Abstract
We sought to examine the potential role of oxidative stress on skeletal muscle function with advancing age. Nuclear magnetic resonance (NMR) was employed to simultaneously assess muscle perfusion (arterial spin labeling) and energetics (31P NMR spectroscopy) in the lower leg of young (26 ± 5 yr, n = 6) and older (70 ± 5 yr, n = 6) healthy volunteers following the consumption of either placebo (PL) or an oral antioxidant (AO) cocktail (vitamins C and E and α-lipoic acid), previously documented to decrease plasma free radical concentration. NMR measurements were made during and after 5 min of moderate intensity (≈5 W) plantar flexion exercise. AO administration significantly improved end-exercise perfusion (AO, 50 ± 5, and PL, 43 ± 4 ml·100 g−1·min−1) and postexercise perfusion area under the curve (AO, 1,286 ± 236, and PL, 866 ± 144 ml/100 g) in older subjects, whereas AO administration did not alter hemodynamics in the young group. Concomitantly, muscle oxidative capacity (time constant of phosphocreatine recovery, τ) was improved following AO in the older (AO, 43 ± 1, and PL, 51 ± 7 s) but not the young (AO, 54 ± 5, and PL, 48 ± 7 s) group. These findings support the concept that oxidative stress may be partially responsible for the age-related decline in skeletal muscle perfusion during physical activity and reveal a muscle metabolic reserve capacity in the elderly that is accessible under conditions of improved perfusion.
Keywords: leg blood flow, oxidative capacity, exercise, nuclear magnetic resonance
with advancing age, structural and functional changes in the peripheral vasculature develop with potentially deleterious consequences, including a diminished perfusion of skeletal muscle both at rest and during exercise (3, 7, 8, 22, 25, 26, 30, 36). The detrimental effects of this age-associated decline in muscle perfusion and the associated decrement in O2 delivery could be closely linked to muscle energetics, as evidenced by the previously demonstrated decline in skeletal muscle oxidative capacity under O2 supply-limited conditions in young, healthy individuals (15, 16). Despite the potentially negative impact of these age-associated changes, the practical consequence of reduced skeletal muscle blood flow in this cohort remains poorly understood.
The paucity of information concerning the effects of reduced perfusion on muscle function with healthy aging may be due in part to the difficulty of simultaneously assessing perfusion and metabolism within skeletal muscle. Using multiparametric nuclear magnetic resonance (NMR) techniques, we have begun to address this issue and have recently documented that the age-related decline in bulk limb blood flow is also evident when perfusion within the skeletal muscle is examined during and following lower limb exercise (38). However, in this study of a healthy, elderly cohort, muscle oxidative capacity was not diminished, suggesting some maintenance of skeletal muscle functional capacity in a relatively hypoperfused state. This finding appears to support the concept that the elderly undergo beneficial adaptations within the skeletal muscle to overcome the age-related decline in perfusion, or that perfusion is not a limiting factor in oxidative phosphorylation, but also raises the question of what effect an intervention capable of restoring perfusion toward that of the young might have on muscle function.
One intervention that may favorably alter peripheral hemodynamics in the elderly is antioxidant (AO) administration. This preclinical intervention has been shown to improve vascular function through the scavenging of plasma free radicals and associated improvement in nitric oxide bioavailability and could thus prove particularly efficacious in the elderly because of the known increase in plasma free radical concentration with advancing age (1, 31). Indeed, in sedentary-aged subjects it has been demonstrated that supraphysiological doses of vitamin C (ascorbic acid) (11, 32) and tetrahydrobiopterin (an endothelial nitric oxide synthase cofactor) (13) transiently restore endothelium-dependent vasodilation, whereas intra-arterial ascorbate has been shown to acutely improve resting (17) and exercising (19) limb blood flow. Recently, our group has quantified the efficacy of an enteral AO cocktail (vitamins C and E and α-lipoic acid) to significantly reduce O2-centered plasma free radical concentration in both young (27) and old (39) and documented the ability of this intervention to acutely improve brachial artery vasodilation during exercise in the elderly (9). Together, these studies provide encouraging evidence for the capacity of an acute AO administration to improve vascular function in older individuals but leave uncertainty concerning muscle metabolic status following this intervention.
