Abstract
Previously we showed that Brown Norway (BN/Mcw) rats are more resistant to myocardial ischemia-reperfusion (I/R) injury than Dahl S (SS/Mcw) rats due to increased nitric oxide (·NO) generation secondary to increased heat shock protein 90 (HSP90) association with endothelial nitric oxide synthase (NOS3). Here we determined whether increased resistance to I/R injury in BN/Mcw hearts is also related to tetrahydrobiopterin (BH4) and GTP cyclohydrolase I (GCH-1), the rate-limiting enzyme for BH4 synthesis. We observed that BH4 supplementation via sepiapterin (SP) and inhibition of GCH-1 via 2,4-diamino-6-hydroxypyrimidine (DAHP) differentially modulate cardioprotection and that SP alters the association of HSP90 with NOS3. BH4 levels were significantly higher and 7,8-dihydrobiopterin (BH2) levels were significantly lower in BN/Mcw than in SS/Mcw hearts. The BH4-to-BH2 ratio in BN/Mcw was more than two times that in SS/Mcw hearts. After I/R, BH4 decreased and BH2 increased in hearts from both strains compared with their preischemia levels. However, the increase in BH2 in SS/Mcw hearts was significantly higher than in BN/Mcw hearts. Real-time PCR revealed that BN/Mcw hearts contained more GCH-1 transcripts than SS/Mcw hearts. SP increased recovery of left ventricular developed pressure (rLVDP) following I/R as well as decreased superoxide (O2•−) and increased·NO in SS/Mcw hearts but not in BN/Mcw hearts. DAHP decreased rLVDP as well as increased O2•− and decreased·NO in BN/Mcw hearts compared with controls but not in SS/Mcw hearts. SP increased the association of HSP90 with NOS3. These data indicate that BH4 mediates resistance to I/R by acting as a cofactor and enhancing HSP90-NOS3 association.
Keywords: nitric oxide, cardioprotection, guanosine 5′-triphosphate cyclohydrolase I
nitric oxide (·NO) is a key signaling molecule in the cardiovascular system (3, 34).·NO, both endogenous and exogenous, has been shown to increase cardioprotection during cardiac ischemia-reperfusion (I/R) injury in recent studies (2, 5, 18).·NO is produced from the guanidine group of L-arginine in a NADPH-dependent reaction catalyzed by a family of nitric oxide synthase (NOS), which requires Ca2+/calmodulin, flavin adenine dinucleotide, flavin mononucleotide, and tetrahydrobiopterin (BH4) as cofactors. Ischemia time-dependently decreases cardiac BH4 content and endothelial NOS (NOS3) activity and increases NOS-derived superoxide (O2•−) production, which is believed to contribute to postischemic endothelial dysfunction and myocardial injury (7). BH4 supplementation has been shown to restore endothelium-dependent coronary flow and decrease I/R injury in isolated perfused rat hearts (26, 32, 33), whereas inhibiting BH4 synthesis has been shown to increase I/R injury (13). These observations suggest the importance of myocardial BH4 bioavailability in cardioprotection and provide support for the notion that BH4 may be a novel therapeutic target for treating I/R injury.
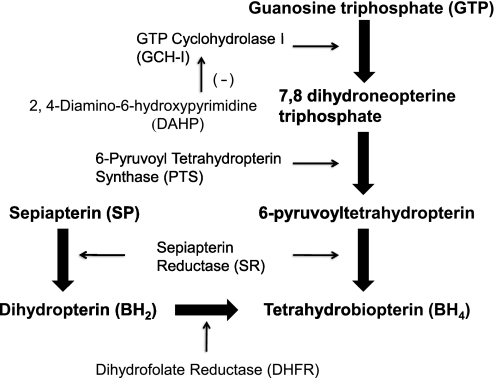
BH4 is synthesized by either the de novo pathway or the salvage pathway (Fig. 1). De novo synthesis starts with GTP in a Mg2+-, Zn2+-, and NADPH-dependent reaction to form 7,8-dihydroneopterin by GTP cyclohydrolase I (GCH-1), which is subsequently converted to 6-pyruvoyl-5,6,7,8-tetrahydropterin by 6-pyruvoyl-tetrahydropterin synthase (PTS), which is then converted to BH4 by sepiapterin reductase (SR) (9). GCH-1 is the rate-limiting enzyme in BH4 synthesis. The salvage pathway metabolizes sepiapterin (SP) and 7,8-dihydrobiopterin (BH2) to BH4 by SR and dihydrofolate reductase (DHFR) (16). Providing SP has been shown to effectively increase BH4 content and thereby cardioprotection (26). In contrast, selective inhibition of GCH-1 with 2,4-diamino-6-hydroxypyrimidine (DAHP) has been shown to either decrease BH4 content and·NO production in a coordinated manner or increase I/R injury in wide variety of experimental models (9, 12, 25, 31). Accordingly, increasing BH4 availability by modulating GCH-1 may be an effective way to restore·NO-mediated endothelial function and to reduce I/R injury to the myocardium (22, 25).
Fig. 1.
Diagram showing the enzymatic pathways for the synthesis of tetrahydrobiopterin (BH4).
Previously, our laboratory showed that hearts from Brown Norway (BN/Mcw) rats were more resistant to ischemia than hearts from Dahl S (SS/Mcw) rats (1, 21). More importantly, we showed that this increase in resistance to ischemia in hearts of BN/Mcw rats was related to an increase in the association of heat shock protein 90 (HSP90) with NOS3 and a subsequent increase in·NO production in BN/Mcw rat hearts (21). Because BH4 is an essential cofactor for NOS3 and a decrease in BH4 bioavailability is associated with uncoupled NOS3 activity and increased NOS3-dependent O2•− production, it is important to determine the role of BH4 in altering resistance to I/R injury in these two rat strains. The goals of the present study were to determine 1) whether BH4 levels are different in the hearts of BN/Mcw and SS/Mcw rats and whether higher BH4 levels contribute to resistance to I/R injury in BN/Mcw rat hearts, 2) whether BH4 levels are modulated by GCH-1 in BN/Mcw and SS/Mcw rat hearts, 3) whether BH4 supplementation or inhibition of GCH-1 differentially modulates resistance to I/R injury in BN/Mcw and SS/Mcw rat hearts, and 4) whether BH4 supplementation alters the association of HSP90 with NOS3. Here we provide the first evidence that BH4 levels are higher in BN/Mcw rat hearts than in SS/Mcw rat hearts. BN/Mcw hearts contained more GCH-1 transcripts than SS/Mcw hearts. Reducing BH4 levels by inhibiting GCH-1 decreased resistance to I/R injury in BN/Mcw rat hearts. In contrast, increasing BH4 levels improved cardiac function and recovery in SS/Mcw rat hearts. In addition, supplementation with SP, the BH4 precursor, enhanced the association of HSP90 with NOS3. These data suggest that BH4 levels mediate the resistance to ischemia in BN/Mcw and SS/Mcw by acting as a cofactor for NOS3 and through altering HSP90-NOS3 association.
