Abstract
We previously demonstrated that the O-linked β-N-acetylglucosamine (O-GlcNAc) posttranslational modification confers cardioprotection at least partially through mitochondrial-dependent mechanisms, but it remained unclear if O-GlcNAc signaling interfered with other mechanisms of cell death. Because ischemia/hypoxia causes endoplasmic reticulum (ER) stress, we ascertained whether O-GlcNAc signaling could attenuate ER stress-induced cell death per se. Before induction of ER stress (with tunicamycin or brefeldin A), we adenovirally overexpressed O-GlcNAc transferase (AdOGT) or pharmacologically inhibited O-GlcNAcase [via O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate] to augment O-GlcNAc levels or adenovirally overexpressed O-GlcNAcase to reduce O-GlcNAc levels. AdOGT significantly (P < 0.05) attenuated the activation of the maladaptive arm of the unfolded protein response [according to C/EBP homologous protein (CHOP) activation] and cardiomyocyte death (reflected by percent propidium iodide positivity). Moreover, pharmacological inhibition of O-GlcNAcase significantly (P < 0.05) mitigated ER stress-induced CHOP activation and cardiac myocyte death. Interestingly, overexpression of GCA did not alter ER stress markers but exacerbated brefeldin A-induced cardiomyocyte death. We conclude that enhanced O-GlcNAc signaling represents a partially proadaptive response to reduce ER stress-induced cell death. These results provide new insights into a possible interaction between O-GlcNAc signaling and ER stress and may partially explain a mechanism of O-GlcNAc-mediated cardioprotection.
Keywords: O-linked β-N-acetylglucosamine, endoplasmic reticulum
proper protein synthesis, folding, and transport are essential for cell survival. The rough endoplasmic reticulum (ER) is involved with maintaining proper synthesis, folding, and transport of proteins targeted to membranes and organelles (4). Conditions that alter the ionic balance, molecular chaperones, protein glyosylation machinery, and/or redox status of the ER lumen cause ER stress and trigger an intracellular signaling system called the unfolded protein response (UPR; Refs. 5, 9, 19, 24, 35). Initially, the UPR promotes adaptation to reestablish normal ER function. Such adaptation occurs by reducing the quantity of protein synthesized in the ER, inducing the expression of genes that enhance the protein folding capacity of the ER, promoting ER-associated protein degradation to remove misfolded proteins, and reestablishing the ER luminal environment to that suitable for new protein synthesis and folding. Such initial responses are designed to resolve the stress and ultimately enhance the chances of survival, but if the ER stress is prolonged, the maladaptive arm of the UPR is activated causing cell death (37).
ER stress has been implicated in the pathogenesis of several diseases including ischemia-reperfusion injury (33, 42), heart failure (14, 26), and diabetes (2, 15, 32). Indeed, one study (16) showed that numerous UPR genes are induced within 24 h of myocardial infarction in mice. Moreover, several studies (31, 33, 38, 39) have shown that interventions that inhibit ER stress in the heart attenuate ischemia-reperfusion injury in mice or hypoxic injury in cultured cardiac myocytes. The present study focused on ER stress because of its importance in dictating cell survival in the cardiovascular system.
O-linked β-N-acetylglucosamine (O-GlcNAc) is a posttranslational sugar modification of nucleocytoplasmic and mitochondrial proteins recently shown by our group (18, 28–30) and others (7, 8, 10, 27) to confer cardioprotection. In such studies, we showed that enhanced O-GlcNAc modification reduced infarct size in mice and attenuated hypoxia- and oxidative stress-induced cardiomyocyte death. In a bid to identify potential mechanisms through which O-GlcNAc confers cytoptrotection, we demonstrated that enhanced O-GlcNAc signaling attenuated mitochondrial Ca2+ overload, mitochondrial permeability transition pore formation, and loss of mitochondrial trans inner membrane potential, all key players in postischemic/posthypoxic injury. Because ischemia-reperfusion injury is multifactoral and because ER stress is activated in ischemia-reperfusion injury, we sought to determine whether O-GlcNAc-mediated cytoprotection occurs at least partially by interfering with the maladaptive aspects of ER stress. Here, we specifically induced ER stress to determine if O-GlcNAc signaling could attenuate the prodeath arm of the UPR response. Our data suggest that augmented O-GlcNAc signaling attenuates ER stress-induced cardiac myocyte death and could represent one mechanism of acute cytoprotection following ischemia/hypoxia.
MATERIALS AND METHODS
All animal studies adhered to the American Physiological Society's “Guiding Principles in the Care and Use of Animals.” All experiments involving animals were conducted in compliance with AVMA guidelines, consistent with federal regulations, and approved by the University of Louisville Institutional Animal Care and Use Committee.
Neonatal rat cardiac myocyte isolation and culture.
Neonatal rat cardiac myocytes (NRCMs) were isolated from 1- to 2-day-old Sprague-Dawley rats and cultured according to a well-characterized protocol (1, 17, 18, 28, 30, 40, 41). Twenty-four hours before experimentation, medium was changed to serum-free DMEM.
