Abstract
The aim of the present study was to test the hypothesis that elevation of prorenin in plasma is sufficient to induce cardiac fibrosis. Normotensive cyp1a1ren-2 transgenic rats with normal plasma prorenin and aldosterone levels were given 0.125% indole-3-carbinol (I3C) orally for a period of 12 wk. Plasma prorenin and aldosterone levels were determined in 4-wk intervals, and cardiac marker enzymes for hypertrophy, fibrosis, and oxidative stress as well as cardiac pathology were investigated. In I3C-treated cyp1a1 ren-2 transgenic rats, plasma prorenin concentrations were >100-fold elevated (≥7.1 ± 2.6 μg ANG I·ml−1·h−1 vs. ≤0.07 ± 0.1; P < 0.001), whereas active renin levels were suppressed (0.09 ± 0.02 vs. 0.2 ± 0.1; P < 0.05). Aldosterone concentrations were elevated three- to fourfold for a period of >4 wk (574 ± 51 vs. 160 ± 68 pg/ml; P < 0.01). After 12 wk of I3C, rats exhibited moderate cardiac hypertrophy (heart weight/body weight 2.5 ± 0.04 vs. 3.1 ± 0.1 mg/g; P < 0.01). There was a slight increase in mRNA contents of endothelin 1 (1.21 ± 0.08 vs. 0.75 ± 0.007; P < 0.001), NADP oxidase-2 (1.03 ± 0.006 vs. 0.76 ± 0.04; P < 0.001), transforming growth factor-β (0.99 ± 0.06 vs. 0.84 ± 0.04; P < 0.05), collagen type I (1.32 ± 0.32 vs. 0.94 ± 0.18; P < 0.05), and intercellular adhesion molecule-1 (1.12 ± 0.12 vs. 0.84 ± 0.08; P < 0.05). These genes are known to be stimulated by the renin-angiotensin system. There were no histological signs of fibrosis in the heart. We found that prorenin and aldosterone alone are not sufficient to induce considerable cardiac fibrosis in the absence of sodium load.
Keywords: prorenin, aldosterone, cardiac fibrosis, renin receptor
prorenin is the inactive precursor of mature (active) renin. The protein is classically activated by proteolytic cleavage of a 43-amino acid prosegment. Alternatively, prorenin may be activated in a nonproteolytic manner by the recently identified (pro)renin receptor (14). Nonproteolytic activation of prorenin has been suggested to induce hypertrophy and proliferation in cultured neonatal rat cardiomyocytes in vitro (28) and to cause cardiac fibrosis in salt-loaded spontaneously hypertensive rats (SHR) (32) as well as stroke-prone SHR (SHRSP) (9) in vivo. Although these actions were presumed to be mediated through the generation of ANG I and II (9, 28), prorenin may have biological effects that are independent of its ANG-generating capacity. Thus it has been reported that prorenin induces intracellular signaling in mesangial cells (14) and cardiomyocytes (27) independently of ANG II in vitro. It is currently unclear whether high circulating prorenin levels are sufficient to cause cardiac fibrosis in the absence of high circulating active renin and ANG levels.
The mineralocorticoid aldosterone has also been implicated in cardiac fibrosis (2, 29, 34). In clinical studies (25, 26) primary hyperaldosteronism was associated with functional and structural alterations of the heart, suggestive of increased myocardial collagen deposition. Furthermore, blockade of aldosterone receptors by spironolactone, in addition to standard therapy, reduced the risk of both morbidity and death among patients with severe heart failure (22). In various rat models with ANG II-dependent hypertension, pharmacological mineralocorticoid receptor blockade (6, 23, 24), removal of aldosterone by adrenalectomy (5, 24), or inhibition of aldosterone synthesis (5) reduced cardiac damage independently of blood pressure. In contrast, chronic hyperaldosteronism failed to induce cardiac fibrosis in a transgenic mouse model with epithelial sodium channel dysfunction (33). Furthermore, inhibition of mineralocorticoid receptor expression in a murine conditional knockdown model led to cardiac fibrosis that was fully reversible when mineralocorticoid receptor expression was normalized (1). Thus, although there is ample evidence that aldosterone is involved in cardiac fibrosis, its exact role is still not completely understood. In particular, there is evidence against the hypothesis that high circulating aldosterone levels are sufficient to cause cardiac fibrosis in the absence of cofactors such as sodium load, high circulating active renin, or high ANG levels (2, 29, 34).