MATERIALS AND METHODS
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Rats used in this study received humane care in compliance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (NIH). Rats were maintained on a low-salt (0.4% NaCl) diet with unlimited access to water. Environmental influences were minimized by maintaining rats in identical housing conditions. Eight-week-old BN/Mcw and SS/Mcw male rats were obtained from Charles River (Wilmington, MA).
Langendorff isolated heart preparation and measurements.
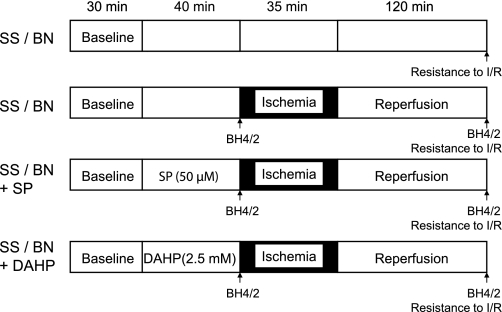
Hearts from BN/Mcw and SS/Mcw rats were isolated and perfused as previously described (21). Briefly, rats were anesthetized and the hearts were excised and perfused in the Langendorff mode at a perfusion pressure equivalent to 80 mmHg. Perfusate and bath temperatures were maintained at 37.2 ± 0.1°C using a thermostatically controlled water circulator (Lauda E100, Lauda Dr. R. Wobser, Pfarrstrasse, Germany). Left ventricular pressure was measured isovolumetrically with a transducer connected to a thin, saline-filled latex balloon inserted into the left ventricle through the mitral valve from an incision in the left atrium. Hearts were subjected to 35 min global ischemia followed by 120 min reperfusion. A three-way stopcock, located immediately above the site of cannulation, allowed the entire perfusate to be diverted away from the heart to produce global, no-flow ischemia. Reperfusion was achieved by repositioning the tap to allow perfusate to be delivered to the heart. The heart was immersed in nongassed physiological buffer solution within temperature-controlled chambers to maintain the myocardium at 37.2°C. Recovery was expressed as a ratio of the postischemic-reperfusion value over the predrug, preischemic value for developed pressure after 120 min of reperfusion. At the end of the experiments, the hearts were freeze-clamped and stored at −80°C until use for BH4 analysis by HPLC or Western blot analysis. The protocol for perfusing isolated hearts with GCH-1 inhibitor DAHP (Sigma-Aldrich, St. Louis, MO) or the BH4 precursor SP (Schircks Laboratories, Jona, Switzerland) is shown in Fig. 2.
Fig. 2.
Schematic illustration of the experimental protocol used in all experiment groups. SS/BN, Dahl S (SS/Mcw) or Brown Norway (BN/Mcw) rat hearts; SP, sepiapterin; DAHP, 2,4-diamino-6-hydroxypyrimidine; I/R, ischemia-reperfusion. BH4/2 marks denote the time points to harvest the tissue samples for HPLC assay for BH4 and BH2, and resistance to I/R means determining functional recovery after 35 min global ischemia and 120 min reperfusion.
Measurement of BH4 and BH2.
The BH4 and BH2 levels were determined as described previously (6). Briefly, 100-mg samples from heart tissue were lysed in 1 ml of 50 mM phosphate buffer (pH 2.6) containing 0.2 mM diethylenetriamine pentaacetic acid (DTPA; Sigma-Aldrich) and 1 mM 1,4-dithioerythritol (DTE; Sigma-Aldrich; freshly added) and then homogenized followed by centrifugation (12,500 rpm × 10 min, 4°C). The supernatants were filtered through a 10-kDa cutoff column (Millipore, Billerica, MA). BH4 and BH2 were quantified on a HPLC with an electrochemical detector (ESA Biosciences CoulArray system Model 542; Chelmsford, MA) using a Synergi Polar-RP column (Phenomenex, Torrance, CA) eluted with argon degassed 50 mM phosphate buffer (pH 2.6). Multichannel coulometric detection was set between 0 and 600 mV. One channel was set at −250 mV to verify the reversibility of BH4 oxidative peak detection. Calibration curves were made by summation of peak areas collected at 0 and 150 mV for BH4 and 280 and 365 mV for BH2. Intracellular concentrations of BH4 and BH2 were calculated using authentic BH4 and BH2 standards. Cellular BH4 and BH2 levels were then normalized to cell protein concentrations.
Cardiomyocyte isolation.
Myocytes were enzymatically isolated from BN/Mcw and SS/Mcw rats by a modified procedure by Mitra and Morad (14). The hearts were excised, mounted on a Langendorff apparatus, and perfused retrogradely via the aorta with oxygenated isolation buffer containing (in mM) 110 Na+, 3.8 K+, 1.2 Mg2+, 25 HEPES, 113 Cl−, 1.2 H2PO4−, and 11 glucose. After blood was washed out of the heart, the buffer was replaced with an enzyme solution containing 2.85 mg/ml collagenase type II (Gibco, Invitrogen, Carlsbad, CA), 0.1 mg/ml protease XIV (Sigma-Aldrich), and 0.1 mM Ca2+ in the isolation buffer at pH 7.35. The temperature was maintained at 37°C. All solutions were continuously bubbled with 95% oxygen-5% carbon dioxide gas mixture. After 25 min of enzyme treatment, the ventricles were excised, minced, and incubated in the same enzyme solution for an additional 5 min in a shaker bath at 37°C. The cell suspension was filtered through a 200-μm mesh and centrifuged (125 g × 30 s; RT). The cell yield percentage (ratio of rods to rounded myocytes) was around 70–80% (Supplemental Fig. 1; all supplemental material can be found with the online version of this article). Approximately 6 × 105 rod shape myocytes were used for BH4 and BH2 assay. After isolation, the myocytes were pelleted and directly lysed in 1 ml of 50 mM phosphate buffer (pH 2.6) containing 0.2 mM DTPA and 1 mM DTE (freshly added) and then analyzed by HPLC for BH4 and BH2.
Real-time-PCR analysis for message levels of essential enzymes in BH4 biosynthesis.
