results in an increase in caspase-3 activity (Fig. 6). Consistent with caspase-3 activity, a 36-h incubation with IL-1 results in the cleavage of caspase-3 [determined by histochemistry, insulin (green), and cleaved caspase-3 (red); Fig. 6C]. In contrast, 24-h incubation with IL-1 or coculture of islets with IL-1 and l-NMMA fails to induce caspase-3 cleavage in rat islet cells (Fig. 6C and data not shown). Caspase-3 activity is also not observed in rat islets following 24-h incubation with IL-1 or 24 h with IL-1 plus 8 (Fig. 6B) or with l-NMMA (data not shown). This is consistent with human islets where caspase-3 is cleaved in response to 36-h cytokine treatment, but caspase-3 activity is not observed at this time point (Fig. 5). The apparent absence of caspase-3 activity following a 36-h cytokine exposure can be attributed to the inhibition of caspase-3 activity by nitric oxide. The inhibition of nitric oxide generation by the addition of l-NMMA to islets treated for 36 h with cytokines and continued culture with cytokines and l-NMMA for 8 (Fig. 6) or 18 h (Fig. 5) results in an increase in caspase-3 activity. These findings indicate that the activation of caspase-3 in response to treatment of rodent islets and insulinoma cells with IL-1 is similar to the actions of cytokines on caspase-3 activation observed in human islets.
Nitric oxide inhibits IL-1-induced caspase-3 activation.
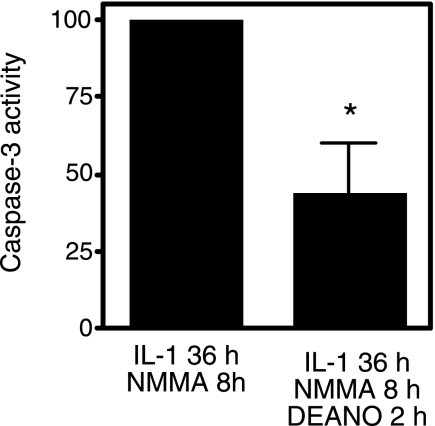
Evidence presented in Figs. 5 and 6 correlates a decrease in nitric oxide production with an increase in caspase-3 activity in islets treated for 36 h with cytokines. To provide further evidence that nitric oxide regulates caspase-3 activity, RINm5F cells were exposed to IL-1 for 36 h, l-NMMA was then added, and the cells were cultured for an additional 8 h. Under these conditions, caspase-3 activity is enhanced twofold compared with the levels stimulated by IL-1 alone (this value is set at 100% caspase activation; Fig. 7). Importantly, the addition of exogenous nitric oxide using the donor DEA-NO followed by an additional 2-h incubation results in ∼60% inhibition of caspase-3 activity (Fig. 7). These findings provide evidence that nitric oxide, exogenously added back to the cultures using the donor DEA-NO, can attenuate increases in caspase-3 activity in cytokine-treated RINm5F cells.
Fig. 7.
DEA-NO attenuates IL-1-induced caspase-3 activity. RINm5F cells (2.0 × 105 cells/400 μl RPMI) were pretreated with 10 U/ml IL-1 for 36 h. l-NMMA (2 mM) was added, and the cells were cultured for an additional 8 h, and caspase activity was determined and set at 100%. To determine whether exogenous nitric oxide inhibits caspase activation under these conditions, DEA-NO (500 μM) was added following the cytokine and l-NMMA incubations, and the cells were cultured for an additional 2 h followed by determination of caspase-3 activity. Results are the average ± SE of 3 independent experiments (statistically significant inhibition; *P < 0.05).
Expression of Bcl-2 attenuates caspase-3 activation.
