Abstract
The production of hyperglycemia-induced mitochondrial reactive oxygen species (mtROS) is a key event in the development of diabetic complications. Because resveratrol, a naturally occurring polyphenol, has been reported to confer vasoprotection, improving endothelial function and preventing complications of diabetes, we investigated the effect of resveratrol on mtROS production in cultured human coronary arterial endothelial cells (CAECs). The measurement of MitoSox fluorescence showed that resveratrol attenuates both steady-state and high glucose (30 mM)-induced mtROS production in CAECs, an effect that was prevented by the knockdown of the protein deacetylase silent information regulator 2/sirtuin 1 (SIRT1), an intracellular target of resveratrol. An overexpression of SIRT1 mimicked the effects of resveratrol, attenuating mtROS production. Similar results were obtained in CAECs transfected with mitochondria-targeted H2O2-sensitive HyPer-Mito fluorescent sensor. Amplex red assay showed that resveratrol and SIRT1 overexpression significantly reduced cellular H2O2 levels as well. Resveratrol upregulated MnSOD expression and increased cellular GSH content in a concentration-dependent manner (measured by HPLC coulometric analysis). These effects were attenuated by SIRT1 knockdown and mimicked by SIRT1 overexpression. We propose that resveratrol, via a pathway that involves the activation of SIRT1 and the upregulation of antioxidant defense mechanisms, attenuates mtROS production, suggesting the potential for new treatment approaches targeting endothelial mitochondria in metabolic diseases.
Keywords: vasoprotection, histone deacetylase, sirtuin 1
endothelial mitochondria have a crucial role in vascular pathophysiology (1, 16, 27, 39). Mitochondrial oxidative stress is frequently observed in diabetes and the metabolic syndrome and is thus likely to contribute to cellular energetic imbalance, activation of inflammatory processes, and endothelial dysfunction in these pathological conditions (19). Since increased mitochondrial production of reactive oxygen species (ROS) appears to be a key event in the development of vascular pathologies both in diabetes and aging (10, 46, 47), an identification of the mechanisms that regulate mitochondrial ROS (mtROS) generation in the endothelial cells may contribute to the development of improved pharmacological approaches to promote vascular health both in patients with diabetes and the elderly.
Resveratrol (3,5,4′-trihydroxystilbene) is a naturally occurring polyphenol found in more than 70 species of plants, including grapevines (Vitis vinifera). Since the original observation that resveratrol prolongs the life span in lower organisms, mimicking the antiaging effects of caloric restriction (49), it became the prototype of a new class of drugs termed caloric restriction mimetics, which are being developed to delay/reverse organ pathologies associated with aging and metabolic diseases (3). Resveratrol was recently shown to extend the life span (2) and to confer vasoprotection in animal models of diabetes mellitus, improving endothelial function and attenuating vascular inflammation (35, 40, 42, 43, 50). Similar protective effects of resveratrol treatment were observed in aged mice (35, 47). Moreover, the consumption of Mediterranean-style diets, which are rich in resveratrol, are associated with a reduced risk of cardiovascular mortality in humans (17, 23). As noted at the outset, both diabetes and aging are characterized by increased mtROS production, yet the effects of resveratrol on mitochondria in the endothelial cells remain incompletely understood.
The present study was conducted to determine whether resveratrol attenuates steady-state mtROS production in primary human coronary arterial endothelial cells (CAECs). The effects of resveratrol treatment on high glucose-induced mitochondrial oxidative stress were also assessed. Since resveratrol activates the NAD+-dependent protein deacetylase silent information regulator 2/sirtuin 1 (SIRT1) (21, 25) and SIRT1 regulates numerous proteins [including peroxisome proliferator-activated receptor coactivator-1α (PGC-1α)] implicated in the regulation of cellular energetics and mitochondrial function in various cell types (5, 18, 25, 30, 33, 41), this study focused on the mechanistic role of SIRT1 in mediating the mitochondrial protective effects of resveratrol in endothelial cells.
METHODS
Cell cultures, SIRT1 knockdown, and SIRT1 overexpression.
