Abstract
Neural crest-specific ablation of BMP type IA receptor (BMPRIA) causes embryonic lethality by embryonic day (E) 12.5, and this was previously postulated to arise from a myocardial defect related to signaling by a small population of cardiac neural crest cells (cNCC) in the epicardium. However, as BMP signaling via cNCC is also required for proper development of the outflow tract cushions, precursors to the semilunar valves, a plausible alternate or additional hypothesis is that heart failure may result from an outflow tract cushion defect. To investigate whether the outflow tract cushions may serve as dynamic valves in regulating hemodynamic function in the early embryo, in this study we used noninvasive ultrasound biomicroscopy-Doppler imaging to quantitatively assess hemodynamic function in mouse embryos with P0-Cre transgene mediated neural crest ablation of Bmpr1a (P0 mutants). Similar to previous studies, the neural crest-deleted Bmpr1a P0 mutants died at ∼E12.5, exhibiting persistent truncus arteriosus, thinned myocardium, and congestive heart failure. Surprisingly, our ultrasound analyses showed normal contractile indices, heart rate, and atrioventricular conduction in the P0 mutants. However, reversed diastolic arterial blood flow was detected as early as E11.5, with cardiovascular insufficiency and death rapidly ensuing by E12.5. Quantitative computed tomography showed thinning of the outflow cushions, and this was associated with a marked reduction in cell proliferation. These results suggest BMP signaling to cNCC is required for growth of the outflow tract cushions. This study provides definitive evidence that the outflow cushions perform a valve-like function critical for survival of the early mouse embryo.
Keywords: bone morphogenetic proteins, endocardial cushions, heart defects congenital, heart development, ultrasound biomicroscopy
bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, transduce signals by binding to heteromeric type I and type II receptor complexes (23, 28). Among three type I receptors for BMPs, type IA receptor (BMPRIA, also known as ALK3) is highly expressed in the developing heart (7). Bmpr1a knockout mice die by embryonic day (E) 8.5 exhibiting failure in mesoderm formation (30). Mutations in genes encoding their ligands, Bmp2 or Bmp4, also cause early embryonic lethality (52, 55). Myocardial- or endocardial-specific disruptions of Bmpr1a result in defects of the endocardial cushions of the atrioventricular canal (AVC; Refs. 10, 33, 46) or malformation of the atrioventricular valves (11). Conditional disruption of Bmp2 or Bmp4 in the myocardium (19, 26) further suggests a role for BMP2 and BMP4 in regulating endocardial epithelial-to-mesenchymal transformation (EMT) in the AVC. BMP signaling also plays an essential role in cardiac outflow tract (OFT) development. This is mediated primarily by the cardiac neural crest cells (cNCC), a subpopulation of NCC derived from the postotic hindbrain neural fold (22). Fate mapping analyses show that cNCC contribute to the aorticopulmonary septum (OFT septum), atrioventricular valves, semilunar valves, and conduction system in the mouse heart (2, 18, 32, 38, 40, 48, 53). Ablation experiments have demonstrated that cNCC are indispensable for OFT septation (14, 41, 54). Although cNCC are found in the semilunar valves of the pulmonary artery and the aorta, the precise function of cNCC in semilunar valve development is unknown.
A recent study (48) showed Wnt1-Cre deletion of Bmpr1a in cNCC resulted in embryonic death by E12.5 due to heart failure. This was associated with thinned myocardial wall and reduced myocardial cell proliferation. It was postulated that BMP signaling mediated by a small population of cNCC in the epicardium was required to support myocardial cell proliferation, with heart failure thought to be elicited by reduced blood flow caused by poor cardiac contractile function associated with ventricular hypoplasia (48). However, given that only a very small number of presumptive cNCC were found in the epicardium and cardiac contractility was not analyzed in these mutants, the role of cNCC-mediated BMP signaling in cardiac development and function remained uncertain. It is significant to note that development of the OFT endocardial cushions is well described to be regulated by the level of BMP (1, 4, 6). Thus a plausible alternative or additional hypothesis is that cNCC expression of BMPRIA is required for OFT cushion development. In the context of this hypothesis, cNCC-targeted deletion of BMPRIA may cause cushion defects such that the cushions may fail to provide the critical valvular function required to sustain normal hemodynamic function. Such “semilunar valvular” insufficiency could secondarily cause the thinning of the myocardium observed in the Wnt1-Cre-deleted Bmpr1a mutant.
To investigate this possibility, we used a P0 promoter-driven Cre mouse line (53) to delete Bmpr1a in NCC (31) (P0-Cre+,Bmpr1afx/−, hereafter referred to as “P0 mutants”) and using noninvasive ultrasound biomicroscopy (UBM)-Doppler imaging, we assessed hemodynamic function in the developing embryos between E11.5 and E12.5 (39). In parallel, histological analyses were carried out to establish structure-function correlations. P0-mutant embryos showed similar phenotypes to those previously described for embryos with Wnt1-Cre deletion of Bmpr1a (48). We found no evidence of a functional deficit in myocardial contractility, since quantitative Doppler assessments showed that contractile indices were normal in the P0 mutants. Rather, our in vivo UBM imaging showed acute heart failure arose from cardiac insufficiency caused by reverse diastolic arterial flow. This was associated with hypoplasia of the OFT cushions, consistent with the known requirement for BMP signaling mediated by cNCC in development of the OFT cushions. Our findings provide direct physiological evidence that the OFT cushions serve a valve-like function in the embryonic heart that is essential for the maintenance of unidirectional blood flow and embryo survival, even at such early developmental stages. Our findings show the elaboration of this valve-like function by the OFT cushions requires cNCC expression of BMPRIA.
MATERIALS AND METHODS
Mice
Mouse strains and genotyping were described previously (31, 53). ROSA26 reporter line (47) was used for detection of Cre activity. Mice were maintained in a mixed 129SvEv and C57BL6/J genetic background. Male mice heterozygous for Bmpr1a knockout allele carrying a P0-Cre transgene were bred with female mice homozygous for the floxed allele of Bmpr1a. Twenty-five percent of the resulting pups will be Bmpr1a deficient in a neural crest-specific manner. All mouse experiments were performed in accordance with National Institutes of Environmental Health Science, National Institutes of Health, guidelines covering the humane care and use of animals in research.