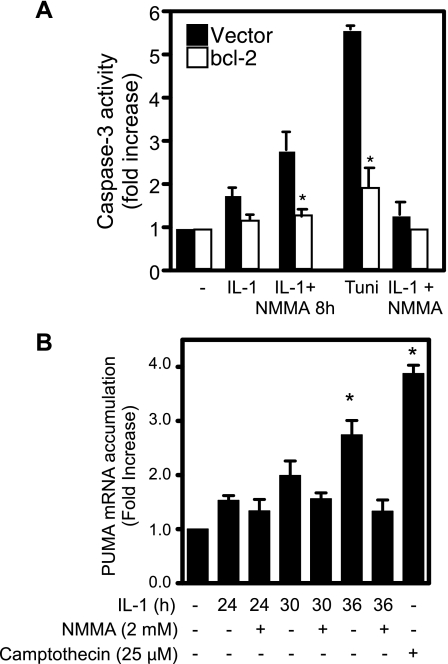
To further explore the mechanisms responsible for caspase-3 activation, the effects of increased Bcl-2 expression on IL-1-induced caspase-3 activation were examined using RINm5F cells. As shown in Fig. 8, IL-1-induced caspase-3 activity in cells treated for 36 h with IL-1 followed by an additional 8 h with both IL-1 and l-NMMA is attenuated in cells transfected with a plasmid expressing Bcl-2. As a positive control, we show that Bcl-2 expression attenuates the activation of caspase-3 in response to 12-h incubation with the ER stress inducer tunicamycin. To further explore the role of mitochondrial-directed apoptosis, the effects of IL-1 on PUMA expression were evaluated. PUMA stimulates mitochondrial-directed apoptosis by sequestering Bcl-2 (53). Consistent with the suppression of caspase-3 activity in cells overexpressing Bcl-2 (Fig. 8A), incubation of INS 832/13 cells with IL-1 for 36 h results in a threefold increase in PUMA mRNA accumulation (Fig. 8B). IL-1 does not stimulate PUMA mRNA accumulation at 24 or 30 h, consistent with an absence of caspase-3 cleavage (Figs. 5 and 6). These results provide additional information that the cellular damage caused by nitric oxide results in the activation of caspase-3 by a pathway consistent with mitochondrial-directed apoptosis.
Fig. 8.
Bcl-2 overexpression attenuates cytokine-induced caspase-3 cleavage. A: RINm5F cells (2.0 × 106 cells) were transiently transfected with 2 μg of pRC-CMV (vector) or pRC-CMV.bcl2 (Bcl-2) and cotransfected with pEGFP to determine transfection efficiency (>70%). Forty-eight hours later, 10 U/ml IL-1 was added, and the cells were incubated for 36 h with IL-1 or 36 h with IL-1 followed by the addition of l-NMMA and continued culture for 8 h (IL-1 + l-NMMA 8h). Following these incubations the cells were harvested, and caspase-3 activity was determined. Cells treated with tunicamycin (2 μg/ml) for 36 h are shown as a positive control for caspase activation, and the inhibitory actions of l-NMMA, when coincubated with IL-1, on caspase activity following a 36-h treatment are shown. B: INS 832/13 cells were treated for the indicated times with 10 U/ml IL-1 in the presence or absence of l-NMMA, the cells were isolated, and PUMA mRNA accumulation was determined by real-time PCR. mRNA levels were normalized to the levels of GAPDH. PUMA induction following 24-h incubation with camptothecin is shown as a positive control. Results are the average ± SE for 3 independent experiments. Statistically significant inhibition of caspase-3 activity by Bcl-2 and induction of PUMA vs. control is indicated (*P < 0.05).
DISCUSSION
There is considerable debate regarding the mechanisms of cytokine-induced β-cell death as well as the general mechanisms by which nitric oxide kills cells. Much of this debate is centered on the type of death (necrosis vs. apoptosis) and the pathways responsible for directing each of these types of cell death (7, 18, 31, 34, 49). In reviewing the literature, it is possible to find reports that support a role for nitric oxide as a mediator of both necrosis and apoptosis in similar cell types and experimental systems (6). Much of this confusion is based on the methodologies used to examine apoptosis and necrosis and the net outcome of each of the death responses. Apoptosis, an active process that requires ATP, results in an ordered degradation and packaging of unwanted cells in a noninflammatory process. Importantly, once activated, the apoptotic program of cell death is primarily irreversible (51). In contrast, necrotic cell death is a proinflammatory process that results in the dissolution of cells and the uncontrolled release of cellular constituents. In contrast to apoptosis, it is possible to recover from insults that stimulate necrotic cell death if the insult is removed and cellular energy balance restored before the loss of membrane integrity (57).
