Abstract
Primary human aging may be associated with augmented Rho kinase (ROCK)-mediated contraction of vascular smooth muscle and ROCK-mediated inhibition of nitric oxide synthase (NOS). We hypothesized that the contribution of ROCK to reflex vasoconstriction (VC) is greater in aged skin. Cutaneous VC was elicited by 1) whole body cooling [mean skin temperature (Tsk) = 30.5°C] and 2) local norepinephrine (NE) infusion (1 × 10−6 M). Four microdialysis fibers were placed in the forearm skin of eight young (Y) and eight older (O) subjects for infusion of 1) Ringer solution (control), 2) 3 mM fasudil (ROCK inhibition), 3) 20 mM NG-nitro-l-arginine methyl ester (NOS inhibition), and 4) both ROCK + NOS inhibitors. Red cell flux was measured by laser-Doppler flowmetry over each site. Cutaneous vascular conductance (CVC) was calculated as flux/mean arterial pressure and normalized to baseline CVC (%ΔCVCbaseline). VC was reduced at the control site in O during cooling (Y, −34 ± 3; and O, −18 ± 3%ΔCVCbaseline; P < 0.001) and NE infusion (Y, −53 ± 4, and O, −41 ± 9%ΔCVCbaseline; P = 0.006). Fasudil attenuated VC in both age groups during mild cooling; however, this reduction remained only in O but not in Y skin during moderate cooling (Y, −30 ± 5; and O, −7 ± 1%ΔCVCbaseline; P = 0.016) and was not altered by NOS inhibition. Fasudil blunted NE-mediated VC in both age groups (Y, −23 ± 4; and O, −7 ± 3%ΔCVCbaseline; P < 0.01). Cumulatively, these data indicate that reflex VC is more reliant on ROCK in aged skin such that approximately half of the total VC response to whole body cooling is ROCK dependent.
Keywords: skin blood flow, aging, temperature regulation, norepinephrine
peripheral cutaneous vessels constrict in response to cold exposure through distinct reflex and locally regulated mechanisms. Localized cooling of skin induces vasoconstriction (VC) that is partly mediated by both stimulation of α2-adrenoreceptors by norepinephrine (NE) (9, 11) and upregulation of the downstream intracellular messenger Rho kinase (ROCK) (30, 31), whereas reflex sympathetically mediated VC relies on NE (∼60% of the VC response) and other coreleased sympathetic neurotransmitters (∼40%) (29, 34).
In aged skin (>60 yr), the reflex VC response is not only blunted but relies entirely on an already compromised adrenergic mechanism (18, 32–34). Furthermore, the vascular signaling pathways that couple adrenoreceptor activation to VC may be altered (20, 31). During localized cooling in aged skin, the magnitude of the VC response is not diminished but relies more on ROCK and less on other adrenergically stimulated protein kinase signaling cascades (31–33). ROCK can be stimulated by NE or mitochondrial superoxide generated in response to localized cooling (2, 28). Activated ROCK elicits VC through two distinct mechanisms: 1) inhibition of myosin light chain (MLC) phosphatase, thereby maintaining MLC phosphorylation without Ca2+ influx (i.e., Ca2+ sensitization) and 2) inducing the translocation of α2C-receptors from the Golgi apparatus to the cell membrane (1, 5, 14). Although ROCK has a clear role in VC during localized cooling, the extent with which ROCK mediates reflex VC remains unclear.
A greater dependence on ROCK in primary aging may parallel that observed in other age-associated vascular pathologies such as atherosclerosis, hypertension, erectile dysfunction, and diabetes (3, 4, 8, 13, 19, 23, 35). From a thermoregulatory standpoint, ROCK may serve as an important mechanism in aged skin to sustain cutaneous VC and prevent excessive heat loss during cold exposure; however, this may occur at the expense of microvascular function since ROCK has a mutually inhibitory influence on endothelial nitric oxide synthase (eNOS) (21–23). In vitro, ROCK decreases eNOS expression and activity and increases arginase activity, thereby reducing NO bioavailability, whereas cGMP-dependent protein kinase, a product of NO metabolism, inhibits Rho activation (21–23). Thus ROCK inhibition may have a vasoprotective effect due in large part to its putative effects on NO bioavailability (23). Collectively, augmented ROCK activity appears to have a deleterious effect on vascular function; whether or not upregulated ROCK during whole body cooling predates the onset of other vascular pathologies has yet to be determined.
