Abstract
Our previous study showed that stimulation of adenosine A1 receptors located in the nucleus of the solitary tract (NTS) exerts counteracting effects on the iliac vascular bed: activation of the adrenal medulla and β-adrenergic vasodilation versus vasoconstriction mediated by neural and unknown humoral factors. In the present study we investigated the relative contribution of three major potential humoral vasoconstrictors: vasopressin, angiotensin II, and norepinephrine in this response. In urethane-chloralose anesthetized rats we compared the integral changes in iliac vascular conductance evoked by microinjections into the NTS of the selective A1 receptor agonist N6-cyclopentyladenosine (CPA; 330 pmol in 50 nl) in intact (Int) animals and following: V1 vasopressin receptor blockade (VX), angiotensin II AT1 receptor blockade (ATX), bilateral adrenalectomy + ganglionic blockade (ADX + GX; which eliminated the potential increases in circulating norepinephrine and epinephrine), ADX + GX + VX and ADX + GX + VX + ATX. In Int animals, stimulation of NTS A1 adenosine receptors evoked typical variable responses with prevailing pressor and vasoconstrictor effects. VX reversed the responses to depressor ones. ATX did not significantly alter the responses. ADX + GX accentuated pressor and vasoconstrictor responses, whereas ADX + GX + VX and ADX + GX + VX + ATX virtually abolished the responses. Stimulation of NTS A1 adenosine receptors increased circulating vasopressin over fourfold (26.4 ± 10.4 vs. 117.0 ± 19 pg/ml). These data strongly suggest that vasopressin is a major vasoconstrictor factor opposing β-adrenergic vasodilation in iliac vascular responses triggered by stimulation of NTS A1 adenosine receptors, whereas angiotensin II and norepinephrine do not contribute significantly to the vasoconstrictor responses.
Keywords: purinergic receptors, V1 receptor blockade, angiotensin II type 1 receptor blockade, ganglionic blockade, adrenalectomy, lumbar sympathectomy, iliac vascular conductance
it is now widely accepted that adenosine operating via A1 and A2a receptors modulates neural cardiovascular control at the level of the nucleus tractus solitarii (NTS) and other brainstem cardiovascular centers (2, 4, 5, 20, 31, 36, 38, 41). Under normal, physiological conditions a natural source of adenosine is ATP released synaptically from neurons as well as from glial cells activated by neighboring neurons (6, 8, 9, 14, 38). This extracellular ATP is catabolized via ectonucleotidases to adenosine, which further acts more broadly as a neuromodulator operating via pre- or postsynaptic A1 and A2a adenosine receptor subtypes (8, 26, 38, 46). Under pathological conditions such as ischemia, hypoxia, and severe hemorrhage, a global release of adenosine from many cell types occurs via the breakdown of intracellular ATP (25, 43, 45). Thus, adenosine, which is generated in or released into the extracellular space under physiological or pathological conditions, acts not via specific synapses in specific neuronal pathways but rather spatially, reaching all adenosine receptor subtypes in the vicinity; this may resemble signaling via diffusive neurotransmitters/neuromodulators like nitric oxide or carbon monoxide. This spatial aspect of the action of naturally released adenosine seems especially important for the NTS, where groups of functionally different neurons usually overlap allowing for adenosine crosstalk. Specific physiological effects exerted via nonselective, spatial spread of adenosine in the NTS may depend on differential expression of adenosine receptor subtypes on functionally distinct NTS neurons/terminals, as our laboratory suggested previously (31, 36).
In the central nervous system adenosine may inhibit or facilitate release of neurotransmitters from synaptic terminals as well as directly inhibit or activate neurons via pre- and postsynaptic A1 or A2a receptors, respectively (8, 26, 31, 36). Since A1 versus A2a adenosine receptors exert contrasting effects on central neurons/terminals, reciprocal effects are usually, although not always, observed in response to selective stimulation of the two receptor subtypes. NTS A1 adenosine receptor stimulation predominately yields differential regional sympathoactivation (adrenal > renal ≥ lumbar) and pressor responses (2, 5, 30, 35). In contrast, NTS A2a receptor stimulation typically evokes depressor responses accompanied by contrasting regional sympathetic responses: decreases in renal sympathetic nerve activity (RSNA), no changes in lumbar sympathetic nerve activity (LSNA), and increases in preganglionic adrenal sympathetic nerve activity (pre-ASNA) (2, 5, 16, 32–34). Note that whereas stimulation of NTS A1 and A2a receptors evoke contrasting changes in RSNA and LSNA, both adenosine receptor subtypes activate the sympathetic output to the adrenal medulla (33, 35).
Recent studies from our laboratory showed that the pressor and sympathoexcitatory responses evoked by stimulation of NTS A1 adenosine receptors are mediated mostly via inhibition of baroreflex mechanisms at the level of the NTS, whereas hemodynamic and differential sympathetic responses evoked by stimulation of NTS A2a adenosine receptors are mediated mostly via activation of nonbaroreflex mechanisms (16, 30, 33–35). It should be stressed that A1 adenosine receptors may also modulate nonglutamatergic, nonbaroreflex mechanisms operating in the NTS. For example, sinoaortic denervation or ionotropic glutamatergic blockade abolished A1-adenosine receptor-mediated increases in RSNA and LSNA, whereas this attenuated, but did not abolish, the increases in pre-ASNA (35). The activation of pre-ASNA, which persisted after sinoaortic and the glutamatergic blockade, was most likely mediated via A1 adenosine receptor modulation of nonglutamatergic pathways descending into the NTS from higher structures, such as from hypothalamic paraventricular and/or dorsomedial nuclei (12, 28, 42). A1 adenosine receptors located on these descending nonglutamatergic pathways and/or NTS interneurons could selectively activate the sympathetic output to the adrenal medulla (but not RSNA and LSNA) via disinhibition of direct NTS-rostral ventrolateral medulla pathways (10). NTS A1 adenosine receptors may also modulate the control of heart rate (HR) via both baroreflex and nonbaroreflex mechanisms (30, 35). Taken together, these observations strongly suggest that A1 adenosine receptors are differentially located on functionally different NTS neurons/terminals, which are mostly, but not exclusively, glutamatergic and involved in the baroreflex arch.
Although selective stimulation of NTS A1 adenosine receptors evokes predominantly pressor responses (2, 5), occasionally biphasic or even depressor responses are observed (35). This variability of the responses is a natural consequence of simultaneous activation of at least two counteracting mechanisms: sympathetic vasoconstriction and β-adrenergic vasodilation mediated via epinephrine released from the activated adrenal medulla. Since β-adrenergic receptors are preferentially located in the muscle vascular bed (44) and both pre-ASNA and LSNA increase upon stimulation of NTS A1 adenosine receptors, it was likely that these two counteracting factors may significantly contribute to the variability of the iliac vascular responses. We confirmed this hypothesis in a recent study from our laboratory by showing that removal of the vasodilatory mechanism (via bilateral adrenalectomy as well as peripheral blockade of β-adrenergic receptors) abolished the variability of the responses normally observed in intact animals and markedly increased the pressor and hindlimb vasoconstrictor responses (21). In contrast, bilateral lumbar sympathectomy tended to increase the vasodilatory component of the responses although the variability still persisted. To our surprise, following combined adrenalectomy plus lumbar sympathectomy, a marked, consistent vasoconstrictor component still persisted, suggesting that some unknown humoral vasoconstrictor factor(s) are involved (21).
The most likely humoral candidates contributing to the iliac vasoconstriction are vasopressin, angiotensin II, and norepinephrine. Since activation of A1 adenosine receptors may inhibit glutamate release in baroreflex pathway at the level of the NTS (30, 35), and given that the NTS is a crucial, primary relay station for tonic baroreflex inhibition of vasopressin release (10, 29, 39), it is likely that the activation of A1 adenosine receptors may inhibit the baroreflex restraint of vasopressin release. When released, vasopressin could evoke powerful peripheral vasoconstriction. However, whether A1 adenosine receptors are present on those NTS baroreflex neurons/terminals, which inhibit the release of vasopressin, is unknown. The present study was designed to test this hypothesis.