Gene transfer.
NRCMs were infected with 100 multiplicity of infection of replication-deficient adenoviruses carrying the rat OGT gene (AdOGT), rat O-GlcNAcase gene (AdGCA), or green fluorescent protein (AdGFP; Vector Biolabs) as described previously (28, 30, 41) for 24 h. Medium was changed to serum-free DMEM, and myocytes were treated with brefeldin A (BfA) or tunicamycin (TM) to induce ER stress. Functional expression was confirmed by immunoblot analysis. Sample size is equal to at least five per group per treatment.
Enzyme inhibition.
NRCMs were treated with O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate (PUGNAc; 200 μmol/l; Refs. 13, 18, 28), an inhibitor of O-GlcNAcase, or vehicle (0.1% ethanol) 3 h before ER stress induction with BfA or TM. Sample size is equal to at least five per group per treatment.
ER stress induction.
NRCMs treated as mentioned above were subjected to ER stress by treatment with 1 μg/ml of BfA or 0.5 μg/ml of TM for 24 h. TM inhibits N-glycosylation of nascent ER proteins by preventing UDP-GlcNAc-dolichol phosphate GlcNAc-phosphate transferase activity (11), while BfA interferes with anterograde protein transport from the ER to the Golgi apparatus by inhibiting transport in Golgi, which leads to proteins accumulating inside the ER (12).
In vitro hypoxia-reoxygenation injury in cardiac myocytes.
Untreated cardiac myocytes were subjected to 6 h of hypoxia in Esumi lethal media in humidified hypoxic chambers (Billups-Rothenberg) as previously described (20, 28, 30). After hypoxia, the media were changed to Esumi control media (28, 30) and the culture dishes were reoxygenated for 6 h in the modular incubator. Normoxic/aerobic controls were achieved by subjecting NRCMs to 6 h of normoxia and 6 h of reoxygenation in Esumi control media. Total cellular protein was isolated and immoblotted for ER stress indicators. Sample size was equal to six per group per treatment.
Cell death.
Cell death was assessed for NRCMs treated as mentioned by staining with the fluorescent DNA-binding dyes Hoechst 33342 (5 μg/ml) and propidium iodide (PI; 5 μg/ml) for 30 min similar to a previous work (3, 28, 30). The stained nuclei were then visualized using a ×20 objective on a Nikon-TE2000E2 fluorescence microscope and Xcite 120 Fluor light source (level of 12%). Filters used included 350/50 nm excitation and 470/40 nm emission filters for Hoechst and 560/40 nm excitation and 630/60 nm emission filters for PI. Neutral density filter setting was set at ND4 and binning of two times for all image acquisition. Exposure duration was set at 100 ms for Hoechst and 200 ms for PI. Four fields per treatment were counted, and data were expressed as percent PI-positive nuclei/total nuclei. Because the nuclear stain Hoechst 33342 is membrane permeable, it was used to determine total cells in each field and not as an index of apoptosis. Sample size is equal to six per group per treatment.
Protein expression.
Total cellular proteins were isolated from NRCMs as described previously (18, 28, 30). Forty micrograms of protein (according to Bradford assay) were applied to each lane of a 7% Tris-acetate gel (Invitrogen) or 10% Bis-Tris gel (Invitrogen) and electroblotted onto PVDF membranes. All blots were incubated with 0.2 μg/ml of anti-O-GlcNAc (CTD110.6), anti-Grp94, anti-Grp78, anti-poly(ADP-ribose) polymerase-1 (anti-PARP), anti-C/EBP homologous protein (anti-CHOP), and anti-calreticulin or with 0.1 μg/ml α-tubulin for 12 min at room temperature. Blots were then incubated for 12 min with 0.05 μg/ml of appropriate horseradish perioxidase-conjugated secondary antibody (goat anti-rat IgG, goat anti-rabbit IgG, goat anti-mouse IgM, donkey anti-goat IgG, or goat anti-mouse IgG) and detected with enhanced chemiluminescent detection reagent (Pierce). All antibodies were purchased from Santa Cruz except CTD110.6 (Covance) and α-tubulin (Sigma).
Apoptosis.
Apoptosis was assessed by measuring caspase-3/7 activity in whole cell lysates using Caspase Glo kit (Promega) according to the manufacturer's instructions. Briefly, equal volumes of whole cell lysate (50 μg) and caspase reagent were mixed and incubated in the dark for 1 h at room temperature. Bioluminescence was measured using a Modulus luminometer (Turner Biosystems) and expressed in relative luminescent units. Sample size is equal to at least five per group per treatment.
Densitometry.
Densitometry was performed using nonsaturated chemiluminescent membranes exposed and quantitated using a Fuji LAS-3000 bioimaging analyzer. Multiple exposures from every experiment were used to confirm that the signal was within the linear range. Densitometric analysis of O-GlcNAc levels using CTD 110.6 antibody was performed on the entire lane and then expressed as a percentage of AdGFP or vehicle (set at 100%). Densitometric analysis for other immunoblots was performed on a single band, and data were normalized to AdGFP or vehicle. α-Tubulin served as loading control for all immunoblots.