Cyp1a1ren-2 transgenic rats (TGR) carry a mouse ren-2 renin transgene, the hepatic expression of which is under the control of the cytochrome P-450 promoter cyp1a1 (10). The latter is activated by various xenobiotics, including the aryl hydrocarbon compound indole-3-carbinol (I3C). When given in the diet, I3C dose-dependently increases circulating prorenin levels and arterial blood pressure. Although cyp1a1ren-2 TGR have been frequently used as a model of malignant hypertension with markedly elevated prorenin as well as active renin concentrations (7, 10 - 12, 15), we have recently developed a protocol using a lower dose of I3C that results in stable nonmalignant hypertension with chronic mean arterial pressure levels around 170 mmHg and markedly elevated prorenin but low active renin concentrations (16). In the present study, we have used this protocol to test the hypothesis that elevated circulating prorenin and aldosterone levels will induce cardiac fibrosis in the presence of low circulating active renin levels.
MATERIALS AND METHODS
Animals.
Cyp1a1ren-2 TGR were bred at the University of Greifswald from stock animals supplied from the University of Edinburgh. Fischer F344 rats obtained from Harlan Winkelmann (Borchen, Germany) served as nontransgenic controls. Animals were housed in a temperature- and humidity-controlled facility with lights on from 6:00 A.M. to 6:00 P.M. They had access to a standard diet (EF 1/80; Ssniff, Soest, Germany) to which NaCl was added to yield a final NaCl content of 1.0% and tap water ad libitum. Body weight and water intake were recorded two times weekly. All procedures were approved by a governmental committee on animal welfare, the guidelines of which are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental protocols.
The study was performed in 24 male cyp1a1ren-2 TGR and 12 age-matched male F344 rats. At the age of 15 wk, 12 cyp1a1ren-2 TGR and 6 F344 rats were placed on an experimental diet containing 0.125% I3C. The remaining rats were placed on a control diet without I3C. The diets were applied for 12 wk. Six rats from each of the four groups were used to study the plasma renin-angiotensin-aldosterone system as well as cardiac gene expression and cardiac histology. These rats were treated as follows. Before application of the experimental diets as well as at weeks 4, 8, and 12 of the diets and at corresponding time points in control animals, 1.5 ml of EDTA-blood were obtained from the retroorbital plexus under light ether anesthesia for measurements of plasma prorenin, plasma active renin, ANG I, and plasma aldosterone concentrations. Before the end of the experiment (2 days), three I3C-treated cyp1a1ren-2 TGR received a femoral artery catheter to determine arterial blood pressure. At the end of the experiment, rats were killed under deep ether anesthesia. The heart was removed and weighted, and the apex of the left ventricle was snap-frozen in liquid nitrogen for RNA extraction. The rest of the heart was fixed in 4% formalin for histological examination. The remaining 12 cyp1a1ren-2 TGR were used for cardiac magnetic resonance imaging (MRI) studies. These rats were killed under deep ether anesthesia at the end of the 12-wk dietary protocol. The heart was removed, fixed in 4% formalin, and examined in a small animal MRI device according to the following protocol.
Determination of left ventricular mass by MRI.