Total RNA was extracted from isolated myocytes or frozen heart tissue homogenates using TRIzol reagent (Invitrogen) and treated with DNase I via a DNA-Free kit (Ambion, Austin, TX). cDNA was generated from 1 μg of total RNA using an iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The primers for real-time-PCR were synthesized by Operon Biotechnologies (Huntsville, AL). The primer sequences for GCH-1 were 5′-GGCCGCTTACTCGTCCATCCT-3′ (forward) and 5′-GGTCTCCTGGTATCCCTTGGTGAA-3′ (reverse); the sequences for PTS were 5′-GCGTTAAAGGGATTGTGAGGTCTGT-3′ (forward) and 5′-CCGGGCTTCAATCCTCAGTTCT-3′ (reverse); the sequences for SR were 5′-CCCACGTGGACTTCTATGAC-3′ (forward) and 5′-CCAGCCCTAAGTTCTCTTCC-3′ (reverse); and the sequences for DHFR were 5′-ACCAGGAAGCCATGAATCAG-3′ (forward) and 5′-AGCAGTAGGACTTGGGAGCA-3′ (reverse). Real-time-PCR was performed on an iCycler iQ real-time PCR instrument (Bio-Rad) in duplicates using iQTM SYBR Green Supermix (Bio-Rad) according to the manufacturer's instruction. The reaction for GAPDH mRNA was amplified for all samples as the internal reference. The amplification conditions were as follows: initial denaturation at 95°C followed by 40 cycles of denaturation at 95°C for 30 s and annealing at 55°C for 30 s and elongation at 72°C for 1 min. With the difference in the cycle threshold (Ct) values between the target gene (GCT-1 or PTS, etc.) and the reference gene (GAPDH) defined as ΔCt, the mRNA expressions were calculated as 2ΔCt. The difference of gene expression in BN/Mcw and SS/Mcw hearts was calculated by 2ΔCt (BN)/2ΔCt (SS) and expressed as a fold increase of BN/Mcw rats over SS/Mcw rats.
Western blot analysis.
Western blot analysis of homogenates from BN/Mcw and SS/Mcw hearts for GCH-1, SR, or DHFR expression was performed using the method described previously (19). Briefly, samples was loaded into and separated by 12% SDS/PAGE. The proteins were transferred to a nitrocellulose membrane, which was then blocked with 5% nonfat milk-PBS buffer and incubated with primary antibodies overnight and secondary antibodies for 1 h. Bands of identity were visualized with a Super Signal West Pico kit (Pierce Biotechnology, Rockford, IL), and bands densities were quantified using UN-SCAN-IT software (Silk Scientific, Orem, UT). Antibodies specific for GCH-1 used in the study were kindly provided by Dr. Gabriele Werner-Felmayer (Division of Biological Chemistry, Biocentre, Innsbruck Medical University). DHFR and SR antibodies were purchased from Fitzgerald (Concord, MA) and Abnova (Taiwan), respectively.
Immunoprecipitation of NOS3.
Heart tissue was homogenized in MOPS buffer containing 20 mM MOPS (pH 7.0), 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 40 mM β-glycerophosphate (pH 7.2), 10 mM sodium pyrophosphate, 0.5% Nonidet P-40, protease inhibitor cocktail, and phosphatase inhibitor cocktail (Sigma-Aldrich). The lysates were precleared with protein A sepharose (Sigma-Aldrich) and then immunoprecipitated with monoclonal anti-NOS3 antibody (Biomol, Plymouth Meeting, PA; 3 μg/400 μg lysate protein) at 4°C overnight as described previously by our laboratory (19). The next day, the protein A beads were added to the mixture and incubated at 4°C for 120 min. The beads were then spun down and washed four times with PBS buffer, and proteins were eluted and separated by SDS-PAGE. Proteins were electroblotted onto nitrocellulose membranes (Bio-Rad). The membrane was blocked in 5% nonfat dry milk in Tris-buffered saline-Tween and immunoblotted for NOS3 (Santa Cruz, CA) and HSP90 (Santa Cruz, CA). Proteins were detected by enhanced chemiluminescence with ECL reagents from Amersham (Pittsburgh, PA). Relative band densities of resulting autoradiograms were quantified with UN-SCAN-IT software.
Reactive oxygen species detection.
Reactive oxygen species (ROS; O2•−) production was detected by using lucigenin-enhanced chemiluminescence. Coronary effluent was collected at 1 min of initial reperfusion time, 500 μl were immediately taken to a 1.5-ml vial, and then 5 μl (500 μM) lucigenin (Sigma) were added for a final concentration of 5 μM. The vial was placed in a luminometer (Turner Biosystems) to measure chemiluminescence for 5 min. The whole process was done in the dark room. The vial containing only lucigenin was read as background luminescence, and this value was subtracted from each sample. The generation of ROS was presented as relative light units.
H2O2 and·NO detection.
One milliliter of coronary effluent that was collected at the first minute of initial reperfusion time was immediately used for determination for concentration of·NO and H2O2 by a·NO electrode and a H2O2 electrode (WPI, Sarasota, FL). Data were normalized by heart wet weight and coronary flow rate. Calibration of the H2O2 and·NO electrode was performed according to the manufacturer's instructions by using 0.5, 1, 2, 4, and 8 μM standard H2O2 solution and NaNO2 and KI + H2SO4 for·NO to generate known amounts of H2O2 and·NO. The calibration curves exhibited a linear correlation coefficient of 0.98 and 0.99.
Statistics.
Data are expressed as means ± SD and were analyzed by two-way ANOVA or by two-tailed t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
BH4 and BH2 levels in BN/Mcw and SS/Mcw rat hearts and cardiac myocytes.
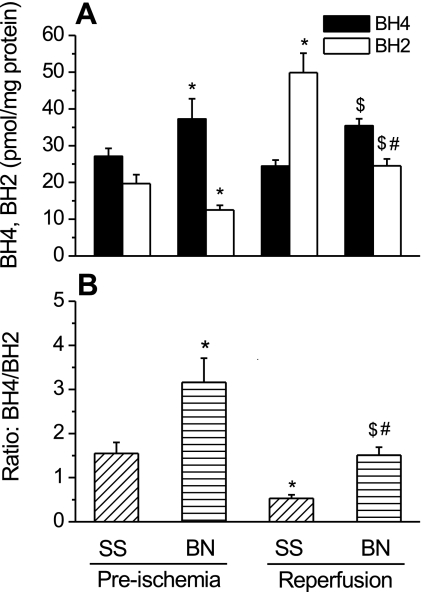
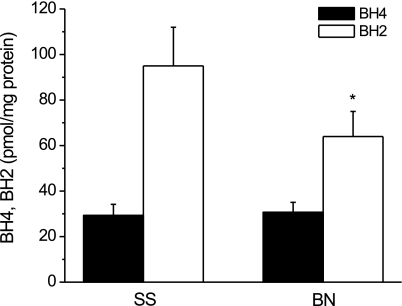
As shown in Fig. 3, before ischemia, BH4 levels were significantly higher and BH2 levels were significantly lower in BN/Mcw rat hearts than that in SS/Mcw rat hearts (BN/Mcw BH4 and BH2: 37.3 ± 5.5 and 12.5 ± 1.3 pmols/mg protein vs. SS/Mcw BH4 and BH2: 27.1 ± 2.2 and 19.7 ± 2.4 pmols/mg protein, respectively; P < 0.05; n = 8/group). The BH4-to-BH2 ratio in BN/Mcw rat hearts was more than two times that in SS/Mcw rat hearts (3.2 ± 0.6 vs. 1.6 ± 0.3). After I/R, BH4 in both BN/Mcw and SS/Mcw rat hearts decreased compared with preischemia levels. BH2 increased in the hearts of both strains and the increase of BH2 was much greater in the SS/Mcw rat hearts than in BN/Mcw rat hearts as evidenced by a dramatic decrease in the BH4-to-BH2 ratio (from 1.55 ± 0.25 to 0.53 ± 0.08; n = 8/group) in SS/Mcw rat hearts compared with the BH4-to-BH2 ratio in BN/Mcw rat hearts, which remained greater than 1 (from 3.16 ± 0.55 to 1.51 ± 0.18; n = 8/group). Since BN/Mcw hearts contain higher BH4 levels than SS/Mcw hearts, we next determined BH4 levels in freshly isolated cardiomyocytes from hearts of both strains of rat. No significant differences in BH4 levels were detected in BN/Mcw and SS/Mcw rat cardiomyocytes (30.7 ± 4.4 vs. 29.3 ± 4.5 pmol/mg protein; n = 4/group). However, BH2 levels were considerably higher in SS/Mcw cardiomyocytes compared with the levels in BN/Mcw cardiomyocytes (95 ± 17 vs. 64 ± 11 pmol/mg protein; P < 0.05; n = 4/group; Fig. 4).