In this report, we address the possibility that cytokines and nitric oxide induce both necrosis and apoptosis in β-cells and that the type of cell death is regulated by the rate of nitric oxide production and the extent of cellular damage mediated by nitric oxide. It has long been known that cytokines (such as IL-1 in the rat and IL-1 + IFNγ in human islets) stimulate β-cell expression of iNOS and production of nitric oxide; however, the role of nitric oxide in cytokine-induced islet cell death has been debated (18, 22, 29, 31). Using biochemical and morphological assays of islet viability, we show that IL-1 induces islet degeneration and β-cell death in a nitric oxide-dependent manner. Importantly, if cytokines were to induce apoptosis, then induction of this type of death would likely be irreversible. In a series of studies, we have shown that pancreatic β-cells have the ability to recover from the inhibitory actions of IL-1 (12, 44, 45). The addition of a NOS inhibitor to islets (rat, mouse, and human) cultured for 24 h with cytokines and continued culture for 8 h (in the presence of the cytokines) results in a complete recovery of metabolic and secretory function (12). Importantly, this recovery occurs in the presence of cytokines, indicating that the damaging actions of cytokines are mediated by nitric oxide. Similar to metabolic function, β-cells have the ability to repair damaged DNA if nitric oxide is removed from the system (in this case using a NOS inhibitor). The ability to repair DNA damage is temporally limited, since β-cells are not capable of repairing damage to islet DNA when incubated with cytokines for periods of 36 h or longer. The irreversible inhibition of function and permanent DNA damage following 36-h incubation correlates with a commitment of islets to degeneration. These findings suggest that nitric oxide mediates cytokine-induced cell death and that the type of cell death early in cytokine treatment (first 24–36 h) is by a process that is reversible, such as the events leading to the progression to necrosis. Consistent with this interpretation, biochemical and molecular evidence support necrosis as the mechanisms by which cytokines induce nitric oxide-dependent β-cell death following short exposures (7, 49). There are a number of reports suggesting that cytokines stimulate β-cell apoptosis (reviewed in Ref. 31); however, most of these studies have not examined the caspase dependence of cell death, nor have these studies included positive controls such as classical apoptosis inducers (camptothecin or etoposide) to compare death by apoptosis with death induced by cytokines. In the few studies where these controls have been performed (5, 7, 49), cell death in response to short exposures to cytokines (IL-1 or IL-1 + IFNγ) for 24 h is prevented by inhibitors of iNOS, occurs in the absence of increases in caspase-3 cleavage or activity, and is associated with reduced ATP content, and the level of cell death is not modified by inhibitors of caspase-3. In contrast, induction of apoptosis in response to camptothecin or etoposide is dependent on caspase-3 activation and is associated with four- to eightfold increases in caspase-3 activity (5, 7, 49).
When the culture with IL-1 continues for periods of 36 h or longer, β-cells lose the ability to recover metabolic function (44) and to repair damaged DNA (Figs. 2 and 3). Since apoptosis is a regulated, primarily irreversible process, we examined whether cytokine-induced death shifts from necrosis to apoptosis by evaluating caspase activation. As discussed above, cytokines fail to stimulate caspase-3 cleavage or activity in rat and human islets or insulinoma cells following a 24-h incubation (7, 49). We now show that caspase-3 is cleaved in human islets treated for 36 h with a combination of IL-1 and IFNγ and rat islets treated with IL-1; however, this caspase cleavage is not associated with an increase in caspase-3 enzymatic activity. In contrast, there is a twofold increase in caspase-3 activity when a NOS inhibitor is added and the islets are cultured for an additional 8–18 h. Nitric oxide is a known inhibitor of caspase activity due to S-nitrosation of active-site cysteine resides (26, 42), thus preventing the detection of caspase-3 activity in cytokine-treated islets. Although the rate of nitric oxide production in islets treated for 36 h with cytokines is reduced sixfold, nitric oxide is still produced to levels sufficient to inhibit caspase-3 activity. In support of this conclusion, the addition of a NOS inhibitor to islets treated for 36 h with cytokines followed by an additional 8–18 h of incubation (with both the cytokine and NOS inhibitor) results in a twofold increase in caspase-3 activity. Under these conditions, caspase-3 activity can be inhibited by the exogenous addition of nitric oxide using DEA-NO.
It is not intuitive to envision that an inflammatory molecule such as nitric oxide would be capable of activating classical apoptotic cascades. However, the absence of caspase activity in islets cotreated for 36 or 48 h with cytokines and l-NMMA indicates that nitric oxide is required for caspase activation. Although nitric oxide itself inhibits caspase activity, we hypothesize that the damage to DNA and the inhibition of metabolic function (42) induced by nitric oxide may participate in the induction of caspase-3 activation and that apoptosis is mitochondrial directed. In support of the later conclusion, we show that Bcl-2 overexpression attenuates caspase-3 activity and that cleavage of caspase-3 correlates with the expression of the proapoptotic factor PUMA (Fig. 8). It is well known that nitric oxide induces oxidative DNA damage in multiple cell types, including pancreatic β-cells (16, 54, 55). In this study, we have correlated caspase-3 activation with irreversible DNA damage and PUMA expression, and this caspase-3 activity can be attenuated by Bcl-2 expression in insulinoma cell lines.