Imaging of formalin-fixed hearts was carried out on a 7T (300 Hz) MRI system (ClinScan; Bruker BioSpin, Rheinstetten, Germany). The system consisted of a horizontal magnet (bore size 130 mm), a console with Syngo surface (V. MRB122; Siemens, Erlangen, Germany), and a shielded gradient system (290 mT/m, slew rate 1,160 T·m−1·s−1). A phased array rat head coil (2 × 2) with an inner diameter of 20 mm was used for transmission. A three-dimensional gradient echo sequence was used (TR = 33 ms, TE 5.1 ms, flip angle 30°). The field of view was 40 × 40 mm, and the acquisition matrix included 256 × 256 pixels, resulting in an in-plane resolution of 313 μm. Short-axis views perpendicular to the two- and four-chamber view were generated with Osirix software. From these short-axis views, heart wall thickness and left ventricular volume were determined using Harp plus (diagnosoft) software.
Determinations of plasma prorenin, active renin, and aldosterone concentrations.
Plasma total renin and plasma active renin concentrations were determined enzymatically by the capacity to generate ANG I from excess substrate with or without prior trypsin activation, respectively, as previously described (16, 18). Prorenin concentrations were calculated as the difference between total and active renin levels (20, 21). Intra- and interassay coefficients of variation were <10% and <15%, respectively, for prorenin and <8% and <8%, respectively, for active renin. Plasma aldosterone concentrations were determined with a commercially available radioimmunoassay (DPC Biermann, Bad Nauheim, Germany).
Histology of the heart.
Formalin-fixed heart tissue was embedded in paraffin, sectioned at a thickness of 4 μm on a microtome, and stained with hematoxylin and eosin. Additionally, myocardial collagen content was quantified using azan-stained tissue sections. Quantification of the fibrous areas was performed by the operator without knowledge of the treatment group. The digital images of Mallory-Azan-stained heart sections were analyzed with Adobe Photoshop CS version 8.0.1 software. For the analysis of interstitial fibrosis, the vessel areas were excluded from measurement. The total cardiac area was calculated from the number of pixels of the total image minus the number of pixels of the background. Next, the number of pixels of the color blue was counted. The ratios of the area affected by fibrosis (pixels of the color blue) to total cardiac area in the samples were expressed as percent fibrosis. For the analysis of perivascular fibrosis, the ratios of perivascular areas affected by fibrosis (pixels of the color blue) to the vessel volume were determined.
Photomicrographs were taken with a digital camera (Color View II Soft Imaging System; Olympus, Berlin, Germany) at the same light intensity without filter. Images of Mallory-Azan-stained sections were subjected to white balance edition.
Analysis of gene expression by quantitative RT-PCR.
Total RNA was isolated with the acid-guanidium-thiocyanate-phenol-chloroform procedure using the TRIzol Reagent (Invitrogen, Paisley, UK). RNA was dissolved in RNA storage solution (Ambion, Huntingdon, UK). To assess the integrity of the prepared RNA, agarose gel electrophoresis followed by ethidium bromide staining was performed. Removal of residual genomic DNA was achieved by treatment of total RNA with DNase I (DNA-free; Ambion, Huntingdon, UK). Concentration and purity of DNase-treated RNA were determined spectrophotometrically by measuring the absorbance at 260 nm and calculating the ratio of absorbance at 260 to 280 nm, respectively. RT was performed with the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). RNA samples of 1 μg were reverse transcribed into cDNA with random hexamer primers according to the manufacturer's instructions.