The purpose of this study was to determine the extent with which ROCK participates in reflex VC during whole body cooling. We hypothesized that ROCK-dependent mechanisms would contribute to the reflex VC response to a greater extent in aged skin. We further hypothesized that ROCK inhibition with local fasudil supplementation would attenuate the VC response to 1) gradual whole body cooling and 2) localized NE perfusion, and that this reduction would be larger in aged than in young skin, which would suggest that ROCK is upregulated or unmasked due to the reduced activity of other adrenergically stimulated protein kinase signaling mechanisms. Finally, we sought to determine whether nitric oxide synthase (NOS) inhibition, which would putatively function to disinhibit ROCK, would have a differential effect on the cutaneous VC response between age groups.
METHODS
Subjects.
With Pennsylvania State University Institutional Review Board approval and after verbal and written informed consent, eight young (20 ± 1 yr; 3 men and 5 women) and eight older (73 ± 2 yr; 5 men and 3 women) subjects participated in the study. Young women were tested in the early follicular phase (days 1–7) of the menstrual cycle, and older women were postmenopausal and not taking hormone replacement therapy. All subjects were healthy, nonobese, normotensive, normal cholesterolemic, nonsmokers, and not taking any medications that would otherwise alter cardiovascular or thermoregulatory function. All procedures conformed to the standards set by the Declaration of Helsinki.
Instrumentation.
On the morning of an experiment, between 8:00 am and 10:00 am, subjects arrived at the laboratory and were instrumented with four microdialysis (MD) fibers (10 mm, 20-kDa cutoff membrane, MD 2000 Bioanalytical Systems, West Lafayette, IN) placed intradermally in the ventral forearm using aseptic technique. Before fiber placement, ice packs were applied to MD sites for 5 min to temporarily anesthetize the skin. For each fiber, a 25-gauge needle guide was inserted horizontally into the dermis such that the entry and exit points were ∼2.5 cm apart. MD fibers were threaded through the needle. The needle was then withdrawn leaving the membrane in place. After all fibers were taped in place, lactated Ringer solution was perfused at 2 μl/min (Bee Hive controller and Baby Bee microinfusion pumps, Bioanalytical Systems) for 60–90 min to allow for local hyperemia due to needle insertion trauma to subside.
To control skin temperature, subjects wore a water-perfused suit that covered the entire body except for the face, feet, hands, and forearms. Copper-constantan thermocouples were placed on the surface of the skin at six sites: calf, thigh, abdomen, chest, back, and upper arm. The unweighted mean of these sites provided an index of mean skin temperature (Tsk).
To obtain an index of skin blood flow, red cell flux was continuously measured with laser-Doppler flowmetry (LDF) probes (MoorLAB, Temperature Monitor SH02, Moor Instruments, Devon, UK). LDF probes were placed in the center of local heaters and positioned directly over each MD fiber site. To specifically isolate reflex mechanisms, local skin temperature was clamped at 33°C throughout the experiment. Arterial blood pressure was measured every 5 min throughout the experiment via brachial auscultation. Mean arterial pressure (MAP) was calculated as the diastolic blood pressure plus one-third pulse pressure. Cutaneous vascular conductance (CVC) was calculated as the ratio of LDF flux to MAP and expressed as percent change from baseline (%ΔCVCbaseline).
Protocol.