Statistical analysis.
Data were analyzed using one-way ANOVA and/or Dunnett's t-tests using GraphPad Prism 4 software. Data are reported as means ± SE with differences between treatment groups accepted as significant when P < 0.05.
RESULTS
Hypoxia induces ER stress.
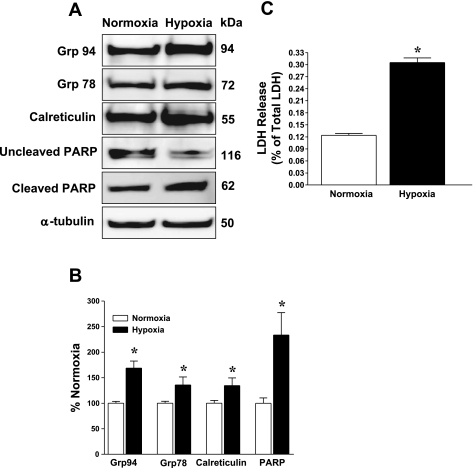
ER stress markers were evaluated to confirm that hypoxia induces ER stress. Cardiac myocytes subjected to 6 h of hypoxia and 6 h of reoxygenation and then immunoblotted for ER stress proteins showed significantly (P < 0.05) augmented UPR-inducible proteins, Grp94, Grp78, and calreticulin, as shown in Fig. 1, A and B. Moreover, hypoxia induced apoptosis according to significant (P < 0.05) PARP cleavage despite no change in α-tubulin protein levels (Fig. 1, A and B). Incidentally, these hypoxia-induced changes were accompanied by significant cardiac myocyte death according to lactate dehydrogenase release (Fig. 1C). These results indicate that hypoxia induces ER stress in cultured neonatal cardiac myocytes and confirms previous findings by other groups (36, 42). Because hypoxia/ischemia exerts multiple insults on cardiac myocytes, we wanted to isolate one such pathology (ER stress) and ask whether O-GlcNAc signaling could interfere with this specific prodeath pathway.
Fig. 1.
Cardiac myocytes were subjected to hypoxia-reoxygenation, and activation of ER stress was evaluated. A: hypoxia-activated endoplasmic reticulum (ER) stress as reflected by immunoblots showing augmented Grp94, Grp78, and calreticulin levels. B: densitometric quantification of immunoblots showed significant posthypoxic upregulation of Grp94, Grp78, calreticulin (Cal), and PARP cleavage [expressed as cleaved/uncleaved poly(ADP-ribose) polymerase-1 (PARP) in bar graph]. C: hypoxia-induced cardiac myocyte death [according to lactate dehydrogenase (LDH) release]. *P < 0.05 vs. normoxia; n = 6/group.
Genetic manipulation of O-GlcNAc signaling alters maladaptive ER stress signaling.
We have previously shown that augmented O-GlcNAc levels reduce infarct size in mice (18) and attenuate posthypoxic cardiac myocyte death (28, 30). Moreover, numerous studies have implicated ER stress in the pathogenesis of postischemia/hypoxic injury (33, 36, 42). Hence, we evaluated whether genetic manipulation of O-GlcNAc signaling affects ER stress-induced cardiac myocyte death, per se, under normoxic conditions.
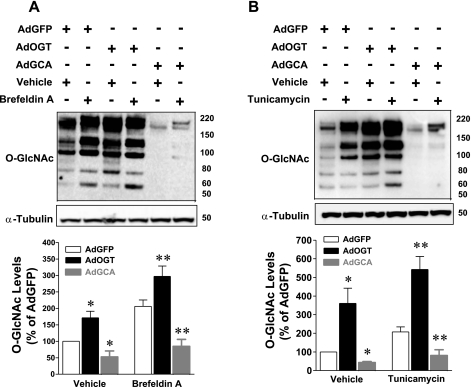
Cardiac myocytes infected with replication-deficient adenovirus carrying the GFP, OGT, or GCA gene for 24 h were treated with the prototypical ER stress inducers TM (11) or BfA (34) for 24 h. O-GlcNAc levels and the levels of several UPR inducible proteins were assessed by immunoblotting. BfA treatment significantly (P < 0.05) augmented O-GlcNAc levels. OGT overexpression significantly increased O-GlcNAc levels over the levels already induced by ER stress (Fig. 2A). Although induction of ER stress in GCA-overexpressed cells augmented O-GlcNAc levels, it was still less than baseline O-GlcNAc levels (Fig. 2A). Similar changes in O-GlcNAc signaling were observed following induction of ER stress with TM (Fig. 2B).
Fig. 2.
