Abstract
A number of type 1 receptor cytokine family members protect the heart from acute and chronic oxidative stress. This protection involves activation of two intracellular signaling cascades: the reperfusion injury salvage kinase (RISK) pathway, which entails activation of phosphatidylinositol 3-kinase (PI3-kinase) and ERK1/2, and JAK-STAT signaling, which involves activation of transcription factor signal transducer and activator of transcription 3 (STAT3). Obligatory for activation of both RISK and STAT3 by nearly all of these cytokines are the kinases JAK1 and JAK2. Yet surprisingly little is known about how JAK1 and JAK2 are regulated in the heart or how they couple to PI3-kinase activation. Although the JAKs are linked to antioxidative stress programs in the heart, we recently reported that these kinases are inhibited by oxidative stress in cardiac myocytes. In contrast, others have reported that cardiac JAK2 is activated by acute oxidative stress by an undefined process. Here we summarize recent insights into the regulation of JAK1 and JAK2. Besides oxidative stress, inhibitory regulation involves phosphorylation, nitration, and intramolecular restraints. Stimulatory regulation involves phosphorylation and adaptor proteins. The net effect of stress on JAK activity in the heart likely represents the sum of both inhibitory and stimulatory processes, along with their dynamic interaction. Thus the regulation of JAKs in the heart, once touted as the paragon of simplicity, is proving rather complicated indeed, requiring a second look. It is our contention that a better understanding of the regulation of this kinase family that is implicated in cardiac protection could translate into effective therapeutic strategies for preventing myocardial damage or repairing the injured heart.
Keywords: Janus kinase, oxidative stress, redox, cardiac remodeling, cytokine, cardiac myocyte
a number of type 1 receptor cytokine family members, namely the interleukin (IL)-6-type cytokines [IL-6, IL-11, leukemia inhibitory factor (LIF), cardiotrophin-1 (CT-1), and oncostatin M (OSM)] (40, 78, 58, 85), growth hormone (GH; Ref. 91) erythropoietin (Epo; Refs. 20, 134, 138), and granulocyte colony-stimulating factor (G-CSF; Refs. 26, 61, 62, 72, 159, 166), as well as insulin (3, 23, 54, 64, 99, 164, 186), have been shown to protect the heart from acute oxidative stress, viz., ischemia-reperfusion injury. LIF (121), GH (56), Epo (77), and insulin (141) have also been reported to protect cardiac myocytes from chronic oxidative stress. Notably, a number of clinical trials have been completed or are underway assessing the efficacy of Epo or G-CSF in treating myocardial infarction and heart failure (13, 87, 88, 93).
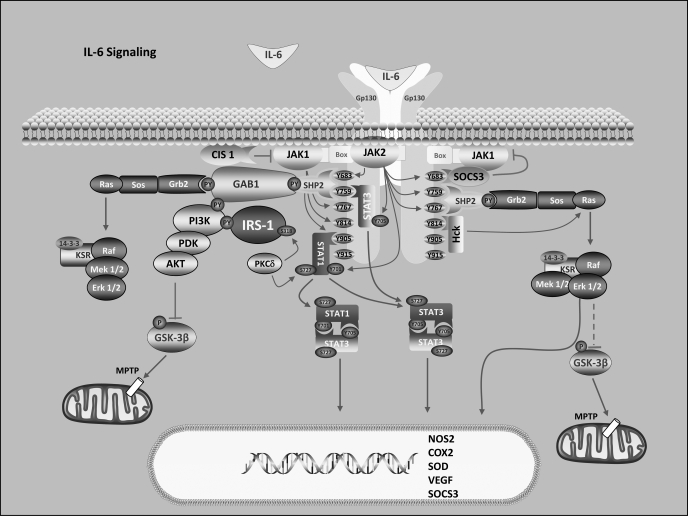
The protective action of the type 1 receptor cytokines (see Fig. 1 for IL-6 cytokine signaling) and insulin has been shown to involve activation of two intracellular signaling cascades: 1) the reperfusion injury salvage kinase (RISK) pathway (17, 27, 40, 41, 64, 66, 77, 99, 101, 102, 108, 121, 126, 128, 133, 134, 142, 156, 159, 160, 161, 166) and 2) JAK-STAT signaling (20, 40, 61, 85, 161, 174). The former, which involves the sequential activation of phosphatidylinositol 3-kinase (PI3-kinase) and AKT, as well as ERK1/2, has as a major target inhibition of the mitochondrial permeability transition pore (MPTP) and plays a dominant role in the mediator phase of both early (classical) preconditioning and postconditioning of the heart (29, 164). The JAK-STAT pathway for the most part involves activation of the transcription factor signal transducer and activator of transcription 3 (STAT3) and upregulation of cyclooxygenase-2 (COX-2) and nitric oxide synthase 2 (NOS2) (involved in MPTP inhibition), vascular endothelial growth factor (VEGF) (angiogenic and cardioprotective agent), the antioxidants manganese superoxide dismutase (MnSOD) and metallothioneins (MT1 and MT2), and matrix metalloproteases that are important in repair or scar formation (85). The JAK-STAT pathway has been shown to be essential to late (delayed) preconditioning of the heart, also referred to as the second window of protection (SWOP), which is initiated in vivo by the autocrine/paracrine production of IL-6 (15, 18, 24, 133, 175–178).
Fig. 1.
Overview of interleukin (IL)-6 cytokine signaling focusing on the basics of cardiac protective signaling. See text for details. Modified from ScienceSlides 2007 software version 2.0 (VisiScience, Chapel Hill, NC).
Obligatory for activation of both the RISK pathway and STAT3 by these cytokines (with the possible exception of G-CSF) (40, 85, 180), and presumably to some extent the former by insulin (144, 146, 169), are the Janus kinases JAK1 and JAK2. Yet surprisingly little is known about how JAK1 and JAK2 are regulated in the heart or how they couple to PI3-kinase activation (85). Although the JAKs are linked to antioxidative stress programs in the heart, we recently reported (86) that these kinases, JAK1 especially, are inhibited by oxidative stress in cardiac myocytes, consistent with evidence by others for a redox-switch in their JH1 catalytic domain (106). In contrast, others have reported that JAK2 is activated by acute oxidative stress in cardiac myocytes or the heart (24, 57, 63, 111, 181), although the mechanism for this activation is unknown. Here we present an update on the regulation of the JAKs with particular focus on the heart.
Activation of the JAKs
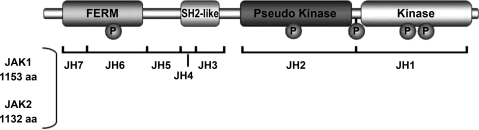
The JAKs are nonreceptor tyrosine kinases and consist of four mammalian members, one of which (JAK3) is strictly expressed in hematopoietic cells (85). For cardiac myocytes, JAK1 and JAK2 are predominant. The JAKs are characterized by seven highly conserved JAK homology (JH) domains (Fig. 2), which, starting at the COOH terminus, are the JH1 kinase domain; the JH2 pseudokinase domain, which appears to serve a complex regulatory function specific for each JAK family member; the JH3–JH4 region, which has some similarity to SH2 domains but does not have phosphotyrosine binding ability; and the JH4–JH7 region, which constitutes a divergent FERM domain (four-point-one, ezrin, radixin, moesin) and is involved in the interactions of JAKs with receptors.
Fig. 2.
Domain structure of JAK1 and JAK2. FERM, four-point-one, ezrin, radixin, moesin domain; JH, JAK homology; P, phosphorylation site. Adapted with permission from J Cardiovasc Pharmacol 50: 126–141, 2007. Copyright 2007 Lippincott Williams & Wilkins.
Of the JAKs, the molecular events underlying JAK2 activation have been best studied. Activation occurs upon dimerization of the JAKs and the transautophosphorylation of conserved tandem tyrosines (Tyr1007/Tyr1008) found in the activation loop of JAK2's catalytic domain (38). JAK2 dimerization and activation is an immediate consequence of cytokine-induced receptor dimerization (172). Without phosphorylation of Tyr1007/Tyr1008, JAK2 exists in a basal activity state characterized by inefficient ATP use and restricted substrate recognition (21). Upon phosphorylation of these tyrosines, JAK2 exists in a high-activity state in which its enzymatic efficiency with respect to ATP increases by at least 4 orders of magnitude and its substrate recognition spectrum broadens. In the inactive state, the JH2 domain is thought to physically interact with the JH1 domain of JAK2 to block its catalytic activity. Recent evidence indicates that for receptor-unbound JAK2, the FERM domain may fold back on the JH1 and JH2 domains to provide an additional physical constraint to activation (45). The JAK1 FERM domain is involved in autoinhibition as well (59).
Canonical JAK activation.
In the classical system, JAKs are activated upon ligand-induced dimerization of receptor subunits, which allows for the transautophosphorylation of the activation loop of juxtapositioned JAKs that are preassociated via their FERM domain with cytoplasmic membrane-proximal Box1/Box2 regions of these receptor subunits. This is the case for the hematopoietin receptor superfamily, which includes receptors for over two dozen different cytokines (80). Upon receptor stimulation, the associated JAK becomes a fully active protein tyrosine kinase and phosphorylates its tyrosines and nearby tyrosines, such as those on the cytoplasmic regions of the receptor, that serve as docking sites for various scaffold or signaling molecules including the STAT (signal transducers and activators of transcription) transcription factors.
Noncanonical JAK activation.
The JAKs can be activated in an atypical fashion, i.e., independently of receptor dimerization or even receptor association. This has been well documented for JAK2, but to the best of our knowledge not for JAK1. Examples of such atypical activation involve 1) G protein-coupled receptors (GPCRs), 2) oxidative stress, and 3) hypertonicity. Given the plethora of autocrine/paracrine factors that are released, and the reactive oxygen species (ROS) produced, both canonical and noncanonical JAK activation likely occur with cardiac ischemia-reperfusion and preconditioning.
g protein-coupled receptors.