After the instrumentation of MD fibers and the resolution of local hyperemia, pharmacological agents were perfused for 45–60 min. MD fiber sites were randomly assigned with respect to the position on the forearm and were perfused with 1) lactated Ringer solution serving as control, 2) 3 mM fasudil (ROCK inhibitor), 3) 20 mM NG-nitro-l-arginine methyl ester (l-NAME), and 4) fasudil + l-NAME. All drugs were mixed just before use, dissolved in lactated Ringer solution, and sterilized using syringe microfilters (Acrodisc, Pall, Ann Arbor, MI). Drug dosages were determined from previous studies where the concentrations of fasudil and l-NAME used maximally inhibited ROCK and NOS, respectively (12, 30, 31). Throughout the baseline period, mean Tsk was held constant at 34°C by perfusing thermoneutral water through the suit.
After baseline measurements, cold water was circulated through the suit to induce reflex VC. Mean Tsk decreased gradually from 34°C to 30.5°C over 30 min and was then clamped for an additional 10 min at 30.5°C (i.e., above the threshold for shivering). Rewarming for ∼30 min followed to return Tsk back to 34°C, after which a 1 × 10−6 M dose of NE was perfused at all sites for 15 min to verify that VC responsivity was preserved postcooling. The CVC established after rewarming was used as a baseline to assess NE-mediated VC. Lastly, 28 mM sodium nitroprusside (SNP) was perfused until a plateau in the vasodilatation response was achieved (∼30 min) at each MD site to ensure that vascular function remained intact postcooling. Because of the VC stimuli used in the protocol, the SNP-induced vasodilation was likely submaximal (Table 1).
Table 1.
Group means ± SE for absolute cutaneous vascular conductance
| Control | Fasudil | Fasudil + l-NAME | l-NAME | |
|---|---|---|---|---|
| Baseline | ||||
| Young | 0.29±0.06 | 1.60±0.24* | 1.28±0.24* | 0.22±0.04 |
| Older | 0.30±0.09 | 1.71±0.41* | 1.16±0.29* | 0.19±0.02 |
| Cooling | ||||
| Young | 0.19±0.05 (−34±3) | 1.16±0.20* (−30±5) | 0.89±0.16* (−28±4) | 0.14±0.02 (−38±4) |
| Older | 0.23±0.06 (−18±3) | 1.59±0.38*† (−7±1) | 1.01±0.25* (−11±4) | 0.15±0.02 (−23±4) |
| Rewarming | ||||
| Young | 0.30±0.06 | 1.31±0.27* | 0.95±0.17* | 0.20±0.04 |
| Older | 0.35±0.07 | 1.64±0.37*† | 1.07±0.32* | 0.20±0.02 |
| NE | ||||
| Young | 0.14±0.03 (−53±4) | 1.06±0.24* (−23±4) | 0.76±0.15* (−21±4) | 0.12±0.02 (−34±3) |
| Older | 0.18±0.03 (−41±9) | 1.52±0.36*† (−7±3) | 0.87±0.29* (−19±5) | 0.15±0.01 (−24±4) |
| SNP | ||||
| Young | 2.17±0.22 | 2.51±0.33* | 2.03±0.23 | 2.53±039* |
| Older | 2.86±0.43† | 2.49±0.47* | 2.31±0.44* | 2.65±036 |
Absolute cutaneous vascular conductance (CVC) values (laser-Doppler flux × mmHg−1) at baseline, maximal cooling (i.e., mean skin temperature = 30.5°C), precooling baseline following rewarming, norepinephrine (NE) perfusion, and sodium nitroprusside (SNP) perfusion are expressed as means ± SE for young (n = 8) and older (n = 8) subjects. Beside each CVC value for cooling and NE (in parentheses) are the corresponding relative values expressed as a percent change from baseline CVC.
P < 0.05 vs. control
P < 0.05 vs. young.
Data acquisition and analysis.
Data were collected at 40 Hz, digitized, recorded, and stored in a personal computer until data analysis (Windaq, Dataq Instruments, Akron, OH). CVC data were averaged over 3-min intervals during baseline and at each 0.5°C drop in mean Tsk during the cooling period. A three-way mixed model repeated-measures analysis of variance was conducted to detect age and treatment differences during both whole body cooling and NE administration. Post hoc comparisons were performed when appropriate to determine where age and treatment differences occurred. The false discovery rate procedure was used to control for multiple comparisons (6). Data relating to subject characteristics were assessed by paired Student's t-test. Statistical significance for all analyses were set at α = 0.05. Values are expressed as means ± SE.