Normoxic cardiac myocytes were infected with adenovirus carrying the green fluorescent protein (AdGFP), rat OGT (AdOGT), or rat O-GlcNAcase (AdGCA) gene and subjected to ER stress [with brefeldin A (BfA) or tunicamycin (TM)]. Whole cell lysates were immunoblotted for O-GlcNAc. A: OGT overexpression significantly augmented O-GlcNAc levels over those induced by BfA as shown in immunoblot and densitometric quantification of immunoblots. The BfA-induced increase in O-GlcNAc levels in GCA-overexpressed cells was less than baseline O-GlcNAc levels. B: OGT overexpression significantly augmented O-GlcNAc levels over those induced by TM. The TM-induced increase in O-GlcNAc levels in GCA-overexpressed cells did not reach baseline O-GlcNAc levels. *P < 0.05 vs. AdGFP; **P < 0.05 vs. AdGFP + BfA or AdGFP + TM; n ≥ 5/group.
Both BfA- and TM-induced ER stress stimulated a significant (P < 0.05) increase in the ER chaperones Grp94, Grp78, and calreticulin as shown in Fig. 3, A –D. Moreover, both BfA and TM treatment activated the maladaptive arm of ER stress response according to PARP cleavage and as shown in Fig. 3, A–D. These results demonstrate that known ER stress inducers (BfA and TM) augment protein levels of three well-characterized UPR-inducible proteins in cardiomyocytes, consistent with other groups (36, 42).
Fig. 3.
Normoxic cardiac myocytes were treated with either AdGFP, AdOGT, or AdGCA and subjected to ER stress (with BfA or TM), and whole cell lysates were immunoblotted for Grp94, Grp78, calreticulin, PARP, and CHOP. A: total protein was isolated from selected cultures and immunoblotted for ER stress indicators. OGT overexpression significantly attenuated, while GCA overexpression did not change, BfA-induced ER stress according to immunoblotting. B: densitometric quantification of immunoblots showed significant reduction in BfA-induction of Grp94, Grp78, and CHOP with OGT overexpression despite no change with GCA overexpression. OGT overexpression did not affect calreticulin expression or PARP cleavage (expressed as cleaved PARP/uncleaved PARP). C: OGT overexpression significantly mitigated, while GCA overexpression did not change, TM-induced ER stress according to immunoblotting. D: densitometric quantification of immunoblots showed significant reduction in TM induction of Grp94, Grp78, and CHOP with OGT overexpression despite no change with GCA overexpression. E: to evaluate cell death, similarly treated cultures were stained with Hoechst and propidium iodide (PI). OGT overexpression significantly attenuated, while GCA overexpression exacerbated ER stress-induced cardiac myocyte death according to PI positivity. F: OGT or GCA overexpression did not change ER stress-induced cardiomyocyte apoptosis. G: OGT overexpression also significantly reduced ER stress-induced cardiac myocyte death despite no change with GCA overexpression according to PI positivity. H: neither OGT nor GCA overexpression affected TM-induced cardiomyocyte apoptosis. **P < 0.05 vs. AdGFP + BfA or TM; n ≥ 5/group.
OGT overexpression significantly reduced BfA-mediated ER stress, as reflected by reduced Grp94 and Grp78 protein levels (Fig. 3, A and B). Furthermore, OGT overexpression significantly (P < 0.05) attenuated BfA-induced activation of maladaptive ER stress response according to diminished CHOP activation. To confirm the protective effects of augmented O-GlcNAc levels (via OGT overexpression) on the activation of maladaptive unfolded protein response, we examined whether OGT overexpression affects ER stress induced by TM, an inhibitor of N-glycosylation of nascent ER proteins. OGT overexpression significantly (P < 0.05) reduced the TM-induced ER stress according to Grp94, Grp78, and CHOP activation as shown in Fig. 3, C and D. In both BfA and TM treatments, OGT overexpression did not affect ER stress-mediated increase in calreticulin protein level or PARP cleavage (Fig. 3, A–D). GCA overexpression did not alter BfA- or TM-mediated increases in the ER chaperones Grp94, Grp78, and calreticulin (Fig. 3, A–D). Moreover, AdGCA did not affect ER stress-mediated apoptosis according to CHOP activation and PARP cleavage (Fig. 3, A–D).
Genetic manipulation of O-GlcNAc signaling affects ER stress-mediated cardiac myocyte death.
During prolonged or severe ER stress, cell death pathways can be activated. Therefore, we questioned whether genetic manipulation of the two enzymes involved with O-GlcNAc signaling affects ER stress-induced cardiac myocyte death. In the absence of ER stress, neither OGT overexpression nor GCA overexpression altered cellular viability according to PI positivity. OGT overexpression significantly (P < 0.05) attenuated, while GCA overexpression exacerbated, BfA-induced cell death according to PI positivity (Fig. 3E). Neither OGT nor GCA overexpression significantly altered BfA-mediated cardiomyocyte apoptosis according to caspase-3/7 activity (Fig. 3F).