Histological Analysis
β-Galactosidase expression in embryos was detected using X-gal as previously described (24). For histological analysis, embryos were fixed in 4% paraformaldehyde in PBS, paraffin embedded, sectioned, and stained with hematoxylin-eosin or eosin alone for X-gal-stained samples. Immunostaining was performed using antibodies: platelet endothelial cell adhesion molecule (PECAM; MEC 13.3 clone, BD Pharmingen, 1:10 dilution), α-smooth muscle actin (clone 1A4; Sigma; 1:50 dilution), phosphohistone H3 (PHH3; Ser10; Cell Signaling; 1:1,000 dilution), and bromodeoxyuridine (Invitrogen; 1:20 dilution) as primary antibodies. Subsequently, species-matched horseradish peroxidase-conjugated secondary antibodies (Jackson; 1:50) were used for PECAM and smooth muscle actin (SMA) followed by the DAB (Sigma) color detection with (dark blue) or without NiCl (brown). Cy3 anti-rabbit IgG (Molecular Probes; 1:500) for PHH3 and Alexa 488 anti-mouse IgG1 (Molecular Probes; 1:500) for bromodeoxyuridine detection in terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assays for apoptotic cells. Imaging was carried out by confocal microscopy (Leica; TCS SP5). To measure PHH3- and TUNEL-positive cells in the OFT cushions, after the immunofluorescence staining was imaged, the same sections were stained with hematoxylin-eosin and rephotographed, and PHH3- and TUNEL-positive cells in the OFT cushions were counted in the merged immunofluorescence and hematoxylin-eosin-stained images of the same slices using Openlab (Improvision, Waltham, MA). At least 7–12 sections from each of the three control and mutant embryos were counted for PHH3 and TUNEL staining.
Electron Microscopy
Embryos were collected and fixed in 2% formaldehyde and 2.5% glutaraldehyde in PBS, pH 7.4, overnight at 4°C. The samples were postfixed for 1 h in potassium ferrocyanide-reduced osmium (45), dehydrated through a graded series of ethanol, and embedded in PolyBed 812 epoxy resin (Polysciences, Warrington, PA). Ultrathin sections were stained with 4% aqueous uranyl acetate followed by Reynolds' lead citrate (43). The sections were imaged using a LEO EM-910 transmission electron microscope (LEO Electron Microscopy, Thornwood, NY) operated at 80 kV, and micrographs were taken using a Gatan Orius SC 1000 CCD Camera (Gatan, Pleasanton, CA).
UBM-Doppler Imaging
All animals used in this study were maintained according to protocols approved by the Institutional Animal Care and Use Committee of the National Institutes of Environmental Health Sciences. Timed pregnant mice were studied at E11.5 and E12.5. Induction was carried out using 2–3% isoflurane, after which 0.7–1.0% was used for maintenance. The abdomen was depilated using a commercial depilatory cream while the mouse lay on a warming pad under heating lamps. The mouse was then transferred to the electrically heated imaging platform of the Vevo 660 UBM-Doppler system (VisualSonics, Toronto, Canada). The body temperature was monitored with a rectal probe and was maintained at 37 ± 1° C via the heated platform and warm air convection from a hair dryer.
Noninvasive UBM-Doppler imaging.
We utilized a Vevo 660 UBM-Doppler system, with a handheld 40-MHz center frequency RMV transducer, which permits high-resolution imaging of in utero mouse embryos for acquisition of physiological data (37, 39). We utilized a noninvasive imaging protocol that allows genotype-phenotype correlation, while permitting the embryos to remain in utero and in vivo, as well as permitting serial imaging over two consecutive days (16). Briefly, warmed acoustic coupling gel was applied to the depilated abdomen. The bladder, in the low midline abdomen, was used as a starting point, and embryos in each uterine horn were then imaged successively in a craniolateral direction. A UBM “map” was established for the entire litter, typically requiring 5–10 min; litters of up to 10 embryos were successfully imaged, and 118/120 (98.3%) living or recently dead embryos were accurately localized, similar to our 100% success rate reported elsewhere (16). After the litter was mapped, each embryo was then imaged for physiological parameters (see below). Scan times were typically 60–90 min. After being imaged, most mice were killed. For serial imaging in a subset of litters, the pregnant mouse was allowed to wake up; no adverse effects of isoflurane anesthesia were noted in these mice.
UBM-Doppler physiological parameters.
As previously reported, we obtained Doppler blood flow data from the middorsal aorta (DA), umbilical artery (UA) and vein, and vitelline artery (36–38). Placement of the pulsed-Doppler gate into the heart also permitted simultaneous acquisition of inflow-outflow flow data (8, 37). In most embryos, we also obtained a four-chamber view of the heart from a transverse imaging plane of the thorax (38); in the minority that could not be imaged as such due to an unfavorable lie, a two-chamber, biventricular view (similar to the short axis view used in clinical echocardiography) was obtained. To gauge ventricular volume, we used the widest end-diastolic (epicardial to epicardial) dimensions and planimetered ventricular areas as surrogates for volumes, similar to our previous study (38); the tiny sizes of embryonic hearts and poor blood-myocardium contrast due to blood echogenicity made true endocardial ventricular measurements impossible. Vessel diameters were not measured, however, because we found that the spatial resolution of the images at these relatively early embryonic stages was worse than in previous studies (38) due to imposed tissue/abdominal wall as a direct result of the noninvasive imaging approach.
Because embryonic orientation with respect to the imaging transducer was highly variable with the noninvasive imaging approach, Doppler data were angle corrected if needed using the Vevo 660's internal capabilities, after attempts were made to align the blood flow as closely (within 20°) as possible with the Doppler beam to minimize the need for angle correction. Mutant data were compared with control data, and the presence of abnormal flow patterns and arrhythmia was determined. Given spatial resolution issues, confounding effects of maternal respiration, and inconsistent orientation, we qualitatively assessed ventricular systolic function from the four- or two-chamber views. Data from injured embryos (e.g., when most of a litter exhibited bradycardia, owing to presumed effects of anesthesia) were omitted from analysis. In addition, those embryos only exhibiting very weak, irregular contractions and poor and/or undetectable blood flow, with unstable hemodynamics (considered “dying”) even within an otherwise healthy litter, were also excluded from analyses; these were embryos from whom reliable physiological data could not be obtained. In total, we imaged 115 embryos, 11 of which were dead or dying, and we obtained stable hemodynamic data from 104 embryos (Table 1).
Table 1.
Summary of ultrasound scanned embryos at E11.5 and E12.5
| Stage | Litters | Number of Embryos |
Phenotype Found in the Mutants |
|||
|---|---|---|---|---|---|---|
| Total | Mutant | Reverse Flow | Dead/Dying | Normal | ||
| E11.5 | 10 | 82 | 23 (28%) | 12 (52%) | 3 (13%) | 8 (35%) |
| E12.5 | 5 | 33 | 11 (33%) | 3 (27%) | 8 (73%) | 0 (0%) |
A total of 3 embryos out of 20 living mutants at embryonic day (E) 11.5 showed arrhythmia: 2 with arrhythmia and reverse blood flow (10%) and 1 with arrhythmia but with normal blood flow (5%). The 3 living mutant embryos at E12.5 showed normal rhythm.