Expression in the heart of the following genes was examined: prorenin receptor, endothelin-1 (ET-1), nicotinamide adenine dinucleotide phosphate oxidase (NOX) subunits 2 and 4, intercellular cell adhesion molecule-1 (ICAM-1), transforming growth factor-β (TGF-β), and collagen type I as well as type III (Col-1 and Col-3, respectively). Expression levels were determined by TaqMan real-time PCR using porphobilinogen deaminase (PBGD) and β-actin as endogenous references. Real-time PCR was performed with ready-to-use TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) using cDNA equivalent to 9 ng DNase I-treated RNA as template and the GenAmp 5700 Sequence Detection System (Applied Biosystems). Design of synthetic oligonucleotide primers and fluorescent probes for each gene investigated was performed on the basis of published sequences (Table 1). Probes were labeled with FAM as reporter and TAMRA as the quencher. In preliminary experiments, concentrations of primers and probes were optimized to give low-threshold values (reporter fluorescence significantly above baseline fluorescence) and high PCR efficiency. The PCR included a 10-min activation of the DNA polymerase at 95°C, 40 cycles at 95°C for 15 s for denaturation, and 1 min at 60°C for annealing and extension. PCRs were carried out in triplicate. Quantification of gene expression was performed with the standard curve method. Standard curves (cycle threshold plotted vs. log of concentration) for target genes and for the endogenous references were generated from a serially diluted cDNA pool of all tested samples. Expression levels are given relative to the geometric mean of the expression of PBGD and β-actin mRNA. In each PCR run, a no-template-control was included to check for any contamination disturbing PCR. It was also confirmed that no amplification occurred when samples were not subjected to reverse transcription, indicating the absence of genomic DNA after DNase treatment. Identity of the gene-specific PCR products was confirmed by size estimation using standard agarose gel electrophoresis and ethidium bromide staining.
Table 1.
Sequences of PCR primers and probes
| Gene (GenBank Accession No.) | Forward Primer, 5′-3′ | Reverse Primer, 5′-3′ | Probe, 5′-3′ |
|---|---|---|---|
| Atp6ap2 (XM_217592.4) | TGGGA AGCGT TATGG AGAAG | CTTCC TCACC AGGGA TGTGT | SYBR Green |
| β-Actin (NM_031144) | ACCCA CACTG TGCCC ATCTA | GCCAC AGGAT TCCAT ACCCA | TTACG CGCTC CCTCA TGCCA TCCTT |
| Col-1a1 (XM_213440.4) | ATCTC CTGGT GCRGA TGGAC | ACGGG AAGCC TCTTT CTCCT | SYBR Green |
| Col-3a1 (XM_343563) | GACAT CCTGG ATCTC CTGGT TCT | GGTCT TCCTG ACTCT CCATC CTTT | AGGTC CTCCT GGTGA ACCGG GT |
| ET-1 M64711) | TGGAC ATCAT CTGGG TCAACA | GCTTA GACCT AGAAG GGCTT CC | TCCCG AGCGC GTCGT CCCGT ATGGA T |
| ICAM-1 (NM_012967) | CTTCT GCCAC CATCA CTGTG TAT | GAGCT GCCTG ACCTC GG | AGAAC CTCAT CCTGC GCTGC CT |
| NOX-2 (NM_023965) | GCCAG TGAAG ATGTG TTCAG CTATG | CCTGC ACAGC CAGTA GAAGT AGATC | CACGC CCTCT GCCTC CATTC TCTCA AGT |
| NOX-4 (NM_053524) | CTGTA CCTCA GTCAA ACAGA TGGGA T | ACTGT TTTCC CTCTG TTACA TTTTG C | TTCCA CCGAG GACGC CCAAT |
| PBGD (X06827) | TGGGC ACCCG GAAGA GT | CCTGT GGTGG ACATA GCAAT GAT | CAGAC CGACA CTGTG GTAGC GATGC T |
| TGF-β1 (NM_021578) | CTAAT GGTGG ACCGC AACA | TTGCT CCACA GTTGA CTTGA ATCTC | CACTG CTTCC CGAAT GTCTG ACGT |
Atp6ap2, ATPase 6a2 (pro)renin receptor; Col-1a1, rat collagen type I, α1; Col-3a1, rat collagen type III, α1; ET-1, endothelin-1; ICAM-1, rat intercellular adhesion molecule-1; NOX-2, nicotinamide adenine dinucleotide phosphate oxidase subunit 2; NOX-4, nicotinamide adenine dinucleotide phosphate oxidase subunit 4; PBGD, porphobilinogen deaminase; TGF-β1, rat transforming growth factor-β1.