Fig. 3.
BH4 and BH2 levels (A) and ratio of BH4 to BH2 (B) in BN/Mcw and SS/Mcw rat hearts. In BN/Mcw or SS/Mcw preischemia group, hearts were harvested before ischemia. In BN/Mcw or SS/Mcw reperfusion group, hearts were harvested after 35 min global ischemia and 120 min reperfusion. The data were shown as means ± SD. *P < 0.05 vs. SS/Mcw preischemia group; #P < 0.05 vs. BN/Mcw preischemia group; $P < 0.05 vs. SS/Mcw reperfusion group (n = 8/group).
Fig. 4.
BH4 and BH2 levels in isolated BN/Mcw and SS/Mcw rat cardiomyocytes (n = 4). The data were shown as means ± SD. *P < 0.05 vs. SS/Mcw rat group.
mRNA levels of the enzymes involved in BH4 biosynthesis in BN/Mcw and SS/Mcw rat hearts and myocytes.
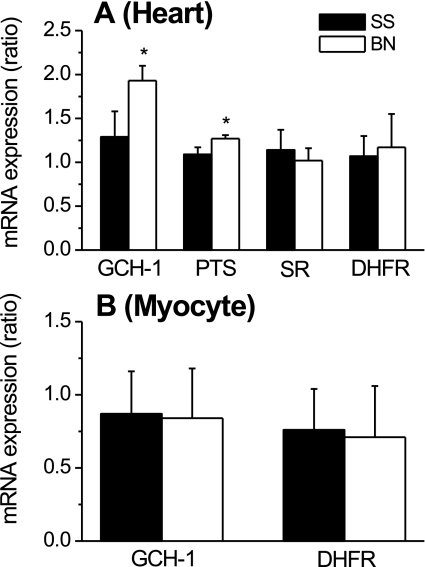
Because BN/Mcw rat hearts produced more BH4 than SS/Mcw rat hearts, we examined mRNA levels for GCH-1, PTS, SR, and DHFR, the key enzymes in both BH4 de novo and salvage biosynthesis pathways. As shown in Fig. 5A, real-time PCR revealed that BN/Mcw rat hearts contained higher mRNA transcript levels for GCH-1 and PTS than did SS/Mcw rat hearts (1.93 ± 0.17 and 1.27 ± 0.04 vs. 1.29 ± 0.29 and 1.09 ± 0.07; P < 0.05; n = 5/group). No differences in DHFR and SR mRNA transcripts were detected between the two strains (1.17 ± 0.38 and 1.02 ± 0.14 vs. 1.07 ± 0.23 and 1.14 ± 0.12). We further examined mRNA levels for GCH-1 and DHFR from freshly isolated cardiomyocytes; however, there were no differences in GCH-1 and DHFR mRNA observed in cardiomyocytes between BN/Mcw and SS/Mcw rats (Fig. 5B; n = 5/group). Agarose gels for real-time PCR products showed only one clear band for GCH-1, PTS, SR, DHFR, and GAPDH, which indicated that the primers for these genes are very specific (supplemental Fig. 2).
Fig. 5.
mRNA levels of GTP cyclohydrolase I (GCH-1), 6-pyruvoyl-tetrahydropterin synthase (PTS), sepiapterin reductase (SR), and dihydrofolate reductase (DHFR) in BN/Mcw and SS/Mcw rat hearts and cardiomyocytes. The results represent means ± SD (n = 5/group). The difference of gene expression in BN/Mcw and SS/Mcw is calculated by 2ΔCt(BN)/2ΔCt(SS) and expressed as fold increase of BN/Mcw rats over SS/Mcw rats. ΔCt represents the difference in the cycle (Ct) values between the target gene (GCT-1 or PTS, etc.) and the reference gene (GAPDH). A and B showed the results from heart tissue (A) and cardiomyocytes (B), respectively. *P < 0.05 vs. SS/Mcw group.
GCH-1 protein levels.
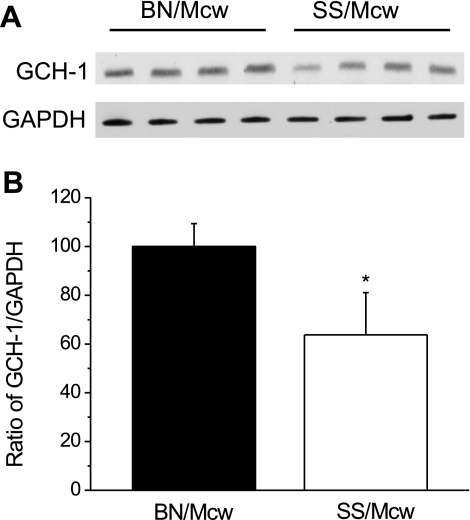
Western blot analysis showed that hearts from BN/Mcw rats have nearly 60% more GCH-1 than hearts from SS/Mcw rats (n = 4/group, normalized to GAPDH protein levels; Fig. 6). There were no differences in DHFR and SR protein levels observed in these two rat strains (data not shown).
Fig. 6.
GCH-1 and GADPH protein levels in BN/Mcw and SS/Mcw rat heart homogenates. GCH-1 and GAPDH were detected by Western blot analysis using lysates of heart homogenates (A). The densimetric analyses of the blot in A were normalized to percentage of SS/Mcw in ratio of GCH-1 vs. GADPH (B; means ± SD; n = 4/group). *P < 0.05 vs. SS/Mcw group.
SP supplementation or DAHP inhibition of GCH-1 and resistance to myocardial ischemia.