To our knowledge, no studies to date have evaluated age-related changes in skeletal muscle perfusion and metabolism under varied levels of oxidative stress. Therefore, using a randomized, crossover design, we employed a multiparametric NMR approach to simultaneously measure muscle perfusion and metabolism during and following exercise after either placebo (PL) or enteral AO administration. We hypothesized that 1) because of a likely age-related elevation in oxidative stress, acute AO consumption would improve end-exercise skeletal muscle perfusion in older individuals to a greater degree than their younger counterparts and that 2) the increased perfusion and concomitant elevation in O2 delivery would improve skeletal muscle oxidative metabolism in the elderly.
METHODS
Subjects.
Six young (26 ± 2 yr) and six older (70 ± 2 yr) volunteers were recruited for this study. Health history and physical activity were assessed using a standard questionnaire. All subjects were normotensive (<140/90 mmHg), normally active (i.e., no regular exercise routine), and free of overt disease. None of the subjects was taking prescription medication or vitamin supplements. The study protocols were approved by Pitié Salpêtrière University Hospital, in accordance with the Declaration of Helsinki, and written informed consent was obtained from all subjects. Exercise work rate was normalized for all subjects according to the cross-sectional area of the gastrocnemius/soleus muscle group, identified from a high-resolution reference image acquired immediately before exercise.
Acute AO administration.
All subjects received both the AO cocktail and PL in a double-blind, balanced, crossover design. The PL and AO trials were separated by 2–7 days. AOs were taken in two doses separated by 30 min, with the first dose ingested 2 h before the initiation of the experiment. The first AO dose consisted of 300 mg of α-lipoic acid, 500 mg vitamin C, and 200 IU vitamin E, and the second dose was 300 mg α-lipoic acid, 500 mg vitamin C, and 400 IU vitamin E (water dispersible). PL microcrystalline cellulose capsules were of similar taste and appearance and were likewise consumed in two doses. We have recently documented the efficacy of this AO cocktail to acutely reduce plasma free radical concentration in young and elderly cohorts (27, 39).
NMR procedures.
Studies were carried out in a 4 T, 46 cm internal bore, superconducting magnet (Magnex 4/60) interfaced to a Bruker Biospec NMR spectrometer. Before the experiments, all subjects were familiarized with the experimental setup and were accustomed to lying supine in the magnet. Subjects arrived to the laboratory in a fasted state, and the calf of the subject's dominant leg was placed inside a 17-cm inner-diameter transversal electromagnetic 1H transmit-and-receive volume coil. Affixed within the volume coil was a circular, 8-cm diameter custom-built 31P surface coil. This volume/surface coil unit was positioned underneath the gastrocnemius muscle, centered at the widest portion of the muscle as verified by sequential reference images.
After a 5-min period of baseline measurements, subjects performed plantar flexion exercise (0.33 Hz, and ≈5 W) for 5 min on a custom-built, nonferrous hydraulic ergometer (10), followed by 10 min of postexercise measurements. Subjects were encouraged to perform muscle contraction as quickly as possible (0.5–1 s) to maximize relaxation time between contractions. To maintain optimal signal-to-noise and minimize motion artifact, all images were collected during this 2-s relaxation phase.
Calf muscle perfusion and energy metabolism were studied by rapidly (3 s) interleaved acquisitions of SATIR arterial spin labeling (ASL) perfusion imaging and 31P spectroscopy of the high-energy phosphate metabolites, as described previously (10). This interleaved acquisition scheme was initially proposed and implemented by our group (5) and was here driven by the multiscan control tool developed and made commercially available by Bruker. A complete dataset was generated every 3 s. The multiscan control tool automatically distributed the raw interleaved data into distinct imaging, 1H, and 31P spectroscopy files, which were immediately ready for processing with standard ParaVision and XWIN NMR Bruker software.