Primary human CAECs (purchased from Cell Applications) in culture were treated with resveratrol (purchased from Sigma-Aldrich) as described (15, 44). To disrupt SIRT1 signaling, the downregulation of SIRT1 was achieved by RNA interference using proprietary small-interfering RNA (siRNA) sequences (Superarray) and the electroporation-bases Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported (9). Cell density at transfection was 30%. Experiments were performed on day 2 after the transfection, when gene silencing was optimal. Specific gene silencing was verified with Western blot analysis as described (9). SIRT1 overexpression was achieved in CAECs by transfection with a SIRT1 full-length cDNA encoding plasmid (Stratagen) as described (13).
Measurement of resveratrol-induced changes in mtROS production in CAECs.
The effect of resveratrol on steady-state mitochondrial O2− production in endothelial cells was assessed by flow cytometry (FAScalibur; BD Bioscience, San Jose, CA) using MitoSox red (Invitrogen, Carlsbad, CA), a mitochondrion-specific hydroethidine-derivative fluorescent dye, as previously reported (11, 31, 32). Cell debris (low forward and side scatter), dead cells (Sytox green and annexin V positive), and apoptotic cells (annexin V positive) were gated out for analysis (31, 32). The data are presented as fold changes in the mean intensity of MitoSox fluorescence when compared with the respective controls. Also, CAECs were treated with high glucose (30 mM for 24 h) to assess the protective effect of resveratrol on mtROS production. In separate experiments, cellular H2O2 production was measured fluorometrically in CAECs using the Amplex red/horseradish peroxidase assay as described (14). The H2O2 generation rate was compared by measuring the time course of the buildup of resorufin fluorescence for 60 min by a Tecan Infinite M200 plate reader. In other experiments, cytoplasmic peroxide levels were measured fluorometrically using the 5(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (C-H2DCFDA) fluorescence assay, as reported (11, 14). Controls included measurements of cellular autofluorescence, time course measurements of dye-only controls, and polyethylene glycol-catalase controls. Calibration curves were generated with exogenous additions of H2O2. The effects of SIRT1 overexpression and SIRT1 knockdown on mtROS production were also determined using both the MitoSox, Amplex red, and C-H2DCFDA assays.
In further experiments, to detect increases in mitochondrial H2O2 generation at the single cell level, CAECs were transfected with mitochondria-targeted HyPer-Mito (Evrogen, www.evrogen.com), which is a fully genetically encoded fluorescent sensor capable for highly specific detection of mitochondrial H2O2 (4). This probe consists of circularly permuted yellow fluorescent protein inserted into the regulatory domain of the prokaryotic H2O2-sensing protein, OxyR (4). The cells were pretreated with resveratrol (10 μmol/l, for 24 h) or vehicle and then exposed to high glucose (30 mmol/l). The green fluorescent signal was observed by fluorescent microscopy. For quantitative purposes, the HyPer-Mito signal in transfected CAECs grown in 96 well plates was assessed using a Tecan Infinite M200 plate reader. Hoechst 33258 fluorescence, representing DNA content/cell mass, was used for normalization as described (11).
SIRT1 activity assay.