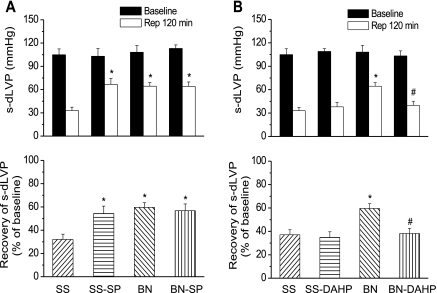
To determine whether BH4 was responsible for increased resistance to ischemia in hearts from BN/Mcw rats compared with SS/Mcw rats, we perfused isolated hearts with either a GCH-1 inhibitor (DAHP, 2.5 mM) or a BH4 donor (SP, 50 μM) for 40 min before 35 min global ischemia. Hearts were then reperfused for 120 min. Left ventricular developed pressure (LVDP) and recovery of LVDP after 120 min reperfusion (expressed as a percentage of its predrug, preischemic value) were used to assess resistance to ischemia. LVDP and recovery of LVDP after I/R in untreated BN/Mcw rats were much higher than in SS/Mcw rats (64.4 ± 4.6 mmHg and 59.6 ± 4.1% vs. 37.8 ± 3.9 mmHg and 37.1 ± 4.3%; P < 0.01), which is consistent with previous findings from our laboratory (21). SP increased LVDP and percent recovery of LVDP after I/R in SS/Mcw rat hearts (66.7 ± 7.6 mmHg and 54.3 ± 6.4%) but had no effect on BN/Mcw hearts (Fig. 7A). DAHP decreased LVDP and recovery of LVDP in BN/Mcw hearts (39.9 ± 5.2 mmHg and 38.2 ± 4.4%) to that observed in untreated SS/Mcw hearts but did not further decrease recovery of LVDP in SS/Mcw rat hearts (Fig. 7B).
Fig. 7.
Resistance to myocardial ischemia in hearts from BN/Mcw and SS/Mcw rats treated with SP and with 2,4-diamino-6-hydroxypyrimidine (DAHP). Isolated rat hearts were perfused with SP (50 μM; A) or DAHP (2.5 mM; B) for 40 min before 35 min global ischemia followed by 120 min reperfusion (Rep). s-dLVP, systolic-diastolic left ventricular pressure. Data are presented as means ± SD (n = 8–10/group). *P < 0.05 vs. SS/Mcw control group; #P < 0.05 vs. BN/Mcw control group.
Effects of SP and DAHP on cardiac BH4 and BH2 levels.
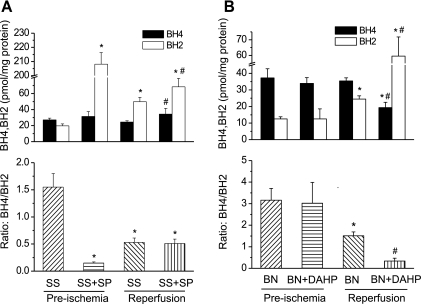
To determine whether SP alters the levels of BH4 and BH2 in SS/Mcw rat hearts or DAHP in BN/Mcw rat hearts, BH4 and BH2 were measured at two time points: one is after perfusion of SP or DAHP for 40 min (right before ischemia) and the other one is after reperfusion for 120 min. The values at these time points were compared with the levels with untreated controls. After 40 min of SP perfusion in SS rat hearts, BH4 increased but was not significantly different when compared with controls (Fig. 8A). The BH2 levels showed a huge increase (208 ± 29 vs. 19.7 ± 2.4 pmol/mg protein; P < 0.001) with SP treatment. After 120 min of reperfusion, BH4 levels were higher in SP treatment group than in the controls (31 ± 6 vs. 24 ± 4 pmol/mg protein; P < 0.05) and BH2 remained high after reperfusion although it was much lower compared with the preischemia value (68 ± 10 vs. 208 ± 29 pmol/mg protein; P < 0.001) in the SP treatment group. The BH4-to-BH2 ratio decreased significantly after perfusion of SP, but the ratio was about the same between control and SP treated groups after 120 min reperfusion. In BN/Mcw rat hearts, DAHP did not alter BH4 or BH2 levels after 40 min perfusion (Fig. 8B). However, after 120 min reperfusion the BH4 levels were much lower and BH2 levels were much higher in DAHP-treated hearts compared with the hearts from the control group (19.3 ± 3.2, 59.5 ± 12.2 vs. 35.4 ± 4.6, 24.5 ± 4.7 pmol/mg protein; P < 0.01). The BH4-to-BH2 ratio was significantly lower in DAHP-treated hearts compared with control hearts after 120 min reperfusion (P < 0.01). These data confirm that BH4 plays an important role in modulating resistance to ischemic injury in BN/Mcw and SS/Mcw rat hearts.
Fig. 8.
BH4 and BH2 levels and ratio of BH4 to BH2 in SS/Mcw and BN/Mcw rat hearts with or without SP (50 μM; A) or DAHP (2.5 mM; B) treatment before and after ischemia and reperfusion. The values were shown as means ± SD (n = 6/group). *P < 0.05 vs. SS/Mcw or BN/Mcw preischemia control group; #P < 0.05 vs. SS/Mcw or BN/Mcw reperfusion control group.
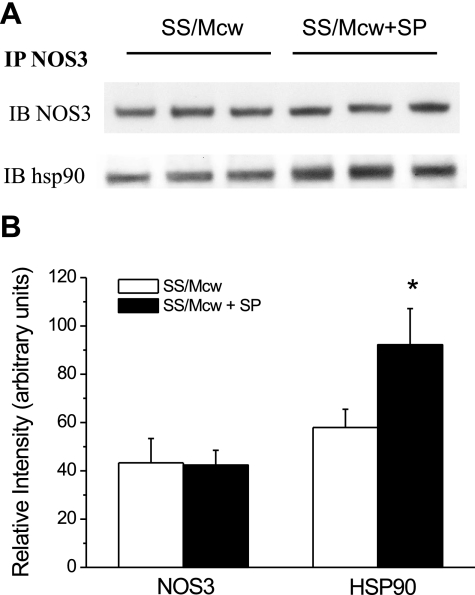
Effects of SP and DAHP treatment on HSP90 association with NOS3.
A previous study from our laboratory demonstrated that the increased association of HSP90 with NOS3 is the major cellular mechanism by which BN/Mcw hearts produce more·NO and less O2•− than SS/Mcw hearts to increase resistance to I/R injury compared with the SS/Mcw heart (21). Here we examined whether the supplement of SP or DAHP affected the association of HSP90 with NOS3. We immunoprecipitated NOS3 from heart homogenates of SS/Mcw hearts with or without SP treatment or BN/Mcw hearts with or without DAHP treatment and probed for NOS3 and HSP90 protein. Neither SP nor DAHP altered NOS3 protein levels. However, immunoblots of the immunoprecipitates showed that the amount of HSP90 bound to NOS3 was increased more than 60% in SP treated SS/Mcw hearts than nontreated hearts (Fig. 9). In contrast, the level of HSP90 associated with NOS3 in BN/Mcw rat hearts treated with DAHP significantly decreased compared with the level in BN/Mcw control hearts (supplemental Fig. 3).