Cell culture and Western blot.
Rat vascular smooth muscle cells were cultured in medium (Lonza, Wuppertal, Germany) containing 2 ng/ml fibroblast growth factor, 0.5 ng/ml epidermal growth factor (both from Biochrome, Berlin, Germany), 5 μg/ml insulin (Sigma, Steinheim, Germany), 5% FCS, and 1% penicillin-streptomycin (both from Biochrome). All experiments were performed under 24-h serum-free conditions. For stimulation, cells were preincubated with the angiotensin type 1 receptor blocker losartan (10 μM) and the angiotensin type 2 receptor blocker PD-123319 (10 μM) for 30 min. Thereafter, cells were treated with recombinant rat prorenin or mouse ren-2-prorenin (1.6 ng/ml each) for 10 min. After stimulation, cells were lysed with lysis buffer containing the protease inhibitor cocktail Complete (Roche, Mannheim, Germany) and a phosphatase inhibitor cocktail (Sigma). For Western blotting, we used anti-extracellular signal-regulated kinase (ERK) 1/2 and anti-phosphorylated ERK1/2 (Cell Signaling, Frankfurt, Germany) antibodies, which were detected by standard techniques. Recombinant rat and mouse prorenin were collected from serum-free media of prorenin-transfected HepG2 cells and purified by ultrafiltration through Centricon C30 membranes (Millipore, Schwalbach, Germany) followed by gel chromatography on a DEAE column.
Statistical analyses.
Data are given as means ± SD. Data were analyzed by two-way ANOVA followed by the Bonferroni post hoc test or by one-way ANOVA for repeated measurements as appropriate. P values of <0.05 were accepted to indicate statistical significance. All statistical analyses were performed using the SigmaStat Software (SPSS, Chicago, IL).
RESULTS
Prorenin, active renin, and aldosterone.
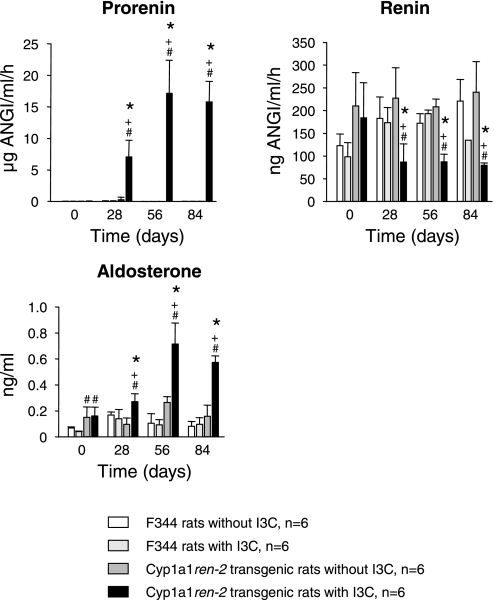
Rats in all groups thrived well and gained weight to a similar extent throughout the study (data not shown). I3C-treated cyp1a1ren-2 TGR showed a strong and sustained increase in plasma prorenin that was associated with a significant decrease in plasma active renin. Plasma aldosterone (Fig. 1) rose markedly, essentially following the time course of plasma prorenin. There were no statistically significant changes in plasma prorenin, active renin, or aldosterone concentrations in either of the three control groups. There were no significant differences among the four groups with respect to plasma sodium [136.0 ± 1, 137.5 ± 1, 137.2 ± 1, and 137.0 ± 1 mmol/l in F344, F344 + I3C, TGR, and TGR + I3C, respectively, not significant (NS)] or potassium (4.1 ± 0.6, 3.8 ± 0.3, 3.5 ± 0.3, and 3.6 ± 0.3 mmol/l in F344, F344 + I3C, TGR, and TGR + I3C, respectively, NS) concentrations at the end of the experiment, indicating that normal electrolyte homeostasis was maintained in all groups.
Fig. 1.