Nuclear SIRT1 activity was measured in cells treated with resveratrol. In brief, cells were suspended in lysis buffer [containing 10 mM Tris·HCl (pH 7.5), 10 mM NaCl, 15 mM MgCl2, 250 mM sucrose, 0.5% Nonidet P-40, and 0.1 mM EGTA], vortexed for 10 s, followed by incubation for 15 min on ice. The cells were spun through 4 ml of sucrose cushion [consisting of 30% sucrose, 10 mM Tris·HCl (pH 7.5), 10 mM NaCl, and 3 mM MgCl2] at 1,300 g for 10 min at 4°C. The nuclear pellet was washed once with cold 10 mM Tris·HCl (pH 7.5) and 10 mM NaCl. The isolated nuclei were suspended in 50 μl of extraction buffer [containing 50 mM HEPES-KOH (pH 7.50), 420 mM NaCl, 0.5 mM EDTA Na2, 0.1 mM EGTA, and 10% glycerol], sonicated for 30 s, and incubated on ice for 30 min, followed by centrifugation (15,000 rpm for 10 min). The nuclear extract was collected and the protein concentration was determined by the Bradford method. SIRT1 was immunoprecipitated from the samples using a rabbit polyclonal antibody directed against the COOH-terminus of SIRT1 (Abcam No. ab28170). SIRT1 activity in the samples was measured using the Cyclex SIRT1 Deacetylase Fluorimetric Assay kit according to the manufacturer's protocol (CycLex, Nagano, Japan). In brief, this assay is based on the principle that upon NAD-dependent deacetylation of the specific substrate by SIRT1 (in the presence of trichostatin A, a potent inhibitor of SIRT1-independent histone deacetylases), the fluorosubstrate peptide is cleaved by a lysyl endopeptidase, separating the quencher from the fluorophore. Specific activity of SIRT1 was assessed by measuring time-dependent changes in fluorescence intensity, normalized to protein concentration. Standard assay controls included the use of a fluoro-deacetylated peptide (to control for lysyl endopeptidase activity), no enzyme control, no NAD+ control, and no inhibitor control. We also assessed resveratrol-induced increases in the specific activity of recombinant SIRT1 in the presence and absence of the specific SIRT1 inhibitor, sirtinol (10−4 mol/l).
Measurement of resveratrol-induced changes in MnSOD expression in CAECs.
To determine the effects of resveratrol on the expression of MnSOD protein in CAECs, Western blot analysis was performed as described using a primary antibody directed against MnSOD (11, 14). The impact of SIRT1 siRNA and SIRT1 overexpression on the effects of resveratrol was also determined. Anti-β-actin (No. 6276, Abcam) was used for normalization purposes.
Determination of glutathione levels in CAECs using HPLC electrochemical detection.
Concentrations of redox-active GSH were measured in homogenates of CAECs pretreated with resveratrol (10−7 to 10−5 mol/l, for 24 h) using a Perkin-Elmer HPLC equipped with an eight-channel coulometric array detector (ESA, Chelmsford, MA) as described (6). In brief, 10-mg aliquots of the samples were washed with ice-cold PBS and homogenized in 5% (wt/vol) metaphosphoric acid. Samples were centrifuged at 10,000 g for 10 min to sediment protein, and the supernatant fraction was saved for the analysis of redox sensitive compounds. Precipitated proteins were dissolved in 0.1 N NaOH and saved for protein determinations by a spectrophotometric quantitation method using BCA reagent (Pierce Chemical, Rockford, IL). Concentrations of GSH in saved supernatant fractions were determined by injecting aliquots onto an Ultrasphere 5 u, 4.6 × 250 mm, C18 column and eluting with mobile phase of 50 mM NaH2PO4, 0.05 mM octane sulfonic acid, and 1.5% acetonitrile (pH 2.62) at a flow rate of 1 ml/min. The eight-channel CoulArray detectors were set at 200, 350, 400, 450, 500, 550, 600, and 700 mV, respectively. Peak areas were analyzed using ESA software, and concentrations of GSH are reported (as nmol/mg protein).
Data analysis.
Data were normalized to the respective control mean values and are expressed as means ± SE. Statistical analyses of data were performed by Student's t-test or by two-way ANOVA followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Resveratrol decreases mtROS production in endothelial cells: role of SIRT1.
To test the effect of resveratrol on steady-state mtROS generation and cellular H2O2 levels, cultured CAECs were treated with resveratrol (10−6 to 10−5 mol/l). The analysis of flow cytometry data showed that resveratrol significantly attenuated basal MitoSox fluorescence in CAECs (Fig. 1A).
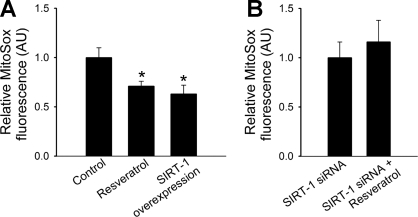
Fig. 1.
Resveratrol (for 48 h) significantly decreases steady-state mitochondrial O2− production (A; assessed by MitoSox fluorescence) in cultured coronary arterial endothelial cells (CAECs). Overexpression of silent information regulator 2/sirtuin 1 (SIRT1) mimics the effects resveratrol. In contrast, resveratrol treatment after SIRT1 small-interfering RNA (siRNA) pretreatment fails to decrease mitochondrial reactive oxygen species generation (B). AU, arbitrary units. *P < 0.05 vs. untreated.