Statistical Analysis
All UBM-Doppler measurements were determined offline in triplicate, with the observer (C. K. L. Phoon) blinded to the genotype. In all, data from 104 E11.5 and E12.5 embryos were available for detailed hemodynamics analysis. A two-tailed Student's t-test (for parametric data with equal variances) or a Mann-Whitney U test (for nonparametric data with unequal variances) was used to compare mutant and control groups (SigmaStat v1.0, San Rafael, CA). Statistical significance was set at P < 0.05, but as in previous studies (38), we did not rely solely on statistical significance but also looked for trends. All data are expressed as means ± SD.
Imaging of Embryonic Heart Structure by Episcopic Fluorescence Image Capture and Computed Tomography Analysis
Episcopic fluorescence image capture (EFIC) was performed as described previously (44). In brief, embryos embedded in paraffin containing Sudan IV, 3% stearin, and up to 20% vybar were sectioned, and fluorescence images of the block face were captured using a Leica MZ FLIII stereomicroscope and Hamamatsu Orca ER CCD camera. Two- and three-dimensional reconstructions were performed using OpenLab 3.17 and Volocity 3.5.1 software (Improvision, Waltham, MA). For computed tomography (CT), embryos fixed in 10% buffered formalin were subsequently stained with 1% osmium tetroxide. Internal structures were visualized by a semitransparent maximum intensity projection and entire embryos were scanned at 6-μm resolution (20).
RESULTS
Neural Crest-Specific Deletion of Bmpr1a Causes Acute Death at E12.5
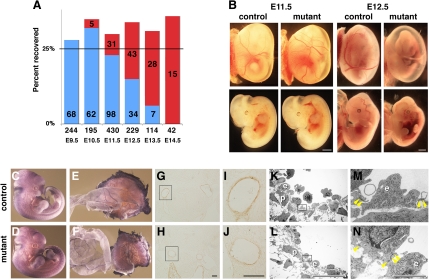
NCC-targeted deletion of Bmpr1a using the P0-Cre transgene (P0-Cre+,Bmpr1a fx/−; P0 mutants) showed no overt phenotype until E11.5, and by E12.5 more than one-half of P0 mutants died from congestive heart failure. This was indicated by severe blood pooling in and near the heart, with no blood in the yolk sac vessels (Fig. 1, A and B). Although P0-mutant embryos comprised more than the expected 25% Mendelian ratio, there are no theoretical reasons for selection against other genotypes; this likely represented a chance occurrence. The survival of some P0-mutant embryos to E12.5–13.5 contrasts with the Wnt1-Cre+/Bmpr1afx/− conditional knockout mouse embryos, which exhibited earlier intrauterine death, with all mutant embryos dying by E12.5 or earlier (48).
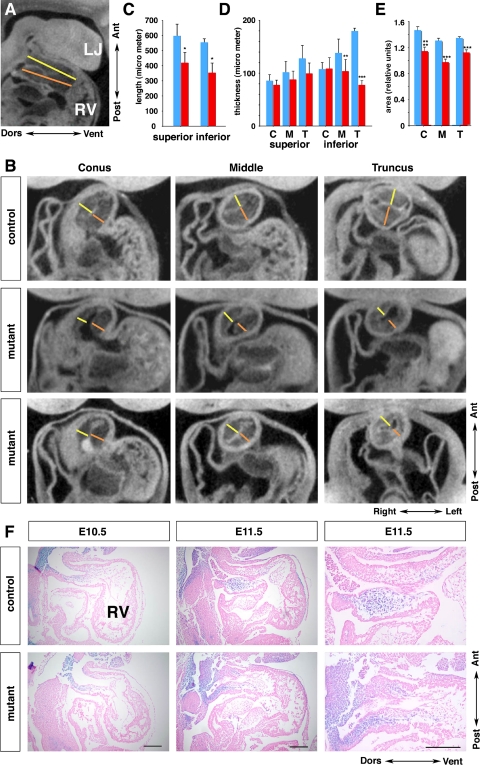
Fig. 1.
Neural crest-specific disruption of Bmpr1a leads to acute death by embryonic day (E) 12.5 without overt abnormality in vessel formation. A: recovery of mutants with no overt abnormalities (blue) vs. those that have died or exhibiting hemorrhage (red) are plotted as a function of gestation age. Actual number of embryos recovered is given in each bar. Total number of embryos is given above gestation age. B: external view of embryos at E11.5 (left 4 panels) and E12.5 (right 4 panels). Top, embryos before removal of yolk sac; bottom, dissected embryos from respective yolk sac. C–F: platelet endothelial cell adhesion molecule staining at E11.5. G–J: α-smooth muscle actin staining at E11.5. G and H: third pharyngeal arch (PhA) artery in control and mutant, respectively. I and J: higher magnification of G and H, respectively. Bar = 100 μm. K–N: transmission electron-microscopy images of vessels. K and L: third PhA artery of control and mutant embryo, respectively. M and N: higher magnification of rectangle areas in K and L, respectively; p, pericyte; e, endothelial cells; yellow arrowhead, junctions of endothelial cells. Bar = 1 mm for B, 20 μm for K and L, and 2 μm for M and N.
To examine whether death of the P0 mutants might be due to vascular defects, we examined the vasculature using PECAM and α-SMA immunostaining. NCC contribute SMA expressing smooth muscle cells that ensheath endothelial tubes in the pharyngeal arch (PhA) arteries. PECAM whole mount immunostaining (Fig. 1, C–F) or immunostaining of vibratome sections (not shown) showed no change in P0 mutants. Similarly, there was no change in the distribution of SMA (Fig. 1, G–J). Examination by transmission electron microscopy showed no vascular defects. The distribution of pericytes, an essential component of vessel maturation, was comparable between mutants and controls (Fig. 1, K–N). These results indicate that acute death is not likely due to defects in vascular development, consistent with the findings of Stottman et al. (48).
Analysis of cNCC Migration and OFT Septation
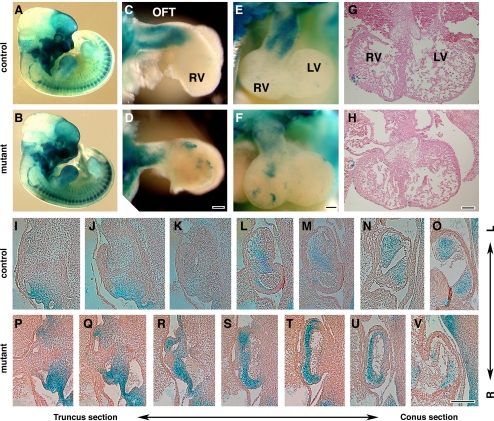
Using the ROSA26 marker and X-gal staining, we examined the distribution of NCC in the P0-mutant embryos. NCC distribution in mutant and control embryos was observed to be identical (Fig. 2, A and B). Histological analyses confirmed the majority of NCC derivatives including those in the peripheral nervous system were properly formed (data not shown). In control embryos, cNCC migrated into the OFT as two streams (Fig. 2, C and E, prongs). However, in P0-mutant embryos, the distribution of cNCC appeared disorganized, and the OFT was shortened (Fig. 2, D and F, n > 5). Notably, in P0-mutant myocardial trabeculations were well formed, but the compact myocardium was somewhat thinner, reminiscent of the compact myocardium observed in Wnt1-Cre-deleted Bmrp1a mutant embryos (48; Fig. 2, G and H). Distribution of cNCC was examined in serial sections of the OFT from the truncus to the conus (from near the PhA to the ventricle) at E11.5 (n = 2 for each). In control embryos, X-gal staining showed cNCC localized in the OFT cushions (Fig. 2, N and O) and in the forming septum (Fig. 2, L and M). In contrast, in P0-mutant embryos, although cardiac cNCC were present, their distribution was abnormal (Fig. 2, S-V). The forming outflow cushions appeared hypoplastic, while tissue that delineates the forming outflow septum was absent, indicating an OFT septation defect. Taken together, our results indicate that in P0 mutants, cNCC migrated into the heart but are abnormally distributed in the OFT septum and endocardial cushions, and this is associated with a failure in OFT septation. These results are similar to the findings reported for the Wnt1-Cre+/Bmpr1afx/− conditional knockout mouse (48).