RESULTS
Age groups were well matched with regard to height [young (Y), 170 ± 3; and older (O), 171 ± 3 cm], weight (Y, 67 ± 3; and O, 71 ± 3 kg), body mass index (Y, 23.3 ± 1.0; and O, 24.5 ± 0.5 kg/m2), resting MAP (Y, 85.6 ± 2.4; and O, 85.7 ± 3.0 mmHg), and cholesterol ratio (total cholesterol to HDL cholesterol) (Y, 3.1 ± 0.2; and O, 3.1 ± 0.3).
The absolute CVC values, calculated as laser-Doppler flux, × mmHg−1 for each MD fiber site are illustrated in Table 1. At the fasudil-treated sites, CVC values differed from the control site at baseline, during cooling, and during NE perfusion (P < 0.01). CVC values at the fasudil site during both cooling and NE perfusion were higher in aged skin (P < 0.01), as was the CVC during SNP perfusion at the control site (P < 0.01). When compared with the control site, CVC during SNP was greater in both fasudil (P = 0.01)- and l-NAME (P < 0.01)-treated sites in young skin, whereas in aged skin CVC was lower in the fasudil (P < 0.01)- and fasudil + l-NAME (P < 0.01)-treated sites.
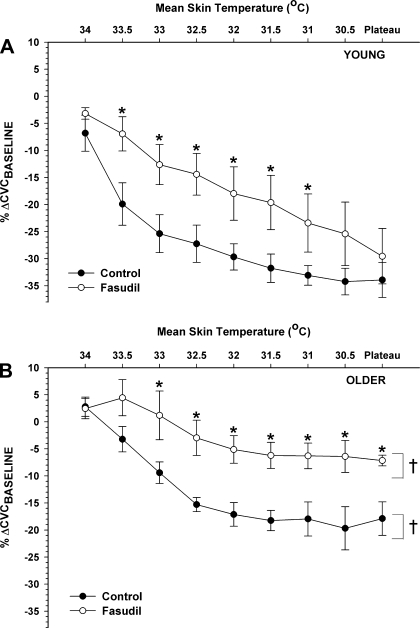
In Fig. 1, the CVC response at every 0.5°C drop in mean Tsk during whole body cooling is illustrated. In young subjects (Fig. 1A), fasudil attenuated the VC response to mild cooling (mean Tsk ≥ 31.0°C; P < 0.05) but VC was unaffected at lower Tsk. In contrast, older subjects exhibited blunted VC at the fasudil site as cooling became more severe (mean Tsk ≤ 33.0°C; P < 0.05) (Fig. 1B).
Fig. 1.
Cutaneous vascular conductance (CVC) responses to 0.5°C incremental decreases in mean skin temperature (Tsk) during whole body cooling in control and Rho kinase (ROCK)-inhibited sites. A: young subjects (n = 8). B: older subjects (n = 8). *P < 0.05 vs. control; †P < 0.05 vs. young. %ΔCVCbaseline, mean percent change from baseline CVC.
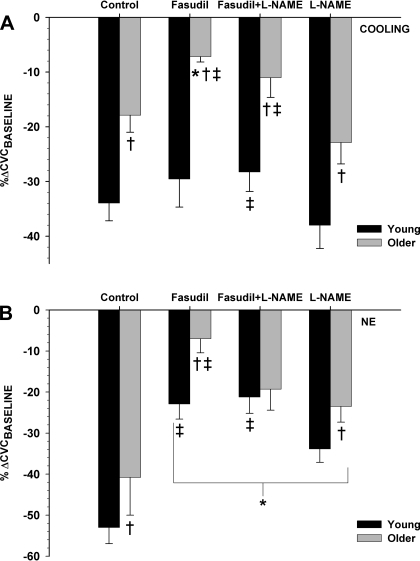
The effect of ROCK inhibition with fasudil on VC function during whole body cooling (Tsk = 30.5°C) is illustrated in Fig. 2A. When compared with young subjects, older subjects exhibited a blunted VC response at the control site (Y, −34 ± 3; and O, −18 ± 3% ΔCVCbaseline; P < 0.01). The local administration of fasudil significantly attenuated VC in older subjects (−7 ± 1% ΔCVCbaseline; P = 0.02) but had no effect in young subjects (−30 ± 5% ΔCVCbaseline; P = 0.33). Similarly, when compared with l-NAME alone (Y, −38 ± 4; and O, −23 ± 4% ΔCVCbaseline), the combined administration of fasudil and l-NAME (Y, −29 ± 4; and O, −11 ± 4% ΔCVCbaseline) blunted the VC response in older (P = 0.01) and young subjects (P = 0.03).