In CAECs, the treatment with resveratrol significantly increased the specific activity of SIRT1 (by ∼50%). Resveratrol (from 10−6 to 10−5 mol/l) also significantly increased the activity of recombinant SIRT1, which could be prevented by sirtinol (not shown), extending our recent findings (12). We found that SIRT1 overexpression effectively attenuated both mtROS production (Fig. 1A) and decreased cellular peroxide levels, as shown by a decreased resorufin fluorescence (fold change: control, 100 ± 2%; and SIRT1 overexpression, 87 ± 1%) and C-H2DCFDA fluorescence (not shown). Endothelial SIRT1 expression was effectively downregulated by siRNA (by ∼90%), which prevented a resveratrol-induced attenuation of mtROS production (Fig. 1B). To test the protective effect of resveratrol against metabolic stress-induced mitochondrial oxidative stress, CAECs were treated with high glucose. The analysis of flow cytometry data showed that high glucose significantly increased mitochondrial O2− production, whereas mannitol was without effect (Fig. 2A). Resveratrol treatment, in a concentration-dependent manner, attenuated high glucose-induced mitochondrial oxidative stress (Fig. 2A).
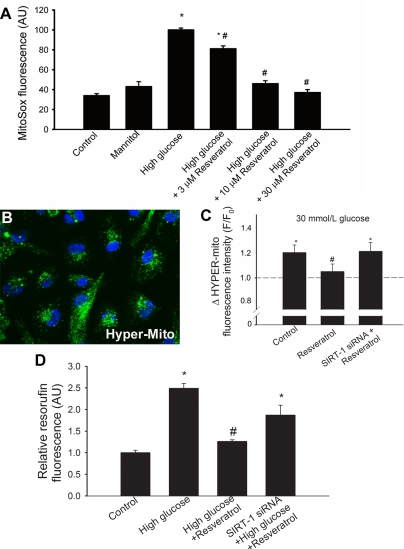
Fig. 2.
A: in CAECs, high glucose (30 mM) induces mitochondrial oxidative stress, as shown by the significant increases in the mean fluorescence intensity of oxidized MitoSox. Resveratrol treatment (for 48 h) significantly attenuates mitochondrial O2− production. Mannitol was used for osmotic control (n = 4 in each group). *P < 0.05 vs. baseline; #P < 0.05 vs. no resveratrol. B: representative fluorescent image showing CAECs transfected with mitochondria-targeted H2O2-sensitive HyPer-Mito fluorescent sensor. C: high glucose (30 mM) significantly increases HyPer-Mito fluorescence, which was prevented by resveratrol pretreatment. Resveratrol is ineffective in siRNA-treated cells that lack the ability to express SIRT1. *P < 0.05 vs. baseline; #P < 0.05 vs. no resveratrol. D: results from Amplex red/horseradish peroxidase assays. In CAECs, high glucose significantly increases H2O2 production, as shown by the significant increases in resorufin fluorescence. Resveratrol treatment significantly attenuates cellular H2O2 levels. The effect of resveratrol is blunted in cells in which SIRT1 was downregulated by siRNA. *P < 0.05 vs. untreated; #P < 0.05 vs. no resveratrol.
For the specific detection of intramitochondrial H2O2, CAECs were transfected with HyPer-Mito, a genetically encoded fluorescent sensor. The transfection efficiency was ∼80%. Fluorescent microscopy showed the perinuclear localization (Fig. 2B) of the HyPer-Mito fluorescence. High-glucose treatment resulted in a significant increase in the green fluorescent HyPer-Mito signal, which was prevented by resveratrol (10 μmol/l) treatment (Fig. 2C). We found that in CAECs resveratrol also significantly attenuated high glucose-induced cellular H2O2 production as shown by a decreased resorufin fluorescence (Fig. 2D). In CAECs, in which SIRT1 was knocked down by siRNA, resveratrol failed to significantly attenuate high glucose-induced increases in both mitochondrial (Fig. 2C) and cellular (Fig. 2D) H2O2 production.