Fig. 9.
Western blot analysis of endothelial nitric oxide synthase (NOS3) and heat shock protein 90 (HSP90) association in heart homogenates from SS/Mcw rats with or without SP (50 μM) treatment (A). B: densimetric analyses of the blot in A. IP, immunoprecipitation; IB, immunoblot. n = 3/group. *P < 0.05 vs. SS/Mcw control group.
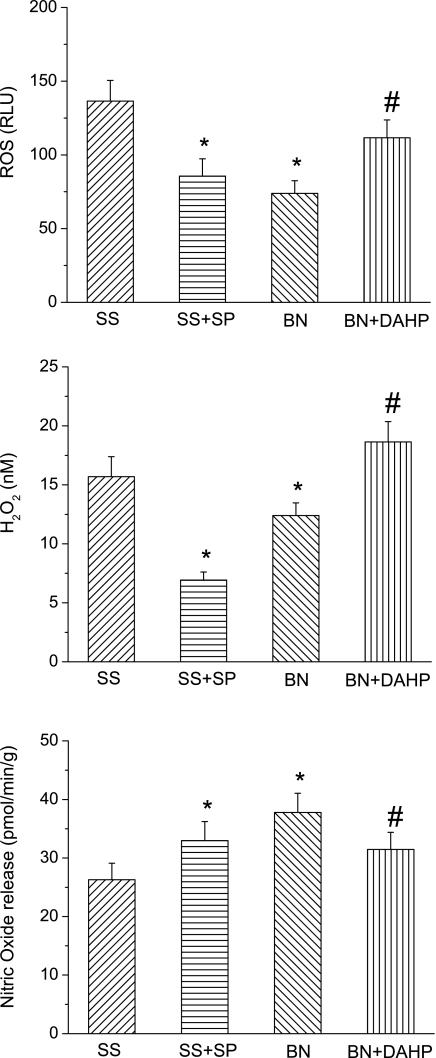
Change of·NO and O2•− levels after ischemia.
To further determine the effects of BH4 in NOS3 coupling, we measured·NO, O2•−, and H2O2 levels in SS/Mcw hearts with SP and BN/Mcw hearts with DAHP treatment (Fig. 10). The results showed that O2•− and H2O2 levels were significantly reduced by SP in SS/Mcw rat hearts and increased by DAHP in BN/Mcw rat hearts after I/R. Moreover, O2•− and H2O2 levels were higher in SS/Mcw control hearts than that in BN/Mcw control hearts after I/R. After SP supplement·NO levels significantly increased compared with control group in SS/Mcw rat hearts after I/R, whereas DAHP treatment significantly decreased the·NO levels in BN rat hearts compared with the control group after I/R.·NO levels in BN/Mcw control hearts were also higher than that in SS/Mcw control hearts after I/R.
Fig. 10.
Superoxide (O2•−), H2O2, and nitric oxide (·NO) release from hearts of SS/Mcw rat with or without SP (50 μM) and BN/Mcw rat with or without DAHP (2.5 mM) treatment at initial reperfusion period (the first minute). The values were shown as means ± SD (n = 6/group). *P < 0.05, compared with SS/Mcw control (SS) group; #P < 0.05, compared with BN/Mcw control (BN) group. ROS, reactive oxygen species; RLU, relative light units.
DISCUSSION
At the physiological level, NOS3 has been shown to play an important role in regulating vascular function (4, 24). Our laboratory previously found that the response of the heart to an identical ischemic insult results in increased resistance to ischemia in the BN/Mcw rat compared with the SS/Mcw rat (21). The underlying mechanism appeared to involve increased·NO production in hearts from BN/Mcw rats compared with SS/Mcw rats mediated by an increase in HSP90 association with NOS3. Since the pteridine cofactor BH4 has been shown to be an essential cofactor for maintaining NOS3·NO production (8, 10, 17, 29), here we examined the role of BH4 and BH4 biosynthesis in the mechanisms governing resistance to ischemia in both BN/Mcw and SS/Mcw rats. The major findings of this study are as follows. First, BH4 levels were significantly higher and BH2 levels were significantly lower in BN/Mcw rat hearts than in SS/Mcw rat hearts. The ratio of BH4 to BH2 in BN/Mcw rat hearts was more than two times the ratio in SS/Mcw rat hearts. Alterations in the absolute BH4 and BH2 levels, as well as the BH4-to-BH2 ratio, were consistent with the recovery of cardiac function in BN/Mcw and SS/Mcw rat hearts. Second, SP supplementation of SS/Mcw hearts or GCH-1 inhibition of BN/Mcw hearts increased and decreased resistance to I/R injury, respectively. Third, BN/Mcw rat hearts had more transcripts for GCH-1 than SS/Mcw rat hearts that together may have contributed to the higher BH4 levels observed in these hearts. Finally, supplementation with the BH4 precursor SP or DAHP, the inhibitor of GCH-1, elevated or decreased the association of HSP90 with NOS3, respectively. Our results clearly indicate that BH4 and ongoing synthesis play crucial roles in modulating the susceptibility of hearts to I/R injury in BN/Mcw and SS/Mcw rats and that BH4 mediates resistance to I/R by acting as a cofactor for NOS3 and enhancing HSP90-NOS3 association.
Reduced BH4 likely represents an important cellular defect involved with both endothelial and cardiomyocyte dysfunction in hearts exposed to I/R (7). The role of BH4 in NOS activity is particularly relevant to cardioprotection. Depletion of BH4 not only prevents·NO formation from NOS3 but also results in NOS3 uncoupling, which is characterized as NOS3 generating O2•− rather than·NO (28). BH4 deficiency resulting from reduced BH4 biosynthesis or increased BH4 oxidation to BH2, which is catalytically inactive, were shown to contribute to I/R injury (7). Previously, our laboratory showed that a higher myocardial·NO production and lower O2•− in BN/Mcw rats correlated to increased resistance to I/R injury compared with SS/Mcw rats (21). We extrapolated on these findings, showing that the level of BH4 in the heart is crucial for maintaining increased resistance to I/R injury in inbred strains of rats. Interestingly, the cellular source that affords different BH4 levels apparently is not from cardiomyocytes. These data were logical since others have shown that vascular endothelium is a major source of BH4 and·NO production in the myocardium under physiological conditions (23, 27). However, the higher BH2 levels in SS/Mcw cardiomyocytes, we observed here, indicate that cardiomyocytes from SS/Mcw rats are under greater oxidative stress than cardiomyocytes from BN/Mcw rats and also contribute to a lower BH4-to-BH2 ratio in SS/Mcw rat hearts. Indeed, an ox-blot (which detects the carbonyl-modified proteins) of BN/Mcw and SS/Mcw rat heart homogenates revealed that the oxidative modification of proteins in SS/Mcw rat hearts was much higher than that in BN/Mcw rat hearts (supplemental Fig. 4). Recent studies in vascular biology have revealed the importance of the BH4-to-BH2 ratio in determining whether BH4 and BH2 bind to NOS3. Since BH4 and BH2 bind to NOS3 with equal affinity, BH2 rapidly and efficiently displaces BH4 in NOS3, resulting in uncoupled NOS3 activity and decreased·NO synthesis (8, 34). Thus, in our study, in addition to lower BH4 levels, the marked decrease in the BH4-to-BH2 ratio in SS/Mcw rat hearts after I/R likely contributed to NOS uncoupling, increased ROS generation, and diminished recovery of cardiac function compared with BN/Mcw rat hearts.