Fig. 2.
Average cutaneous vasoconstriction (VC) in response to moderate whole body cooling (Tsk = 30.5°C; A) or norepinephrine (NE; 1 × 10−6; B) at each microdialysis site in young and older subjects. The %ΔCVCbaseline at control, fasudil (ROCK inhibited)-, NG-nitro-l-arginine methyl ester (l-NAME)-, and fasudil + l-NAME-pretreated sites (n = 16, 8 young and 8 older) subjects is shown. *P < 0.05 vs. control; †P < 0.05 vs. young; ‡P < 0.05 vs. l-NAME.
During NE (1 × 10−6 M) perfusion (Fig. 2B), VC at the control site was significantly lower in aged skin (Y, −53 ± 4; and O, −41 ± 9% ΔCVCbaseline; P < 0.01). When compared with control, fasudil attenuated the VC response in both young and older subjects (Y, −23 ± 4; and O, −7 ± 3% ΔCVCbaseline; P < 0.01). Moreover, NE-mediated VC at l-NAME (Y, −34 ± 3; and O, −24 ± 4% ΔCVCbaseline; P < 0.01) and fasudil + l-NAME (Y, −21 ± 4; and O, −19 ± 5% ΔCVCbaseline; P < 0.01) sites were also reduced relative to the VC at the control site. When compared with l-NAME alone, adding fasudil to l-NAME blunted the VC response in young (P < 0.01) but not older subjects (P = 0.35).
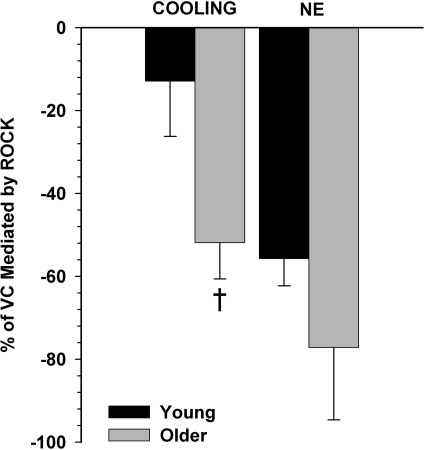
Figure 3 illustrates the ROCK contribution to the VC response, expressed as a percentage of the total VC elicited by moderate whole body cooling (mean Tsk = 30.5°C) or by NE (1 × 10−6 M), and calculated as the percentage of VC mediated by ROCK = [(%ΔCVCbaseline at control site − %ΔCVCbaseline at fasudil site)/%ΔCVCbaseline at control site]. During whole body cooling, ROCK contributed to reflex VC to a greater extent in aged (52 ± 9%; P < 0.01) than in young skin (13 ± 13%). However, the ROCK contribution to NE-mediated VC was not different between age groups (Y, 56 ± 7; and O, 77 ± 17%).
Fig. 3.
The proportion of the VC response that is ROCK mediated, expressed as percentage of the total VC response during moderate whole body cooling (Tsk = 30.5°C) or NE. The percentage of VC mediated by ROCK is calculated as (%ΔCVCbaseline at control site − %ΔCVCbaseline at fasudil site)/%ΔCVCbaseline. †P < 0.05 vs. young.