SATIR perfusion images.
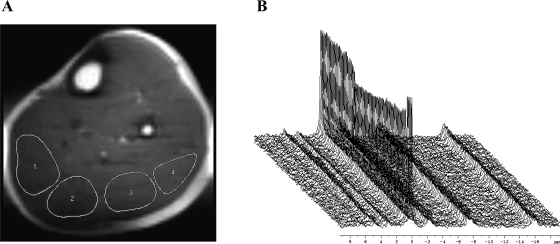
A temporary perfusion map was extracted by summing the differences between pairs of images (tagged and untagged) acquired during and after plantar flexion exercise. Four regions of interest (ROIs) of 2 to 3 cm2 were traced inside the sections of the gastrocnemius and soleus muscles, carefully excluding voxels containing lipids or vessels (Fig. 1). Similar ROIs were selected in all the images of the series, and perfusion (f) was calculated according to the following:
where M stands for the image intensity in muscle ROI after slice-selective (SS) and nonselective (NS) inversion, T is the ASL time (0.82 s), λ is the tissue/blood partition coefficient, and r1 is the tissue spin-lattice relaxation rate. In muscle, we assume r1 = 0.66 s−1 and λ = 0.9.
Fig. 1.
A: example 1H image of the lower leg (Ultrafast spin echo imaging, 6 × 6 mm; field of view, 22 × 11 cm; and acquisition matrix, 128 × 36) used for perfusion mapping. Four regions of interest have been highlighted from medial (left side of image) to lateral (right side of image). B: stack plot of 31P spectra (−7 to 20 parts/million) with visible changes in the phosphocreatine (PCr) peak (−1.5–1.5 parts/million) during plantar flexion exercise.
Perfusion data are presented as the average from all ROIs (9 to 10 cm2). End-exercise perfusion was determined using the averaged values from the 10 s immediately preceding the cessation of exercise. Postexercise hyperemic decay was determined using cumulative perfusion area under the curve (AUC, trapezoidal rule) for 5 min following the cessation of exercise, according to the equation:
31P spectra of high-energy phosphates.
The 31P free induction decays were summed four by four and were processed in a similar fashion to the 1H spectra, except for an 8-Hz line-broadening exponential multiplication. Pi and phosphocreatine (PCr) integrals were calculated with integration limits set to 5.6/3.5 and 1.5/−1.5 parts/million, respectively (Fig. 1). No zero filling was used. Muscle intracellular pH was calculated from the chemical shift (δ) between the Pi and PCr peaks (34).
PCr recovery was fitted monoexponentially to determine the PCr time constant.
Statistical analyses.
Statistics were performed with the use of commercially available software (SigmaStat 3.10, Systat Software, Point Richmond, CA). Student's t-tests were used to identify between-group differences in end-exercise perfusion, PCr depletion, perfusion AUC, and PCr time constant. Repeated-measure analysis of variance was used to identify between-group differences in the Pi-to-PCr ratio, with the Bonferroni test used for post hoc analysis when a significant main effect was found. All group data are expressed as means ± SE. Significance was established at P < 0.05.
RESULTS
Young (26 ± 5 yr) and older (70 ± 5 yr) subjects exhibited similar physical characteristics (body mass index, 170 ± 5 vs. 166 ± 5 cm and 69 ± 7 vs. 66 ± 7 kg; and young vs. older, 23 ± 2 vs. 24 ± 3 kg/m2). Gastrocnemius/soleus cross-sectional area was not different between young and old, and thus work rate (young, 4.7 ± 0.05, and old, 4.8 ± 0.1 W) and total work performed (young, 1,444 ± 32, and old, 1,422 ± 19 W) were similar between groups. None of the subjects participated in a regular physical activity program (International Physical Activity Questionnaire Category 1).
Oxidative stress and ASL perfusion.