Resveratrol upregulates MnSOD and increases GSH levels in cultured endothelial cells: role of SIRT1.
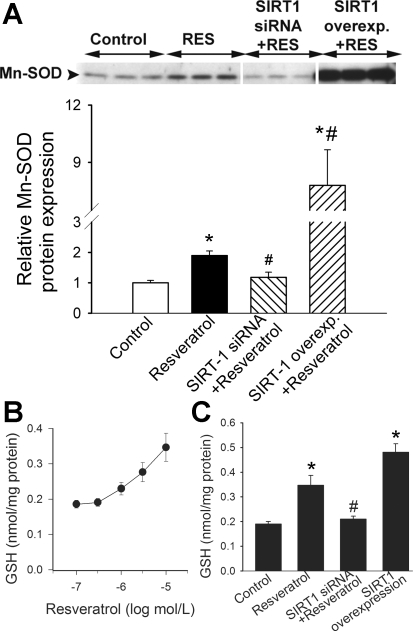
Western blot analysis showed that in CAECs, resveratrol elicited significant increases in the expression of MnSOD (Fig. 3A). A knockdown of SIRT1 prevented the resveratrol-induced induction of MnSOD (Fig. 3A), whereas an overexpression of SIRT1 significantly potentiated the effect of resveratrol on cellular MnSOD expression (Fig. 3A). HPLC coulometric analysis revealed that resveratrol elicited concentration-dependent increases in GSH content in CAECs (Fig. 3B). A knockdown of SIRT1 significantly attenuated resveratrol-induced increases in cellular GSH levels (Fig. 3C). By contrast, an overexpression of SIRT1 elicited significant increases in cellular GSH content (Fig. 3C).
Fig. 3.
A: original Western blot and densitometric results show that resveratrol (RES) induces MnSOD expression in CAECs. Knockdown of SIRT1 (siRNA) prevents the effect of resveratrol, whereas SIRT1 overexpression (Overexp) substantially augments expression. Data are means ± SE. *P < 0.05; #P < 0.05 vs. untreated. B: summary data for HPLC coulometric analysis of glutathione (GSH) content in homogenates of CAECs. Resveratrol in concentration-dependent manner elicits significant increases in cellular GSH content. C: resveratrol increases cellular content of GSH vs. control, whereas knockdown of SIRT1 using siRNA blocks resveratrol-enhancing effect. By contract, overexpression of SIRT1 amplifies endogenous GSH levels. Data are means ± SE. *P < 0. 05 vs. control; #P < 0.05 vs. no resveratrol.
DISCUSSION
Pathways that regulate mitochondrial function and ROS production have recently emerged as potential therapeutic targets for the amelioration of endothelial dysfunction and prevention of vascular disease in diabetes and other pathological conditions (39). Our studies show that resveratrol attenuates mitochondrial oxidative stress in CAECs. A reduction of mtROS production is associated with the activation of SIRT1 and the induction of mitochondrial antioxidant systems.
Resveratrol is a promising new therapeutic approach for preventing cardiovascular diseases in type 2 diabetes and aging (3, 35). Previous studies focused on the direct effects of resveratrol on proinflammatory pathways and antioxidant defense mechanisms in endothelial cells (13, 15, 44) but provided little information on its effects on endothelial mitochondria. Our data support the finding that resveratrol significantly attenuates steady-state levels of mitochondrial O2− production in CAECs (Fig. 1A).
To test whether resveratrol treatment can also prevent mitochondrial oxidative stress induced by metabolic stress, we exposed endothelial cells to high glucose to mimic diabetic conditions. In CAECs, high glucose in vitro is known to elicit substantial increases in mitochondrial O2− generation (32, 34, 36). We found that resveratrol attenuates high glucose-induced mitochondrial oxidative stress in the endothelial cells (Fig. 2, A–C), suggesting that it effectively increases cellular metabolic stress resistance. The concentrations of resveratrol required to reduce mtROS generation are achievable in the plasma in vivo when resveratrol is used as a dietary supplement (3), suggesting that the attenuation of mitochondrial oxidative stress may contribute to the vasoprotective effects of resveratrol treatment under pathophysiological conditions (35). We recently demonstrated that in mice with type 2 diabetes, resveratrol treatment effectively attenuated oxidative stress in the aorta, protecting endothelial function (35). Mitochondria-derived O2− is membrane impermeable (except in the protonated perhydroxyl radical form, which represents only a small fraction of total O2− produced), whereas H2O2 easily penetrates the mitochondrial membranes. As shown in Fig. 2D, resveratrol prevents high glucose-induced increases in resorufin fluorescence in the Amplex red assay, suggesting that decreased mtROS production also results in lower cytoplasmic H2O2 levels in resveratrol-treated cells.