Several extracellular stimuli that directly activate GPCR pathways have been reported to stimulate JAK2 and to require JAK2 activation in order to evoke a complete biological response. These GPCRs include angiotensin II type 1 (AT1) receptor (137), platelet-activating factor receptor (105), CXCR4 (SDF-1α receptor; Refs. 170, 187), CCK2 (cholecystokinin receptor; Ref. 39), CCR2B (MCP-1 receptor; Ref. 118), Mas [angiotensin-(1–7) receptor; Ref. 49], and—notably—receptors for two agonists that have been linked to ischemic preconditioning, viz., bradykinin B2 receptors (94, 183) and opioid receptors (55).
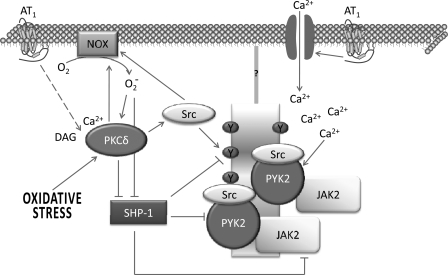
The exact mechanism by which GPCRs activate JAK2 remains poorly defined but has been best studied with the AT1 receptor. Evidence has shown that AT1-mediated JAK2 activation occurs before, and is not dependent on, association with AT1 (4, 50); nor does it involve EGF receptor transactivation (42). At least in vascular smooth muscle cells (VSMCs), AT1-mediated JAK2 activation involves PKCδ, preassociated PYK (a nonreceptor tyrosine kinase), and most likely a Src family member (42). Superoxide generation from NADPH oxidase is required (151), while conflicting results on the role of intracellular calcium have been reported (42, 150). In any event, the likely scenario for GPCR-mediated JAK2 activation involves the intermediacy of a nonreceptor tyrosine kinase, without a requirement for JAK2-receptor association (Fig. 3). This model likely applies to insulin-induced JAK2 activation also (146). Whether atypical JAK2 activation is part of the repertoire of IL-6-type cytokine signaling is unknown but is a possibility given that these cytokines also activate JAK2, although the JAK associated with their receptor subunits is JAK1 (85, 143). One feature that distinguishes GPCR-mediated, and likely noncanonical, JAK2 activation from cytokine-mediated activation is an absolute requirement for Tyr972 autophosphorylation, which is postulated to stabilize the active conformation of the protein (117).
Fig. 3.
Noncanonical JAK2 activation by G protein-coupled receptors (GPCRs) and oxidative stress. Evidence implicates PKCδ, the tyrosine kinase PYK2, and an NADPH oxidase (NOX). A likely scenario to explain their involvement in JAK2 activation is proposed in this figure. Redox-sensitive PKCδ can be activated via GPCR angiotensin II type 1 (AT1) receptor-induced phospholipase C activation or through oxidative stress. In turn, PKCδ can activate NOX directly or through a Src family member. NOX stimulation may further enhance PKCδ activity. Both reactive oxygen species (ROS) and PKCδ can inhibit SHP-1, a protein tyrosine phosphatase that dephosphorylates and thereby inactivates PYK2 and JAK2. PYK2 and JAK2 are preassociated. SHP-1 inhibition, PYK2 activation, or Src activation may create docking sites on some scaffold or membrane receptor protein that recruits preassociated PYK2-JAK2, thereby bringing two JAK2 molecules into close position to allow for their transautophosphorylation and activation. Src may also associate with PYK2. Increased cellular Ca2+ may enhance PYK2 activity, but its importance in noncanonical JAK2 activation is not clear.
oxidative stress.
ROS (most commonly hydrogen peroxide) have been documented to stimulate JAK2 activity (114, 155). No direct molecular mechanism would seem to explain how this occurs; most likely the effect is indirect, possibly involving other biomolecules that interact with JAK2. Supporting this conclusion is the demonstration that JAK2-mediated signaling following hydrogen peroxide exposure is dependent on the cell line used (76). Indirect activation of JAK2 may result in part from oxidative inhibition of the active site cysteine in protein tyrosine phosphatases (53, 119, 165, 168), which dramatically increases phosphotyrosine content of JAK2 (60, 92). Alternatively, indirect activation of JAKs may require intervention of a transphosphorylating Src kinase family member (1), as H2O2-stimulated JAK2 activity was inhibited in Fyn−/− cells. Finally, JAK2 activation has also been reported to cause ROS generation in atypical GPCR-mediated systems (149), but for the most part these studies relied on the so-called JAK2 inhibitor AG490. Not only is AG490 not a specific inhibitor of JAK2 (135), but AG490 may itself act as a ROS scavenger (52).
High levels of glucose, as occur in diabetes, have been shown to enhance basal activity of JAK2 in aortic VSMCs and perhaps cardiac myocytes and to potentiate JAK2 activation by angiotensin II and endothelin-1 (5, 9, 10, 122, 153). Like GPCR-mediated JAK2 activation, the effect of high glucose involves a PKC family member (in this case activated via the polyol pathway) and subsequent activation of NADPH oxidase (153). Evidence is reported suggesting that subsequent ROS generation enhances JAK2 activity by inhibiting the tyrosine phosphatase SHP-1, which mediates JAK2 dephosphorylation (153). Whether a similar scenario explains AT1-mediated JAK2 activation is unclear. Although angiotensin II was reported to induce a rapid decrease in SHP-1 activity (within 5 min) in VSMCs, the decrease was very transient and followed by increased SHP-1 activity (109). Furthermore, the transient decrease in SHP-1 activity was accompanied by decreased association of JAK2 with SHP-1, while the angiotensin II-induced increase in SHP-1 activity was accompanied by increased association. Overall, evidence would indicate that SHP-1 is responsible for JAK2 deactivation in response to AT1 stimulation.
In contrast, oxidative stress has been shown to attenuate or inhibit JAK activity in several “classical” cytokine systems, including IL-6 and LIF (85), leptin (74), IL-3 (32), IL-2 (14, 116), ciliary neurotrophic factor (CNTF) (76), and interferon-α (28). Important evidence provided by the Halvorsen lab (76, 123, 124) showed that neurons treated with oxidative stress reagents do not effectively respond to JAK2-stimulating cytokines such as CNTF. Besides the possible cell type-specific differences in the contribution of phosphatases to JAK2 activation, we think it highly likely that structural/molecular differences between JAK1 and JAK2 that impact on their regulation, together with relative differences in their contributions to a particular signaling system, contribute to the confusion in the literature as to the impact of oxidative stress on JAK activity.
osmotic stress.
There are two reports that osmotic stress induced by hyperosmolarity can activate the JAKs in various cell lines; the basis for this activation is unexplained, but it does not appear to involve a Src kinase family member (47, 48). Demonstration of the in vivo occurrence of this mechanism has not been reported.
Additional Control Mechanisms Regulating JAK Activity
Superimposed on the basic process of transautophosphorylation that activates JAK1 and JAK2 are several distinct mechanisms, as discussed here.
Redox sensitivity.
Oxidants such as nitric oxide (NO) can inhibit Janus kinases under cellular conditions, and the oxidative inhibition of Janus kinases is reversible under in vitro conditions (32). Recently, individual and combinatorial cysteine-to-serine mutagenesis led to the identification of four cysteine residues in the catalytic domain (Cys866, Cys917, Cys1094, and Cys1105) that cooperatively maintain JAK2's catalytic competence, an observation that might explain why the enzyme is catalytically inactive when oxidized and maximally active when reduced (106). On the basis of their positions in the JAK2 catalytic domain, one might speculate that Cys866 and Cys917 coordinate the metal center of the magnesium/manganese ATP and therefore have a direct role in catalysis. The two sulfur centers of these cysteine residues are ∼9 Å apart and might be able to form a disulfide bond in an oxidizing environment. Because of the high homology between JAK1 and JAK2 in their JH1 domain, it is likely that Cys891 and Cys943 perform a similar function in JAK1.
Phosphorylation and nitration.
An early study reported evidence for direct PKCδ-mediated phosphorylation of JAK2 on serine and threonine residues, which was inhibitory; however, the residues involved were not identified (84). Subsequent studies have shown that cytokine-induced Ser523 phosphorylation, within the linker region between the JH3 and JH2 domains, most likely by ERK1/2, inhibits JAK2 activity (73, 115).
Cytokine-induced JAK2 phosphorylation of Tyr570 within the JH2 domain is also inhibitory and would seem to function as a classical negative feedback mechanism (37). Autophosphorylation of Tyr913, which was observed as a result of Epo stimulation, also seems to serve as a negative feedback mechanism (46). Whether Tyr913 phosphorylation is a general feature of JAK2 activation or specific for certain activators is not known. In contrast, JAK2-mediated phosphorylation of Tyr813 within the JH1 domain enhances its catalytic activity by creating a docking site for the cytoplasmic adaptor protein SH2B1 (89). Finally, two recent reports documented that agonist-induced nitration of Tyr1007/Tyr1008 within the regulatory epitope of JAK2 inhibits its catalytic activity (35, 36). Nitration was studied in the context of GH signaling of liver cells and was interpreted as the basis for endotoxin-induced resistance and as a natural counterpoise to JAK2 activation that was linked to a parallel signaling pathway. Whether nitration has functional relevance for JAK activation in the heart awaits exploration.
JH2 domain and SH2B1.