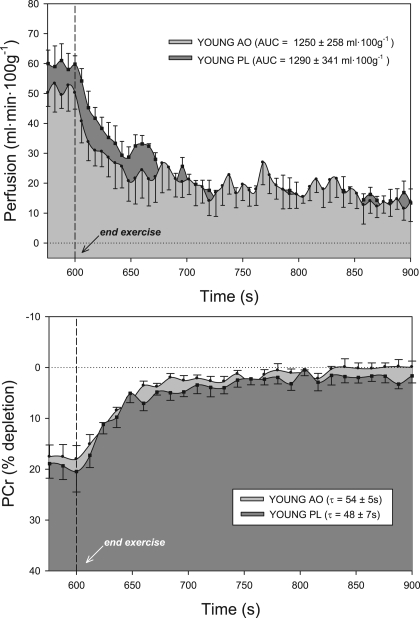
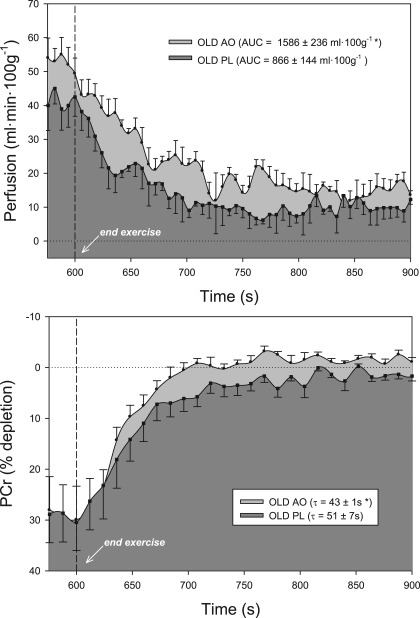
At the end of plantar flexion exercise, lower-leg muscle perfusion increased significantly following AO consumption versus PL in older subjects (AO, 50 ± 5, and PL, 43 ± 4 ml·100 g−1·min−1) (Fig. 2). In the young, end-exercise perfusion was not statistically different than PL following the consumption of AO, though there was a tendency for a decrease in perfusion (AO, 50 ± 5, and PL, 59 ± 3 ml·100 g−1·min−1) (Fig. 3). Likewise, hyperemic decay following cessation of exercise (perfusion AUC) was prolonged in older subjects following AO (AO, 1,286 ± 236, and PL, 866 ± 144 m/100 g; Fig. 2) but unchanged in the young group (AO, 1,250 ± 258, and PL, 1,290 ± 341 ml/100 g; Fig. 3).
Fig. 2.
Lower leg perfusion kinetics (top) and PCr resynthesis (bottom) at the end of 5-min plantar flexion exercise in young subjects following consumption of either antioxidant (AO) cocktail (light gray) or placebo (PL; dark gray). AO consumption did not significantly alter perfusion or metabolism in this group. Dashed line indicates the cessation of exercise. Values are means ± SE. AUC, area under the curve; τ, time constant.
Fig. 3.
Lower leg perfusion kinetics (top) and PCr resynthesis (bottom) at the end of 5-min plantar flexion exercise in older subjects following consumption of either AO cocktail (light gray) or PL (dark gray). AO consumption significantly improved both perfusion and metabolism in this cohort. Dashed line indicates the cessation of exercise. Values are means ± SE. *P < 0.05, significantly different than PL.
Oxidative stress and 31P energetics.