Since RSNA directed to the kidney has been shown to increase following stimulation of NTS A1 adenosine receptors (35), this may facilitate the renin/angiotensin mechanism leading to humoral vasoconstriction of the hindlimb vasculature mediated via AT1 angiotensin II receptors (11). In addition, since stimulation of NTS A1 adenosine receptors increases the activity of various sympathetic outputs (35), it is also possible that circulating norepinephrine released from other synaptic terminals may reach the iliac vasculature and cause vasoconstriction. Therefore, in the present study we investigated the extent to which these three potential humoral vasoconstrictors (vasopressin, angiotensin II, and/or norepinephrine) contribute to NTS-A1 adenosine receptor-elicited iliac vasoconstriction (21).
MATERIALS AND METHODS
All protocols and surgical procedures employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee and were performed in accordance with the Guiding Principles in the Care and Use of Animals endorsed by the American Physiological Society and published by the National Institutes of Health.
Design
This study investigates further the mechanisms responsible for the consistent variability of hemodynamic responses elicited by activation of adenosine A1 receptors in the NTS (21, 35). Previously, our laboratory showed that the variability of the pressor/depressor and iliac vasoconstrictor/vasodilator responses is not a simple effect of competitive interactions between sympathetic vasoconstriction versus β-adrenergic vasodilation, but some unknown, powerful, humoral vasoconstrictor factor(s) are also involved (21). Therefore, the present study assessed the relative contribution of potential humoral vasoconstricting factors to the iliac vascular responses evoked by selective stimulation of NTS A1 adenosine receptors. Experiments were performed on a total of 102 male Sprague-Dawley rats. In 63 rats, we compared the relative vasoconstrictor effects potentially mediated via vasopressin, angiotensin II, norepinephrine, and sympathetic innervation of the hindquarters, the effects normally opposed by simultaneous β-adrenergic vasodilation mediated via activation of the adrenal medulla. In an additional 20 rats, respective time controls were performed, and in six rats the effectiveness of vasopressin and angiotensin II receptor blockades was assessed. These functional experiments strongly suggested that the major vasoconstrictor factor triggered by activation of adenosine A1 receptors in the NTS may be vasopressin. Therefore, in an additional group of 13 animals, the levels of circulating vasopressin were evaluated before and following microinjections into the NTS of the selective A1 adenosine receptor agonist N6-cyclopentyladenosine (CPA) or vehicle control.
Instrumentation and measurements.
All the procedures were described in detail previously (4, 19, 21, 32–35). Briefly, male Sprague-Dawley rats (350–400 g; Charles River) were anesthetized with a mixture of α-chloralose (80 mg/kg) and urethane (500 mg/kg ip), tracheotomized, connected to a small animal respirator (SAR-830; CWE, Ardmore, PA), and artificially ventilated with 40% oxygen-60% nitrogen mixture. Catheterization of the right femoral artery and vein were performed to monitor arterial blood pressure and infuse drugs, respectively. Arterial blood gases were tested occasionally for appropriate experimental values (Radiometer, ABL500, OSM3). Averaged values measured at the end of each experiment were the following: pH = 7.38 ± 0.01, Po2 = 140.1 ± 3.8 mmHg, and Pco2 = 36.2 ± 0.7 mmHg.
From a midabdominal incision, the left iliac artery was exposed. A pulse Doppler blood flow velocity transducer was placed around the artery and connected to the flowmeter (Baylor Electronics). From the same midabdominal incision in some animals, bilateral adrenalectomy or lumbar sympathectomy (L1-L6) was performed. The intermesenteric nerves were also severed in sympathectomized animals.
Arterial blood pressure and iliac flow signals were digitized and recorded with an analog-digital converter (Modular Instruments) interfaced to a laboratory computer. The signals were recorded continuously using Biowindows software (Modular Instruments), averaged over 5-s intervals and stored on hard disk for subsequent analysis.
Microinjections into the NTS.
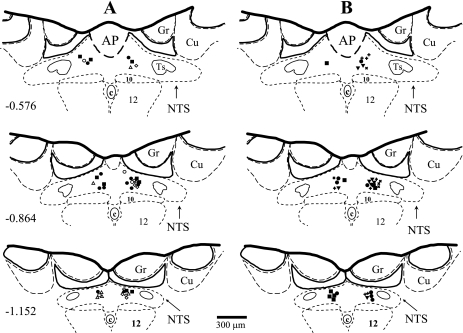
Animals were placed in stereotaxic frame with head tilted down at 45°. After the exposure of the brainstem via dissected atlantoccipital membrane, the animals were allowed to stabilize for at least 30 min before microinjections. Unilateral microinjections of the selective A1 adenosine receptor agonist CPA (Tocris, 330 pmol) dissolved in 50 nl of artificial cerebrospinal fluid (ACF) were made through multibarrel, glass micropipettes into the medial region of the caudal subpostremal NTS as described previously (4, 19, 32–35). This dose of CPA produced the most consistent, predominantly pressor responses in a previous study from our laboratory (35). The CPA was dissolved in ACF, and the pH was adjusted to 7.2. In several previous studies our laboratory has shown that microinjections of the same amount of ACF into the same site of the NTS did not markedly affect mean arterial pressure (MAP), HR, RSNA, LSNA, and pre-ASNA and vascular flows in iliac, renal, and mesenteric arteries (4, 32–35). The changes in all these variables were either not different from zero or smaller than natural, random fluctuations of these variables over the time of measurements. To avoid the effect of desensitization of A1 adenosine receptors, in all experiments only one dose of the agonist was microinjected into left or right side of the NTS. All microinjection sites were marked with fluorescent dye (DiI; Molecular Probes) and verified histologically (Fig. 1) as described previously (4, 19, 32–35).
Fig. 1.
Microinjection sites in the caudal subpostremal nucleus tractus solitarii (NTS) for all experiments. Schematic diagrams of transverse sections of the medulla oblongata from a rat brain are shown. AP, area postrema; c, central canal; 10, dorsal motor nucleus of the vagus nerve; 12, nucleus of the hypoglossal nerve; ts, tractus solitarius; Gr, gracile nucleus; Cu, cuneate nucleus. Scale is shown at the bottom; the number on the left side of the schematic diagram denotes the rostro-caudal position in millimeters of the section relative to the obex according to the atlas of the rat subpostremal NTS by Barraco et al. (Ref. 1). Microinjection sites were marked with fluorescent dye and are denoted with filled symbols for the pressor responses to N6-cyclopentyladenosine (CPA) and corresponding open symbols for the depressor responses. A: microinjections of CPA in intact animals (●, ○), after vasopressin V1 receptor blockade (VX; ◊), lumbar sympathectomy plus VX (LX + VXi; ▴, ▵), and after angiotensin II AT1 receptor blockade (ATX; ■, □). B: microinjections of CPA after bilateral adrenalectomy (ADX) plus ganglionic blockade (GX) (▴), following ADX + GX + VX (dotted closed circle, dotted open circle), after ADX + GX + VX + ATX (dotted closed square, dotted open square). In experiments where vasopressin assay was performed, the microinjection sites were denoted: + and x for CPA and artificial cerebrospinal fluid (ACF) microinjections, respectively.
We believe that the microinjection technique mimics natural, spatial (not strictly synaptic) action of adenosine in the central nervous system since adenosine is naturally produced in the intracellular space by ectonucleotidases from extracellular ATP (released from neurons and glial cells under physiological conditions) (6, 8, 14, 38, 46) or is directly released into the intracellular space from ischemic/hypoxic neurons and glial cells under pathological conditions (25, 43, 45).