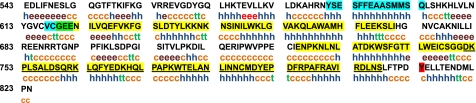
The JH2 domain of JAK2, which is highly conserved among human, mouse, and rat (95.7% identity), is thought to function like a pseudokinase domain to maintain basal JAK2 in a low-activity state (147, 188). Exactly how this is accomplished is not known. On the basis of dimer formation in crystals of the tyrosine kinase domain of the FGF receptor, Lindauer et al. (98) postulated two regions of interaction between JH1 and JH2. Interface 1 would involve the region Tyr590 to Gln603 of JH2, which is predicted to form an α-helix, and interface 2 would comprise JH2 residues Val617 to Glu621 (Fig. 4). Interface 1 was predicted to form a helix-helix interaction with the analogous region of JH1, while interface 2 was predicted to interact with the activation loop of JH1. Using a deletion mutational strategy, Saharinen et al. (148) identified three inhibitory regions (IR) of JH2 against JH1 catalytic activity. IR3 (Asp758–Ser807) directly inhibited JH1 activity (indicating a likely physical interaction with JH1) by lowering the Vmax for enzymatic activity with no effect on substrate affinity (Fig. 4). IR3 is predicted to comprise three α-helices and lies proximal to Tyr813, the phosphorylation of which enhances JAK2 activity. IR1 (Gly619–Ile670) and IR2 (Glu725–Gly757) were found to enhance the inhibitory actions of IR3.
Fig. 4.
Mouse JAK2 JH2 domain. The amino acids comprising the JH2 domain are shown in black. Regions postulated to be important in the inhibition of JAK2 catalytic activity are highlighted. Highlighted in blue (from NH2 to COOH terminus) are interface 1 and interface 2. Highlighted in yellow (from NH2 to COOH terminus) are inhibitory regions (IR)1, 2, and 3. Tyr813, which is important in recruiting SH2B1, is highlighted in red. h, α-Helix; e, extended strand; t, β-turn; c, random coil. Analysis was by the secondary structure prediction method (SOPM) at http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_server.html (21a).
SH2B1 (previously named SH2-B) is a ubiquitously expressed cytoplasmic adaptor protein that selectively enhances catalytic activity of JAK2 (113, 132). SH2B1 contains an NH2-terminal dimerization domain, a central pleckstrin homology (PH) domain, and a COOH-terminal SH2 domain. The latter binds phosphorylated Tyr813 in the JH2 domain and somehow enhances JAK2 activity. Contrary to original thinking, SH2B1 dimerization, with subsequent JAK2-JAK2 apposition, is not how SH2B1 enhances JAK2 activity. Rather, the SH2 domain of SH2B1 alone appears to be sufficient in this regard (89). One explanation for this observation would be that SH2B1 physically prevents JH2 from reassociating with and inhibiting the JH1 catalytic domain.
Recent evidence indicates that in the case of leptin and insulin signaling, insulin receptor substrate (IRS)1 and IRS2 are recruited to the SH2B1·JAK2 complex to be phosphorylated by JAK2, thereby promoting their association with the p85 regulatory subunit of PI3-kinase and subsequent AKT activation (30, 95, 97, 140). A similar scenario may occur with opioid receptors in the heart (55).
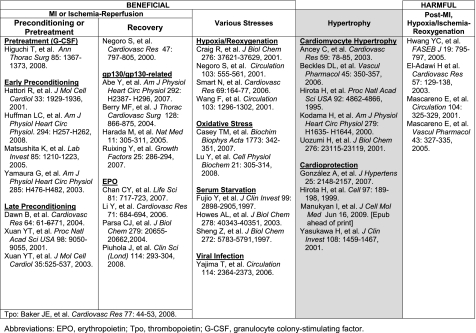
Role of JAKs in the Heart
To date, no cardiac myocyte-specific JAK1 or JAK2 knockout mice have been generated (85). Therefore, no definitive conclusions can be made as to what role(s) the JAKs play in the stressed heart. However, JAK-STAT (in particular STAT3) signaling has been associated with the responses of the heart and isolated cardiac myocytes to various stresses (Fig. 5), and most evidence indicates that JAK signaling linked to STAT3 activation elicits a protective response. Transgenic and knockout mouse models have clearly shown that STAT3 activation in the heart is protective (85). Since class I cytokine receptors that activate STAT3 initially activate JAK1 and/or JAK2, it would seem that the JAKs are protective as well. In fact, the JAKs that are associated with class I cytokine receptors activate other protective signaling pathways besides STAT3, such as PI3-kinase/Akt (156).
Fig. 5.
Role of the JAKs or JAKs-signal transducer and activator of transcription 3 (STAT3) in the heart.
Preconditioning or pretreatment.
Pretreatment of rats in vivo with G-CSF (65) or IL-6 (112) improved hypothermic preservation ex vivo and reduced infarct size and apoptosis immediately after ischemia-reperfusion, respectively. The former response was blocked by AG490 and was associated with increased cardiac capillary density. JAK activation was also implicated by means of AG490 in early preconditioning in several studies using the isolated rat heart (63, 70, 181). Blocking JAK2 attenuated preconditioning-induced improved contractile function and reduced infarct size, and this was associated with reduced upregulation and downregulation of antiapoptotic (bcl-2) and apoptotic (Bax) genes, respectively. Using knockout mice, Bolli and colleagues (24, 175, 176) found that IL-6 plays an essential role in the late phase of preconditioning, although the lack of IL-6 did not modulate infarct size in naïve myocardium. IL-6 was found to be obligatory for the upregulation of NOS2 and COX-2, comediators of late preconditioning. With AG490, JAK2 was implicated in the upregulation of COX-2.
Recovery.
Evidence indicates that the JAKs contribute to the recovery of the heart from myocardial infarction (MI) or ischemia-reperfusion. An early study done on an acute rat MI model reported that blocking JAK activation with AG490 resulted in deterioration of myocardial viability (130). A number of cytokines that signal via gp130 and/or a receptor closely related to gp130 have been shown to improve cardiac function and reduce myocardial damage when introduced at the time of MI in either mouse or rat. These include leptin (2), LIF (12), CT-1 (145), and G-CSF (61). Of note, leptin treatment preserved systolic function but caused eccentric dilatation of the heart. A number of studies either in vivo or on isolated hearts have demonstrated that Epo delivered acutely during ischemia or reperfusion or chronically after MI reduces infarct size, oxidative stress, inflammation, and apoptosis and improves cardiac function (20, 96, 136, 138). Thrombopoietin (Tpo), another class I hematopoietic cytokine that shares sequence homology with Epo, was shown to preserve cardiac structure and function when given before ischemia-reperfusion assault of the rat heart and to reduce infarct size when given at the onset of ischemia or at reperfusion (8).
There are several reports that JAK2 activation is bad in rat hearts (isolated and in vivo) subjected to ischemia (and reperfusion) or in isolated adult rat cardiac myocytes treated with angiotensin II or subjected to hypoxia-reoxygenation (Fig. 5; Refs. 34, 71, 110, 111). Besides increased infarct size and worsened functional hemodynamics, adverse effects were associated with increased angiotensinogen gene and Bax protein expression, increased caspase and protein phosphatase 1 activity, and decreased levels of p16-phospholamban. The discrepancy between these studies and those showing a beneficial role for JAK signaling after MI may perhaps be explained by differences in experimental approach. However, those studies reporting that JAK activity is detrimental relied solely on the use of the inhibitor AG490 to draw their conclusions, and because of the demonstrable lack of specificity of this inhibitor these conclusions cannot be viewed as definitive.
Various stresses.
Multiple in vitro studies have shown that JAK-STAT protects cardiac myocytes against various stresses. Most of these studies have utilized neonatal rat ventricular myocytes (19, 22, 44, 69, 104, 128, 154, 156, 179), although a few have used adult [e.g., mouse (179) and cat (171)] cardiac myocytes. The gp130-related cytokines IL-6 (156), LIF (129, 171), and CT-1 (22) were shown to protect against hypoxia-reoxygenation-induced cell damage via the activation of a number of signaling molecules downstream of the JAKs, including p38 MAP kinase, the ERKs, AKT, NF-κB, and NO. Specifically how these cytokines mediated protection was not defined, although one study reported an upregulation of MnSOD (129). LIF also protects cardiac myocytes against oxidative stress initiated by H2O2 (19), which may represent a manifestation of an innate JAK2 apoptosis resistance mechanism in cardiac muscle (104). An innate protective mechanism against viral infection linked to gp130 has been postulated as well (179). Finally, LIF and CT-1 protect cultured cardiac myocytes against serum deprivation through survival signaling involving, for example, the ERKs and AKT and by upregulating bcl-xL expression (44, 69, 154).
Hypertrophy.
STAT3 and the JAKs have been implicated as well in cardiac hypertrophy (Fig. 5). Whether this is good or bad is arguable, and consequently we note it as a gray area Fig. 5. Pathological hypertrophy as observed during hypertension eventually proves detrimental to heart function and may lead to heart failure (16). gp130 stimulation was shown to induce hypertrophy of isolated human atrial myocytes (6) and cultured neonatal rat ventricular myocytes (see, e.g., Ref. 83) and to lead to left ventricular hypertrophy in a transgenic mouse model of chronic gp130 stimulation (68). Furthermore, the JAK2 inhibitor AG490 was reported to block development of concentric hypertrophy in mice subjected to transverse aortic constriction (11). Which signaling pathway (ERK, STAT3, or PI3-kinase) links gp130 to cardiac hypertrophy is less than clear; however, that issue is irrelevant, as JAK1 and JAK2 (to a lesser extent) are critically important in gp130 signaling overall (85, 143). Nonetheless, Kodama et al. (83) reported that JAK2 inhibition with AG490 markedly attenuated LIF-induced myofilament reorganization and atrial natriuretic peptide (ANP) expression but had minimal effects on protein synthesis itself.
Some have suggested that gp130 and JAK signaling in the heart activates a response that is protective during cardiac hypertrophy. With the use of human transvenous endomyocardial biopsies, an association was found between depression of gp130 protein expression and myocardial apoptosis in hypertensive patients who develop heart failure, but not in those who did not (51). Unlike wild-type mice that develop compensatory cardiac hypertrophy in response to aortic pressure overload, mice with cardiac myocyte-restricted gp130 deletion developed rapid-onset dilated cardiomyopathy and heart failure marked by marked myocardial apoptosis (67). But is this primarily due to the absence of hypertrophy or the loss of cardiac protective signaling? Finally, the hypertrophic agonist endothelin-1 was recently reported to upregulate the cytoprotective stress protein αβ-crystallin in neonatal rat cardiac myocytes, and JAK2 was implicated in this effect through the use of AG490 (107). As suggested by others, gp130 signaling, and by extension JAK activation, may be viewed as a precarious balance between compensatory (but ultimately) maladaptive hypertrophy and cytoprotection (67).