Plasma prorenin, active renin, and aldosterone concentrations in F344 control rats and cyp1a1ren-2 transgenic rats with and without indol-3-carbinol (I3C). P < 0.05 vs. day 0 (*) and vs. without I3C (+). #P < 0.01 vs. F344.
Cardiac hypertrophy.
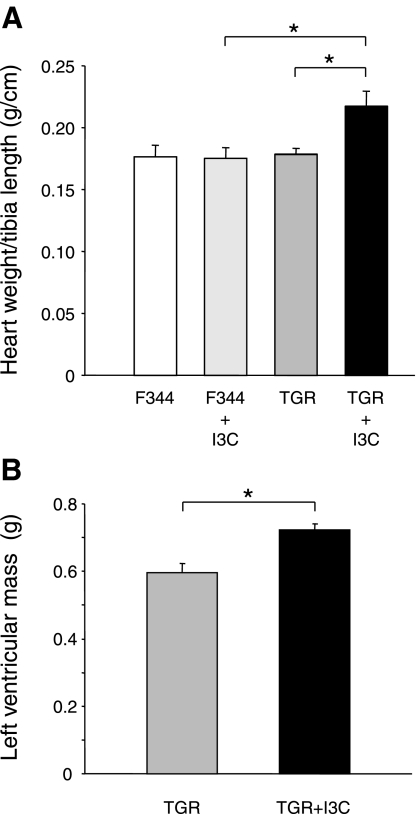
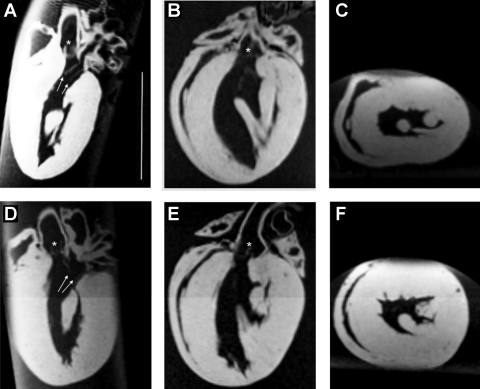
After 12 wk of treatment with the transgene inducer I3C, cyp1a1ren-2 TGR showed a significant increase in cardiac weight-to-tibia length ratio (Fig. 2 A), heart-to-body weight ratio (cyp1a1ren-2 induced: 3.12 ± 0.1 vs. controls: 2.56 ± 0.2, 2.6 ± 0.2, and 2.5 ± 0.1, respectively), and clear signs of cardiac hypertrophy in MRT images (Figs. 2B and 3) with an increased left ventricular volume in I3C-treated cyp1a1ren-2 rats. At the end of the experiment, mean arterial pressure in three I3C-treated cyp1a1ren-2 TGR was 169 ± 9 mmHg (16). These data agree well with previously published results (17).
Fig. 2.
A: heart weight normalized to tibia length in F344 control rats and cyp1a1ren-2 transgenic rats (TGR) with and without I3C; n = 6 rats/group, *P < 0.01. B: left ventricular volume as determined by magnetic resonance imaging (MRI) analysis of formalin-fixed hearts of TGR with and without I3C; n = 6 rats/group. *P < 0.01.
Fig. 3.
MRI images of formalin-fixed hearts from cyp1a1ren-2 TGR without (A–C) and with (D–F) I3C. A and D: vertical long axis view. B and E: horizontal long axis view. C and F: long axis and midventricular short axis view. Arrows, mitral valve leaflets; star, aortic valve. Bar = 1 cm.
Histology of the heart.