Experimental Protocols
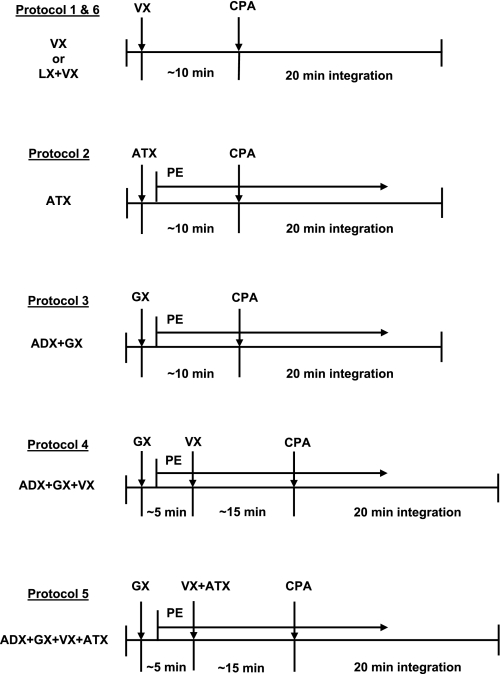
In a previous study from our laboratory we showed that in addition to sympathetic iliac vasoconstriction and β-adrenergic vasodilation, some unknown humoral vasoconstrictor(s) contribute to the consistent variability of hemodynamic responses evoked by selective stimulation of adenosine A1 receptors located in the NTS (21). This conclusion was based on comparing of the responses observed in intact animals and following four experimental protocols: 1) β-adrenergic blockade, 2) adrenalectomy, 3) lumbar sympathectomy, and 4) combined adrenalectomy plus lumbar sympathectomy. In the last experimental condition a powerful iliac vasoconstriction was observed, indicating that nonsympathetic, humoral vasoconstrictors are involved (21). The present study is a direct extension of our previous findings and focuses on the relative contribution to the responses of three potential humoral vasoconstrictors: vasopressin, angiotensin II, and norepinephrine. Six experimental protocols were designed, according to the diagrams presented in Fig. 2. Data collected in each protocol were compared with responses observed in the intact group. In protocols 1 and 2, the contribution of vasopressin and angiotensin II was assessed by comparing hemodynamic responses elicited by stimulation of NTS A1 adenosine receptors in intact animals with those obtained following selective blockade of vasopressin V1 receptors and angiotensin II AT1 receptors via intravenous injections of selective antagonists: [β-mercapto-β,β-cyclopentylmethylenepropionyl,1-O-Me-Tyr2,Arg8 ]-vasopressin (20 μg/kg; Sigma) and losartan (5 mg/kg; Merck), respectively. To evaluate the potential contribution of circulating norepinephrine to the responses, ganglionic blockade (hexamethonium bromide, 25 mg/kg iv; Sigma) was combined with adrenalectomy (protocol 3), and these data and the responses observed previously following adrenalectomy alone (21) are discussed together. This indirect evaluation of norepinephrine contribution to the responses was necessary because total sympathetic denervation is impossible and ganglionic blockade, which prevents secretion of norepinephrine from sympathetic terminals, also impairs/abolishes the effects of activation of the adrenal medulla; thus ganglionic blockade removes β-adrenergic vasodilation simultaneously. Therefore, the appropriate reference point for the responses obtained following the ganglionic blockade (protocol 3) were the responses obtained following adrenalectomy alone, which has been already performed in a previous study from our laboratory (21). Protocol 4 removed the combined contribution of norepinephrine and vasopressin to the responses (ganglionic blockade + vasopressin V1 receptor blockade), whereas protocol 5 removed the combined effect off all three potential vasoconstrictors considered via ganglionic blockade + vasopressin V1 receptor blockade + angiotensin AT1 receptor blockade. Protocols 3–5 were performed in adrenalectomized animals to clarify the experimental conditions by removing any residual adrenal responses, which may potentially persist following the ganglionic blockade. Since preliminary results of the above five experimental protocols strongly suggested that only vasopressin has a marked contribution to the responses, in protocol 6 the magnitude of β-adrenergic vasodilation alone, not opposed by major vasoconstrictor factors (sympathetic vasoconstriction and vasopressin), was assessed. In this protocol bilateral lumbar sympathectomy was combined with blockade of V1 vasopressin receptors, and these data were compared with data following V1 vasopressin receptor blockade alone (protocol 1) and discussed together with previous data obtained following bilateral lumbar sympathectomy alone (21).
Fig. 2.
Time line of how experimental protocols are executed. Abbreviations are as in Fig. 1. Time control experiments were performed for protocols including ATX and/or GX (protocols 2–5) according to the respective diagrams; however, microinjections of CPA were omitted. PE, phenylephrine.
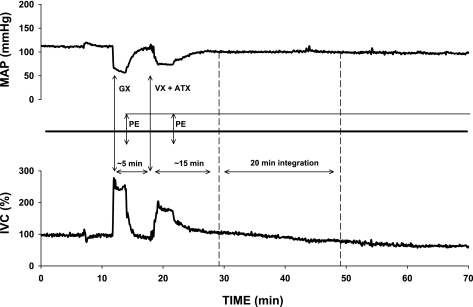
The effectiveness of vasopressin V1 and angiotensin AT1 receptor blockades were tested in separate groups of animals (n = 3 for each blockade) with intravenous injections of arginine-vasopressin (50 mU/kg; Sigma) and angiotensin II (300 ng/kg; Sigma), respectively, before and after the blockade. Both blockades remained effective for over 1 h. Blockade of V1 vasopressin receptors caused relatively small decreases in MAP and increases in iliac vascular conductance (IVC; Table 1), which spontaneously returned toward resting values in ∼10 min; therefore, ∼10 min after V1 vasopressinergic blockade the microinjection of CPA was performed in protocols 1 and 6 (Fig. 2). However, following blockade of angiotensin AT1 receptors and ganglionic blockade, marked and sustained decreases in MAP and increases in IVC were observed (Table 1). Therefore, in protocols 2–5, where these blockades were performed, intravenous infusions of phenylephrine (PE; 200 μg/ml; Sigma) were used to return the hemodynamic parameters toward baseline, preblockade values. Table 2 presents the rates of PE infusion needed for the compensation. No differences were observed in PE infusion rates between protocols 2 and 4. However, significantly greater PE infusion rates were required when angiotensin AT1 receptor antagonist, losartan, was combined with ganglionic and V1 vasopressin receptor blockades in protocol 5 (Table 2). During the responses to stimulation of NTS A1 adenosine receptors, PE infusion was continued at the same rates as needed to compensate for the altered hemodynamic values in protocols 2–5. The effect of PE infusion on baseline hemodynamic values was estimated in respective time controls for protocols 2–5 (Table 2). In the time-control experiments, all procedures except microinjections of CPA were performed in the same time pattern as in experimental protocols 2-5. Figure 3 shows an example of a time control for the most complex experimental protocol 5. PE infusion rates were similar in the experimental protocols and respective time controls (Table 2).
Table 1.
Maximal hemodynamic responses evoked by blockade of V1 vasopressin receptors, AT1 angiotensin II receptors, and ganglionic blockade
| Experimental Groups | n | Δ% Mean Arterial Pressure, mmHg | Δ% Heart Rate, beats/min | Δ% Iliac Blood Flow | Δ% Iliac Vascular Conductance |
|---|---|---|---|---|---|
| VX | 16 | −4.7±1.8* | 1.2±0.9 | 10.2±1.6* | 15.3±2.7* |
| GX | 39 | −39.7±1.9* | 3.1±1.9 | 16.6±3.3* | 99.9±9.9* |
| ATX | 15 | −49.2±2.8* | −13.9±3.5* | 8.6±6.1 | 125.1±18.2* |
Values are means ± SE. Abbreviations are as in Fig. 1. Numbers of responses to VX, GX, and ATX were combined from those protocols where these blockades were applied as a first pharmacological manipulation: nVX = nVX + nLX+VX; nGX = nADX+GX + nADX+GX+VX + nADX+GX+VX+ATX + n of respective controls; nATX = nATX + n of respective control (see Table 2). The small changes in mean arterial pressure and iliac vascular conductance caused by VX were allowed to return spontaneously toward resting levels, whereas the large, sustained changes in these variables caused by ATX and GX were compensated via intravenous infusion of phenylephrine.
P < 0.05 vs. zero.
Table 2.
Infusion rates of phenylephrine used to maintain mean arterial pressure and iliac vascular conductance at preblockade levels in experimental and respective time control groups in which ATX and/or GX were performed
| Protocol Number | Experimental Procedure | n | Infusion Rate, ml·h−1·kg−1 | Time Controls | n | Infusion Rate, ml·h−1·kg−1 |
|---|---|---|---|---|---|---|
| 2 | ATX | 10 | 3.18±0.12* | ATX | 5 | 2.39±0.25* |
| 3 | ADX + GX | 8 | 3.02±0.24* | ADX + GX | 5 | 3.20±0.33* |
| 4 | ADX + GX + VX | 8 | 3.45±0.4* | ADX + GX + VX | 5 | 3.94±0.62 |
| 5 | ADX + GX + VX + ATX | 8 | 6.33±0.24 | ADX + GX + VX + ATX | 5 | 5.29±0.62 |
Values are means ± SE; n = number of rats. Abbreviations are as in Fig. 1. There were no differences in infusion rates between experimental groups versus respective time control groups (P > 0.05).