Evidence that acute oxidative stress activates JAK2 in cardiac myocytes.
Ischemia-reperfusion and preconditioning have been shown to activate JAK2 in vivo and in the isolated working heart (24, 63, 111, 160, 190). Moreover, JAK2/JAK1 signaling has been implicated in the protective effects of classical (63, 157, 181) and delayed (24) preconditioning. The basis for this activation is not established but could result from the generation of ROS and/or from the local release of angiotensin II, growth factors, or cytokines. In cultured human cardiac myocytes, angiotensin II was reported to couple to JAK2 activation via ROS generation (122). Overall, however, evidence that JAK2 is in fact activated by ROS per se in cardiac myocytes is scant; at this time a more accurate statement would be that JAK2 may be active during exposure of the heart or cardiac myocytes to ROS.
Evidence that chronic oxidative stress inhibits JAK1 and JAK2.
We recently reported (85) that parthenolide-induced oxidative stress inhibits JAK1, and to a lesser degree JAK2, activation by the IL-6-type cytokines in isolated cardiac myocytes. Perhaps JAK2 is less affected by ROS because of the opposing actions of ROS on JH2 and JH1. Consistent with the possibility that chronic oxidative stress inhibits JAK activation in the human heart is a report that JAK2 phosphorylation in the left ventricles is markedly reduced (by 72%) in patients with dilated cardiomyopathy (139).
Recent evidence for a role of JAK2 activation in cardiac protection: Epo and G-CSF.
Involvement of the JAKs in the cardioprotective action of the IL-6 family cytokines in the heart was reviewed recently elsewhere (85) and is not further discussed here. Rather, we focus on two agents that also activate receptors belonging to the type 1 cytokine receptor family and that have lately generated much clinical interest, namely, Epo and G-CSF.
Besides its ability to increase the number of red blood cells and consequently oxygen delivery to underperfused organs, Epo has other actions that are beneficial under conditions of perfusion dysfunction, including stimulation of new blood vessel formation and a direct protective action on cells, most notably cardiac myocytes (13, 93). Epo is able to protect the heart against ischemia-reperfusion injury and reduce infarct size via activation of its receptor on cardiac myocytes. Epo-initiated intracellular signaling involves formation of a receptor homodimer with subsequent activation via transphosphorylation of juxtaposed JAK2 molecules. This preconditioning-like effect of Epo involves in part Akt-mediated targeting of mitochondrial glycogen synthase kinase (GSK)-β (82, 134). Although postinfarct ventricular remodeling impairs this protective mechanism, the protective actions of Epo are retained because of a compensatory ERK-mediated mechanism (120). In addition, STAT3 activation likely contributes to the protective effects of Epo on cardiac myocytes (81, 138). Another way Epo protects the heart is through increased expression and activation by phosphorylation of NOS3 (33). The subsequent increase in NO acts to improve blood flow and oxygen delivery, as well as to quench superoxide formation.
Although it is also a member of the hematopoietin cytokine receptor superfamily, signaling via the G-CSF receptor involves prominently not only JAK2 but also Lyn kinase (189). Both of these nonreceptor kinases are recruited to the G-CSF homodimer and couple independently to distinct signaling pathways. Cardiac myocytes have been shown to express the G-CSF receptor, which directly couples to protective signaling. In addition, G-CSF, which is produced by many cells including endothelial and immune cells, indirectly promotes cardiac repair by mobilizing bone marrow-derived stem cells after myocardial infarction (87, 88).
JAK-AKT interface.
Numerous studies have reported that the IL-6-type cytokines couple to PI3-kinase and AKT activation, although the exact mechanism is unknown (101, 133, 156). LIF in particular has been shown to activate AKT in adult (41) and neonatal (83, 128) rat ventricular myocytes. Available evidence suggests a possible role for the adapter-protein GRB2 associated binder 1 (Gab1; Refs. 127, 160), which contains several highly conserved JAK2 substrate motifs as well as binding motifs for the PI3-kinase regulatory domain (although the 2 sets of motifs are distinct). In addition, activated JAK2 has been shown to form a complex with protein phosphatase 2A (PP2A), resulting in tyrosine phosphorylation and inactivation of this serine/threonine phosphatase (7, 25, 43, 79, 100, 152, 184, 185). PP2A appears to be the primary phosphatase downregulating AKT activity (142, 190). Both the receptors for Epo and G-CSF are also linked to Akt activation (134, 189).
An alternative inhibitory mediator of JAK2 action on PI3-kinase/AKT signaling is the protein tyrosine phosphatase SHP-1, which binds and is activated by JAK2 (75). It was reported that mice expressing functionally deficient SHP-1 are more glucose tolerant and insulin sensitive because of enhanced insulin receptor signaling in liver and muscle via the IRS-PI3-kinase-AKT pathway (31). SHP-1 has been shown to attenuate insulin signaling by binding and dephosphorylating the insulin receptor (31). Also, SHP-1 might attenuate PI3-kinase signaling to AKT by binding and dephosphorylating the phosphodiesterase PTEN, thus enhancing its binding to membrane phosphatidylinositols and effectiveness in inhibiting the AKT pathway (103). A recent report linked heightened JAK2 activity and insulin resistance in L6 myotubes to reduced AKT activity/phosphorylation at some level downstream of PI3-kinase (163). AKT activation (separate or together with downstream ERK1/2 activation) may in turn lead to attenuation of JAK2 activity, as well as STAT3 activity, although the details are not well defined. Finally, JAK2 and AKT signaling are likely to interact as well in cardiac myocytes downstream at the transcriptional level (104). AKT activation may lead to increased STAT3 expression via the intermediacy of NF-κB activation, while STAT3 activation increases AKT expression.
Summary and Perspectives
The regulation of the activities of JAK1 and JAK2 is now known to be more complicated than once thought only a few years ago. Our recent studies have revealed that the activity of these kinases in cardiac myocytes is dependent on cellular redox status and is inhibited by oxidative stress. A potential redox switch has been localized within the catalytic JH1 domain of JAK2. Based on primary sequence similarities, this redox switch is likely present in JAK1 as well. At the same time, the work of others has revealed a role for nitration and phosphorylation as well as scaffolding proteins in controlling JAK2 kinase activity. Altogether, recent studies support the conclusion that JAK1 and JAK2 function as part of integrated signaling networks. This raises several questions that challenge the limitations of current approaches. For instance, how do the various input signals that control JAK activity interact, and how does JAK signaling regulation change with cellular context and with changes in cellular environment that occur with cardiac remodeling? The answers will ultimately address the overarching question of whether the JAK kinases are a viable drug target relevant to cardiac remodeling and heart failure.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (1-R01-HL-088101, G. W. Booz) and Lebanese University (M. Kurdi).
ACKNOWLEDGMENTS
We thank Dr. Roy Duhé for his ongoing collaboration and discussions.