There are multiple mechanisms by which resveratrol-induced reduction of mitochondrial oxidative stress may promote vascular health. Mitochondrion-derived ROS have important signaling functions, such as the activation of NF-κB-dependent inflammatory pathways in aging (47) and metabolic diseases. Thus resveratrol-induced attenuation of mitochondrial oxidative stress is likely to confer anti-inflammatory effects. Indeed, we have found that resveratrol treatment significantly decreases NF-κB-dependent gene expression in aortas of aged mice and mice with type 2 diabetes (35). Resveratrol also inhibits NF-κB-driven gene expression in vessels of aged rats (47). Furthermore, resveratrol effectively inhibits high glucose-induced NF-κB activation and inflammatory gene expression in cultured endothelial cells (A. Csiszar and Z. Ungvari, unpublished observation). Recent studies also suggest that a link exists between mitochondrial oxidative stress, mtDNA depletion, and development of pathological vascular phenotypes in diabetes (37). Increased ROS levels in the mitochondria are known to inactivate critical enzymes involved in mitochondrial metabolism (e.g., α-ketoglutarate dehydrogenase and aconitase). Dysfunctional mitochondria may diminish ATP production, thereby impairing the synthesis and secretion of endothelium-derived factors that serve as paracrine signals in the vascular wall and affecting the transport functions of the vascular endothelium. A Resveratrol-induced attenuation of mitochondrial oxidative stress would correct these impairments.
The NAD+-dependent protein deacetylase SIRT1 plays a critical role in resveratrol-induced effects in endothelial cells. Accordingly, resveratrol, similar to that observed in other cell types (21, 25), induces SIRT1 in endothelial cells (13). Resveratrol also lowers the Km of SIRT1 for the acetylated substrate and for NAD+ (21). A knockdown of SIRT1 prevents a resveratrol-induced reduction in mtROS production (Fig. 1B) and cellular H2O2 levels (Fig. 2D). An overexpression of SIRT1 also attenuates mtROS generation (Fig. 1A) and cellular H2O2 levels in CAECs, mimicking the effects of resveratrol. These findings are in accord with previous studies that showed that resveratrol and SIRT1 regulate mitochondrial function in skeletal muscle and liver (18, 25). SIRT1 likely regulates multiple pathways involved in mtROS generation in the endothelial cells, among which the upregulation of mitochondrial antioxidant systems appear to play a key role. Accordingly, resveratrol and SIRT1 overexpression increases MnSOD expression in CAECs (Fig. 3A). We attribute the resveratrol-induced reduction of mitochondrial O2− levels, at least in part, to this effect. Furthermore, resveratrol also significantly increases cellular GSH levels (Fig. 3B). In addition, resveratrol was previously shown to upregulate glutathione peroxidase and catalase in the endothelial cells (44). GSH, glutathione peroxidase, and catalase are important components in the cellular antioxidant system involved in the detoxification of H2O2, which play an important role in oxidative stress resistance in the mitochondria. Because increased MnSOD per se would increase mitochondrial H2O2 release, we attribute the reduction of H2O2 levels in resveratrol-treated cells to the upregulation of the aforementioned H2O2 detoxification systems. The effects of resveratrol on MnSOD and GSH are prevented by knockdown of SIRT1 (Fig. 3, A and B) and mimicked/potentiated by SIRT1 overexpression (Fig. 3, A and C), suggesting that SIRT1 activation plays a key role in inducing mitochondrial antioxidant defense mechanisms in endothelial cells. In addition, resveratrol, via a SIRT1-dependent pathway, increases mitochondrial content in the vascular endothelium (12) as well as in the liver and skeletal muscle (2, 25). Mitochondrial proliferation reduces the flow of electrons per unit mitochondria, thus resveratrol-induced mitochondrial biogenesis may also contribute to the reduction of mitochondrial oxidative stress in endothelial cells.