P < 0.05 vs. ADX + GX + VX + ATX.
Fig. 3.
An example of time control experiment following ADX + GX + VX + ATX; no CPA was microinjected in this experiment. The dashed arrow denotes a potential microinjection of CPA and the subsequent 20-min integration of the response. Note that although the infusion of PE did not fully compensate for the decrease in mean arterial pressure (MAP), the iliac vascular conductance (IVC) gradually declined constricting iliac vasculature slightly below the baseline level.
Vasopressin Assay
Since the hemodynamic experiments (protocols 1–6) suggested that vasopressin plays a dominant role in the iliac vascular responses to stimulation of NTS A1 adenosine receptors, in an additional group of animals the effect of microinjections into the NTS of CPA (n = 8) or respective volume control (50 nl of ACF; n = 5) on circulating vasopressin was evaluated. We compared the levels of plasma vasopressin measured 30 min before and ∼8 min after the microinjections (the average time when maximal hemodynamic responses to stimulation of NTS A1 receptors occur). Arterial blood samples (∼1 ml) were slowly withdrawn from the femoral artery into prechilled, heparinized tubes. Blood volume was kept unchanged via simultaneous infusion of the same volume of donor blood into the femoral vein. The samples were immediately placed on ice and centrifuged at 5,000 g for 10 min at 4°C. Plasma was collected and stored at −70°C. Plasma vasopressin concentration was assessed via standard radioimmunoassay procedures in our laboratory as described previously (13, 24, 27). The sensitivity of the vasopressin assay was 0.1 pg/ml and 50% displacement was 4.1 pg/tube. Intra- and interassay variability was 7.0% and 13.4%, respectively.
Data Analysis
Hemodynamic responses were analyzed over a 20-min period following the microinjections, similar to a previous study from our laboratory (21). The responses were quantified as an integration of the differences between the baseline and response values averaged in 1-min periods and summed for 20 min of the response, i.e., when the majority of the responses occur. The integral reflects the predominant trend of the responses despite transient, sometimes large, bidirectional fluctuations in each variable. Because hemodynamic effects evoked by stimulation of NTS A1 adenosine receptors were variable, often biphasic, or even polyphasic, as we previously reported (21, 35), we used the integral values for the comparison between the experimental groups. The absolute values of blood flow depend to some extent on positioning of the probe around the iliac artery; therefore, the comparison between the relative changes in MAP, iliac blood flow (IBF), and IVC was more reliable. The HR responses, calculated from pulse intervals through the flow probe, were expressed in absolute values (beats/min). IVC was calculated by dividing IBF, expressed as a Doppler shift (in Hz), by MAP (in mmHg). In experimental protocols 2-5, where PE was infused to compensate for the decreased MAP and increased IVC, the direct effect of PE on baseline hemodynamic variables was evaluated in respective time control experiments and subtracted from experimental data. Specifically, changes occurring in each variable during time-control experiments were integrated for 20 min and subtracted from the respective 20-min integral values obtained in each animal of the experimental groups (protocols 2–5).
One-way ANOVA for independent measures was used to compare hemodynamic responses versus experimental conditions. Differences observed were further evaluated by t-test with Bonferroni adjustment for independent measures. Differences between circulating vasopressin levels measured before and after microinjections of ACF or CPA were evaluated using paired t-test; the differences in vasopressin levels between the groups (ACF vs. CPA) were evaluated using unpaired t-test. The changes in all recorded variables were also compared with zero by means of SYSTAT univariate F test. An α-level of P < 0.05 was used to determine statistical significance.
RESULTS
Resting values of MAP, HR, IBF, and IVC for each experimental group, measured just before stimulation of NTS A1 adenosine receptors, are presented in Table 3. The resting MAP and IVC for all the groups where blockades were performed were not different from those for intact animals; this provided reliable comparison between the experimental protocols. The direct effects of ganglionic blockade, and blockade of V1 vasopressin and AT1 angiotensin II receptors on all hemodynamic variables, are presented in Table 1. The large decreases in MAP and increases in IVC evoked by blockade of AT1 angiotensin II receptors and/or ganglionic blockade required additional compensation with PE, whereas the small decreases in MAP and increases in IVC evoked by V1 vasopressin receptor blockade were allowed to partially recover without compensation.
Table 3.
Resting values of hemodynamic parameters in each experimental group
| Protocol Number | Experimental Procedure | n | Mean Arterial Pressure, mmHg | Heart Rate, beats/min | Iliac Blood Flow, Hz | Iliac Vascular Conductance, Hz/mmHg |
|---|---|---|---|---|---|---|
| Intact | 13 | 98.1±.1 | 353.9±4.7 | 1030.2±3.6 | 10.9±3.3 | |
| 1 | VX | 8 | 105.3±4.9 | 359.7±11.2 | 1091.0±204.2 | 10.6±2.0 |
| 2 | ATX | 10 | 95.3±4.9 | 342.2±8.2 | 992.8±122.5 | 10.8±1.5 |
| 3 | ADX + GX | 8 | 93.1±5.1 | 383.6±11.4 | 1190.4±167.2 | 13.2±2.1 |
| 4 | ADX + GX + VX | 8 | 88.7±1.3 | 383.3±9.0 | 1394.0±222.7 | 15.8±2.7 |
| 5 | ADX + GX + VX + ATX | 8 | 88.1±2.1 | 405.0±10.9* | 1271.0±102.9 | 14.4±1.1 |
| 6 | LX + VX | 8 | 95.8±4.1 | 340.6±7.0 | 1336.4±132.8* | 14.3±1.7 |
| (2) | Control (ATX) | 5 | 99.3±2.8 | 330.0±11.8 | 973.7±173.2 | 10.0±2.0 |
| (3) | Control (ADX + GX) | 5 | 84.2±2.8 | 365.8±17.2 | 1073.1±144.7 | 12.8±1.8 |
| (4) | Control (ADX + GX + VX) | 5 | 88.1±3.5 | 381.6±7.8 | 1121.6±141.1 | 12.7±1.4 |
| (5) | Control (ADX + GX + VX + ATX) | 5 | 90.2±4.7 | 398.9±13.3 | 1144.6±302.0 | 12.2±2.4 |
Values are means ± SE; n = number of rats. Numbers in parentheses show time controls for respective protocols. Abbreviations are as in Fig. 1.
P < 0.05 vs. Intact.