REFERENCES
- 1.Abe J, Berk BC. Fyn and JAK2 mediate Ras activation by reactive oxygen species. J Biol Chem 274: 21003–21010, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Abe Y, Ono K, Kawamura T, Wada H, Kita T, Shimatsu A, Hasegawa K. Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am J Physiol Heart Circ Physiol 292: H2387–H2396, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Albacker TB, Carvalho G, Schricker T, Lachapelle K. Myocardial protection during elective coronary artery bypass grafting using high-dose insulin therapy. Ann Thorac Surg 84: 1920–1927, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ali MS, Sayeski PP, Safavi A, Lyles M, Bernstein KE. Janus kinase 2 (Jak2) must be catalytically active to associate with the AT1 receptor in response to angiotensin II. Biochem Biophys Res Commun 249: 672–677, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Amiri F, Venema VJ, Wang X, Ju H, Venema RC, Marrero MB. Hyperglycemia enhances angiotensin II-induced janus-activated kinase/STAT signaling in vascular smooth muscle cells. J Biol Chem 274: 32382–32386, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Ancey C, Menet E, Corbi P, Fredj S, Garcia M, Rücker-Martin C, Bescond J, Morel F, Wijdenes J, Lecron JC, Potreau D. Human cardiomyocyte hypertrophy induced in vitro by gp130 stimulation. Cardiovasc Res 59: 78–85, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Avdi NJ, Malcolm KC, Nick JA, Worthen GS. A role for protein phosphatase-2A in p38 mitogen-activated protein kinase-mediated regulation of the c-Jun NH2-terminal kinase pathway in human neutrophils. J Biol Chem 277: 40687–40696, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Baker JE, Su J, Hsu A, Shi Y, Zhao M, Strande JL, Fu X, Xu H, Eis A, Komorowski R, Jensen ES, Tweddell JS, Rafiee P, Gross GJ. Human thrombopoietin reduces myocardial infarct size, apoptosis, and stunning following ischaemia/reperfusion in rats. Cardiovasc Res 77: 44–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banes-Berceli AK, Ketsawatsomkron P, Ogbi S, Patel B, Pollock DM, Marrero MB. Angiotensin II and endothelin-1 augment the vascular complications of diabetes via JAK2 activation. Am J Physiol Heart Circ Physiol 293: H1291–H1299, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Banes-Berceli AK, Ogobi S, Tawfik A, Patel B, Shirley A, Pollock DM, Fulton D, Marrero MB. Endothelin-1 activation of JAK2 in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Vascul Pharmacol 43: 310–319, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Beckles DL, Mascareno E, Siddiqui MA. Inhibition of Jak2 phosphorylation attenuates pressure overload cardiac hypertrophy. Vascul Pharmacol 45: 350–357, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Berry MF, Pirolli TJ, Jayasankar V, Morine KJ, Moise MA, Fisher O, Gardner TJ, Patterson PH, Woo YJ. Targeted overexpression of leukemia inhibitory factor to preserve myocardium in a rat model of postinfarction heart failure. J Thorac Cardiovasc Surg 128: 866–875, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Binbrek AS, Mittal B, Rao KN, Sobel BE. The potential of erythropoietin for conferring cardioprotection complementing reperfusion. Coron Artery Dis 18: 583–585, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol 160: 5729–5734, 1998 [PubMed] [Google Scholar]
- 15.Bolli R, Dawn B, Xuan YT. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc Med 13: 72– 79, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Booz GW. Lip YH, Hall JE. Left ventricular physiology in hypertension. Comprehensive Hypertension, Philadelphia, PA:Mosby, 2007 [Google Scholar]
- 17.Brar BK, Stephanou A, Pennica D, Latchman DS. CT-1 mediated cardioprotection against ischaemic re-oxygenation injury is mediated by PI3 kinase, Akt and MEK1/2 pathways. Cytokine 16: 93–96, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Butler KL, Huffman LC, Koch SE, Hahn HS, Gwathmey JK. STAT-3 activation is necessary for ischemic preconditioning in hypertrophied myocardium. Am J Physiol Heart Circ Physiol 291: H797–H803, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Casey TM, Arthur PG, Bogoyevitch MA. Necrotic death without mitochondrial dysfunction-delayed death of cardiac myocytes following oxidative stress. Biochim Biophys Acta 1773: 342–351, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Chan CY, Chen YS, Lee HH, Huang HS, Lai YL, Chen CF, Ma MC. Erythropoietin protects post-ischemic hearts by preventing extracellular matrix degradation: role of Jak2-ERK pathway. Life Sci 81: 717–723, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Chatti K, Farrar WL, Duhé RJ. Tyrosine phosphorylation of the Janus kinase 2 activation loop is essential for a high-activity catalytic state but dispensable for a basal catalytic state. Biochemistry 43: 4272–4283, 2004 [DOI] [PubMed] [Google Scholar]
- 21a.Combet C, Blanchet C, Geourjon C, Deléage G. NPS@: network protein sequence analysis. Trends Biochem Sci 25: 147–150, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Craig R, Wagner M, McCardle T, Craig AG, Glembotski CC. The cytoprotective effects of the glycoprotein 130 receptor-coupled cytokine, cardiotrophin-1, require activation of NF-κB. J Biol Chem 276: 37621–37629, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Davidson SM, Hausenloy D, Duchen MR, Yellon DM. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol 38: 414–419, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Dawn B, Xuan YT, Guo Y, Rezazadeh A, Stein AB, Hunt G, Wu WJ, Tan W, Bolli R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res 64: 61–71, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Torre P, Diaz-Sanjuan T, Garcia-Ruiz I, Esteban E, Canga F, Munoz-Yague T, Solis-Herruzo JA. Interleukin-6 increases rat metalloproteinase-13 gene expression through Janus kinase-2-mediated inhibition of serine/threonine phosphatase-2A. Cell Signal 17: 427–435, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, Hoefer IE, Mueller-Hoecker J, Franz WM. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J 20: 956–958, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Demyanets S, Kaun C, Rychli K, Rega G, Pfaffenberger S, Afonyushkin T, Bochkov VN, Maurer G, Huber K, Wojta J. The inflammatory cytokine oncostatin M induces PAI-1 in human vascular smooth muscle cells in vitro via PI3-kinase and ERK1/2-dependent pathways. Am J Physiol Heart Circ Physiol 293: H1962–H1968, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Di Bona D, Cippitelli M, Fionda C, Cammà C, Licata A, Santoni A, Craxμ A. Oxidative stress inhibits IFN-α-induced antiviral gene expression by blocking the JAK-STAT pathway. J Hepatol 45: 271–279, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem 279: 43684–43691, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois MJ, Bergeron S, Kim HJ, Dombrowski L, Perreault M, Fournes B, Faure R, Olivier M, Beauchemin N, Shulman GI, Siminovitch KA, Kim JK, Marette A. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat Med 12: 549–556, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Duhé RJ, Evans GA, Erwin RA, Kirken RA, Cox GW, Farrar WL. Nitric oxide and thiol redox regulation of Janus kinase activity. Proc Natl Acad Sci USA 95: 126–131, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.d'Uscio LV, Smith LA, Santhanam AV, Richardson D, Nath KA, Katusic ZS. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension 49: 1142–1148, 2007 [DOI] [PubMed] [Google Scholar]
- 34.El-Adawi H, Deng L, Tramontano A, Smith S, Mascareno E, Ganguly K, Castillo R, El-Sherif N. The functional role of the JAK-STAT pathway in post-infarction remodeling. Cardiovasc Res 57: 129–138, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Elsasser TH, Kahl S, Li CJ, Sartin JL, Garrett WM, Rodrigo J. Caveolae nitration of Janus kinase-2 at the 1007Y-1008Y site: coordinating inflammatory response and metabolic hormone readjustment within the somatotropic axis. Endocrinology 148: 3803–2813, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Elsasser TH, Li CJ, Caperna TJ, Kahl S, Schmidt WF. Growth hormone (GH)-associated nitration of Janus kinase-2 at the 1007Y-1008Y epitope impedes phosphorylation at this site: mechanism for and impact of a GH, AKT, and nitric oxide synthase axis on GH signal transduction. Endocrinology 148: 3792–3802, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Feener EP, Rosario F, Dunn SL, Stancheva Z, Myers MG Jr. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol Cell Biol 24: 4968–4978, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol 17: 2497–2501, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrand A, Kowalski-Chauvel A, Bertrand C, Escrieut C, Mathieu A, Portolan G, Pradayrol L, Fourmy D, Dufresne M, Seva C. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem 280: 10710–10715, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT axis. Basic Res Cardiol 102: 393–411, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Florholmen G, Thoresen GH, Rustan AC, Jensen J, Christensen G, Aas V. Leukaemia inhibitory factor stimulates glucose transport in isolated cardiomyocytes and induces insulin resistance after chronic exposure. Diabetologia 49: 724–731, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Frank GD, Saito S, Motley ED, Sasaki T, Ohba M, Kuroki T, Inagami T, Eguchi S. Requirement of Ca2+ and PKCδ for Janus kinase 2 activation by angiotensin II: involvement of PYK2. Mol Endocrinol 16: 367–377, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Fuhrer DK, Yang YC. Complex formation of JAK2 with PP2A, P13K, and Yes in response to the hematopoietic cytokine interleukin-11. Biochem Biophys Res Commun 224: 289–296, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Fujio Y, Kunisada K, Hirota H, Yamauchi-Takihara K, Kishimoto T. Signals through gp130 upregulate bcl-x gene expression via STAT1-binding cis-element in cardiac myocytes. J Clin Invest 99: 2898–2905, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Funakoshi-Tago M, Pelletier S, Moritake H, Parganas E, Ihle JN. Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity. Mol Cell Biol 28: 1792–1801, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funakoshi-Tago M, Tago K, Kasahara T, Parganas E, Ihle JN. Negative regulation of Jak2 by its auto-phosphorylation at tyrosine 913 via the Epo signaling pathway. Cell Signal 20: 1995–2001, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Garnovskaya MN, Mukhin YV, Vlasova TM, Raymond JR. Hypertonicity activates Na+/H+ exchange through Janus kinase 2 and calmodulin. J Biol Chem 278: 16908–16915, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Gatsios P, Terstegen L, Schliess F, Häussinger D, Kerr IM, Heinrich PC, Graeve L. Activation of the Janus kinase/signal transducer and activator of transcription pathway by osmotic shock. J Biol Chem 273: 22962–22968, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Giani JF, Gironacci MM, Muñoz MC, Peña C, Turyn D, Dominici FP. Angiotensin-(1–7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am J Physiol Heart Circ Physiol 293: H1154–H1163, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Godeny MD, Sayyah J, VonDerLinden D, Johns M, Ostrov DA, Caldwell-Busby J, Sayeski PP. The N-terminal SH2 domain of the tyrosine phosphatase, SHP-2, is essential for Jak2-dependent signaling via the angiotensin II type AT1 receptor. Cell Signal 19: 600–609, 2007 [DOI] [PubMed] [Google Scholar]