Similarly, OGT overexpression significantly mitigated TM-induced cardiomyocyte death. GCA overexpression did not alter TM-mediated cardiomyocyte death (Fig. 3G). Moreover, both OGT and GCA overexpression did not significantly alter TM-mediated cardiomyocyte apoptosis according to caspase-3/7 activity (Fig. 3H). Taken together, these results indicate that augmented O-GlcNAc levels are protective against ER stress-induced cell death.
O-GlcNAcase inhibition reduces prodeath UPR signaling.
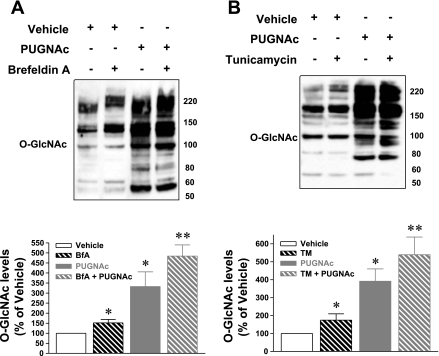
Next, we used a complementary approach to elevate O-GlcNAc levels by inhibiting O-GlcNAcase with PUGNAc (13). Cardiac myocytes were treated with PUGNAc or vehicle (0.1% ethanol) 3 h before ER stress induction (with TM or BfA, 24 h), and whole cell lysates were immunoblotted for O-GlcNAc levels and ER stress indicators. Inhibition of O-GlcNAcase significantly augmented O-GlcNAc levels above those induced by BfA (Fig. 4A) or TM (Fig. 4B) alone. Moreover, O-GlcNAcase inhibition significantly (P < 0.05) reduced BfA-induced ER stress according to Grp94, Grp78, and calreticulin (shown in Fig. 5, A and B). Additionally, O-GlcNAcase inhibition significantly (P < 0.05) blocked the activation of maladaptive ER stress response as reflected by diminished CHOP activation (Fig. 5, A and B). Similar findings were observed with TM. PUGNAc treatment significantly attenuated TM-induced ER stress according to Grp94, Grp78, and calreticulin as represented in Fig. 5, C and D. Finally, O-GlcNAcase inhibition reduced TM-induced CHOP activation but did not affect BfA- or TM-induced PARP cleavage (Fig. 5, A–D).
Fig. 4.
Normoxic cardiac myocytes were treated with either vehicle or O-GlcNAcase inhibitor [O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate (PUGNAc)] and subjected to ER stress (with BfA or TM). A: Western blots of whole cell lysates showed augmented O-GlcNAc levels with O-GlcNAcase inhibition compared with vehicle. O-GlcNAcase inhibition augmented O-GlcNAc levels above those induced by BfA. B: O-GlcNAcase inhibition significantly increased O-GlcNAc levels above those induced by TM. *P < 0.05 vs. vehicle, ** P < 0.05 vs. BfA or TM; n = 4/group.
Fig. 5.
Normoxic cardiac myocytes were pretreated with either vehicle or O-GlcNAcase inhibitor (PUGNAc) and subjected to ER stress with BfA or TM. A: Western blots of whole cell lysates showed significant reduction in BfA-mediated activation of Grp94, Grp78, calreticulin, and CHOP protein levels with O-GlcNAcase inhibition. B: densitometric quantification of immunoblots showed significantly reduced Grp94, Grp78, calreticulin, and CHOP protein levels with O-GlcNAcase inhibition. PUGNAc treatment did not affect the BfA-mediated increase in calreticulin expression or PARP cleavage (expressed as cleaved PARP/uncleaved PARP). O-GlcNAcase inhibition also significantly reduced the TM-mediated increase in Grp78, Grp94, and CHOP protein levels compared with BfA alone according to representative immunoblots (C) and densitometric quantification (D). E: similarly treated cultures were stained with Hoechst and PI for cell death. O-GlcNAcase inhibition attenuated BfA-induced cardiomyocyte death. F: O-GlcNAcase inhibition did not affect BfA-induced cardiomyocyte apoptosis. G: O-GlcNAcase inhibition attenuated TM-induced cardiomyocyte death. H: O-GlcNAcase inhibition did not affect TM-induced cardiomyocyte apoptosis. *P < 0.05 vs. control; **P < 0.05 vs. BfA or TM; n ≥ 5/group.
O-GlcNAcase inhibition mitigates ER stress-induced cardiac myocyte death.
Like OGT overexpression, O-GlcNAcase inhibition (with PUGNAc) has been shown by our group and others to be cytoprotective during hypoxia, ischemia, and oxidative stress (18, 22, 28). Here, O-GlcNAcase inhibition with PUGNAc significantly attenuated BfA-induced cardiomyocyte death (18 ± 7% for PUGNAc + BfA vs. 41 ± 7% for BfA alone, P < 0.05; Fig. 5E). Such results were further supported by significantly attenuated TM-induced ER stress with O-GlcNAcase inhibition with PUGNAc (15 ± 3% PUGNAc + TM vs. 33 ± 6% for TM alone, P < 0.05; Fig. 5G). Thus shifting the cellular balance in favor of O-GlcNAcylation via genetic or pharmacologic means mitigates ER stress-induced cell death. Inhibition of O-GlcNAcase did not significantly alter BfA- or TM-induced apoptosis according to caspase-3/7 activity (Fig. 5, F and H).