Effects of V1, AT1, and Ganglionic Blockades on Responses to Stimulation of NTS A1 Adenosine Receptors
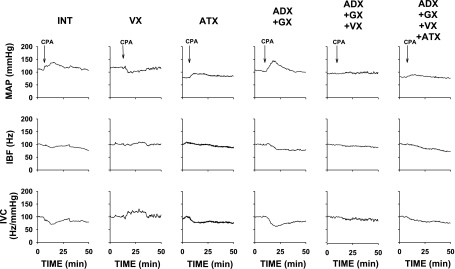
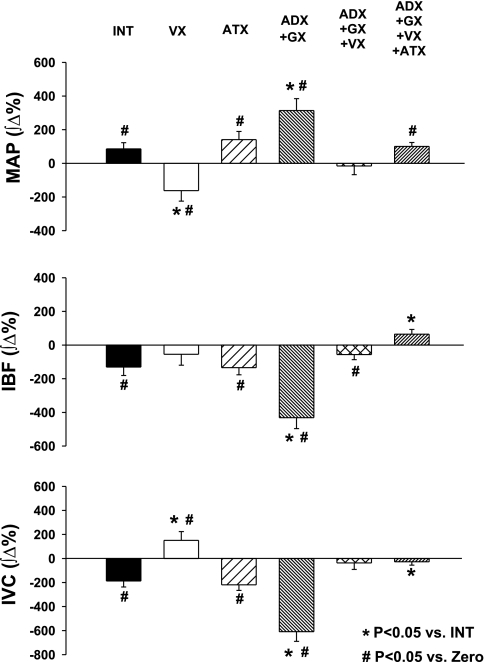
Figure 4 presents examples of the responses evoked by selective stimulation of NTS A1 adenosine receptors, which were observed most often under each experimental condition. The average integral responses for each experimental group are presented in Fig. 5. In intact animals the typical variability in the responses to stimulation of NTS A1 adenosine receptors was observed: the pressor and vasoconstrictor responses prevailed (Table 4 and Figs. 4 and 5), although biphasic, polyphasic, or, more rarely, depressor and vasodilatory responses were also observed. As we previously demonstrated, these patterns and variability of the responses reflected counteracting effects of β-adrenergic vasodilation versus sympathetic and humoral vasoconstriction (21, 35). V1 vasopressin receptor blockade reversed iliac vasoconstrictor responses observed in the intact group into slight iliac vasodilation (P = 0.0001 vs. intact). Blockade of angiotensin II AT1 receptors alone did not significantly alter the responses compared with the intact group (P > 0.05 for all variables). Elimination of adrenal and sympathetic neural effects on the iliac vasculature (protocol 3) increased the iliac vasoconstrictor responses almost fourfold compared with the intact group, indicating that other humoral factor(s) different than circulating norepinephrine play a crucial role in the iliac vasoconstrictor responses. Subsequent blockade of vasopressin V1 receptors (protocol 4) virtually abolished the exaggerated iliac vasoconstriction observed following adrenalectomy plus ganglionic blockade alone (protocol 3; Figs. 4 and 5), indicating that the humoral iliac vasoconstriction evoked by stimulation of NTS A1 adenosine receptors is mediated mostly via the release of vasopressin. Combined blockade of neural and all considered humoral factors (adrenalectomy + ganglionic + V1 vasopressinergic + AT1 angiotensinergic blockades; protocol 5) had very similar effects on the responses to that observed in protocol 4 (adrenalectomy + ganglionic + V1 vasopressinergic blockades); there were no significant differences between these two groups with respect to MAP, HR, and IVC responses (P > 0.05 for all comparisons). The lack of differences between responses observed in protocols 4 and 5 additionally confirmed that vasopressin is the dominant humoral vasoconstrictor factor triggered by stimulation of NTS A1 adenosine receptors. The analysis of absolute values of the integral responses presented in Table 5 leads to the same conclusions as those based on the relative responses (Fig. 5). The absolute values of the responses, presented in Table 5, show residual iliac vasoconstrictor effects in protocols 4 and 5. However, this vasoconstriction was abolished when the respective time control values were subtracted from the direct experimental values presented in Table 5.
Fig. 4.
MAP, iliac blood flow (IBF), and IVC responses to microinjection of adenosine A1 receptor agonist (CPA; 330 pmol/50 nl) into the subpostremal NTS in intact rats (INT) and each experimental group. Abbreviations are as in Fig. 1. Microinjections of CPA, marked by vertical arrows, were applied ∼10–20 min after the blockades, when baseline levels of all variables stabilized (see Fig. 2). Note that VX reversed pressor and vasoconstrictor responses most often observed in Int group into depressor and vasodilatory responses. ADX + GX exaggerated the pressor and vasoconstrictor response. These exaggerated vasoconstrictor responses were virtually abolished following ADX + GX + VX and ADX + GX + VX + ATX.
Fig. 5.
Integral responses of MAP, IBF, and IVC evoked by microinjections of CPA (330 pmol/50 nl) into the caudal subpostremal NTS. Abbreviations are as in Fig. 1. Data are means ± SE. In groups ATX, ADX + GX, ADX + GX + VX, and ADX + GX + VX + ATX, respective time control values were subtracted. *Different vs. intact group (P < 0.05); #different vs. zero (P < 0.05). VX reversed iliac vasoconstrictor responses observed in intact group into vasodilation and virtually abolished exaggerated vasoconstrictor responses observed following ADX + GX.
Table 4.
Number of individual experiments where overall increments or decrements were observed for each recorded hemodynamic parameter based on its integral values
| Protocol Number | Experimental Procedure | n | Mean Arterial Pressure, mmHg |
Heart Rate, beats/min |
Iliac Blood Flow, Hz |
Iliac Vascular Conductance, Hz/mmHg |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incr | Decr | Incr | Decr | Incr | Decr | Incr | Decr | |||
| Intact | 13 | 10 | 3 | 1 | 12 | 1 | 12 | 1 | 12 | |
| 1 | VX | 8 | 0 | 8 | 0 | 8 | 3 | 5 | 7 | 1 |
| 2 | ATX | 10 | 9 | 1 | 4 | 6 | 2 | 8 | 0 | 10 |
| 3 | ADX + GX | 8 | 8 | 0 | 1 | 7 | 0 | 8 | 0 | 8 |
| 4 | ADX + GX + VX | 8 | 5 | 3 | 2 | 6 | 0 | 8 | 1 | 7 |
| 5 | ADX + GX + VX + ATX | 8 | 7 | 1 | 2 | 6 | 1 | 7 | 0 | 8 |
| 6 | LX + VX | 8 | 1 | 7 | 1 | 7 | 5 | 3 | 8 | 0 |
Number of increments (Incr) and decrements (Decr) observed in each experimental group. Abbreviations are as in Fig. 1.
Table 5.
Absolute values of integral changes in mean arterial pressure, heart rate, illiac blood flow, and illiac vascular conductance evoked by microinjections of 330 pmol in 50 nl N6-cyclopentyladenosine into the nucleus tractus solitarii in each experimental group
| Protocol Number | Experimental Procedure | n | Mean Arterial Pressure, mmHg | Heart Rate, beats/min | Iliac Blood Flow, Hz | Iliac Vascular Conductance, Hz/mmHg |
|---|---|---|---|---|---|---|
| Intact | 13 | 86.0±37.7† | −379.3±96.4† | −1480.3±588.7† | −23.1±7.1† | |
| 1 | VX | 8 | −195.2±63.9*† | −411.9±99.3† | 59.6±784.5 | 20.8±9.3*† |
| 2 | ATX | 10 | 198.4±47.3† | −70.6±126.0 | −1585.8±445.4† | −34.8±6.4† |
| 3 | ADX + GX | 8 | 312.3±67.1*† | −139.0±48.6† | −5758.2±1284.4*† | −91.6±20.2*† |
| 4 | ADX + GX + VX | 8 | 7.1±46.3 | −237.0±137.5 | −2752.3±715.7† | −31.3±11.5† |
| 5 | ADX + GX + VX + ATX | 8 | 74.9±19.0† | −249.8±134.8 | −1784.9±447.1† | −30.9±5.5† |
| 6 | LX + VX | 8 | −162.0±71.5*† | −502.0±177.3† | 2127.6±1489.5* | 57.5±11.4*† |
| (2) | Control (ATX) | 5 | 80.0±37.6 | 504.1±91.8*† | −303.2±573.6 | −9.8±3.6† |
| (3) | Control (ADX + GX) | 5 | 22.3±36.2 | 54.5±104.8* | −166.1±364.4 | −5.9±3.8 |
| (4) | Control (ADX + GX + VX) | 5 | 15.6±39.7 | 163.3±54.5*† | −1224.1±380.7† | −13.8±5.1† |
| (5) | Control (ADX + GX + VX + ATX) | 5 | −11.4±23.7 | 206.5±61.6*† | −2694.9±1428.2 | −24.7±13.0 |
Values are means ± SE. Numbers in parentheses show time controls for respective protocols. Abbreviations are as in Fig. 1.
P < 0.05 vs. intact;
P < 0.05 vs. zero.
Data collected in protocols 1–5 (Figs. 4 and 5) strongly suggested that the only significant humoral vasoconstrictor contributing to iliac vascular responses evoked by selective stimulation of NTS A1 adenosine receptors is vasopressin. Therefore, in protocol 6 vasopressin and lumbar sympathetic vasoconstrictor components of the responses were eliminated to unmask β-adrenergic vasodilation alone, not opposed by neural and humoral vasoconstriction. Following V1 vasopressin receptor blockade combined with bilateral lumbar sympathectomy (protocol 6), the iliac vasodilation response doubled compared with that observed following V1 vasopressin receptor blockade alone (405.1 ± 58.4 ∫Δ% vs. 195.8 ± 51.2 ∫Δ%; P = 0.0175).