Fig. 2.
Cardiac neural crest cells migrate into the outflow tract (OFT) with altered distribution in mutant. A and B: visualization of neural crest cells derivatives at E11.5 by X-gal staining (blue) to visualize ROSA26-derived β-gal expression. C–F: whole-mount view of the X-gal stained heart after removal of atrium. C and D: lateral view. E and F: anterior view. G and H: transverse sections of E11.5 hearts stained with hematoxylin-eosin. I–V: frontal sections of OFTs from X-gal-stained embryos counterstained with eosin. LV, left ventricle; RV, right ventricle. Bar = 100 μm.
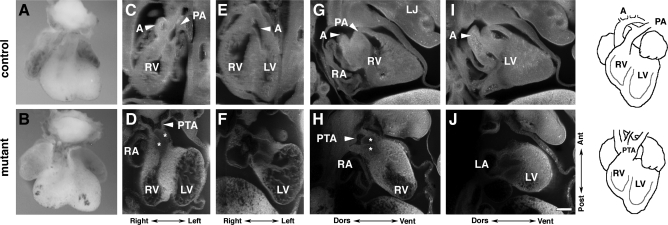
Further analysis of the OFT of P0-mutant embryos at E12.5 using EFIC imaging confirmed an outflow septation defect (Fig. 3). With EFIC imaging, serial sections of the embryo can be digitally resectioned in arbitrary planes (44), allowing detailed examination and comparison of the forming outflow septum and cushion tissues that comprise the semilunar valve primordia. In control embryos, two distinct outflow channels were observed, with proper pulmonary artery-right ventricle (Fig. 3, C and G) and aorta-left ventricle connections (Fig. 3, E and I; supplemental data for this article are available online at the Am J Physiol Heart Circ Physiol website; Supplemental Movie 1). However, P0-mutant embryos show only a single outflow channel arising from the right ventricle (Fig. 3, D and H; Supplemental Movie 2), indicating persistent truncus arteriosus.
Fig. 3.
OFT septation is deficient in mutants. A and B: external ventral view of isolated embryonic hearts at E12.5. C–H: episcopic fluorescence image capture analysis in frontal (C–F) and sagittal sections (G–J). OFT of the mutant (D, F, H, and J) only connects to the RV, with no connection to the LV [persistent truncus arteriosus (PTA) over RV]. A, aorta; LJ, lower jaw; RA, right atrium; PA, pulmonary artery. *Semilunar valve primordia (SLVP). Bar = 200 μm for C–H.
UBM-Doppler Imaging Analysis Reveals Abnormal Blood Flow in the Mutants
As outflow septation defects such as persistent truncus arteriosus do not preclude survival to term, this is not likely the cause for heart failure and death at E12.5 (see for example, refs. 21, 34, 41). To assess cardiac function in the P0 mutants, we used noninvasive fetal ultrasound biomicroscopy-Doppler imaging. This allowed us to evaluate circulatory physiology and hemodynamic function in the same embryos serially at E11.5 and E12.5.
Pathophysiological hallmark is diastolic retrograde (reverse) arterial flow.
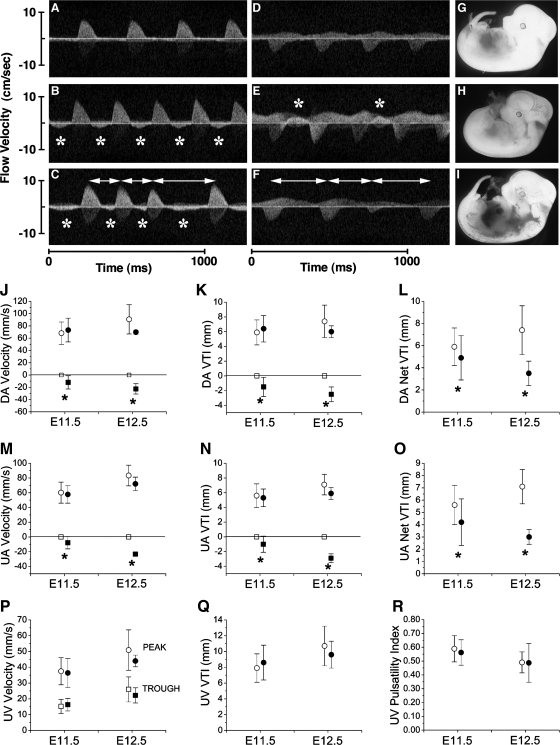
The hallmark hemodynamic abnormality of the P0-mutant embryos was retrograde, or reverse, arterial flow during diastole, in both the DA (Fig. 4, B and C) and UA (Fig. 4E). Of the 79 E11.5 living embryos interrogated, 20 (25%) were P0-mutant embryos, representing the expected Mendelian ratio, and 60% of these (12/20) exhibited reversed arterial flow. Only 3 of the 25 E12.5 living embryos (12%) were P0 mutants, all of which exhibited abnormal reversed arterial flow. No control embryos ever exhibited reversed arterial blood flow. None of the viable embryos with reversed arterial flow exhibited blood pooling either at E11.5 or E12.5 (data not shown and Fig. 4H). In contrast, severe blood pooling (Fig. 4I) and bloodless yolk sac (Fig. 1B) were observed in dead or dying embryos.
Fig. 4.
Blood circulation is abnormal in the mutant. Representative Doppler profiles of blood flow in the dorsal aorta (DA; A–C) and umbilical artery (UA; D–F) at E11.5 are shown. Data are means ± SD. *Reversed arterial flow. Double arrows are cardiac arrhythmia. These embryos were serially imaged at E11.5 and E12.5 and harvested at E12.5 (G–I). A, D, and G: control. B, C, E, F, H, and I: mutant. J–O: summary of hemodynamic analyses. Peak Doppler blood flow velocity, velocity-time integral (VTI), and net VTI in DA and UA are shown as indicated. P: umbilical vein (UV) velocities [systolic (peak) and diastolic (trough)]. Q: UV VTI. R: UV pulsatility index = (systolic − diastolic)/systolic. ○ and □, control; ● and ■, mutant; ○ and ●, forward flow; □ and ■, reverse flow.*P < 0.01.