DISCUSSION
Recent work from this (18, 28, 30) and other (7, 8, 21–23) laboratories supports the role of O-GlcNAc as an important survival signal. These collective studies indicate that O-GlcNAc can attenuate posthypoxic cellular injury in vitro and in vivo. However, the question remains regarding the precise mechanism. Because O-GlcNAcylation occurs in many proteins, it is not surprising that multiple targets/mechanisms may be operative during the mitigation of pathologies as complex as hypoxia/ischemia. Recent work (8, 18, 28, 30) indicates that mitochondria are critical targets of O-GlcNAc-mediated cytoprotection. Moreover, O-GlcNAc signaling can directly attenuate oxidative stress-induced cellular dysfunction (18) and calcium overload (28). Oxidative stress and calcium overload are hallmarks of postischemic cardiomyocyte damage. In addition to such standards of cellular damage, ER stress has emerged as a potentially critical element of ischemia/hypoxia. Consequently, we focused on the potential ability of O-GlcNAc signaling to directly attenuate ER stress-induced cell death (in the absence of the confounding condition of hypoxia). Here, we show that 1) ER stress induces O-GlcNAc signaling, 2) overexpression of OGT or inhibition of O-GlcNAcase attenuates activation of the maladaptive ER stress response, 3) overexpression of OGT or inhibition of O-GlcNAcase mitigates ER stress-induced cardiac myocyte death, 4) overexpression of GCA does not alter BfA- or TM-mediated activation of maladaptive ER stress response, and 5) manipulation of O-GlcNAc signaling does not affect ER stress-mediated cardiomyocyte apoptosis.
It is abundantly clear that O-GlcNAcylation represents a significant mechanism in the ability of the cell to successfully respond to a variety of stressors (29, 44). A notable finding in the present study is the augmentation of O-GlcNAc signaling during ER stress. It will be interesting to ascertain the cause for augmented O-GlcNAcylation during ER stress, but the focus of the present study was to identify whether O-GlcNAc signaling affected ER stress-induced cell death. One might conjecture that augmented O-GlcNAc levels may be due to elevated OGT and/or reduced O-GlcNAcase activities. Regardless of the mechanism, we hypothesized that such a response was a proadaptive but insufficient response to the induction of ER stress. Consequently, we designed a series of experiments testing whether multiple approaches to augment O-GlcNAc signaling would subsequently improve the ability of the cardiac myocytes to withstand ER stress-induced cell death.
Similar to previous findings, our data support hypoxic activation of ER stress in cardiomyocytes. Thuerauf et al. (42) showed that hypoxia induced XBP1 splicing and ER chaperones in cardiomyocytes. Likewise, Szegezdi et al. (36) subjected cardiomyocytes to hypoxia and found induction of Grp78 mRNA and protein expression. The induction of O-GlcNAc signaling during ER stress might be predicted from the significant literature supporting the role of O-GlcNAc as an acute stress or alarm signal (29, 44). The present demonstration of O-GlcNAc signaling may reflect a partially proadaptive response during ER stress. Clearly, augmentation of O-GlcNAc signaling rescued cardiomyocytes from ER stress-induced cell death. The new question relates to the mechanism operative during O-GlcNAc signaling-mediated rescue of cells undergoing the UPR.
The data related to OGT overexpression and O-GlcNAcase inhibition (via PUGNAc) are clear in that there is robust attenuation of ER stress-mediated cell death (with either BfA or TM). In addition, it also appears that such maneuvers designed to augment O-GlcNAc signaling might limit the severity of the induction of ER stress, as indicated by the general limitation of the markers of ER stress activation. In this regard, augmented O-GlcNAc signaling might function as a regulator of the initiation/propagation of ER stress and indirectly attenuate cell death. In addition, enhanced O-GlcNAc signaling may directly attenuate cell death, which may be independent of ER stress mitigation. Conversely, the data from O-GlcNAcase could initially be viewed as ambiguous. Overexpression of O-GlcNAcase (AdGCA) did not exaggerate any of the ER stress indicators. Interestingly, AdGCA exacerbated ER stress-mediated cell death when BfA, but not TM, was used as the inducer of ER stress. Although the precise reason for the apparently discrepant response is not clear, we hypothesize that the dose of TM used in our system produces a somewhat greater induction of ER stress (as evidenced by the larger increase in the ER stress markers in the TM experiments) and that AdGCA is unable to exacerbate this response.