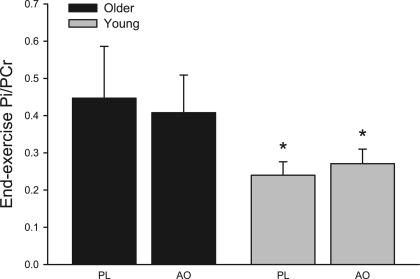
A clear age-related difference in PCr depletion during exercise was observed, which has been reported previously (38). However, AO consumption did not alter end-exercise PCr depletion in either young (AO, 18 ± 3%, and PL, 19 ± 3%; Fig. 2) or older (AO, 29 ± 7%, and PL, 28 ± 6%; Fig. 3) subjects. The repletion of PCr at the cessation of exercise, an index of muscle oxidative capacity, improved following AO consumption in the older group (AO, 43 ± 1, and PL, 51 ± 7 s) but was not different between PL and AO trials in young subjects (AO, 54 ± 5, and PL, 48 ± 7 s). The ratio of Pi to PCr was not affected by AO consumption in either group but was significantly greater in older subjects in both PL and AO conditions (Fig. 4). In the elderly, the shortening of PCr recovery time following AO correlated well (r2 = 0.84) with the AO-induced improvement in perfusion recovery. Resting intracellular pH did not differ between young and older subjects in either PL (young, 7.04 ± 0.02, and older, 7.00 ± 0.02) or AO (young, 7.01 ± 0.01, and older, 7.00 ± 0.01) conditions. Likewise, end-exercise pH did not differ between young and older subjects in either PL (young, 7.03 ± 0.07, and older, 7.04 ± 0.02) or AO (young, 7.04 ± 0.02, and older, 7.03 ± 0.02) conditions, values which were not significantly changed from rest in either group or condition.
Fig. 4.
End-exercise Pi-to-PCr ratio (Pi/PCr) in older (black) and young (gray) subjects. Values are means ± SE. *P < 0.05, significantly different than older group.
DISCUSSION
The present study has identified the capacity of oxidative stress to blunt exercise hyperemia with advancing age and demonstrated the ability of an AO intervention with known efficacy to acutely remediate this age-related decline in exercising skeletal muscle perfusion. The AO-mediated restoration of perfusion and the associated increase in O2 availability were accompanied by a significant improvement in skeletal muscle energetics in elderly individuals, providing evidence for a hemodynamic and metabolic reserve in this cohort that is accessible under conditions of reduced oxidative stress.
Novel NMR-based approach to the study of muscle function.
In the present study, a unique interleaved pulse sequence was employed to acquire perfusion and 31P data every 3 s, resulting in a highly resolved set of data collected in vivo. Measurements of perfusion within the skeletal muscle tissue were made using the ASL method (Fig. 1A). Skeletal muscle energetics were assessed through 31P NMR spectroscopy (Fig. 1B), and from this spectra PCr depletion and recovery kinetics were evaluated as an index of muscle oxidative demand and capacity, respectively (6, 14, 24). Both of these measurements are unique in that they allow for the probing of perfusion and metabolism in a noninvasive manner with very high-spatial and temporal resolution and do so in a focused ROI within the exercising muscle tissue. The simultaneous acquisition of ASL and PCr provides the truly unique opportunity to examine the real-time interplay between perfusion and metabolism in the active skeletal muscle, providing new information concerning the matching of these parameters in the human leg.
Oxidative stress and skeletal muscle perfusion.
The present study provides striking new evidence of the capacity of an acute AO intervention to improve skeletal muscle perfusion in the elderly. In the PL condition, perfusion within the gastrocnemius and soleus muscle groups during plantar flexion exercise was attenuated by ≈20% in older subjects (Fig. 3) compared with young (Fig. 2). Following acute AO administration, exercise-induced perfusion and postexercise hyperemia were 30–40% greater in the elderly (Fig. 3), whereas these hemodynamic parameters were unaffected by AO consumption in younger individuals (Fig. 2). Viewed together, it appears that AO consumption effectively restores muscle perfusion to that of the younger group, ablating the peripheral hypoperfusion in the lower leg of elderly subjects.
In a similar cohort, Jablonski et al. (17) recently documented the ability of a high-dose (2 g over 20 min) intravenous infusion of ascorbic acid to improve resting leg blood flow in the elderly. The present study extends these findings to exercise, demonstrating a similar magnitude of effect on hemodynamics but with a much lower dose, over-the-counter AO cocktail known to reduce plasma free radical concentration in the elderly (39). To our knowledge, this is the first study to identify the capacity of acute oral AO administration to restore the age-related decline in skeletal muscle perfusion during leg exercise. This new finding not only provides convincing evidence that oxidative stress plays a significant role in the well-documented decline of skeletal muscle blood flow with advancing age but also demonstrates an encouraging plasticity for this age-related adaptation.