- 51.González A, Ravassa S, Loperena I, López B, Beaumont J, Querejeta R, Larman M, Díez J. Association of depressed cardiac gp130-mediated antiapoptotic pathways with stimulated cardiomyocyte apoptosis in hypertensive patients with heart failure. J Hypertens 25: 2148–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Gorina R, Sanfeliu C, Galitó A, Messeguer A, Planas AM. Exposure of glia to pro-oxidant agents revealed selective Stat1 activation by H2O2 and Jak2-independent antioxidant features of the Jak2 inhibitor AG490. Glia 55: 1313–1324, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Groen A, Lemeer S, van der Wijk T, Overvoorde J, Heck AJ, Ostman A, Barford D, Slijper M, den Hertog J. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem 280: 10298–10304, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Gross ER, Gross GJ. Pharmacologic therapeutics for cardiac reperfusion injury. Expert Opin Emerg Drugs 12: 367–388, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3β. Am J Physiol Heart Circ Physiol 291: H827–H834, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Gu Y, Zou Y, Aikawa R, Hayashi D, Kudoh S, Yamauchi T, Uozumi H, Zhu W, Kadowaki T, Yazaki Y, Komuro I. Growth hormone signalling and apoptosis in neonatal rat cardiomyocytes. Mol Cell Biochem 223: 35–46, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Guaiquil VH, Golde DW, Beckles DL, Mascareno EJ, Siddiqui MA. Vitamin C inhibits hypoxia-induced damage and apoptotic signaling pathways in cardiomyocytes and ischemic hearts. Free Radic Biol Med 37: 1419–1429, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Gwechenberger M, Moertl D, Pacher R, Huelsmann M. Oncostatin-M in myocardial ischemia/reperfusion injury may regulate tissue repair. Croat Med J 45: 149–157, 2004 [PubMed] [Google Scholar]
- 59.Haan S, Margue C, Engrand A, Rolvering C, Schmitz-Van de Leur H, Heinrich PC, Behrmann I, Haan C. Dual role of the Jak1 FERM and kinase domains in cytokine receptor binding and in stimulation-dependent Jak activation. J Immunol 180: 998–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Haque SJ, Wu Q, Kammer W, Friedrich K, Smith JM, Kerr IM, Stark GR, Williams BR. Receptor-associated constitutive protein tyrosine phosphatase activity controls the kinase function of JAK1. Proc Natl Acad Sci USA 94: 8563–8568, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 11: 305–311, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Hasegawa H, Takano H, Iwanaga K, Ohtsuka M, Qin Y, Niitsuma Y, Ueda K, Toyoda T, Tadokoro H, Komuro I. Cardioprotective effects of granulocyte colony-stimulating factor in swine with chronic myocardial ischemia. J Am Coll Cardiol 47: 842–849, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Hattori R, Maulik N, Otani H, Zhu L, Cordis G, Engelman RM, Siddiqui MA, Das DK. Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol 33: 1929–1936, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev 12: 217–234, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Higuchi T, Yamauchi-Takihara K, Matsumiya G, Fukushima N, Ichikawa H, Kuratani T, Maehata Y, Sawa Y. Granulocyte colony-stimulating factor prevents reperfusion injury after heart preservation. Ann Thorac Surg 85: 1367–1373, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Hiraoka E, Kawashima S, Takahashi T, Rikitake Y, Hirase T, Yokoyama M. PI 3-kinase-Akt-p70 S6 kinase in hypertrophic responses to leukemia inhibitory factor in cardiac myocytes. Kobe J Med Sci 49: 25–37, 2003 [PubMed] [Google Scholar]
- 67.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J Jr, Müller W, Chien KR. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 97: 189–198, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA 92: 4862–4866, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howes AL, Arthur JF, Zhang T, Miyamoto S, Adams JW, Dorn GW 2nd, Woodcock EA, Brown JH. Akt-mediated cardiomyocyte survival pathways are compromised by Gαq-induced phosphoinositide 4,5-bisphosphate depletion. J Biol Chem 278: 40343–40351, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Huffman LC, Koch SE, Butler KL. Coronary effluent from a preconditioned heart activates the JAK-STAT pathway and induces cardioprotection in a donor heart. Am J Physiol Heart Circ Physiol 294: H257–H262, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Hwang YC, Shaw S, Kaneko M, Redd H, Marrero MB, Ramasamy R. Aldose reductase pathway mediates JAK-STAT signaling: a novel axis in myocardial ischemic injury. FASEB J 19: 795–797, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Ieishi K, Nomura M, Kawano T, Fujimoto S, Ikefuji H, Noda Y, Nishikado A, Ito S. The effect of G-CSF in a myocardial ischemia reperfusion model rat. J Med Invest 54: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Ishida-Takahashi R, Rosario F, Gong Y, Kopp K, Stancheva Z, Chen X, Feener EP, Myers MG Jr. Phosphorylation of Jak2 on Ser523 inhibits Jak2-dependent leptin receptor signaling. Mol Cell Biol 26: 4063–4073, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jang EH, Park CS, Lee SK, Pie JE, Kang JH. Excessive nitric oxide attenuates leptin-mediated signal transducer and activator of transcription 3 activation. Life Sci 80: 609–617, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Jiao H, Berrada K, Yang W, Tabrizi M, Platanias LC, Yi T. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol Cell Biol 16: 6985–6992, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaur N, Lu B, Monroe RK, Ward SM, Halvorsen SW. Inducers of oxidative stress block ciliary neurotrophic factor activation of Jak/STAT signaling in neurons. J Neurochem 92: 1521–1530, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther 324: 160–169, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Kimura R, Maeda M, Arita A, Oshima Y, Obana M, Ito T, Yamamoto Y, Mohri T, Kishimoto T, Kawase I, Fujio Y, Azuma J. Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine 38: 107–115, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Kins S, Kurosinski P, Nitsch RM, Gotz J. Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice. Am J Pathol 163: 833–843, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kirken RA, Rui H, Howard OM, Farrar WL. Involvement of JAK-family tyrosine kinases in hematopoietin receptor signal transduction. Prog Growth Factor Res 5: 195–211, 1994 [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi H, Minatoguchi S, Yasuda S, Bao N, Kawamura I, Iwasa M, Yamaki T, Sumi S, Misao Y, Ushikoshi H, Nishigaki K, Takemura G, Fujiwara T, Tabata Y, Fujiwara H. Post-infarct treatment with an erythropoietin-gelatin hydrogel drug delivery system for cardiac repair. Cardiovasc Res 79: 611–620, 2008 [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi H, Miura T, Ishida H, Miki T, Tanno M, Yano T, Sato T, Hotta H, Shimamoto K. Limitation of infarct size by erythropoietin is associated with translocation of Akt to the mitochondria after reperfusion. Clin Exp Pharmacol Physiol 35: 812–819, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Kodama H, Fukuda K, Pan J, Sano M, Takahashi T, Kato T, Makino S, Manabe T, Murata M, Ogawa S. Significance of ERK cascade compared with JAK/STAT and PI3-K pathway in gp130-mediated cardiac hypertrophy. Am J Physiol Heart Circ Physiol 279: H1635–H1644, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Kovanen PE, Junttila I, Takaluoma K, Saharinen P, Valmu L, Li W, Silvennoinen O. Regulation of Jak2 tyrosine kinase by protein kinase C during macrophage differentiation of IL-3-dependent myeloid progenitor cells. Blood 95: 1626–1632, 2000 [PubMed] [Google Scholar]
- 85.Kurdi M, Booz GW. Can the protective actions of JAK-STAT in the heart be exploited therapeutically? Parsing the regulation of interleukin-6-type cytokine signaling. J Cardiovasc Pharmacol 50: 126–141, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Kurdi M, Booz GW. Evidence that IL-6-type cytokine signaling in cardiomyocytes is inhibited by oxidative stress: parthenolide targets JAK1 activation by generating ROS. J Cell Physiol 212: 424–431, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Kurdi M, Booz GW. G-CSF-based stem cell therapy for the heart—unresolved issues part B: stem cells, engraftment, transdifferentiation, and bioengineering. Congest Heart Fail 13: 347–351, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Kurdi M, Booz GW. G-CSF-based stem cell therapy for the heart—unresolved issues part A: paracrine actions, mobilization, and delivery. Congest Heart Fail 13: 221–227, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Kurzer JH, Argetsinger LS, Zhou YJ, Kouadio JL, O'Shea JJ, Carter-Su C. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol Cell Biol 24: 4557–4570, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurzer JH, Saharinen P, Silvennoinen O, Carter-Su C. Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol Cell Biol 26: 6381–6394, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kusano K, Tsutsumi Y, Dean J, Gavin M, Ma H, Silver M, Thorne T, Zhu Y, Losordo DW, Aikawa R. Long-term stable expression of human growth hormone by rAAV promotes myocardial protection post-myocardial infarction. J Mol Cell Cardiol 42: 390–399, 2007 [DOI] [PubMed] [Google Scholar]