The UPR initially attempts to support restoration of ER homoeostasis and protect cells from stress. In fact, Vitadello et al. (43) first demonstrated that overexpression of Grp94 reduces ischemia-induced cardiomyocyte death. Moreover, Thuerauf et al. (42) showed that subjecting cardiac myocytes to hypoxia-activated XBP1 and XBP1 contributed to protecting posthypoxic cardiomyocytes. However, prolonged ER stress activates cell death. Induction of the proapoptotic transcription factor CHOP is a hallmark of ER stress-mediated cell death. Here, BfA- or TM-induced CHOP expression was attenuated by genetic or pharmacologic elevation of O-GlcNAc levels (via OGT overexpression and O-GlcNAcase inhibition). The exact mechanism through which O-GlcNAc signaling blocks CHOP activation is unknown. It is known that p38 MAPK phosphorylates CHOP, which increases its activity (37). Accordingly, O-GlcNAc signaling may block CHOP phosphorylation, independent of absolute reductions in protein expression, thereby reducing its activity and subsequent cardiomyocyte death.
Targeting eukaryotic initiation factor-2 (eIF-2) represents another potential mechanism in the context of ER stress. Its activity is regulated by phosphorylation and indirectly by O-GlcNAcylation. A 67-kDa polypeptide (p67) binds to eIF-2 blocking its phosphorylation and subsequently inhibits protein synthesis (6). It is plausible that enhanced p67 glycosylation (i.e., with AdOGT) binds to and blocks eIF-2 phosphorylation by eIF-2 kinase. Although transient reduction in protein synthesis may be proadaptive during the early stages of ER stress, prolonged blockade of protein synthesis is not viable. Therefore, one might predict that augmenting O-GlcNAc levels promotes p67 glycosylation and subsequent blockade of eIF-2 phosphorylation, as one potential mechanism in the present study. Such possibilities remain to be seen, and the present data do not directly support or refute such a possibility.
PUGNAc, a GlcNAc analog, is the most widely studied inhibitor of O-GlcNAcase and prevents the binding of O-GlcNAcase to GlcNAc. Thus PUGNAc prevents the removal of O-GlcNAc leading to a rapid increase in O-GlcNAc levels. PUGNAc inhibits other lysosomal hydrolases and shows limited specificity for O-GlcNAcase over β-hexosaminidase (25). AdOGT data in conjunction with the PUGNAc data provide sufficient evidence that O-GlcNAc signaling can attenuate ER stress-induced cell death, per se, although the limitations of the PUGNAc experiments are clear.
By design, the present study was executed entirely in neonatal rat cardiomyocytes. Although there are key differences between neonatal and adult cells, the concept that O-GlcNAc signaling could directly antagonize ER stress-induced cell death was the point of this study. We and others have amassed significant data supporting the acute cardioprotective effects of O-GlcNAc signaling in the heart both in vitro and in vivo (i.e., adult cardiac myocytes). In summary, the present study demonstrates that augmented O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death and represents a unique mechanism through which O-GlcNAc confers cardioprotection. Future studies will delineate the exact mechanism through which O-GlcNAc mitigates ER stress-induced cell death.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-083320 and R01-HL-094419, American Heart Association National Center Grant 0535270N, and Kentucky Science and Engineering Foundation Grant KSEF-1667-RDE-011 to S. P. Jones. T. Hamid is supported by American Heart Association Scientist Development Grant 0835456N. G. A. Ngoh was an American Heart Association Predoctoral Fellow (0715493B–Great Rivers Affiliate). S. D. Prabhu and S. P. Jones are supported by the National Institutes of Health Diabetes and Obesity Center (P20-RR-024489).
REFERENCES
- 1.Akao M, O'Rourke B, Kusuoka H, Teshima Y, Jones SP, Marban E. Differential actions of cardioprotective agents on the mitochondrial death pathway.Circ Res 92: 195–202,2003 [DOI] [PubMed] [Google Scholar]
- 2.Araki E, Oyadomari S, Mori M. Endoplasmic reticulum stress and diabetes mellitus.Intern Med 42:7–14,2003 [DOI] [PubMed] [Google Scholar]
- 3.Bishopric NH, Zeng GQ, Sato B, Webster KA. Adenovirus E1A inhibits cardiac myocyte-specific gene expression through its amino terminus.J Biol Chem 272:20584–20594,1997 [DOI] [PubMed] [Google Scholar]
- 4.Blobel G. Protein targeting.Biosci Rep 20:303–344,2000 [DOI] [PubMed] [Google Scholar]
- 5.Booth C, Koch GL. Perturbation of cellular calcium induces secretion of luminal ER proteins.Cell 59:729–737,1989 [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty A, Saha D, Bose A, Chatterjee M, Gupta NK. Regulation of eIF-2 alpha-subunit phosphorylation in reticulocyte lysate.Biochemistry 33:6700–6706,1994 [DOI] [PubMed] [Google Scholar]
- 7.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc.Am J Physiol Cell Physiol 292:C178–C187,2007 [DOI] [PubMed] [Google Scholar]
- 8.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2.Am J Physiol Cell Physiol 294:C1509–C1520,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SC, Wooden SK, Nakaki T, Kim YK, Lin AY, Kung L, Attenello JW, Lee AS. Rat gene encoding the 78-kDa glucose-regulated protein GRP78: its regulatory sequences and the effect of protein glycosylation on its expression.Proc Natl Acad Sci USA 84:680–684,1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma.Shock 29:431–440,2008 [DOI] [PubMed] [Google Scholar]