DISCUSSION
The primary finding from this study was that ROCK inhibition diminished the VC response to mild cooling in both age groups, and this reduction remained during more severe cooling in aged but not young skin. In fact, fasudil attenuated ∼50% of the VC response to more severe whole body cooling (mean Tsk = 30.5°C) in aged skin. Similar reductions in the VC response due to ROCK inhibition were observed at NOS-inhibited sites. In contrast, the VC response to an exogenous physiological dose of NE was blunted by fasudil in both age groups. Cumulatively, these data suggest that ROCK mediates approximately half of the reflex VC response to whole body cooling in aged skin.
The data from the present investigation are consistent with previous studies demonstrating that reflex VC to whole body cooling is not only attenuated in aged skin but relies almost entirely on a compromised adrenergic mechanism (7, 10, 18, 32–34). In addition, the second messenger responses coupling adrenoreceptor activation to reflex VC are altered in aged skin such that VC relies more on ROCK (31–34). This may be due in part to how Ca2+ is handled in vascular smooth muscle. NE induces VC through Ca2+-dependent and Ca2+-independent mechanisms; the latter is stimulated by ROCK and sensitizes vascular smooth muscle to extant intracellular Ca2+ by deactivating MLC phosphatase. In aged rats, small vessels demonstrate a reduced sensitivity of contractile proteins to Ca2+ as well as a greater NE-evoked VC when placed in a Ca2+-free medium. Thus it is plausible that compromised Ca2+-induced signaling explains the augmented ROCK component of cutaneous VC in older humans.
In addition to altered Ca2+ handling in vascular smooth muscle, ROCK may be unmasked during reflex VC in light of absent cotransmitter function in aged skin. At milder skin temperatures (>31.0°C), ROCK inhibition attenuated reflex VC in both young and older skin, which suggests not only that ROCK contributes to this response but that the ROCK-mediated component of reflex VC is not different between age groups at these temperatures. However, with more severe cooling (<31.0°C), ROCK had no appreciable affect on the VC response in younger skin but remained a considerable component in older skin. Interestingly, it is in this skin temperature range that cotransmitter-mediated VC significantly contributes to the reflex VC response in younger skin (29, 34). Because cotransmitter and noradrenergic-mediated VC are absent or reduced, respectively, in aged skin, it is plausible that the ROCK component is what functionally remains to contribute to reflex VC. In contrast, sympathetic cotransmitters may be inhibiting the ROCK pathway in young skin; thus the VC differences with fasudil may actually be a consequence of absent cotransmitter function, or disinhibited ROCK, in aged skin rather than differences in the ROCK-mediated component. However, the interaction between ROCK and the cotransmitter function during whole body cooling requires further investigation.
Much of what is known about how ROCK implements cold-induced VC comes from in vivo localized cooling studies in humans (30, 31) and in vitro work using mouse tail arteries. The in vitro studies revealed that localized cooling of vessels increases the generation of mitochondrial superoxide which directly stimulates ROCK (2). Elevated ROCK activity augments VC through two distinct mechanisms: 1) Ca2+ sensitization by inhibiting MLC phophatase and maintaining MLC phosphorylation in the absence of Ca2+ influx and 2) translocation of α2C-receptors from the Golgi apparatus to the plasma membrane, thereby augmenting adrenoreceptor binding sites for NE by as much as fivefold (1, 5, 14). The in vivo studies have examined the functional role of ROCK in the VC response to a locally applied cold stimulus, demonstrating that ∼60% of the VC response is ROCK mediated in young subjects, and the VC becomes more dependent on ROCK with age (30, 31). In contrast to local cooling, our data demonstrate that during moderate whole body cooling (Tsk = 30.5°C), young subjects exhibited little ROCK-mediated VC (∼10%), whereas over half of the VC response in older skin was ROCK dependent. The collective results from both localized and whole body cooling in humans suggest that cutaneous VC is more reliant on ROCK with primary human aging.