Of the 104 total living embryos imaged (Table 1), 15 with stable hemodynamics were serially imaged from E11.5 to E12.5 to address the hypothesis that abnormal hemodynamics observed at E11.5 may lead to death by E12.5. Three of these embryos exhibited reversed arterial blood flow at E11.5, with two of these dying by E12.5. An additional eight embryos were also serially imaged at E11.5–12.5, but the data were excluded from the analyses, as the mothers appeared sick at E12.5, possibly from anesthesia. Two of the eight embryos exhibiting reversed flow at E11.5 from the “sick litters” were found dead at E12.5. The advanced necrotic state of these two embryos suggested death likely occurred much earlier and not related to the effects of the anesthetic. Therefore, these studies suggest four of five embryos exhibiting reversed flow at E11.5 were dead by E12.5, indicating reverse arterial flow is likely the underlying cause of death.
Reduced net cardiac output despite normal contractile function in P0 mutants.
Heart rate and all indices of contractile function were found to be normal in all viable mutants at both E11.5 and E12.5 (Fig. 4, J–R; Table 2). Notably, OFT regurgitation did not lead to progressive ventricular dilatation or the typical course of initially augmented systolic function followed by progressive systolic deterioration and dilatation, typical for a mature adult heart subjected to volume overload (27). However, P0-mutant embryos failed to increase their blood flow velocities and velocity-time integral (VTI) in DA and UA (Fig. 4, L and O). The failure to increase peak blood flow velocities at E12.5 was the one hemodynamic parameter that suggested failing contractile ability, but this may be secondary to the effects of valvular insufficiency and low cardiac output. Moreover, the degree of diastolic reversed flow worsened from E11.5 to E12.5 (Fig. 4, J, K, M, and ). Indeed, at E12.5, the mean reversed VTI was 2.5 vs. 6.0 mm forward VTI; thus 42% of the stroke volume regurgitated back into the heart. This worsening diastolic reversed blood flow caused a significantly reduced net (forward minus retrograde) VTI in both the DA and UA compared with control embryos and a decreasing net VTI from E11.5 to E12.5 (Fig. 4, L and O). Importantly, these data were derived from embryos that survived, therefore, corresponding to P0-mutant embryos with more robust circulation. There were no significant pericardial effusions in any embryos. Taken together, the data show that P0-mutant embryos not only failed to increase their cardiac output in concert with growth, but rather there is a significant decrease in cardiac output from E11.5 to E12.5.
Table 2.
Summary of ultrasound biomicroscopy-Doppler hemodynamic data
| Functional Parameter | Stage |
|
|---|---|---|
| E11.5 | E12.5 | |
| Heart rate, beats/min | ||
| Control | 192±22 | 211±22 |
| Mutant | 184±24 | 208±34 |
| End-diastolic dimension*, mm | ||
| Control | 1.72±0.11 | 1.89±0.16 |
| Mutant | 1.75±0.08 | 1.89±0.17 |
| End-diastolic area*, mm2 | ||
| Control | 1.55±0.21 | 1.92±0.39 |
| Mutant | 1.58±0.08 | 1.91±0.14 |
| Fractional area shortening* | ||
| Control | 0.44±0.05 | 0.41±0.04 |
| Mutant | 0.44±0.04 | 0.52±0.04 |
| P-R interval, msec | ||
| Control | 85±6 | 85±7 |
| Mutant | 82±10 | 84±16 |
| DA ET, msec | ||
| Control | 129±16 | 125±12 |
| Mutant | 134±16 | 129±16 |
| DA CL, msec | ||
| Control | 318±44 | 288±30 |
| Mutant | 334±61 | 293±51 |
| ET-to-CL ratio | ||
| Control | 0.408±0.043 | 0.436±0.032 |
| Mutant | 0.406±0.043 | 0.442±0.026 |
| DA acceleration time, msec | ||
| Control | 40±6 | 41±5 |
| Mutant | 42±8 | 37±6 |
| VA peak flow velocity, mm/s | ||
| Control | 30.8±6.6 | 34.1±10.2 |
| Mutant | 31.4±6.5 | 33.8±3.2 |
| VA velocity-time integral, mm | ||
| Control | 2.6±0.6 | 2.6±0.7 |
| Mutant | 2.6±0.7 | 2.8±0.3 |
Data are means ± SD. Data from mutants did not differ significantly from control data. Total embryos imaged: E11.5 control (59), E11.5 mutant (20), E12.5 control (22), and E12.5 mutant (3). CL, cycle length; DA, dorsal aorta; ET, ejection time; VA, vitelline artery.
Because of inadequate resolution and variable fetal lie/positioning within the maternal abdomen, fewer embryos were imaged for ventricular dimensions, but n ≥ 7 except for E12.5 P0 mutants.
Arrhythmia in a subset of P0 mutants.
Three of twenty (15%) E11.5 P0 mutants exhibited cardiac arrhythmias, whereas none of the control embryos did. One displayed an irregular heart rhythm with pauses, another one with premature beats, and the third with premature beats, as well as a three-beat run of tachycardia at 286 beats/min. Atrioventricular conduction, as determined by the mechanical P-R interval (8), was intact and normal in mutants (Table 2).
Extraembryonic vascular beds appear normal in P0 mutants.
We also examined placental vascular physiology by interrogating umbilical venous Doppler hemodynamics (36). P0-mutant embryos were found to have normal umbilical venous hemodynamics (Fig. 4, P–R). PECAM staining of the placenta also showed normally developed vasculature (Fig. 1F). Taken together, our data suggest that the placenta itself is normal in the P0 mutants. Although not statistically significant, blood flow in the UA and umbilical vein did not increase as much in P0 mutants as in controls, while the UA net VTI decreased from E11.5 to E12.5. Therefore, we speculate that altered placental blood flow might compromise embryonic oxygenation and nutrition in P0 mutants. Bloodless yolk sacs further suggested that yolk sac blood flow may be decreased in P0 mutants. However, PECAM staining showed normal vascular development in the yolk sac (Fig. 1). Our Doppler imaging in vivo showed normal vitelline arterial peak blood flow velocities and VTI at E11.5 and E12.5 (Table 2). Together, these findings suggest normal yolk sac blood flow in P0-mutant embryos.