The mitochondrial theory of aging, originally proposed by Harman in the early 1970s (20), postulates that mitochondrial oxidative stress and consequential free radical reactions underlie aging. According to this theory, an increased production of ROS results in a variety of macromolecular oxidative modifications with age and the accumulation of such oxidative damage of proteins, lipids, and DNA is the primary causal factor in the aging process. There is clear evidence that aging in mammals is associated with mitochondrial oxidative stress in virtually every tissue studied, including blood vessels (8, 22, 28, 29, 38, 46–48). Moreover, recent studies suggest that longevity is associated with increased vascular resistance to high glucose-induced mitochondrial oxidative stress (24). Thus the protection of mitochondria as mediated through exposure to resveratrol is likely to contribute to its antiaging action (2, 41). These theories are converging with those involving caloric restriction in that in most organisms, the extension of life span and cardiovascular protection can be achieved through feeding calorie-restricted diets (45), which also induce SIRT1 (7) and attenuate mtROS production (26).
In conclusion, resveratrol reduces mtROS production in endothelial cells via the activation of SIRT1. We propose that SIRT1 increases mitochondrial antioxidant capacity, via the upregulation of MnSOD and other antioxidant systems that attenuate mitochondrial oxidative stress. Our findings suggest the potential for use of caloric restriction mimetics to target endothelial mitochondria in metabolic diseases.
GRANTS
This work was supported by grants from the American Federation for Aging Research (to A. Csiszar) and the American Diabetes Association (to Z. Ungvari); by American Heart Association Grant 110350047A (to C. Zhang) and National Institutes of Health (NIH) Grants HL-077256 and HL-43023 (to Z. Ungvari and A. Csiszar), CA-111842 (to J. PT. Pinto), and RO1-HL-077566 and RO1-HL-085119 (to C. Zhang); and by the Intramural Research Program of the NIH (to P. Pacher).
REFERENCES
- 1.Addabbo F, Ratliff B, Park HC, Kuo MC, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol 174: 34–43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One 3: e1759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem 282: 4634–4642, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Corral-Debrinski M, Shoffner JM, Lott MT, Wallace DC. Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat Res 275: 169–180, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol 168: 629–638, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csiszar A, Labinskyy N, Orosz Z, Ungvari Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med Hypotheses 67: 904–908, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol 295: H1882–H1894, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson KJ, de Cabo R, Pacher P, Zhang C, Ungvari ZI. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297: H13–H20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100: 1128–1141, 2007 [DOI] [PubMed] [Google Scholar]
- 17.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 99: 779–785, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res 99: 924–932, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc 20: 145–147, 1972 [DOI] [PubMed] [Google Scholar]
- 21.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 19: 419–421, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, Kromhout D, Nedeljkovic S, Punsar S, Seccareccia F, Toshima H. The diet and 15-year death rate in the seven countries study. Am J Epidemiol 124: 903–915, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol 296: H946–H956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 103: 1768–1773, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 100: 460–473, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 127: 298–306, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Melov S, Shoffner JM, Kaufman A, Wallace DC. Marked increase in the number and variety of mitochondrial DNA rearrangements in aging human skeletal muscle. Nucleic Acids Res 23: 4122–4126, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450: 712–716, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2: 2295–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, Obrosova IG, Pacher P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol 293: H610–H619, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocic P, Wilson G, Shokolenko I, Joseph J, Kalyanaraman B, Chilian WM. The importance of mitochondrial oxidative stress and integrity of mitochondrial DNA in coronary arteriogenesis (Abstract). Circulation 116: II-174–II-175, 2007 [Google Scholar]
- 38.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909–1911, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation 118: 1347–1357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 76: 69–75, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol 3: 31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 290: E1339–E1346, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med 43: 720–729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Van Remmen H, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol 36: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006 [DOI] [PubMed] [Google Scholar]