The above observations are supported by comparison of the frequency of which we observed increases versus decreases in hemodynamic variables in each experiment for all experimental groups (Table 4). In intact animals, decreases in IVC prevailed, whereas following V1 vasopressinergic blockade the increases in IVC prevailed. Combined V1 vasopressinergic blockade and lumbar sympathectomy completely eliminated iliac vasoconstrictor responses (protocol 6). AT1 angiotensinergic blockade increased the number of pressor and vasoconstrictor events compared with the intact group (Table 4), although there were no significant differences between the averaged responses observed in this versus intact groups (Fig. 5). Adrenalectomy plus ganglionic blockade completely eliminated the depressor and iliac vasodilatory responses as expected (protocol 3). When V1 vasopressinergic blockade was added to adrenalectomy and ganglionic blockade (protocol 4) or all the blockades were performed in adrenalectomized animals (protocol 5), again variable vasoconstrictor/vasodilatory responses were observed; however, this variability reflected rather random variations of IVC since vascular responses were virtually abolished in these groups (changes in IVC were not different from zero; Fig. 5). Prevailing pressor and depressor responses were consistent with prevailing vasoconstrictor and vasodilatory responses in each experimental group. However, the decreases in HR dominated in all experimental groups despite the different experimental conditions.
Release of Vasopressin in Response to Stimulation of NTS A1 Adenosine Receptors
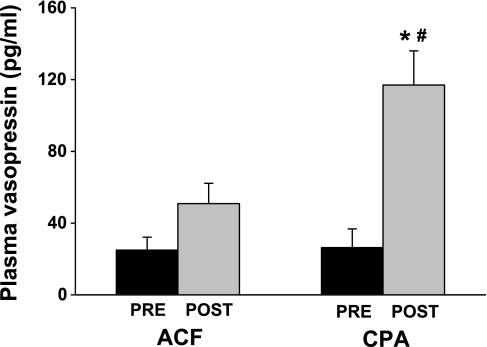
The above results, obtained via pharmacological blockade approaches, strongly suggested that vasopressin is a dominant vasoconstrictor factor released into the circulation following the selective stimulation of NTS A1 adenosine receptors. Therefore, in an additional two groups of animals, we tested this hypothesis more directly by measuring whether the plasma levels of vasopressin indeed increase as a result of stimulation of NTS A1 adenosine receptors (Fig. 6). The resting levels of vasopressin were similar in both groups (P = 0.931), and they were moderately elevated compared with vasopressin levels normally observed in intact conscious animals (7, 13, 15, 23, 24), consistent with levels associated with anesthesia and surgical stress (7, 15). Stimulation of NTS A1 adenosine receptors increased circulating vasopressin levels over fourfold (P = 0.0006). Microinjection of ACF into the NTS also tended to increase the level of circulating vasopressin; however, the increase did not reach statistical significance (P = 0.083). Notably, the difference between vasopressin levels measured across the groups, i.e., following microinjections of CPA versus ACF, was also significant (P = 0.041).
Fig. 6.
Comparison of plasma vasopressin levels measured before (pre) and after (post) microinjections into the NTS of vehicle (ACF; n = 5) or selective A1 adenosine receptor agonist (CPA; n = 8). *Differences between pre- vs. postmicroinjections (P < 0.05); #differences between the groups (P < 0.05). CPA increased circulating vasopressin levels over 4-fold, whereas ACF did not significantly increase the level of circulating vasopressin.
DISCUSSION
The present study assessed the relative roles of three major humoral vasoconstrictor factors in mediating the responses to stimulation of A1 adenosine receptors located in the NTS. The release of humoral vasoconstrictor factor(s) had been implied by a previous study from our laboratory, which showed that the large iliac vasoconstriction, triggered by stimulation of NTS A1 adenosine receptors, persisted after bilateral lumbar sympathectomy and adrenalectomy (21). The major finding of the present study is that vasopressin is released into the circulation upon stimulation of NTS A1 adenosine receptors and that it is the primary humoral factor contributing to the iliac vasoconstriction. The potential release of norepinephrine and angiotensin II did not contribute significantly to the iliac vascular responses.
NTS A1 Adenosine Receptors Are Involved in Control of Vasopressin Release
Our previous studies strongly suggested that A1 adenosine receptors may modulate vasopressin release at the level of the NTS similarly as they modulate baroreflex control of regional sympathetic outputs and HR (30, 35). However, with the consideration of differential localization/expression of adenosine receptor subtypes on functionally different NTS neurons (31, 36), it remained unknown whether A1 adenosine receptors are indeed located on these baroreflex neurons controlling vasopressin release. The present study showed that activation of A1 adenosine receptors in the NTS disinhibits vasopressin release. Therefore, pre- and/or postsynaptic A1 adenosine receptors are likely located on those NTS baroreflex neurons/terminals, which control vasopressin release. This hypothesis based on integrative, systemic data may be confirmed in future studies at the cellular level.
The most likely mechanism responsible for the release of vasopressin into the circulation in response to stimulation of NTS A1 adenosine receptors is the inhibition of baroreflex transmission in the NTS resulting in the disinhibition of vasopressin release from hypothalamic nuclei (paraventricular and supraoptic) (10). The role of NTS in baroreflex control of vasopressin release has been well established (29, 39). Bilateral inhibition, anesthetization, or lesioning of the NTS, which removes baroreflex mechanisms, results in 10-fold increases of circulating vasopressin levels and vasopressin-mediated hypertension (39). Sinoaortic baroreceptor denervation prevents this response (29). In the present study the unilateral inhibition of NTS baroreflex mechanisms via unilateral activation of A1 adenosine receptors resulted in over fourfold increases in circulating vasopressin.
Relative Roles of Vasoactive Factors Triggered by Activation of A1 Adenosine Receptors in the NTS
A previous study from our laboratory showed that activation of A1 adenosine receptors in the NTS evokes neural and humoral iliac vasoconstriction opposed by β-adrenergic vasodilation (21). The counteracting vascular effects contributed to the variability of the overall pressor/depressor responses with prevailing vasoconstriction and increases in MAP. These conclusions were based on comparison of iliac vascular responses observed in intact animals and following β-adrenergic blockade, bilateral adrenalectomy, lumbar sympathectomy, and combined adrenalectomy plus lumbar sympathectomy. The present study investigated potential humoral vasoconstrictors (vasopressin, angiotensin II, and norepinephrine) by assessing the effects of vasopressin V1 receptor and angiotensin AT1 receptor blockade as well as ganglionic blockade performed in adrenalectomized animals. Perhaps the most compelling results were that the blockade of peripheral V1 vasopressin receptors reversed the normal iliac vasoconstriction into marked vasodilation. This vasodilation was further enhanced by adding lumbar sympathectomy to the V1 receptor blockade. It is likely that sympathetic nerves have a smaller role than vasopressin in mediating this iliac vasoconstriction since lumbar sympathectomy alone, performed in a previous study from our laboratory (21), had a relatively smaller effect on the IVC responses then V1 vasopressinergic blockade alone, performed in the present study. Collectively, these data indicate that vasopressin alone can override epinephrine-induced iliac vasodilation and induce vasoconstriction, but that sympathetic nerves alone do not. The roles of vasopressin and sympathetic nerves appear additive since the sum of the individual effects approximate that of the combined effects. Vasopressin together with increases in LSNA can induce significant vasoconstriction without which a powerful β-adrenergic vasodilation is revealed. We conclude that the variability often seen in the arterial pressure response to NTS A1 adenosine receptor stimulation is the direct result of these push-pull counteracting mechanisms.
Circulating norepinephrine potentially released upon stimulation of NTS A1 adenosine receptors likely has little role since ganglionic blockade did not lessen the intense iliac vasoconstriction seen after bilateral adrenalectomy (21). Probably the amount of norepinephrine released from sympathetic terminals was too small to exert measureable effects beyond that of circulating norepinephrine already likely to be elevated as a result of anesthesia and surgical stress. A previous study from our laboratory showed that pre-ASNA increased much more than RSNA and LSNA in response to stimulation of NTS A1 adenosine receptors (35). It is possible that other sympathetic outputs responded even less or might even be inhibited with the stimulation. This could cause the increase of circulating norepinephrine in the present study to be functionally irrelevant.