- 92.Lamb P, Haslam J, Kessler L, Seidel HM, Stein RB, Rosen J. Rapid activation of the interferon-γ signal transduction pathway by inhibitors of tyrosine phosphatases. J Interferon Res 14: 365–373, 1994 [DOI] [PubMed] [Google Scholar]
- 93.Latini R, Brines M, Fiordaliso F. Do non-hemopoietic effects of erythropoietin play a beneficial role in heart failure? Heart Fail Rev 13: 415– 423, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Lefler D, Mukhin YV, Pettus T, Leeb-Lundberg LM, Garnovskaya MN, Raymond JR. Jak2 and Ca2+/calmodulin are key intermediates for bradykinin B2 receptor-mediated activation of Na+/H+ exchange in KNRK and CHO cells. Assay Drug Dev Technol 1: 281– 289, 2003 [DOI] [PubMed] [Google Scholar]
- 95.Li M, Li Z, Morris DL, Rui L. Identification of SH2B2β as an inhibitor for SH2B1- and SH2B2α-promoted Janus kinase-2 activation and insulin signaling. Endocrinology 148: 1615–1621, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T, Fujiwara H. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res 71: 684–694, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Li Z, Zhou Y, Carter-Su C, Myers MG Jr, Rui L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol 21: 2270–2281, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Lindauer K, Loerting T, Liedl KR, Kroemer RT. Prediction of the structure of human Janus kinase 2 (JAK2) comprising the two carboxy-terminal domains reveals a mechanism for autoregulation. Protein Eng 14: 27–37, 2001 [DOI] [PubMed] [Google Scholar]
- 99.Liu HT, Zhang HF, Si R, Zhang QJ, Zhang KR, Guo WY, Wang HC, Gao F. Insulin protects isolated hearts from ischemia/reperfusion injury: cross-talk between PI3-K/Akt and JNKs. Sheng Li Xue Bao 59: 651–659, 2007 [PubMed] [Google Scholar]
- 100.Liu Q, Hofmann PA. Modulation of protein phosphatase 2a by adenosine A1 receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol Heart Circ Physiol 285: H97–H103, 2003 [DOI] [PubMed] [Google Scholar]
- 101.López N, Diez J, Fortuño MA. Characterization of the protective effects of cardiotrophin-1 against non-ischemic death stimuli in adult cardiomyocytes. Cytokine 30: 282–292, 2005 [DOI] [PubMed] [Google Scholar]
- 102.Lu C, Schwartzbauer G, Sperling MA, Devaskar SU, Thamotharan S, Robbins PD, McTiernan CF, Liu JL, Jiang J, Frank SJ, Menon RK. Demonstration of direct effects of growth hormone on neonatal cardiomyocytes. J Biol Chem 276: 22892–22900, 2001 [DOI] [PubMed] [Google Scholar]
- 103.Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurray JS, Fang X, Yung WK, Siminovitch KA, Mills GB. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem 278: 40057–40066, 2003 [DOI] [PubMed] [Google Scholar]
- 104.Lu Y, Zhou J, Xu C, Lin H, Xiao J, Wang Z, Yang B. JAK/STAT and PI3K/AKT pathways form a mutual transactivation loop and afford resistance to oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem 21: 305–314, 2008 [DOI] [PubMed] [Google Scholar]
- 105.Lukashova V, Chen Z, Duhé RJ, Rola-Pleszczynski M, Stanková J. Janus kinase 2 activation by the platelet-activating factor receptor (PAFR): roles of Tyk2 and PAFR C terminus. J Immunol 171: 3794–3800, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Mamoon NM, Smith JK, Chatti K, Lee S, Kundrapu K, Duhé RJ. Multiple cysteine residues are implicated in Janus kinase 2-mediated catalysis. Biochemistry 46: 14810–81481, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Manukyan I, Galatioto J, Mascareno E, Bhaduri S, Siddiqui MA. Cross-talk between calcineurin/NFAT and Jak/STAT signaling induces cardioprotective αB-crystallin gene expression in response to hypertrophic stimuli. J Cell Mol Med( June16,2009) doi: 10.1111/j.1582-4934.2009.00804.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, Clerk A, Sugden PH. Glycogen synthase kinases 3α and 3β in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal 20: 206–218, 2008 [DOI] [PubMed] [Google Scholar]
- 109.Marrero MB, Venema VJ, Ju H, Eaton DC, Venema RC. Regulation of angiotensin II-induced JAK2 tyrosine phosphorylation: roles of SHP-1 and SHP-2. Am J Physiol Cell Physiol 275: C1216–C1223, 1998 [DOI] [PubMed] [Google Scholar]
- 110.Mascareno E, Beckles DL, Siddiqui MA. Janus kinase-2 signaling mediates apoptosis in rat cardiomyocytes. Vascul Pharmacol 43: 327–335, 2005 [DOI] [PubMed] [Google Scholar]
- 111.Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, Das DK, Siddiqui MA. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation 104: 325–329, 2001 [DOI] [PubMed] [Google Scholar]
- 112.Matsushita K, Iwanaga S, Oda T, Kimura K, Shimada M, Sano M, Umezawa A, Hata J, Ogawa S. Interleukin-6/soluble interleukin-6 receptor complex reduces infarct size via inhibiting myocardial apoptosis. Lab Invest 85: 1210–1223, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Maures TJ, Kurzer JH, Carter-Su C. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab 18: 38–45, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Mazière C, Conte MA, Mazière JC. Activation of JAK2 by the oxidative stress generated with oxidized low-density lipoprotein. Free Radic Biol Med 31: 1334–1340, 2001 [DOI] [PubMed] [Google Scholar]
- 115.Mazurkiewicz-Munoz AM, Argetsinger LS, Kouadio JL, Stensballe A, Jensen ON, Cline JM, Carter-Su C. Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor. Mol Cell Biol 26: 4052–4062, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol 168: 689–695, 2002 [DOI] [PubMed] [Google Scholar]
- 117.McDoom I, Ma X, Kirabo A, Lee KY, Ostrov DA, Sayeski PP. Identification of tyrosine 972 as a novel site of Jak2 tyrosine kinase phosphorylation and its role in Jak2 activation. Biochemistry 47: 8326–8334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mellado M, Rodríguez-Frade JM, Aragay A, del Real G, Martín AM, Vila-Coro AJ, Serrano A, Mayor F, Jr, Martínez-AC The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol 161: 805–813, 1998 [PubMed] [Google Scholar]
- 119.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9: 387–399, 2002 [DOI] [PubMed] [Google Scholar]
- 120.Miki T, Miura T, Tanno M, Nishihara M, Naitoh K, Sato T, Takahashi A, Shimamoto K. Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res Cardiol 102: 163–170, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ 15: 521–529, 2008 [DOI] [PubMed] [Google Scholar]
- 122.Modesti A, Bertolozzi I, Gamberi T, Marchetta M, Lumachi C, Coppo M, Moroni F, Toscano T, Lucchese G, Gensini GF, Modesti PA. Hyperglycemia activates JAK2 signaling pathway in human failing myocytes via angiotensin II-mediated oxidative stress. Diabetes 54: 394–401, 2005 [DOI] [PubMed] [Google Scholar]
- 123.Monroe RK, Halvorsen SW. Cadmium blocks receptor-mediated Jak/STAT signaling in neurons by oxidative stress. Free Radic Biol Med 41: 493–502, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Monroe RK, Halvorsen SW. Mercury abolishes neurotrophic factor-stimulated Jak-STAT signaling in nerve cells by oxidative stress. Toxicol Sci 94: 129–138, 2006 [DOI] [PubMed] [Google Scholar]
- 125.Morisco C, Marrone C, Trimarco V, Crispo S, Monti MG, Sadoshima J, Trimarco B. Insulin resistance affects the cytoprotective effect of insulin in cardiomyocytes through an impairment of MAPK phosphatase-1 expression. Cardiovasc Res 76: 453–464, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Mudalagiri NR, Mocanu MM, Di Salvo C, Kolvekar S, Hayward M, Yap J, Keogh B, Yellon DM. Erythropoietin protects the human myocardium against hypoxia/reoxygenation injury via phosphatidylinositol-3 kinase and ERK1/2 activation. Br J Pharmacol 153: 50–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, Sugiyama S, Matsuda S, Koyasu S, Yamauchi-Takihara K, Hirano T, Kawase I, Hirota H. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res 93: 221–229, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, Kishimoto T, Yamauchi-Takihara K. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction. Circulation 103: 555–561, 2001 [DOI] [PubMed] [Google Scholar]
- 129.Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 104: 979–981, 2001 [DOI] [PubMed] [Google Scholar]
- 130.Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, Yamauchi-Takihara K. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res 47: 797–805, 2000 [DOI] [PubMed] [Google Scholar]
- 131.Nishihara M, Miura T, Miki T, Tanno M, Yano T, Naitoh K, Ohori K, Hotta H, Terashima Y, Shimamoto K. Modulation of the mitochondrial permeability transition pore complex in GSK-3β-mediated myocardial protection. J Mol Cell Cardiol 43: 564–570, 2007 [DOI] [PubMed] [Google Scholar]
- 132.O'Brien KB, O'Shea JJ, Carter-Su C. SH2-B family members differentially regulate JAK family tyrosine kinases. J Biol Chem 277: 8673–8681, 2002 [DOI] [PubMed] [Google Scholar]
- 133.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem 273: 9703–9710, 1998 [DOI] [PubMed] [Google Scholar]
- 134.Ohori K, Miura T, Tanno M, Miki T, Sato T, Ishikawa S, Horio Y, Shimamoto K. Ser9 phosphorylation of mitochondrial GSK-3beta is a primary mechanism of cardiomyocyte protection by erythropoietin against oxidant-induced apoptosis. Am J Physiol Heart Circ Physiol 295: H2079–H2086, 2008 [DOI] [PubMed] [Google Scholar]
- 135.Osherov N, Gazit A, Gilon C, Levitzki A. Selective inhibition of the epidermal growth factor and HER2/neu receptors by tyrphostins. J Biol Chem 268: 11134–11142, 1993 [PubMed] [Google Scholar]
- 136.Parsa CJ, Kim J, Riel RU, Pascal LS, Thompson RB, Petrofski JA, Matsumoto A, Stamler JS, Koch WJ. Cardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblasts. J Biol Chem 279: 20655–20662, 2004 [DOI] [PubMed] [Google Scholar]
- 137.Paxton WG, Heerdt L, Berk BC, Delafontaine P, Bernstein KE. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature 375: 247–250, 1995 [DOI] [PubMed] [Google Scholar]
- 138.Piuhola J, Kerkelä R, Keenan JI, Hampton MB, Richards AM, Pemberton CJ. Direct cardiac actions of erythropoietin (EPO): effects on cardiac contractility, BNP secretion and ischaemia/reperfusion injury. Clin Sci (Lond) 114: 293–304, 2008 [DOI] [PubMed] [Google Scholar]
- 139.Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation 107: 798–802, 2003 [DOI] [PubMed] [Google Scholar]
- 140.Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest 117: 397–406, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ricci C, Pastukh V, Mozaffari M, Schaffer SW. Insulin withdrawal induces apoptosis via a free radical-mediated mechanism. Can J Physiol Pharmacol 85: 455–464, 2007 [DOI] [PubMed] [Google Scholar]