Interestingly, AO administration did not improve hemodynamics in the young, which is most likely attributable to better endogenous AO capacity and therefore a lesser degree of oxidative stress in these subjects (33). In fact, despite evidence for the ability of the AO cocktail to reduce plasma free radical concentration both at rest and during exercise (27), end-exercise perfusion tended to be lower after AO consumption in the young group (Fig. 2). This is in agreement with recent work from our group identifying a negative effect of AO administration on exercise-induced vasodilation in young subjects (9), adding credence to the concept that some level of free radical concentration is essential for normal function during exercise in young, healthy individuals.
This positive outcome on the peripheral circulation following an AO-mediated reduction in vascular oxidative stress in the elderly is in contrast to the largely disappointing results from interventional trials concerning the effects of AO supplementation on many indicators of cardiovascular health (37). Such equivocal findings may be due, at least in part, to a tendency for clinical trials to focus on specific patient populations and also to a general lack of knowledge concerning in vivo AO status and efficacy. The current study, reporting favorable vascular and metabolic effects in the elderly following acute AO administration, has attempted to address both of these potentially confounding influences. Specifically, recruiting a focused cohort of subjects in good overall health with no diagnosed disease or use of prescription medication provided the opportunity to examine the impact of oxidative stress on muscle function in a preclinical context. Regarding efficacy, the AO intervention used in the present study was selected based on previous studies from our group, using electron paramagnetic resonance spectroscopy which has quantified the capacity of this AO cocktail to reduce plasma O2-centered free radical levels in both young (27) and older (39) subjects. This previous work thus confirms the ability of the AO intervention to significantly attenuate vascular oxidative stress in an acute manner and lends confidence to the apparent link between reduced oxidative stress and improved muscle perfusion in the present study.
Oxidative stress and skeletal muscle energetics.
In the PL condition, PCr depletion and Pi-to-PCr ratio were 30–40% greater in older compared with young subjects at a similar work rate, indicative of a greater metabolic stress during exercise in the elderly (Figs. 3 and 4). In contrast to the pronounced effect of AO administration on perfusion in the elderly, this intervention did not alter PCr depletion or Pi-to-PCr ratio, suggesting that age-related differences in muscle energetics during exercise was due to inherit differences in skeletal muscle metabolism or simply greater relative stress due to a diminished exercise capacity in the older group. To our knowledge, no prior studies have been undertaken to specifically examine the impact of oxidative stress on these parameters in younger and older individuals.
PCr recovery was significantly faster in the elderly following AO consumption, suggestive of improved skeletal muscle energetics in this group (Fig. 3). This remarkable and acute effect of AO on skeletal muscle energetics in the elderly could be the result of either an AO-mediated change in muscle metabolism or a secondary consequence of the AO-mediated improvement in perfusion and thus O2 availability. Regarding the prior, animal studies have identified a profound effect of oxidative stress on cardiac muscle energetics in the context of ischemic preconditioning (40), suggesting that repeated exposure to reactive oxygen species may directly affect mitochondrial function. However, the time course for the observed effect (2 to 3 h postconsumption) in the present study makes this an unlikely mechanism for the AO-mediated improvement in PCr recovery. Alternately, there is already evidence supporting the influence of perfusion on muscle energetics from NMR-based studies, with a clear association between O2 availability and the rate of PCr recovery at the end of exercise in animals (23) and normal, healthy subjects (15, 16). Furthermore, while recognizing the limitation of correlation analysis with small sample sizes, a clear relationship (r2 = 0.84) between metabolism and perfusion was noted in the present study, such that the greatest AO-induced increase in perfusion AUC also yielded the largest improvement in PCr recovery time. Based on this previous and current work, we speculate that the AO-induced increase in muscle perfusion observed in the present study augmented O2 delivery to the muscle tissue, which may be viewed as effectively improving O2 availability for oxidative metabolism within the skeletal muscle.