Angiotensin II blockade alone markedly decreased baseline MAP and increased IVC. However, the normal pressor and vasoconstrictor responses to microinjections of CPA did not decrease but rather tended to increase. This indicates that angiotensin II, similarly as circulating norepinephrine, does not contribute to the iliac vasoconstriction elicited by stimulation of NTS A1 adenosine receptors. Although increases of RSNA may cause an increase in the release of renin from the kidney (11), the total time from activation of the renal nerve to the subsequent release of renin and conversion of angiotensinogen to angiotensin I and then to angiotensin II may have exceeded the time of the analyzed responses. In addition, losartan used to block AT1 receptors in this study most likely crossed the blood-brain barrier and might evoke central effects counteracting the peripheral actions. Nevertheless, our data show that circulating angiotensin II had no functional effect as a potential humoral iliac vasoconstrictor triggered by stimulation of NTS A1 adenosine receptors.
Vasoactive Effects of NTS A1 versus A2a Adenosine Receptor Subtypes
The predominately pressor but often variable responses to selective stimulation of NTS A1 adenosine receptors are mediated most likely via inhibition of baroreflex glutamatergic transmission in the NTS and resetting of baroreflex functions toward higher MAP (30, 35). In contrast, selective stimulation of NTS A2a adenosine receptors evokes decreases in MAP and preferential iliac vasodilation mediated via nonbaroreflex, nonglutamatergic mechanism(s) (16, 33, 34). These responses were mediated mostly via activation of the adrenal medulla and β-adrenergic mechanism and to a lesser extent via lumbar sympathetic nerves (19). However, no other humoral mechanisms were involved since bilateral adrenalectomy and lumbar sympathectomy abolished the responses (19). How do these two adenosine receptor subtypes contribute to the responses mediated by adenosine operating in the NTS under physiological (9, 38) or pathological (25, 37, 43, 45) conditions? It is well known that microinjections of adenosine into the NTS result in depressor responses similar to those observed following selective activation of A2a adenosine receptors (3, 22, 40). However, both adenosine receptor subtypes may contribute to the depressor responses since they both activate the adrenal medulla and facilitate β-adrenergic vasodilation via baroreflex and unknown, nonbaroreflex mechanisms (33, 35). Other components of the responses elicited by NTS A2a receptors (decreases in RSNA and HR) may further contribute to the depressor responses, whereas A1 receptor-mediated sympathoactivation and vasopressin release would oppose these responses. Under physiological conditions adenosine may contribute to the pressor effects evoked by stimulation of the hypothalamic defense area (9, 38) via A1 receptor-mediated inhibition of baroreflex mechanisms and resulting sympathoactivation and vasopressin release (30, 35). In contrast, under pathological conditions naturally released adenosine, acting via both A1 and A2a adenosine receptor subtypes, may contribute to the paradoxical sympathoinhibition and cardiac slowing observed during hypovolemic phase of hemorrhagic shock (37).
Limitations of the Method
In the central nervous system adenosine is not released synaptically but rather produced in the extracellular space via the degradation of ATP, which is released at synaptic terminals from neurons as well as released from glial cells activated by extracellular glutamate diffused from nearby active nerve terminals (6, 8, 14, 46). In addition, under pathological conditions adenosine is released into the extracellular space in a global, nonsynaptic manner from hypoxic/hypoperfused neurons and glial cells (25, 43, 45). Therefore, the natural, spatial (not strictly synaptic) action of adenosine in the NTS may be simulated well via microinjections of selective agonists of adenosine receptor subtypes. Importantly, although adenosine is released nonselectively in the NTS, it does produce specific differential responses in regional sympathetic outputs and vascular beds as shown by numerous studies from our laboratory (4, 30, 32–35). We believe that these specific, differential effects evoked by nonspecific, spatial activation of adenosine receptors result from differential location/expression of adenosine receptor subtypes on NTS neurons and synaptic terminals controlling different sympathetic outputs and involved in different mechanisms integrated in the NTS (31, 36). To confirm this hypothesis, based on systemic, integrative approaches, further studies at the cellular level are required.
The experiments were performed in anesthetized animals, and these conditions most likely attenuated baroreflex mechanisms, which may have contributed to the increased vasopressin levels. In the conscious rat circulating the vasopressin level is ∼2 pg/ml (7, 13, 15, 23, 24, 27). With even limited surgery under anesthesia, baseline vasopressin rises to ∼6–10 pg/ml (7, 15, 29, 39). In our study after extensive surgery under anesthesia, baseline vasopressin levels were ∼25 pg/ml. However, despite the increased baseline vasopressin levels, activation of NTS A1 adenosine receptors triggered marked release of vasopressin into the circulation (Fig. 6), and a powerful V1 receptor-mediated vasoconstrictor effect in the hindlimb vasculature was apparent (Fig. 5). In contrast, no functional effects of potential, A1 adenosine receptor-mediated increases in circulating norepinephrine or angiotensin II were observed.
Glucocorticoid deficiency, which may be observed following chronic adrenalectomy, increases circulating levels of vasopressin (18). However, this effect seems to be mediated at the level of transcription of the vasopressin gene and not an immediate release (18). Therefore, in the animals in which the adrenal glands were removed acutely, as in the present study, increased resting levels of vasopressin were most likely due to anesthesia and surgical stress.
The large and sustained changes in resting hemodynamic variables following AT1 angiotensinergic and ganglionic blockades required compensation with intravenous infusions of phenylephrine to make the relative responses comparable across the experimental groups. It was extremely difficult to return both MAP and IVC to the preblockade levels. Since we tried to compensate most accurately for IVC, the resting MAP, preceding the microinjections into the NTS in the groups where ganglionic blockade was combined with V1 vasopressinergic and with AT1 angiotensinergic blockades (protocols 4 and 5), was slightly lower compared with other experimental groups. The long lasting compensatory infusions of phenylephrine in protocols 2–5 could facilitate central baroreflex mechanisms (17). Therefore, the inhibition of enhanced baroreflex activity via stimulation of NTS A1 adenosine receptors could result in relatively greater pressor and vasoconstrictor responses compared with those observed in intact animals and following V1 vasopressinergic blockade alone (protocol 1) and V1 vasopressinergic blockade combined with lumbar sympathectomy (protocol 6). In fact, pressor and vasoconstrictor responses tended to increase following AT1 receptor blockade although this tendency did not reach statistical significance. The pressor and vasoconstrictor responses observed after adrenalectomy combined with ganglionic blockade (protocol 3) were not different from those observed following adrenalectomy alone in a previous study from our laboratory (21). Finally, these responses (even if slightly exaggerated) were virtually abolished by the vasopressinergic blockade (protocols 4 and 5). Therefore, the potential central effects of infusions of phenylephrine on responses to stimulation of NTS A1 adenosine receptors were likely negligible and did not affect the primary conclusions of the present study. The infused phenylephrine activated α1-adrenergic receptors located on vascular smooth muscles, including those in the iliac vascular bed. The occupation of α1-adrenergic receptors by an exogenous agonist could mask the effect of circulating endogenous norepinephrine, which may be potentially increased following stimulation of NTS A1 adenosine receptors. The peripheral interactions between simultaneously activated V1, α1-, and AT1 receptors could further complicate the comparison of relative roles of humoral vasoconstrictor factors, obscuring the smaller ones. Nevertheless, the marked effects of vasopressin V1 receptor blockade were evident in experimental groups with and without phenylephrine compensation (protocols 1 and 6 vs. protocols 4 and 5). This indicates that vasopressin contribution to the iliac vasoconstriction was much larger than the contribution (if any) of other potential vasoconstrictors (norepinephrine and/or angiotensin II).