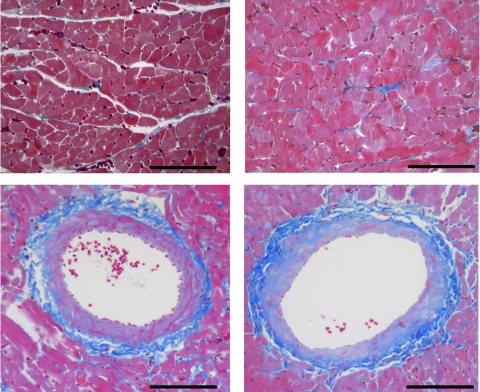
Histological examinations of cardiac tissue sections failed to reveal any signs of myocardial fibrosis, degeneration, or necrosis in I3C-treated rats of either strain. In cyp1a1ren-2 transgenic control rats, interstitial fibrosis amounted to 1.03 ± 0.24 vs. 0.97 ± 0.14% in I3C-treated TGR (NS) (representative photomicrographs: Fig. 4); perivascular fibrosis was 1.4 ± 0.4% in controls vs. 1.2 ± 0.2% in I3C-treated TGR (NS) (Fig. 4).
Fig. 4.
Azan-stained myocardial sections from cyp1a1ren-2 TGR without (left) and with (right) I3C. Bar = 100 μm.
Cardiac gene expression.
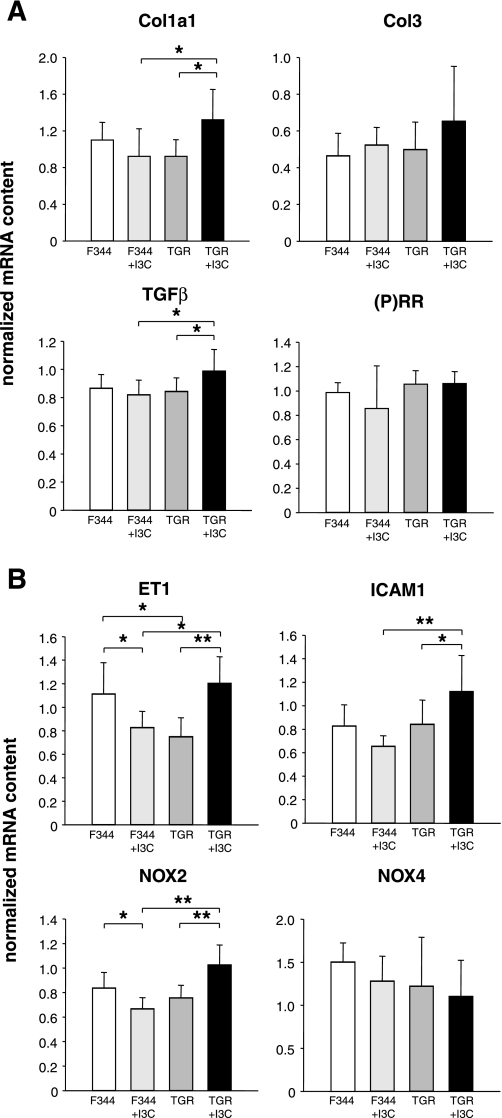
F344 and cyp1a1ren-2 TGR showed no significant differences in the abundances of the investigated genes except for ET-1, which was less abundant in transgenic than in control rat hearts (Fig. 5). I3C-induced induction of the transgene in cyp1a1ren-2 TGR was associated with significant increases in cardiac transcript abundances for ET-1, NOX-2, ICAM-1, TGF-β and Col-1, whereas transcript abundances for NOX-4, Col-3, and the (pro)renin receptor were not significantly altered. Feeding of I3C to nontransgenic controls led to significant reductions in cardiac transcript abundances for ET-1 and NOX-2, whereas transcript abundances for the other genes investigated remained unaltered (Fig. 5).
Fig. 5.
Cardiac mRNA abundances of the (pro)renin receptor [(P)RR] marker genes for fibrosis [transforming growth factor-β (TGF-β), collagen type Ia1 (Col1a1), collagen type III (Col-3)] (A), hypertrophy [endothelin-1 (ET-1)], oxidative stress [NADP oxidase (NOX)-2, NOX-4], and inflammation [intercellular adhesion molecule-1 (ICAM-1)] (B) in F344 control rats and cyp1a1ren-2 TGR with and without I3C. The mRNA expression values were normalized to the geometric mean of the expression of porphobilinogen deaminase (PBGD) and β-actin mRNA; n = 6 rats/group. *P < 0.05 and **P < 0.01.