Consistent with BH4 levels in hearts, real-time PCR data showed that BN/Mcw rat hearts contained higher mRNA transcripts for GCH-1 and PTS than did SS/Mcw rat hearts. However, there were no differences of GCH-1 mRNA levels observed between BN/Mcw and SS/Mcw rat cardiomyocytes. Further studies to determine GCH-1 levels in endothelial cells and other cells in myocardium are needed to identify the exact source of the increase of GCH-1 in BN/Mcw rat hearts compared with SS/Mcw rat hearts and to explain the discrepancy between GCH-1 mRNA levels in the whole heart versus in isolated cardiomyocytes. Nevertheless, our results demonstrated that GCH-1 message levels in BN/Mcw and SS/Mcw rat hearts regulate·NO production, and this regulation may be limited to vascular endothelium. Recently, Ionova et al. (11) reported that DHFR mRNA was not detectable in either unstimulated adult cardiac myocytes or after stimulation with inflammatory cytokines although the constitutive expression of DHFR was observed in neonate rat cardiomyocytes of the same Sprague-Dawley strain. In our study DHFR mRNA was detectable in freshly isolated BN/Mcw and SS/Mcw cardiomyocytes (without culture) but showed no differences between these two strains. Thus the salvage pathway, which has the functional capacity to convert BH2 to BH4, does not play a crucial role in maintaining the high levels of BH4 in BN/Mcw rat hearts.
In a recent review, increasing BH4 availability by modulating GCH-1 has been suggested as a new strategy to protect the heart under conditions of stress, such as postinfarction remodeling, dilated myopathic remodeling, and hypertrophy (15). It has been shown that the administration of exogenous BH4 or SP, the precursor of BH4, restores·NO production from NOS and enhances postischemic recovery of NOS-dependent coronary flow (7, 26). In our study, SP significantly increased the recovery of cardiac function in SS/Mcw rat hearts. Interestingly, SP was unable to increase resistance to ischemia further in BN/Mcw hearts, suggesting that the myocardium of BN/Mcw hearts is already at a maximal state of protection. Resistance to ischemia in BN/Mcw hearts was decreased to the level of ischemic injury observed in SS/Mcw hearts simply by inhibiting GCH-1 with DAHP. In contrast, the recovery of function after ischemia in SS/Mcw rats was unaffected by DAHP but was increased by SP supplementation to levels present in BN/Mcw hearts. This reciprocal relationship provides strong evidence that BH4 correlates with cardioprotection and increased GCH-1 expression plays an important role in cardioprotection. To examine the relationship between BH4, BH2 metabolism and cardiac function in DAHP treated SS/Mcw rat hearts and SP treated BN/Mcw rat hearts, we measured BH4 and BH2 in these hearts after 120 min reperfusion. The results showed that DAHP increased BH2 levels and decreased BH4 levels in SS/Mcw rat hearts, whereas SP significantly increased BH2 levels but did not change BH4 levels in BN rat hearts (supplemental Fig. 5). Since the functional recovery of the SS/Mcw hearts was not altered by DAHP inhibition (Fig. 7), these results indicated that NOS3 is fully uncoupled in SS/Mcw hearts after I/R injury; thus a further decrease in BH4-to-BH2 ratio will not affect functional recovery, which was already significantly suppressed at the maximal level by I/R in SS/Mcw rat hearts. On the other hand, in BN/Mcw rat hearts, the BH4 levels after I/R may still be sufficient for maintaining NOS3 function. BH4 was not depleted enough to induce DHFR enzyme activity to convert BH2 to BH4, thus SP supplementation could only result a higher BH2 level. Consequently, SP was not able to increase functional recovery in BN/Mcw hearts where the function recovery was already high.
The cellular levels of BH4 are a key factor in modulating NOS3 activity,·NO production, O2•− production, and vascular reactivity in the postischemic heart. In our findings, O2•− and H2O2 release from SS/Mcw rat hearts were significantly reduced by SP, whereas O2•− and H2O2 release from BN/Mcw rat hearts were increased by DAHP. Moreover, there was more O2•− and H2O2 released from SS/Mcw rat hearts than that from BN/Mcw rat hearts. After SP supplement·NO release was increased in SS/Mcw rat hearts. In contrast, DAHP significantly decreased·NO production by BN/Mcw rat hearts compared with controls. Moreover, BN/Mcw rat hearts released more·NO than SS/Mcw rat hearts. These results indeed confirm that there are good correlations between BH4 levels and NOS-dependant·NO and O2•− production. Previously, BH4 was reported to structurally stabilize NOS dimers (30), one of the mechanisms for promoting coupled NOS activity. Using Western blot analysis, we examined NOS3 dimers and monomers in SS/Mcw or BN/Mcw rats treated with SP or DAHP (supplemental Fig. 6). However, NOS3 dimer-to-monomer ratios were not altered in either SS/Mcw or BN/Mcw rat hearts after SP treatment. There was a slight decrease in NOS3 dimer-to-monomer ratios after SS/Mcw rat hearts were treated with DAHP. Thus these data suggest that NOS3 dimer-to-monomer ratios may not play a big role in NOS-coupled activity in our animal model.
A previous study from our laboratory indicated that protein-protein interactions play an important role in regulating·NO production with HSP90 modulating the balance of·NO and O2•− generation from NOS3 (21). An increased association of HSP90 with NOS3 is a major mechanism by which BN/Mcw hearts are more resistant to ischemia than SS/Mcw hearts. The advantage this mechanism affords is that the heart could rapidly respond to stressful conditions of oxygen deprivation, i.e., ischemia or hypoxia, without resorting to increased gene expression. The association of HSP90 with NOS3 may represent a universal mechanism by which the heart increases·NO generation. An interesting finding in this study is that the association of NOS3 with HSP90 is increased in SS/Mcw rat hearts after SP supplementation compared with control hearts, whereas DAHP decreased the association of NOS3 with HSP90. The importance of this observation is that it demonstrates for the first time that BH4 improves NOS3 activity and function by a mechanism beyond its role as a cofactor for NOS3. Furthermore, although some consider BH4 to be important for improving vascular function, our data suggest that the mechanisms by which BH4 and HSP90 increase NOS3-coupled activity may actually be interdependent.