Hypoplastic OFT Cushions in the P0 Mutants
The finding of retrograde arterial flow indicated that the valve-like function of OFT cushions is compromised in the P0 mutants. Consistent with this, OFT cushion defects were noted in the Wnt1-Cre+/Bmpr1afx/− mouse model (48), as well as other models of BMP ablation or modulation of NCC activity (4, 6, 17, 21, 25, 28). In fact, in the Wnt1-Cre Bmpr1a-ablated mutants, OFT cushions were poorly developed, although nonobstructive (48). Our EFIC imaging analysis of E12.5 P0-mutant hearts showed the proximal OFT cushions appear to coapt (Fig. 3, D and H). To further quantitatively assess development of the OFT cushions in the P0 mutants, we carried out CT of E11.5 embryos (20).
The CT analyses indicated the OFT cushions were hypoplastic, with wide variation in the thickness of the cushion tissue. To quantitate these changes, we made measurements of the thickness of the superior and inferior OFT cushions at the junction of the right ventricle (conus), middle, and anterior end of the OFT subjacent to the PhA (truncus; Fig. 5, B and D). These measurements showed significant thinning of the inferior OFT cushion (middle and truncus measurements; Fig. 5D), but no significant difference was found for the superior cushions at any level (Fig. 5D). Given the wide variability in thickness of the cushion tissue in the P0 mutants (Fig. 5B), we also made area measurements of the cushion tissue over these same regions. This analysis showed a significant reduction for all three regions of the OFT cushion in the P0-mutant embryos (Fig. 5E). Hematoxylin-eosin staining of sagittal sections supported the CT data: the OFT cushions of the mutant embryos at E10.5 and E11.5 were thinner and reduced in size compared with the controls (Fig. 5F). Thus, although the OFT cushions were formed in the P0 mutants, they were hypoplastic and this could account for the retrograde flow observed by Doppler ultrasound imaging.
Fig. 5.
Outflow tract cushions are abnormally short and hypoplastic at E11.5. A and B: reconstructed sagittal and frontal sections by computed topography scan with lines delineating the length (A) and thickness (B) measurements, respectively. Yellow and orange lines indicate the superior and inferior OFT cushion, respectively. C–E: summary of the length (C) and thickness (D) measurements; area measurements of the cushion tissue over these same regions (E). Blue: control; red: mutant; n = 4 for each group. C, conus (closer to RV); M, middle; T, truncus (closer to PhA). *P < 0.02, **P < 0.01, ***P < 0.0001, ****P < 0.0002. F: sagittal sections stained with X-gal showing NCC (blue) in the OFT at E10.5 and E11.5, counterstained with eosin (pink). Right: higher magnifications of middle. Bar = 200 μm. LJ, lower jaw; Ant, anterior, Post, posterior; Dors, dorsal; Vent, ventral.
Examination of reconstructed three-dimensional CT images also revealed significant shortening of the OFT (Fig. 5, A and C; n = 4 for each genotype), consistent with the whole mount view (Fig. 2, C and D). In the mutants, the OFT rotation was reduced to <45° as it ascended away from the right ventricle (Fig. 5B, middle and bottom; n = 4), compared with the ∼90° rotation observed in the control embryos (Fig. 5B, top; n = 4). Together these findings show in the P0 mutants, there is marked hypoplasia of the OFT cushions associated with a shortened and malrotated OFT.
Reduced Cell Proliferation in the OFT Cushions
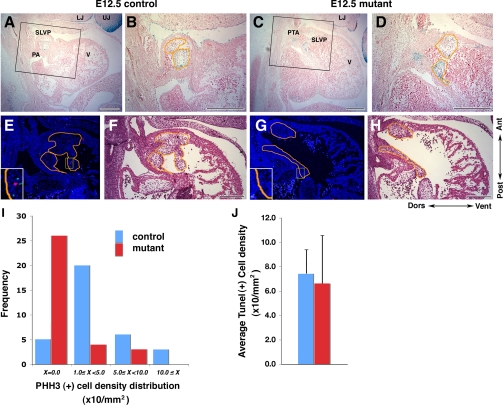
To examine if loss of BMP signaling via P0-Cre-targeted Bmpr1a deletion may affect the growth or survival of OFT cushion mesenchymal cells, we quantitated cell proliferation and apoptosis in the OFT cushions of E11–12 P0-mutant embryos. These studies were carried out using two P0-mutant embryos shown via UBM-Doppler imaging to have reverse blood flow at E12.5 and one additional P0-mutant embryo. Immunostaining for phosphohistone H3 revealed a marked reduction in cell proliferation in the OFT cushions in the P0 mutants (Fig. 6, E –I). TUNEL assays showed no difference in apoptosis (Fig. 6J). Using the ROSA26 lacZ reporter, we also confirmed that cNCC migrated into the OFT cushions in these P0 mutants as in controls (Fig. 6, A–D). These findings suggest that hypoplasia of the OFT cushions may be caused by a reduction in cell proliferation.
Fig. 6.
Proliferation in the SLVP at E12.5 is reduced in mutant embryos. A–D: sagittal sections of heart stained with X-gal (blue) to delineate ROSA26 reporter expression in NCC. B and D: higher magnification of the rectangle areas in A and C. E–H: sagittal sections stained with phosphohistone H3 (PHH3; red), terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL; green), and Topro3 (blue) for nucleus. F and H: same sections as in E and G stained with hematoxylin-eosin after darkfield fluorescence imaging. Orange lined areas indicate SLVP, where signals were counted. E and G, insets: higher magnification images. I: summary of PHH3 density distribution per area of SLVP. Average of PHH3 density in control and mutant was 4.2 and 1.2, respectively (P = 0.001). J: summary of TUNEL-positive cell density in the SLVP. V, ventricle; red, control; blue, mutant. Bar = 200 μm.
DISCUSSION
Our analysis of mouse embryos with P0-Cre-mediated Bmpr1a deletion in NCC showed persistent truncus arteriosus and hypoplasia of the OFT cushions, phenotypes that are consistent with the well-documented requirement for BMP signaling in OFT septation and cushion development. Previous studies (6, 21, 25, 29, 48) of mouse embryos with targeted disruption of genes encoding BMP ligands or receptors have shown OFT cushion hypoplasia. Severe hypoplasia of the OFT cushions was also found with gene-targeted disruption of Smad4, an activator Smad required for transcriptional activation in response to signaling initiated by TGF-β superfamily ligands (17). Conversely, hyperplastic cardiac valves were found in neonatal or adult mice homozygous for a null allele of Smad6, an inhibitory Smad (9), and hyperplastic cushions also were found in mice with null alleles of Noggin, a BMP antagonist (4). In the latter study, increased migration of neural crest cells was shown to contribute to the cushion hyperplasia.