Conclusion
Selective stimulation of NTS A1 adenosine receptors triggers the release of vasopressin into the circulation, strongly suggesting that A1 adenosine receptors are likely located on afferent terminals and/or NTS interneurons mediating baroreflex control of vasopressin release. Vasopressin is a major humoral vasoconstrictor factor contributing to the iliac vascular responses. The natural variability of MAP and IVC responses to stimulation of NTS A1 adenosine receptors observed in intact animals is a result of the simultaneous triggering of sympathetic and vasopressinergic vasoconstriction counteracted by β-adrenergic vasodilation resulting mainly from epinephrine release in response to the large increase in adrenal preganglionic sympathetic activity.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-67814 (to D. S. O'Leary and T. J. Scislo) and a Merit Review Award by Department of Veterans Affairs (to N. F. Rossi and T. J. Scislo).
ACKNOWLEDGMENTS
We thank Andrew Baumgartner for technical assistance. We acknowledge the generous gift of losartan by Merck.
REFERENCES
- 1.Barraco R, el Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull 29: 703–765, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Barraco RA, el Ridi MR, Ergene E, Phillis JW. Adenosine receptor subtypes in the brainstem mediate distinct cardiovascular response patterns. Brain Res Bull 26: 59–84, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Barraco RA, Janusz CJ, Polasek PM, Parizon M, Roberts PA. Cardiovascular effects of microinjection of adenosine into the nucleus tractus solitarius. Brain Res Bull 20: 129–132, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Barraco RA, O'Leary DS, Ergene E, Scislo TJ. Activation of purinergic receptor subtypes in the nucleus tractus solitarius elicits specific regional vascular response patterns. J Auton Nerv Syst 59: 113–124, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Barraco RA, Phillis JW. Subtypes of adenosine receptors in the brainstem mediate opposite blood pressure responses. Neuropharmacology 30: 403–407, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Bennett MR, Buljan V, Farnell L, Gibson WG. Purinergic junctional transmission and propagation of calcium waves in cultured spinal cord microglial networks. Purinergic Signal 4: 47–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonjour JP, Malvin RL. Plasma concentrations of ADH in conscious and anesthetized dogs. Am J Physiol 218: 1128–1132, 1970 [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dale N, Gourine AV, Llaudet E, Bulmer D, Thomas T, Spyer KM. Rapid adenosine release in the nucleus tractus solitarii during defence response in rats: real-time measurement in vivo. J Physiol 544: 149–160, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 11.DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol 279: R1517–R1524, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol 280: H2891–H2901, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86: 1009–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Howard RL, Summer S, Rossi N, Kim JK, Schrier RW. Short-term hypothyroidism and vasopressin gene expression in the rat. Am J Kidney Dis 19: 573–577, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Ichinose TK, O'Leary DS, Scislo TJ. Activation of NTS A2a adenosine receptors differentially resets baroreflex control of renal vs. adrenal sympathetic nerve activity. Am J Physiol Heart Circ Physiol 296: H1058–H1068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imaizumi T, Brunk SD, Gupta BN, Thames MD. Central effect of intravenous phenylephrine on baroreflex control of renal nerves. Hypertension 6: 906–914, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Summer SN, Wood WM, Schrier RW. Role of glucocorticoid hormones in arginine vasopressin gene regulation. Biochem Biophys Res Commun 289: 1252–1256, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kitchen AM, Scislo TJ, O'Leary DS. NTS A2a purinoceptor activation elicits hindlimb vasodilation primarily via a β-adrenergic mechanism. Am J Physiol Heart Circ Physiol 278: H1775–H1782, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol 48: 21–53, 1996 [DOI] [PubMed] [Google Scholar]
- 21.McClure JM, O'Leary DS, Scislo TJ. Stimulation of NTS A1 adenosine receptors evokes counteracting effects on hindlimb vasculature. Am J Physiol Heart Circ Physiol 289: H2536–H2542, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Mosqueda-Garcia R, Tseng CJ, Appalsamy M, Beck C, Robertson D. Cardiovascular excitatory effects of adenosine in the nucleus of the solitary tract. Hypertension 18: 494–502, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Mueller PJ, Sullivan MJ, Grindstaff RR, Cunningham JT, Hasser EM. Regulation of plasma vasopressin and renin activity in conscious hindlimb-unloaded rats. Am J Physiol Regul Integr Comp Physiol 291: R46–R52, 2006 [DOI] [PubMed] [Google Scholar]
- 24.O'Leary DS, Rossi NF, Churchill PC. Muscle metaboreflex control of vasopressin and renin release. Am J Physiol Heart Circ Physiol 264: H1422–H1427, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Phillis JW, Walter GA, O'Regan MH, Stair RE. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. J Cereb Blood Flow Metab 7: 679–686, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 27.Rossi NF, Schrier RW. Anti-calmodulin agents affect osmotic and angiotensin II-induced vasopressin release. Am J Physiol Endocrinol Metab 256: E516–E523, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205: 260–272, 1982 [DOI] [PubMed] [Google Scholar]
- 29.Schreihofer AM, Sved AF. Nucleus tractus solitarius and control of blood pressure in chronic sinoaortic denervated rats. Am J Physiol Regul Integr Comp Physiol 263: R258–R266, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Scislo TJ, Ichinose TK, O'Leary DS. Stimulation of NTS A1 adenosine receptors differentially resets baroreflex control of regional sympathetic outputs. Am J Physiol Heart Circ Physiol 294: H172–H182, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Scislo TJ, Kitchen AM, Augustyniak RA, O'Leary DS. Differential patterns of sympathetic responses to selective stimulation of nucleus tractus solitarius purinergic receptor subtypes. Clin Exp Pharmacol Physiol 28: 120–124, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Scislo TJ, O'Leary DS. Activation of A2a adenosine receptors in the nucleus tractus solitarius inhibits renal but not lumbar sympathetic nerve activity. J Auton Nerv Syst 68: 145–152, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Scislo TJ, O'Leary DS. Differential control of renal vs. adrenal sympathetic nerve activity by NTS A2a and P2x purinoceptors. Am J Physiol Heart Circ Physiol 275: H2130–H2139, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Scislo TJ, O'Leary DS. Differential role of ionotropic glutamatergic mechanisms in responses to NTS P2x and A2a receptor stimulation. Am J Physiol Heart Circ Physiol 278: H2057–H2068, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Scislo TJ, O'Leary DS. Mechanisms mediating regional sympathoactivatory responses to stimulation of NTS A1 adenosine receptors. Am J Physiol Heart Circ Physiol 283: H1588–H1599, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Scislo TJ, O'Leary DS. Purinergic mechanisms of the nucleus of the solitary tract and neural cardiovascular control. Neurol Res 27: 182–194, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Scislo TJ, O'Leary DS. Adenosine receptors located in the NTS contribute to renal sympathoinhibition during hypotensive phase of severe hemorrhage in anesthetized rats. Am J Physiol Heart Circ Physiol 291: H2453–H2461, 2006 [DOI] [PubMed] [Google Scholar]
- 38.St Lambert JH, Thomas T, Burnstock G, Spyer KM. A source of adenosine involved in cardiovascular responses to defense area stimulation. Am J Physiol Regul Integr Comp Physiol 272: R195–R200, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Sved AF, Imaizumi T, Talman WT, Reis DJ. Vasopressin contributes to hypertension caused by nucleus tractus solitarius lesions. Hypertension 7: 262–267, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Tao S, Abdel-Rahman A. Neuronal and cardiovascular responses to adenosine microinjection into the nucleus tractus solitarius. Brain Res Bull 32: 407–417, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Thomas T, Spyer KM. A novel influence of adenosine on ongoing activity in rat rostral ventrolateral medulla. Neuroscience 88: 1213–1223, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol 376: 143–173, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Van Wylen DG, Park TS, Rubio R, Berne RM. Cerebral blood flow and interstitial fluid adenosine during hemorrhagic hypotension. Am J Physiol Heart Circ Physiol 255: H1211–H1218, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Vanhoutte B. Heterogeneity in vascular smooth muscle. In: Microcirculation, edited by Kaley G, Altura BM. Baltimore, MD: : University Park, 1978, p. 181– 310 [Google Scholar]
- 45.Yan S, Laferriere A, Zhang C, Moss IR. Microdialyzed adenosine in nucleus tractus solitarii and ventilatory response to hypoxia in piglets. J Appl Physiol 79: 405–410, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol 49: 589–618, 1996 [DOI] [PubMed] [Google Scholar]