Evidence of hemodynamic and metabolic reserve in aging skeletal muscle.
The observed improvement in perfusion and the subsequent beneficial effect of muscle oxidative capacity following AO administration raise the question of whether age-related changes in these parameters should be viewed as a detrimental or adaptive. Theoretically, Fick's equation dictates that any decline in perfusion will decrease O2 delivery and may jeopardize O2 availability to the exercising muscle tissue. However, we have previously reported an age-related decline in limb blood flow that is accompanied by improved O2 extraction, apparently compensating for the reduction in O2 delivery and thereby maintaining O2 consumption (22). In terms of metabolism, there is little consensus regarding the impact of age on muscle energetics (28). In the present study we did not observe an age-related difference in PCr repletion following moderate-intensity exercise in the PL condition (Figs. 2 and 3), supporting the body of evidence against a decline in metabolic capacity in leg skeletal muscle with healthy aging (6, 21, 35). Together, these previous and current findings suggest that healthy aging is associated with a decline in exercising peripheral blood flow but that this adaptation does not result in skeletal muscle dysfunction in this cohort.
With these previous studies demonstrating an apparent maintenance of skeletal muscle function with advancing age, it is noteworthy that AO administration unmasked such a marked improvement in both hemodynamic and metabolic parameters, which may suggest a reserve capacity in the elderly. In pathophysiological states such as peripheral vascular disease characterized by chronic ischemia, the detrimental effects of reduced perfusion on muscle function and energetics are well documented (4, 18, 20, 29). In this population, there is some evidence for improved peripheral blood flow following AO interventions (2), which may presumably also improve muscle energetics through improved O2 supply. Though less severe, perfusion and metabolic data from the present study in the elderly parallel these clinical findings, suggesting that the aging skeletal muscle may operate in a functional but somewhat depressed state due to elevated oxidative stress, which may be acutely restored in the presence of a more favorable AO environment.
Experimental considerations.
In the present study, a select group of carefully screened volunteers was recruited to participate in a crossover paradigm with multiple study days. A sufficient number of subjects were enrolled to achieve adequate statistical power (β ≥ 0.8) in the major variables while ensuring compliance of all subjects for the duration of the study. While we did not collect blood samples for analysis, all volunteers were considered to be healthy based on health histories and physical examination. Additionally, we acknowledge the known disparity between the acute and chronic effects of AO administration on vascular function (11). Whether the acute reversal of chronic vasoconstriction reported here could be maintained with longer-term AO supplementation cannot be ascertained from the present findings but is an intriguing possibility that awaits further study.
GRANTS
This study was funded in part by the Tobacco-Related Disease Research Program Grant 15RT-0100 (to R. S. Richardson); the Francis Family Foundation (Parker B. Francis Fellowship) (to D. W. Wray); American Heart Association Scientist Development Grant 0835209N (to D. W. Wray); National Heart, Lung, and Blood Institute Grant 1-P01-HL-091830-01A1 (to R. S. Richardson); and the Association Française contre les Myopathies (to R. S. Richardson).