- 11.Dorner AJ, Wasley LC, Raney P, Haugejorden S, Green M, Kaufman RJ. The stress response in Chinese hamster ovary cells. Regulation of ERp72 and protein disulfide isomerase expression and secretion.J Biol Chem 265:22029–22034,1990 [PubMed] [Google Scholar]
- 12.Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum.J Biol Chem 263:18545–18552,1988 [PubMed] [Google Scholar]
- 13.Haltiwanger RSGK, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate.J Biol Chem 273:3611–3617,1998 [DOI] [PubMed] [Google Scholar]
- 14.Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Nishino T, Nakaya H, Koseki H, Aoe T. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice.Mol Cell Biol 24:8007–8017,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk −/− mice reveals a role for translational control in secretory cell survival.Mol Cell 7:1153–1163,2001 [DOI] [PubMed] [Google Scholar]
- 16.Harpster MH, Bandyopadhyay S, Thomas DP, Ivanov PS, Keele JA, Pineguina N, Gao B, Amarendran V, Gomelsky M, McCormick RJ, Stayton MM. Earliest changes in the left ventricular transcriptome postmyocardial infarction.Mamm Genome 17:701–715,2006 [DOI] [PubMed] [Google Scholar]
- 17.Jones SP, Teshima Y, Akao M, Marban E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes.Circ Res 93:697–699,2003 [DOI] [PubMed] [Google Scholar]
- 18.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins.Circulation 117:1172–1182,2008 [DOI] [PubMed] [Google Scholar]
- 19.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls.Genes Dev 13:1211–1233,1999 [DOI] [PubMed] [Google Scholar]
- 20.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1.Circ Res 93:1074–1081,2003 [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels.J Mol Cell Cardiol 42:177–185,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion leads to improved functional recovery and reduced calpain proteolysis.Am J Physiol Heart Circ Physiol 293:H1391–H1399,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia.J Mol Cell Cardiol 40:303–312,2006 [DOI] [PubMed] [Google Scholar]
- 24.Lodish HF, Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum.J Biol Chem 265:10893–10899,1990 [PubMed] [Google Scholar]
- 25.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors.J Biol Chem 280:25313–25322,2005 [DOI] [PubMed] [Google Scholar]
- 26.Nadanaka S, Okada T, Yoshida H, Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress.Mol Cell Biol 27:1027–1043,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagy T, Champattanachai V, Marchase RB, Chatham JC. Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-linked N-acetylglucosamine.Am J Physiol Cell Physiol 290:C57–C65,2006 [DOI] [PubMed] [Google Scholar]
- 28.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury.Circ Res 104:41–49,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngoh GA, Jones SP. New insights into metabolic signaling and cell survival: the role of beta-O-linkage of N-acetylglucosamine.J Pharmacol Exp Ther 327:602–609,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition.J Mol Cell Cardiol 45:313–325,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickson P, Toth A, Erhardt P. PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress.Cardiovasc Res 73:48–56,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes.Science 306:457–461,2004 [DOI] [PubMed] [Google Scholar]
- 33.Qi X, Vallentin A, Churchill E, Mochly-Rosen D. DeltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart.J Mol Cell Cardiol 43:420–428,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway.J Biol Chem 277:21836–21842,2002 [DOI] [PubMed] [Google Scholar]
- 35.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress.Trends Cell Biol 14:20–28,2004 [DOI] [PubMed] [Google Scholar]
- 36.Szegezdi E, Duffy A, O'Mahoney ME, Logue SE, Mylotte LA, O'Brien T, Samali A. ER stress contributes to ischemia-induced cardiomyocyte apoptosis.Biochem Biophys Res Commun 349:1406–1411,2006 [DOI] [PubMed] [Google Scholar]
- 37.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis.EMBO Rep 7:880–885,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takagi H, Matsui Y, Sadoshima J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart.Antioxid Redox Signal 9:1373–1381,2007 [DOI] [PubMed] [Google Scholar]
- 39.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress.Mol Cell Biol 25:9554–9575,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teshima Y, Akao M, Jones SP, Marban E. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway.Circulation 108:2275–2281,2003 [DOI] [PubMed] [Google Scholar]
- 41.Teshima Y, Akao M, Jones SP, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes.Circ Res 93:192–200,2003 [DOI] [PubMed] [Google Scholar]
- 42.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes.Circ Res 99:275–282,2006 [DOI] [PubMed] [Google Scholar]
- 43.Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabo R, Di Lisa F, Gorza L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia.FASEB J 17:923–925,2003 [DOI] [PubMed] [Google Scholar]
- 44.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells.J Biol Chem 279:30133–30142,2004 [DOI] [PubMed] [Google Scholar]