The mechanism of how ROCK participates in localized versus reflex VC may differ. In contrast to whole body cooling, the VC to local cooling in aged skin 1) does not differ in magnitude from the VC response observed in young skin (31, 33), 2) is independent of efferent sympathetic reflex activity (9, 24), and 3) is only modestly attenuated by adrenoreceptor blockade during more prolonged (i.e., “late phase”) cooling (16, 31). However, the VC to whole body cooling in aged skin is completely abolished in response to bretylium tosylate and to adrenoreceptor blockade, indicating that reflex VC is entirely dependent on axonal release of NE from sympathetic adrenergic nerves (24, 34). Moreover, we found that fasudil substantially reduced the VC in response to exogenous NE (10−6 M) in older subjects (i.e., ROCK contributed to ∼80% of the NE-mediated VC response). Cumulatively, these data suggest that NE relies primarily on ROCK to elicit VC in aged skin.
Oxidative stress may also play a role in NE-induced stimulation of ROCK. In rat renal arteries, phenylephrine (α-adrenoreceptor agonist) infusion can rapidly stimulate superoxide production (17). Subsequently, superoxide induces VC through ROCK-mediated pathways (15). Perhaps the globalized increase in reactive oxygen species that occurs with primary aging tonically augments ROCK activity and increases the gain of the ROCK response under adrenoreceptor stimulation. However, this mechanism requires further study.
In addition to its effects on vascular smooth muscle, ROCK also reciprocally inhibits NOS (21–23). ROCK can decrease NO bioavailability by inhibiting eNOS transcription and activity (22) and by augmenting arginase activity (13, 21). As a result, increased ROCK activity may precede more serious age-related clinical pathologies such as atherosclerosis (19), diabetes (8), endothelial dysfunction (3, 4), cerebral and coronary vasospasm (25), and hypertension (13, 35). As such, inhibition of ROCK may be beneficial in mitigating these vascular pathologies (23). Much of the underlying protective effects of ROCK inhibition are mediated by the upregulation of eNOS (3, 4, 21–23).
We examined the interactive role of NOS and ROCK during whole body cooling and found that the reduction in VC associated with fasudil was similar at NOS-inhibited and NOS-intact sites. Thus NOS inhibition does not appear to affect the ROCK contribution to VC during whole body cooling in aged skin. However, in young skin, the VC response was reduced when comparing the affect of fasudil across the NOS-blocked sites but was unaffected when comparing fasudil with control. This would suggest that NOS inhibition is required to fully express the ROCK-mediated component of reflex VC. In contrast, l-NAME blunted the VC response to NE in both young and older subjects. This was counter to what we hypothesized based on previous evidence that NOS inhibition augments cutaneous VC (26, 27). Possible explanations for the discrepancy with l-NAME may be that it is dose related or that there is an order effect of perfusing NE following cooling. Additionally, baseline CVC at the l-NAME site tended to be lower than other sites (Table 1), which may limit the signal gain during a VC stimulus, resulting in an artificially blunted response. Nevertheless, ROCK inhibition clearly attenuated NE-mediated VC in both young and older subjects, suggesting that ROCK has an important role in NE-mediated VC.
Limitations.
The baseline vasodilation observed in the fasudil sites may be a source of concern. Although fasudil is a selective inhibitor of ROCK, it may be acting on other signaling pathways in addition to ROCK. It is apparent that at least a portion of the dilatory response to fasudil was NO-mediated. However, more selective inhibitors could not be substituted as they are not available for in vivo administration in humans. As a result, it is difficult to determine the influence of ROCK on resting tone. Because normalization occurred at higher baseline CVCs, it is possible that the prior dilation to fasudil may have blunted the subsequent VC to cooling or NE. This is refuted in part by the observation that the VC to whole body cooling at the fasudil site did not differ from the control site in young skin.
In summary, the present study suggests that VC is more dependent on ROCK during more severe whole body cooling in aged but not young skin, which was largely unaffected by NOS inhibition. Furthermore, much of the VC to exogenous NE relies on ROCK, particularly in aged skin. Thus reflex VC in aged skin relies entirely on noradrenergic function, and approximately half of this response is ROCK mediated. Greater dependence on ROCK to elicit VC in primary aging may predate more serious clinical pathologies due primarily to its inhibitory effects on NOS.