REFERENCES
- 1.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90: 7915–7922, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arosio E, De Marchi S, Zannoni M, Prior M, Lechi A. Effect of glutathione infusion on leg arterial circulation, cutaneous microcirculation, and pain-free walking distance in patients with peripheral obstructive arterial disease: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 77: 754–759, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100: 1085–1094, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Carlier PG, Bertoldi D. In vivo functional NMR imaging of resistance artery control. Am J Physiol Heart Circ Physiol 288: H1028–H1036, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Carlier PG, Brillault-Salvat C, Giacomini E, Wary C, Bloch G. How to investigate oxygen supply, uptake, and utilization simultaneously by interleaved NMR imaging and spectroscopy of the skeletal muscle. Magn Reson Med 54: 1010–1013, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chilibeck PD, Paterson DH, McCreary CR, Marsh GD, Cunningham DA, Tohmpson RT. The effects of age on kinetics of oxygen uptake and phosphocreatine in humans during exercise. Exp Physiol 83: 107–117, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Dinenno FA, Seals DR, DeSouza CA, Tanaka H. Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol 531: 573–579, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Donato AJ, Wray DW, Bailey DM, Uberoi A, Richardson RS. Exercise-induced brachial vasodilation: effects of age, free radicals, and exercise training (Abstract). FASEB J 19: A1231, 2005 [Google Scholar]
- 10.Duteil S, Bourrilhon C, Raynaud JS, Wary C, Richardson RS, Leroy-Willig A, Jouanin JC, Guezennec CY, Carlier PG. Metabolic and vascular support for the role of myoglobin in humans: a multiparametric NMR study. Am J Physiol Regul Integr Comp Physiol 287: R1441–R1449, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haseler LJ, Hogan MC, Richardson RS. Oxygen uptake and 31P response to square wave and ramp exercise: implications for oxygen deficit, debt, and metabolic control (Abstract). Soc Magn Reson Med Proc 6: 1790, 1998 [Google Scholar]
- 15.Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol 86: 2013–2018, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol 97: 1077–1081, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol 103: 1715–1721, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kemp GJ. Mitochondrial dysfunction in chronic ischemia and peripheral vascular disease. Mitochondrion 4: 629–640, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer CM. Peripheral arterial disease assessment: wall, perfusion, and spectroscopy. Top Magn Reson Imaging 18: 357–369, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutsuzawa T, Shioya S, Kurita D, Haida M, Yamabayashi H. Effects of age on muscle energy metabolism and oxygenation in the forearm muscles. Med Sci Sports Exerc 33: 901–906, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Lawrenson L, Poole JG, Kim J, Brown CF, Patel PM, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: the effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Marro KI, Olive JL, Hyyti OM, Kushmerick MJ. Time-courses of perfusion and phosphocreatine in rat leg during low-level exercise and recovery. J Magn Reson Imaging 25: 1021–1027, 2007 [DOI] [PubMed] [Google Scholar]
- 24.McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT. Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol 81: 1331–1338, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol 292: H1516–H1522, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Russ DW, Kent-Braun JA. Is skeletal muscle oxidative capacity decreased in old age? Sports Med 34: 221–229, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Schunk K, Romaneehsen B, Rieker O, Duber C, Kersjes W, Schadmand-Fischer S, Schmiedt W, Thelen M. Dynamic phosphorus-31 magnetic resonance spectroscopy in arterial occlusive disease: effects of vascular therapy on spectroscopic results. Invest Radiol 33: 329–335, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582: 63–71, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, aging. Science 273: 59–63, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of no availability and oxidative stress in humans. Hypertension 38: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda G. Bioenergetics of intact human muscle: a 31P nuclear magnetic resonance study. Mol Biol Med 1: 77–94, 1983 [PubMed] [Google Scholar]
- 35.Taylor DJ, Crowe M, Bore PJ, Styles P, Arnold DL, Radda GK. Examination of the energetics of aging skeletal muscle using nuclear magnetic resonance. Gerontology 30: 2–7, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest 33: 79–86, 1974 [DOI] [PubMed] [Google Scholar]
- 37.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol 101: 75D–86D, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil S, Carlier PG, Richardson RS. Multiparametric NMR-based assessment of skeletal muscle perfusion and metabolism during exercise in elderly persons: preliminary findings. J Gerontol A Biol Sci Med Sci 64: 968–974, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 116: 433–441, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Yaguchi Y, Satoh H, Wakahara N, Katoh H, Uehara A, Terada H, Fujise Y, Hayashi H. Protective effects of hydrogen peroxide against ischemia/reperfusion injury in perfused rat hearts. Circ J 67: 253–258, 2003 [DOI] [PubMed] [Google Scholar]