Our findings suggest that it is possible that cytokines are capable of killing islet cells by both necrotic and apoptotic pathways. The induction of death responses is mediated by nitric oxide, because cytokines do not damage β-cells in the absence of nitric oxide production. Early in response to cytokines, nitric oxide is produced at high levels, leading to disruption in the energy balance of β-cells and the induction of a necrotic death process. As nitric oxide-mediated damage becomes more extensive, and as the cellular damage becomes irreversible, the process by which islet cells die shifts from necrosis to apoptosis. This shift correlates with extensive and irreversible DNA damage (current study), irreversible inhibition of oxidative metabolism, protein synthesis, and insulin secretion (44). This hypothesis may explain why some laboratories have observed β-cell apoptosis following prolonged incubation (3–7 days) of islets with cytokines (31), and other laboratories support necrosis as the mechanism of cytokine-induced β-cell death in response to short exposures of 24 h (7, 49). A key step in unraveling the mechanisms of cytokine-induced nitric oxide-dependent β-cell death will be to determine how this shift in the form of death is regulated. We hypothesize that understanding the molecular regulation of DNA repair following nitric oxide-mediated damage will provide insights into the mechanisms controlling this shift from necrosis to apoptosis. One potential target may be p53, a DNA repair enzyme that participates in the regulation of cell fate (19, 36). In support of this target, nitric oxide has been shown to stimulate p53 expression/activation (24, 35), and recent evidence suggests that nitric oxide-dependent apoptosis may be p53 dependent (46).
GRANTS
This work was supported by National Institutes of Health Grants DK-52194 and AI-44458 (J. A. Corbett) and an American Heart Association predoctoral fellowship award (K. T. Chambers).
ACKNOWLEDGMENTS
We thank Colleen Kelly Bratcher for expert technical assistance and Dr. Michael Moxley and Abdual Waheed for helpful discussion related to these studies. We thank Dr. David Andrews (McMaster University) for providing Bcl-2 constructs.
REFERENCES
- 1.Arnush M, Scarim AL, Heitmeier MR, Kelly CB, Corbett JA. Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J Immunol 160: 2684–2691, 1998 [PubMed] [Google Scholar]
- 2.Bendtzen K, Barington T, Mandrup-Poulsen T, Pedersen JG, Svenson M. Measurement of human IL-1 by LIF induction, pancreatic islet-cell cytotoxicity, and bone resorption. Lymphokine Res 5, Suppl 1: S93–S98, 1986 [PubMed] [Google Scholar]
- 3.Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic Biol Med 33: 1440–1450, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Brune B. The intimate relation between nitric oxide and superoxide in apoptosis and cell survival. Antioxid Redox Signal 7: 497–507, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA. The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes 57: 124–132, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54, Suppl 2: S97–S107, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Collier JJ, Fueger PT, Hohmeier HE, Newgard CB. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes 55: 1398–1406, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Comens PG, Wolf BA, Unanue ER, Lacy PE, McDaniel ML. Interleukin 1 is potent modulator of insulin secretion from isolated rat islets of Langerhans. Diabetes 36: 963–970, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Corbett JA, Kwon G, Misko TP, Rodi CP, McDaniel ML. Tyrosine kinase involvement in IL-1β-induced expression of iNOS by β-cells purified from islets of Langerhans. Am J Physiol Cell Physiol 267: C48–C54, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Corbett JA, Lancaster JR, Jr, Sweetland MA, McDaniel ML. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem 266: 21351–21354, 1991 [PubMed] [Google Scholar]
- 11.Corbett JA, McDaniel ML. Intraislet release of interleukin 1 inhibits beta cell function by inducing beta cell expression of inducible nitric oxide synthase. J Exp Med 181: 559–568, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett JA, McDaniel ML. Reversibility of interleukin-1 beta-induced islet destruction and dysfunction by the inhibition of nitric oxide synthase. Biochem J 299: 719–724, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA 90: 1731–1735, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbett JA, Wang JL, Hughes JH, Wolf BA, Sweetland MA, Lancaster JR, Jr, McDaniel ML. Nitric oxide and cyclic GMP formation induced by interleukin 1 beta in islets of Langerhans. Evidence for an effector role of nitric oxide in islet dysfunction. Biochem J 287: 229–235, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbett JA, Wang JL, Sweetland MA, Lancaster JR, Jr, McDaniel ML. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest 90: 2384–2391, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delaney CA, Green MH, Lowe JE, Green IC. Endogenous nitric oxide induced by interleukin-1 beta in rat islets of Langerhans and HIT-T15 cells causes significant DNA damage as measured by the “comet” assay. FEBS Lett 333: 291–295, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Eizirik DL, Björklund A, Welsh N. Interleukin-1-induced expression of nitric oxide synthase in insulin-producing cells is preceded by c-fos induction and depends on gene transcription and protein synthesis. FEBS Lett 317: 62–66, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Eizirik DL, Flodstrom M, Karlsen AE, Welsh N. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia 39: 875–890, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Gatz SA, Wiesmuller L. p53 in recombination and repair. Cell Death Differ 13: 1003–1016, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14: 619–633, 1965 [DOI] [PubMed] [Google Scholar]