In summary, our results show, for the first time, that BH4 and GCH-1 differentially regulate resistance to I/R injury in the hearts of BN/Mcw and SS/Mcw rats. The mRNA transcription of GCH-1 regulates rates of BH4 biosynthesis that are critical for increased·NO production in BN/Mcw rat hearts, which affords increased resistance to myocardial ischemia. The different BH4-to-BH2 ratios between BN/Mcw and SS/Mcw rat hearts also may explain the difference in the coupling state of NOS3 in these hearts. SS/Mcw rats have increased oxidation of BH4, which appears to contribute directly to decreased resistance to I/R injury in these hearts compared with the hearts from BN/Mcw rats. Furthermore, the mechanisms by which BH4 and HSP90 regulate NOS3 activity and function to modulate cardioprotection may be interdependent.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-080468 (to Y. Shi) and HL-71214 (to K. A. Pritchard, Jr.) from the National Institutes of Health (Bethesda, MA).
ACKNOWLEDGMENTS
The administrative assistance of Anne Laulederkind and Meghann Sytsma is gratefully acknowledged.
REFERENCES
- 1.Baker JE, Konorev EA, Gross GJ, Chilian WM, Jacob HJ. Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardioprotection? Am J Physiol Heart Circ Physiol 278: H1395–H1400, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 33: 1897–1918, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63: 175–195, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee A, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol 49: 134–140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MV, Yang XM, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 70: 231–239, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Du J, Wei N, Guan T, Xu H, An J, Pritchard KA Jr, Shi Y. Inhibition of CDKs by roscovitine suppressed LPS-induced·NO production through inhibiting NF-κB activation and BH4 biosynthesis in macrophages. Am J Physiol Cell Physiol297: H742–H749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA 104: 15081–15086, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorren AC, List BM, Schrammel A, Pitters E, Hemmens B, Werner ER, Schmidt K, Mayer B. Tetrahydrobiopterin-free neuronal nitric oxide synthase: evidence for two identical highly anticooperative pteridine binding sites. Biochemistry 35: 16735–16745, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Gross SS, Levi R. Tetrahydrobiopterin synthesis: an absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem 267: 25722–25729, 1992 [PubMed] [Google Scholar]
- 10.Hemmens B, Mayer B. Enzymology of nitric oxide synthases. Methods Mol Biol 100: 1–32, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Ionova IA, Vasquez-Vivar J, Whitsett J, Herrnreiter A, Medhora M, Cooley BC, Pieper GM. Deficient BH4 production via de novo and salvage pathways regulates NO responses to cytokines in adult cardiac myocytes. Am J Physiol Heart Circ Physiol 295: H2178–H2187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasai K, Hattori Y, Banba N, Hattori S, Motohashi S, Shimoda S, Nakanishi N, Gross SS. Induction of tetrahydrobiopterin synthesis in rat cardiac myocytes: impact on cytokine-induced NO generation. Am J Physiol Heart Circ Physiol 273: H665–H672, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Kawahara K, Takase M, Yamauchi Y. Increased vulnerability to ischemia/reperfusion-induced ventricular tachyarrhythmias by pre-ischemic inhibition of nitric oxide synthase in isolated rat hearts. Cardiovasc Pathol 12: 49–56, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol Heart Circ Physiol 249: H1056–H1060, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 26: 2439–2444, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Sawabe K, Yamamoto K, Harada Y, Ohashi A, Sugawara Y, Matsuoka H, Hasegawa H. Cellular uptake of sepiapterin and push-pull accumulation of tetrahydrobiopterin. Mol Genet Metab 94: 410–416, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt K, Werner ER, Mayer B, Wachter H, Kukovetz WR. Tetrahydrobiopterin-dependent formation of endothelium-derived relaxing factor (nitric oxide) in aortic endothelial cells. Biochem J 281: 297–300, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res 61: 402–413, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Baker JE, Zhang C, Tweddell JS, Su J, Pritchard KA Jr. Chronic hypoxia increases endothelial nitric oxide synthase generation of nitric oxide by increasing heat shock protein 90 association and serine phosphorylation. Circ Res 91: 300–306, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Hutchins W, Ogawa H, Chang CC, Pritchard KA, Jr, Zhang C, Khampang P, Lazar J, Jacob HJ, Rafiee P, Baker JE. Increased resistance to myocardial ischemia in the Brown Norway vs. Dahl S rat: role of nitric oxide synthase and Hsp90. J Mol Cell Cardiol 38: 625–635, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Shinozaki K, Nishio Y, Yoshida Y, Koya D, Ayajiki K, Masada M, Kashiwagi A, Okamura T. Supplement of tetrahydrobiopterin by a gene transfer of GTP cyclohydrolase I cDNA improves vascular dysfunction in insulin-resistant rats. J Cardiovasc Pharmacol 46: 505–512, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Thomas SR, Chen K, Keaney JF Jr. Oxidative stress and endothelial nitric oxide bioactivity. Antioxid Redox Signal 5: 181–194, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 10: 1713–1765, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Tiefenbacher CP, Chilian WM, Mitchell M, DeFily DV. Restoration of endothelium-dependent vasodilation after reperfusion injury by tetrahydrobiopterin. Circulation 94: 1423–1429, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Tiefenbacher CP, Lee CH, Kapitza J, Dietz V, Niroomand F. Sepiapterin reduces postischemic injury in the rat heart. Pflügers Arch 447: 1–7, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circ Res 87: 1108–1117, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res 37: 121–127, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werner-Felmayer G, Golderer G, Werner ER. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab 3: 159–173, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Xie L, Smith JA, Gross SS. GTP cyclohydrolase I inhibition by the prototypic inhibitor 2,4-diamino-6-hydroxypyrimidine. Mechanisms and unanticipated role of GTP cyclohydrolase I feedback regulatory protein. J Biol Chem 273: 21091–21098, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Yamashiro S, Noguchi K, Kuniyoshi Y, Koja K, Sakanashi M. Role of tetrahydrobiopterin on ischemia-reperfusion injury in isolated perfused rat hearts. J Cardiovasc Surg (Torino) 44: 37–49, 2003 [PubMed] [Google Scholar]
- 33.Yamashiro S, Noguchi K, Matsuzaki T, Miyagi K, Nakasone J, Sakanashi M, Koja K. Beneficial effect of tetrahydrobiopterin on ischemia-reperfusion injury in isolated perfused rat hearts. J Thorac Cardiovasc Surg 124: 775–784, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol 45: 625–632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]