In our study, hypoplasia of the OFT cushions was associated with a reduction in cell proliferation in the P0-Cre-deleted Bmpr1a mutants. As P0-Cre specifically targets Bmpr1a deletion in NCC lineage, our results would suggest BMP signaling to NCC is required for supporting the growth of the OFT cushions. In agreement with this, we note that with Nkx2.5-Cre-mediated Bmp4 deletion hypoplastic outflow cushions were observed in conjunction with a severe reduction in cell proliferation in the cushion tissue and that cushion hypoplasia is synergistically worsened by a compound Bmp4/Bmp7 mutation (25). Hypoplastic OFT cushions were also observed with Bmp4 deletion mediated by an anterior heart field (AHF)-specific Cre transgenic line, Mef2c-AHF-Cre. The OFT cushions in these mutant embryos showed no change in either cell proliferation or cell death (29). The findings from the Nkx2.5 and Mef2c-AHF-Cre experiments would suggest the ligand(s) for the responding NCC is likely derived from the OFT myocardium. We note formation of the OFT cushions is also known to be dependent on the endocardium, which undergoes an EMT to generate the cushion mesenchyme. TGF-β superfamily members are known to play an important role in regulating endocardial EMT (3, 26, 49). Studies using endocardial explant cultures showed increased endocardial EMT in the Noggin knockout mouse embryos, suggesting that enhanced EMT also may contribute to the cushion hyperplasia in the Noggin knockout mutants. We note Noggin is expressed in the endocardium and myocardium. However, Mef2c-AHF-Cre-deleted Bmp4 mutants showed no change in endocardial EMT (29). Overall, our results together with the previous studies lend further support to the concept that tight control of BMP signaling is important in regulating OFT cushion development. In particular, our results suggest BMP signaling to NCC is essential for normal growth of the OFT cushions.
Our in vivo physiological analyses of cardiovascular function show P0 mutants with Bmpr1a deficiency in NCC develop reverse blood flow in the aorta and peripheral arterial system as early as E11.5, with acute heart failure and death ensuing at E12.5. Cardiac contractile function was surprisingly normal. Also unexpected was the failure to detect progressive ventricular dilatation. It is notable in NFATc1−/− mutant embryos similar OFT regurgitation was observed in the absence of ventricular dilation (38). PECAM staining and analysis with light and electron microscopy showed no vascular defects in the P0 mutants. These physiological findings together with the observed hypoplasia of the OFT cushions would suggest that the failure of the OFT cushions to provide a valve-like function may underlie the reverse aortic blood flow and subsequent circulatory failure. In this setting, the thinning of the myocardium may be secondary to the hemodynamic perturbation caused by the OFT cushion defect. However, we cannot entirely rule out the thinned myocardium being a primary defect elicited by Bmpr1a conditional deletion in neural crest cells, as suggested by Stottman et al. (48). If this were to cause decreased cardiac output over a very short window of time, its contribution to acute circulatory failure by E12.5 may not have been detected by our UBM-Doppler study. Overall, these hemodynamic data constitute the first in vivo physiological demonstration that unidirectional blood flow and embryonic survival may be predicated on OFT cushions functioning as dynamic valves in the E11.5 embryo before remodeling of the OFT cushions to form mature semilunar valves. We note the Wnt1-Cre-deleted Bmpr1a conditional knockout mouse embryos exhibited 100% lethality by E12.5, earlier than our P0 mutants. Hypoplasia of the OFT cushions was also more severe, which may accelerate heart failure and death with earlier onset and more severe reversal of arterial blood flow (48). Although both models represent “neural crest-specific” conditional knockouts, we speculate that earlier timing of recombination and slight differences in expression patterns likely account for the differences in the severity of the cushion defects observed between the Wnt1-Cre and P0 mutants.
While unidirectional blood flow has been observed at the earliest stages of the functioning heart, and well before proper valve development (15, 36, 50), evidence for its requirement for survival, while intuitive, has been scant. We note that the importance of valve-like functioning of dynamically apposing endocardial cushions has been recognized for decades (35). Patten et al. (35) obtained experimental evidence in the chick embryo of valve-like action provided by dynamically apposing endocardial cushions, both in the atrioventricular inflow and in the truncal outflow. In NFATc1 mutants, OFT septation and OFT cushions are normally developed at E12.5; however, the OFT cushions fail to develop into semilunar valves at E13.5, resulting in reverse blood flow at E13.5 and lethality by E14.5–15.5 (5, 38, 42). As Bmpr1a mutants have reverse blood flow at E11.5 and E12.5, BMP signaling in NCC is required earlier for cushion development than the endocardial requirement for NFATc1 in semilunar valve morphogenesis. Our study indicates cNCC-specific BMPRIA-mediated signaling is required to support cell proliferation and growth of the OFT cushions. Overall, our findings indicate that the survival of early midgestation embryos requires the critical valve-like function provided by the OFT cushions before the emergence of mature semilunar valves. Interestingly, the P0-Cre-deleted Bmpr1a mutants and the NFATc1 knockout embryos (38) both exhibited normal contractile function, and yet neither knockout embryos exhibited ventricular dilatation despite an expected substantial volume overload. While it is possible the short time course of OFT regurgitation did not provide an opportunity for ventricular dilatation, these findings also raise interesting questions regarding possible differences in the hemodynamic forces at play and/or the biomechanical properties of embryonic myocardium compared with the adult myocardium (27).
Overall, these findings show the importance of examining fetal circulatory physiology in evaluating cardiovascular phenotypes in the developing embryo. The P0-Cre-deleted Bmpr1a knockout mouse is now the second mouse model shown to have OFT regurgitant blood flow before precipitous death at midgestation. These findings suggest this pathophysiology may be an important mechanism that could contribute to early human intrauterine demise. Of note, the prenatal incidence of congenital cardiac malformations is estimated at 10 times the incidence observed postnatally (12). How an embryonic heart will respond to pressure overloads is another question entirely. While it has been established that obstruction to flow with presumed pressure overload leads to functional and structural defects (13, 51), the impact on the myocardium and how it may compare to volume overloads is not known. Our study provides the first definitive evidence that the OFT cushions in the early embryonic heart serve as dynamic valves required for survival of the embryo, even before complete septation of the heart. Further analyses of molecular pathways and tissue interactions in OFT cushion and valve development may help to elucidate the etiology of human congenital heart diseases associated with the disruption of unidirectional blood flow.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants K08-HL-04414 and American Heart Association (Heritage Affiliate) Grant-in-Aid No. 655907T (to C. K. L. Phoon) and Grant Z01-HL-005701 (to C. W. Lo), the Intramural Research Program of the National Institute of Environmental Health Sciences Grant ES071003-10, and a conditional gift from RIKEN-Brain Science Institute to Y. Mishina. S. Kishigami was supported in part by the Japanese Science Promotion Society. K. Yamamura was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology (Monkasho, Japan).
Supplementary Material
ACKNOWLEDGMENTS
We thank Linda Leatherbury for a discussion of EFIC data, Xiao Qing Zhao and Shigehito Yamada for computational reconstruction of EFIC data, and Takashi Uchimura for help with the histological analyses.