- 21.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126: 131–138, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Heitmeier MR, Corbett JA. Cytotoxic role of nitric oxide in diabetes. In: Nitric Oxide Biology and Pathobiology San Diego, CA: Academic, 2000, p. 785–810 [Google Scholar]
- 23.Heitmeier MR, Scarim AL, Corbett JA. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem 272: 13697–13704, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, Jhappan C, Higashimoto Y, He P, Linke SP, Quezado MM, Zurer I, Rotter V, Wink DA, Appella E, Harris CC. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci USA 100: 143–148, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly CB, Blair LA, Corbett JA, Scarim AL. Isolation of islets of Langerhans from rodent pancreas. Methods Mol Med 83: 3–14, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 272: 31138–31148, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Konca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Gozdz S, Koza Z, Wojcik A. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res 534: 15–20, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell 100: 645–654, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Pavlovic D, Chen MC, Flodström M, Sandler S, Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS−/−). Diabetes 49: 1116–1122, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Maggi LB, Jr, Heitmeier MR, Scheuner D, Kaufman RJ, Buller RM, Corbett JA. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J 19: 3630–3638, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol 66: 1433–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 39: 1005–1029, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Mandrup-Poulsen T, Bendtzen K, Nielsen JH, Bendixen G, Nerup J. Cytokines cause functional and structural damage to isolated islets of Langerhans. Allergy 40: 424–429, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Mathews CE, Suarez-Pinzon WL, Baust JJ, Strynadka K, Leiter EH, Rabinovitch A. Mechanisms underlying resistance of pancreatic islets from ALR/Lt mice to cytokine-induced destruction. J Immunol 175: 1248–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin LM, Demple B. Nitric oxide-induced apoptosis in lymphoblastoid and fibroblast cells dependent on the phosphorylation and activation of p53. Cancer Res 65: 6097–6104, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Menendez D, Inga A, Jordan JJ, Resnick MA. Changing the p53 master regulatory network: ELEMENTary, my dear Mr Watson. Oncogene 26: 2191–2201, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Misler S, Barnett DW, Gillis KD, Pressel DM. Electrophysiology of stimulus-secretion coupling in human beta-cells. Diabetes 41: 1221–1228, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 98: 10845–10850, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer JP, Helqvist S, Spinas GA, Molvig J, Mandrup-Poulsen T, Andersen HU, Nerup J. Interaction of beta-cell activity and IL-1 concentration and exposure time in isolated rat islets of Langerhans. Diabetes 38: 1211–1216, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol 55: 1139–1149, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Rössig L, Fichtlscherer B, Breitschopf K, Haendeler J, Zeiher AM, Mülsch A, Dimmeler S. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem 274: 6823–6826, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Scarim AL, Heitmeier MR, Corbett JA. Heat shock inhibits cytokine-induced nitric oxide synthase expression by rat and human islets. Endocrinology 139: 5050–5057, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Scarim AL, Heitmeier MR, Corbett JA. Irreversible inhibition of metabolic function and islet destruction after a 36-hour exposure to interleukin-1beta. Endocrinology 138: 5301–5307, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Scarim AL, Nishimoto SY, Weber SM, Corbett JA. Role for c-Jun N-terminal kinase in beta-cell recovery from nitric oxide-mediated damage. Endocrinology 144: 3415–3422, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N, Thomas B, Dawson TM, Dawson VL, Snyder SH, Sawa A. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol 10: 866–873, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175: 184–191, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Southern C, Schulster D, Green IC. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an l-arginine-dependent nitric oxide generating mechanism. FEBS Lett 276: 42–44, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med 3: e17, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes 51: 311–316, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 281: 1312–1316, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Welsh N, Eizirik DL, Bendtzen K, Sandler S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology 129: 3167–3173, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol 17: 617–625, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS, Keefer LK. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 254: 1001–1003, 1991 [DOI] [PubMed] [Google Scholar]
- 55.Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25: 434–456, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Zhu W, Cowie A, Wasfy GW, Penn LZ, Leber B, Andrews DW. Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. EMBO J 15: 4130–4141, 1996 [PMC free article] [PubMed] [Google Scholar]
- 57.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev 20: 1–15, 2006 [DOI] [PubMed] [Google Scholar]