GRANTS
The research was supported by the Carl V. Gisolfi Memorial Research Fund from the American College of Sports Medicine and by National Institutes of Health Grants RO1-AG-07004-18 and General Clinical Research Center M01-RR-10732.
ACKNOWLEDGMENTS
We gratefully acknowledge the subjects who participated in the study. We also specially thank Jane Pierzga for technical assistance and the General Clinical Research Center for medical assistance.
REFERENCES
- 1.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation.Circ Res 94:1367–1374, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries.Am J Physiol Heart Circ Physiol 289:H243–H250, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction.Proc Natl Acad Sci USA 101:9121–9126, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, Mills TM. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway.Nat Med 7:119–122, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent α2C-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries.Am J Physiol Heart Circ Physiol 278:H1075–H1083, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Curran-Everett D. Multiple comparisons: philosophies and illustrations.Am J Physiol Regul Integr Comp Physiol 279:R1–R8, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Degroot DW, Kenney WL. Impaired defense of core temperature in aged humans during mild cold stress.Am J Physiol Regul Integr Comp Physiol 292:R103–R108, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes.Stroke 36:342–347, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Ekenvall L, Lindblad LE, Norbeck O, Etzell BM. α-Adrenoceptors and cold-induced vasoconstriction in human finger skin.Am J Physiol Heart Circ Physiol 255:H1000–H1003, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in humans.Am J Physiol Regul Integr Comp Physiol 279:R349–R354, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Freedman RR, Sabharwal SC, Moten M, Migaly P. Local temperature modulates α1- and α2-adrenergic vasoconstriction in men.Am J Physiol Heart Circ Physiol 263:H1197–H1200, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans.J Physiol 574:849–857, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans.J Physiol 581:863–872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells.Mol Pharmacol 60:1195–1200, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta.Am J Physiol Heart Circ Physiol 287:H1495–H1500, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling.Am J Physiol Heart Circ Physiol 288:H1573–H1579, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide.Am J Physiol Heart Circ Physiol 292:H83–H92, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kenney WL, Armstrong CG. Reflex peripheral vasoconstriction is diminished in older men.J Appl Physiol 80:512–515, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice.Circ Res 93:884–888, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Matz RL, Alvarez de Sotomayor M, Schott C, Andriantsitohaina R. Preservation of vascular contraction during ageing: dual effect on calcium handling and sensitization.Br J Pharmacol 138:745–750, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction.Circulation 110:3708–3714, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells.Mol Cell Biol 22:8467–8477, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system.Am J Physiol Cell Physiol 290:C661–C668, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pergola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin.Am J Physiol Heart Circ Physiol 265:H785–H792, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm.Circ Res 87:195–200, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Shibasaki M, Durand S, Davis SL, Cui J, Low DA, Keller DM, Crandall CG. Endogenous nitric oxide attenuates neutrally mediated cutaneous vasoconstriction.J Physiol 585:627–634, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibasaki M, Low DA, Davis SL, Crandall CG. Nitric oxide inhibits cutaneous vasoconstriction to exogenous norepinephrine.J Appl Physiol 105:1504–1508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II.J Physiol 522:177–185, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men.Am J Physiol Heart Circ Physiol 280:H1496–H1504, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin.Am J Physiol Heart Circ Physiol 292:H1700–H1705, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Rho kinase-mediated local cold-induced cutaneous vasoconstriction is augmented in aged human skin.Am J Physiol Heart Circ Physiol 293:H30–H36, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Thompson CS, Holowatz LA, Kenney WL. Attenuated noradrenergic sensitivity during local cooling in aged human skin.J Physiol 564:313–319, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans.Am J Physiol Regul Integr Comp Physiol 288:R1108–R1113, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin.J Physiol 558:697–704, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension.Nature 389:990–994, 1997 [DOI] [PubMed] [Google Scholar]