Present addresses: A. Nomura-Kitabayashi, Laboratory of Molecular Cellular Biology, Sunnybrook Research Centre, 2075 Bayview Avenue, S207B, Toronto, Ontario M4N 3M5, Canada; S. Kishigami, School of Biology-Oriented Science and Technology, Kinki University, Wakayama, Japan; K. Abe, BioResource Center, RIKEN Tsukuba Institute, 3-1-1 Koyadai, Tsukuba, Ibaraki 305-0074, Japan; C. W. Lo, Dept. of Developmental Biology, John G. Rangos Sr. Research Center, RANCH 8120, Children's Hospital Drive, 45th Street and Penn Avenue, Pittsburgh, PA 15201.
REFERENCES
- 1.Armstrong EJ, Bischoff J. Heart valve development. Endothelial cell signaling and differentiation. Circ Res 95: 459–470, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol 18: 67–76, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science 23: 2080–2082, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Choi M, Stottman RW, Yang YP, Meyers EN, Klingensmith J. The bone morphogenetic protein antagonist Noggin regulates mammalian cardiac morphogenesis. Circ Res 100: 220–228, 2007 [DOI] [PubMed] [Google Scholar]
- 5.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392: 182–186, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Delot EC, Bahamonde ME, Zhao M, Lyons M. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development 130: 209–220, 2003 [DOI] [PubMed] [Google Scholar]
- 7.DeWulf N, Verschueren K, Lonnoy O, Morén A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology 136: 2652–2663, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CKL, Glickstein JS, Buyon JP, Investigators PRIDE Utility of cardiac monitoring in fetuses at risk for congenital heart block. The PR Interval and Dexamethasone Evaluation (PRIDE) Prospective Study. Circulation 117: 485–493, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr, Falb D, Huszar D. A role for Smad6 in development and homeostasis of the cardiovascular system. Nature 24: 171–174, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci USA 99: 2878–2883, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaussin V, Morley GE, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway SJ, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishman GI, Burch JB, Vatner SF. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res 97: 219–226, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman JIE. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol 16: 155–165, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Hove JR, Köster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421: 172–177, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hutson MR, Kirby ML. Model systems for the study of heart development and disease: cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol 18: 101–110, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji RP, Phoon CKL, Aristizábal O, McGrath KE, Palis J, Turnbull DH. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ Res 92: 133–135, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ji RP, Phoon CKL. Noninvasive localization of nuclear factor of activated T cells c1−/− mouse embryos by ultrasound biomicroscopy-Doppler allows genotype-phenotype correlation. J Am Soc Echocardiogr 18: 1415–1421, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Jia Q, McDill BW, Li SZ, Deng C, Chang CP, Chen F. Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Dev Biol 311: 172–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development 127: 1607–1616, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev 17: 2362–2367, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson JT, Hansen MS, Wu I, Healy LJ, Johnson CR, Jones GM, Capecchi MR, Keller C. Virtual histology of transgenic mouse embryos for high-throughput phenotyping. PLoS Genet 2: e61, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SK, Solloway MJ, Robertson EJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol 235: 449– 466, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science 220: 1059–1061, 1983 [DOI] [PubMed] [Google Scholar]
- 23.Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev 16: 265–273, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kishigami S, Komatsu Y, Takeda H, Nomura-Kitabayashi A, Yamauchi Y, Abe K, Yamamura K, Mishina Y. Optimized beta-galactosidase staining method for simultaneous detection of endogenous gene expression in early mouse embryos. Genesis 44: 57–65, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci USA 101: 4489–4494, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132: 5601–5611, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Magid NM, Opio G, Wallerson DC, Young MS, Borer JS. Heart failure due to chronic experimental aortic regurgitation. Am J Physiol Heart Circ Physiol 267: H556–H562, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Massague J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 103: 295–309, 2000 [DOI] [PubMed] [Google Scholar]
- 29.McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn 237: 3200–3209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9: 3027–3037, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32: 69–72, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res 98: 1547–1554, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor type IA signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development 133: 3473–3484, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development 133: 2419–2433, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patten BM, Kramer TC, Barry A. Valvular action in the embryonic chick heart by localized apposition of endocardial masses. Anat Rec 102: 299–311, 1948 [DOI] [PubMed] [Google Scholar]
- 36.Phoon CKL, Aristizábal O, Turnbull DH. 40 MHz Doppler characterization of umbilical and dorsal aortic blood flow in the early mouse embryo. Ultrasound Med Biol 26: 1275–1283, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Phoon CKL, Turnbull DH. Ultrasound biomicroscopy-Doppler in mouse cardiovascular development. Physiol Genomics 14: 3–15, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Phoon CKL, Ji RP, Aristizábal O, Worrad DM, Zhou B, Baldwin HS, Turnbull DH. Embryonic heart failure in NFATc1−/− mice: novel mechanistic insights from in utero ultrasound biomicroscopy. Circ Res 95: 92–99, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Phoon CKL. Imaging tools for the developmental biologist: ultrasound biomicroscopy of mouse embryonic development. Pediatr Res 60: 14–21, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Poelmann RE, Jongbloed MR, Molin DG, Fekkes ML, Wang Z, Fishman GI, Doetschman T, Azhar M, Gittenberger-de Groot AC. The neural crest is contiguous with the cardiac conduction system in the mouse embryo: a role in induction? Anat Embryol (Berl) 208: 389–393, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Porras D, Brown CB. Temporal-spatial ablation of neural crest in the mouse results in cardiovascular defects. Dev Dyn 237: 153–162, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 392: 186–190, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenthal J, Mangal V, Walker D, Bennett M, Mohun TJ, Lo CW. Rapid high resolution three dimensional reconstruction of embryos with episcopic fluorescence image capture. Birth Defects Res C Embryo Today 72: 213–223, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Russell LD. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec 190: 99–111, 1978 [DOI] [PubMed] [Google Scholar]
- 46.Song L, Fässler R, Mishina Y, Jiao K, Baldwin HS. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol 301: 276–286, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Soriano P. Generalized lacZ expression with ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Stottman RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development 131: 2205–2218, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugi Y, Uamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol 269: 505–518, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Teichert AM, Scott JA, Robb GB, Zhou YQ, Zhu SN, Lem M, Keightley A, Steer BM, Schuh AC, Adamson SL, Cybulsky MI, Marsden PA. Endothelial nitric oxide synthase gene expression during murine embryogenesis: commencement of expression in the embryo occurs with the establishment of a unidirectional circulatory system. Circ Res 103: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, Colan SD, Benson CB, Lock JE, Perry SB. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation 110: 2125–2131, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9: 2105–2116, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, Kuratani S, Yamamura K. A novel transgenic technique that allows specific marking of the neural crest lineage in mice. Dev Biol 212: 191–203, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Yelbuz TM, Waldo KL, Zhang X, Zdanowicz M, Parker J, Creazzo TL, Johnson GA, Kirby ML. Myocardial volume and organization are changed by failure of addition of secondary heart field myocardium to the cardiac outflow tract. Dev Dyn 228: 152–160, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122: 2977–2986, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.