- 142.Rocher G, Letourneux C, Lenormand P, Porteu F. Inhibition of B56-containing PP2As by the early response gene IEX-1 leads to control of Akt activity. J Biol Chem 282: 5468–5477, 2007 [DOI] [PubMed] [Google Scholar]
- 143.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM Jr, Schreiber RD. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 93: 373–383, 1998 [DOI] [PubMed] [Google Scholar]
- 144.Rojas FA, Carvalho CR, Paez-Espinosa V, Saad MJ. Regulation of cardiac Jak-2 in animal models of insulin resistance. IUBMB Life 49: 501–509, 2000 [DOI] [PubMed] [Google Scholar]
- 145.Ruixing Y, Jinzhen W, Dezhai Y, Jiaquan L. Cardioprotective role of cardiotrophin-1 gene transfer in a murine model of myocardial infarction. Growth Factors 25: 286–294, 2007 [DOI] [PubMed] [Google Scholar]
- 146.Saad MJ, Carvalho CR, Thirone AC, Velloso LA. Insulin induces tyrosine phosphorylation of JAK2 in insulin-sensitive tissues of the intact rat. J Biol Chem 271: 22100–22104, 1996 [DOI] [PubMed] [Google Scholar]
- 147.Saharinen P, Silvennoinen O. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J Biol Chem 277: 47954–47963, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Saharinen P, Vihinen M, Silvennoinen O. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol Biol Cell 14: 1448–1459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sandberg EM, Sayeski PP. Jak2 tyrosine kinase mediates oxidative stress-induced apoptosis in vascular smooth muscle cells. J Biol Chem 279: 34547–34552, 2004 [DOI] [PubMed] [Google Scholar]
- 150.Sayeski PP, Ali MS, Bernstein KE. The role of Ca2+ mobilization and heterotrimeric G protein activation in mediating tyrosine phosphorylation signaling patterns in vascular smooth muscle cells. Mol Cell Biochem 212: 91–98, 2000 [PubMed] [Google Scholar]
- 151.Schieffer B, Luchtefeld M, Braun S, Hilfiker A, Hilfiker-Kleiner D, Drexler H. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ Res 87: 1195–1201, 2000 [DOI] [PubMed] [Google Scholar]
- 152.Shanley TP, Vasi N, Denenberg A, Wong HR. The serine/threonine phosphatase, PP2A: endogenous regulator of inflammatory cell signaling. J Immunol 166: 966–972, 2001 [DOI] [PubMed] [Google Scholar]
- 153.Shaw S, Wang X, Redd H, Alexander GD, Isales CM, Marrero MB. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J Biol Chem 278: 30634–30641, 2003 [DOI] [PubMed] [Google Scholar]
- 154.Sheng Z, Knowlton K, Chen J, Hoshijima M, Brown JH, Chien KR. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem 272: 5783–5791, 1997 [DOI] [PubMed] [Google Scholar]
- 155.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol Cell Physiol 275: C1640–C1652, 1998 [DOI] [PubMed] [Google Scholar]
- 156.Smart N, Mojet MH, Latchman DS, Marber MS, Duchen MR, Heads RJ. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc Res 69: 164–177, 2006 [DOI] [PubMed] [Google Scholar]
- 157.Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, Sack MN. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res 63: 611–616, 2004 [DOI] [PubMed] [Google Scholar]
- 158.Stein AB, Tang XL, Guo Y, Xuan YT, Dawn B, Bolli R. Delayed adaptation of the heart to stress: late preconditioning. Stroke 35, Suppl 1: 2676–2679, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takahama H, Minamino T, Hirata A, Ogai A, Asanuma H, Fujita M, Wakeno M, Tsukamoto O, Okada K, Komamura K, Takashima S, Shinozaki Y, Mori H, Mochizuki N, Kitakaze M. Granulocyte colony-stimulating factor mediates cardioprotection against ischemia/reperfusion injury via phosphatidylinositol-3-kinase/Akt pathway in canine hearts. Cardiovasc Drugs Ther 20: 159–165, 2006 [DOI] [PubMed] [Google Scholar]
- 160.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol 18: 4109–4117, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takano H, Ueda K, Hasegawa H, Komuro I. G-CSF therapy for acute myocardial infarction. Trends Pharmacol Sci 28: 512–517, 2007 [DOI] [PubMed] [Google Scholar]
- 162.Takeishi Y, Huang Q, Wang T, Glassman M, Yoshizumi M, Baines CP, Lee JD, Kawakatsu H, Che W, Lerner-Marmarosh N, Zhang C, Yan C, Ohta S, Walsh RA, Berk BC, Abe J. Src family kinase and adenosine differentially regulate multiple MAP kinases in ischemic myocardium: modulation of MAP kinases activation by ischemic preconditioning. J Mol Cell Cardiol 33: 1989–2005, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Thirone AC, JeBailey L, Bilan PJ, Klip A. Opposite effect of JAK2 on insulin-dependent activation of mitogen-activated protein kinases and Akt in muscle cells: possible target to ameliorate insulin resistance. Diabetes 55: 942–951, 2006 [DOI] [PubMed] [Google Scholar]
- 164.Tissier R, Berdeaux A, Ghaleh B, Couvreur N, Krieg T, Cohen MV, Downey JM. Making the heart resistant to infarction: how can we further decrease infarct size? Front Biosci 13: 284– 301, 2008 [DOI] [PubMed] [Google Scholar]
- 165.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell 121: 667– 670, 2005 [DOI] [PubMed] [Google Scholar]
- 166.Ueda K, Takano H, Hasegawa H, Niitsuma Y, Qin Y, Ohtsuka M, Komuro I. Granulocyte colony stimulating factor directly inhibits myocardial ischemia-reperfusion injury through Akt-endothelial NO synthase pathway. Arterioscler Thromb Vasc Biol 26: e108–e113, 2006 [DOI] [PubMed] [Google Scholar]
- 167.Uozumi H, Hiroi Y, Zou Y, Takimoto E, Toko H, Niu P, Shimoyama M, Yazaki Y, Nagai R, Komuro I. gp130 plays a critical role in pressure overload-induced cardiac hypertrophy. J Biol Chem 276: 23115–23119, 2001 [DOI] [PubMed] [Google Scholar]
- 168.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature 423: 773–777, 2003 [DOI] [PubMed] [Google Scholar]
- 169.Velloso LA, Carvalho CR, Rojas FA, Folli F, Saad MJ. Insulin signalling in heart involves insulin receptor substrates-1 and -2, activation of phosphatidylinositol 3-kinase and the JAK 2-growth related pathway. Cardiovasc Res 40: 96–102, 1998 [DOI] [PubMed] [Google Scholar]
- 170.Vila-Coro AJ, Rodríguez-Frade JM, Martín De Ana A, Moreno-Ortíz MC, Martínez-AC, Mellado M. The chemokine SDF-1α triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J 13: 1699–1710, 1999 [PubMed] [Google Scholar]
- 171.Wang F, Seta Y, Baumgarten G, Engel DJ, Sivasubramanian N, Mann DL. Functional significance of hemodynamic overload-induced expression of leukemia-inhibitory factor in the adult mammalian heart. Circulation 103: 1296–1302, 2001 [DOI] [PubMed] [Google Scholar]
- 172.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell 94: 277–280, 1998 [DOI] [PubMed] [Google Scholar]
- 173.Wu KK. Regulation of endothelial nitric oxide synthase activity and gene expression. Ann NY Acad Sci 962: 122–130, 2002 [DOI] [PubMed] [Google Scholar]
- 174.Xu X, Sonntag WE. Growth hormone-induced nuclear translocation of Stat-3 decreases with age: modulation by caloric restriction. Am J Physiol Endocrinol Metab 271: E903–E909, 1996 [DOI] [PubMed] [Google Scholar]
- 175.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA 98: 9050–9055, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, Bolli R. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol 35: 525–537, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R. Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase Cε p44/42 mitogen-activated protein kinase pSer-signal transducers and activators of transcription 1/3 pathway. Circulation 116: 535–544, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, Bolli R. Role of the protein kinase C-ε-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation 112: 1971–1978, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yajima T, Yasukawa H, Jeon ES, Xiong D, Dorner A, Iwatate M, Nara M, Zhou H, Summers-Torres D, Hoshijima M, Chien KR, Yoshimura A, Knowlton KU. Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation 114: 2364–2373, 2006 [DOI] [PubMed] [Google Scholar]
- 180.Yamaoka K, Saharinen P, Pesu M, Holt VE 3rd, Silvennoinen O, O'Shea JJ. The Janus kinases (Jaks). Genome Biol 5: 253, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamaura G, Turoczi T, Yamamoto F, Siddqui MA, Maulik N, Das DK. STAT signaling in ischemic heart: a role of STAT5A in ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H476– H482, 2003 [DOI] [PubMed] [Google Scholar]
- 182.Yasukawa H, Hoshijima M, Gu Y, Nakamura T, Pradervand S, Hanada T, Hanakawa Y, Yoshimura A, Ross J Jr, Chien KR. Suppressor of cytokine signaling-3 is a biomechanical stress-inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. J Clin Invest 108: 1459–1467, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yin H, Chao J, Bader M, Chao L. Differential role of kinin B1 and B2 receptors in ischemia-induced apoptosis and ventricular remodeling. Peptides 28: 1383–1389, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yokoyama N, Reich NC, Miller WT. Determinants for the interaction between Janus kinase 2 and protein phosphatase 2A. Arch Biochem Biophys 417: 87–95, 2003 [DOI] [PubMed] [Google Scholar]
- 185.Yokoyama N, Reich NC, Miller WT. Involvement of protein phosphatase 2A in the interleukin-3-stimulated Jak2-Stat5 signaling pathway. J Interferon Cytokine Res 21: 369–378, 2001 [DOI] [PubMed] [Google Scholar]
- 186.Yu QJ, Si R, Zhou N, Zhang HF, Guo WY, Wang HC, Gao F. Insulin inhibits β-adrenergic action in ischemic/reperfused heart: a novel mechanism of insulin in cardioprotection. Apoptosis 13: 305–317, 2008 [DOI] [PubMed] [Google Scholar]
- 187.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1α-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood 97: 3342–3348, 2001 [DOI] [PubMed] [Google Scholar]
- 188.Zhao J, Moch H. Absence of JH2 domain mutation of the tyrosine kinase JAK2 in renal cell carcinomas. Acta Oncol 47: 474–476, 2008 [DOI] [PubMed] [Google Scholar]
- 189.Zhu QS, Robinson LJ, Roginskaya V, Corey SJ. G-CSF-induced tyrosine phosphorylation of Gab2 is Lyn kinase dependent and associated with enhanced Akt and differentiative, not proliferative, responses. Blood 103: 3305–3312, 2004 [DOI] [PubMed] [Google Scholar]
- 190.Zuluaga S, Alvarez-Barrientos A, Gutierrez-Uzquiza A, Benito M, Nebreda AR, Porras A. Negative regulation of Akt activity by p38alpha MAP kinase in cardiomyocytes involves membrane localization of PP2A through interaction with caveolin-1. Cell Signal 19: 62–74, 2007 [DOI] [PubMed] [Google Scholar]