Angiotensin-independent phosphorylation of Erk by endogeneous rat and transgenic ren-2 prorenin.
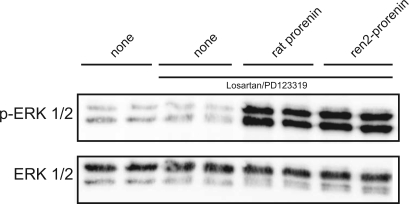
To confirm that mouse ren-2 prorenin is able to activate the Erk pathway in rat tissue, we incubated rat vascular smooth muscle cells expressing the rat (pro)renin receptor with purified recombinant rat and mouse prorenin and monitored phosphorylation of Erk (Fig. 6). Rat and mouse ren-2 prorenin preparations stimulated Erk phosphorylation to a similar extent and in an angiotensin-independent manner.
Fig. 6.
Western blot for extracellular signal-regulated kinase (ERK) 1/2 and phosphorylated Erk1/2 (p-ERK1/2) in rat vascular smooth muscle cells in the absence (none) or presence of rat or mouse ren-2 prorenin (1.6 ng/ml for 10 min). ANG II receptors were blocked with the angiotensin type 1 receptor blocker losartan and the angiotensin type 2 receptor blocker PD-123319 (30 min before and during stimulation) as indicated (solid line).
DISCUSSION
The major finding of the present study is the lack of cardiac fibrosis in hypertensive cyp1a1ren-2 TGR despite massively increased circulating prorenin levels and moderately elevated plasma aldosterone concentrations. This finding argues against the hypothesis that increased circulating prorenin and aldosterone levels alone or in combination are sufficient to cause cardiac fibrosis in otherwise healthy adult rats. At the same time, the study shows that prorenin induces cardiac hypertrophy, either directly or via prorenin-induced hypertension. Our finding that the degree of cardiac hypertrophy was similar to that observed in other rat models of hypertension with corresponding blood pressures (4) tends to support the latter mechanism.
Prorenin and cardiac fibrosis.
Although there is circumstantial evidence from in vitro studies (18, 27, 28) that prorenin may promote the development of cardiac damage, there is little direct evidence from in vivo studies to support this notion. In two recent studies, chronic subcutaneous infusion of a decapeptide that binds to prorenin, thereby inhibiting its interaction with the (pro)renin receptor, attenuated the development of cardiac fibrosis in salt-loaded SHR (32) and SHRSP (9), but not in SHR on a normal salt diet (32). The lack of effect in SHR on a normal salt diet together with a recent report (13) indicating that blockade of the (pro)renin receptor did not improve target organ damage in rats with renovascular hypertension led to the suggestion that the contribution of prorenin to end-organ damage may be limited to acute and more severe forms of hypertension, such as in salt-loaded models (32). In support of this notion, we did not find any histological signs of cardiac fibrosis in our transgenic rat model of chronic nonmalignant hypertension despite circulating prorenin levels that were several orders of magnitude higher than those in salt-loaded SHRSP in which blockade of the (pro)renin receptor has been reported to improve cardiac damage (9).
The lack of significant cardiac end-organ damage in our model may indicate that prorenin alone does not play an important role in the pathogenesis of cardiac fibrosis. Alternative explanations that must be considered include 1) the potential failure of circulating prorenin to reach relevant targets within the heart, 2) the potential failure of mouse prorenin to stimulate the rat (pro)renin receptor, and 3) potential downregulation of the cardiac (pro)renin receptor in the presence of high-circulation prorenin levels (30). With respect to the first hypothesis, we have been able to demonstrate that circulating prorenin is taken up by several tissues, including the heart (18). The local uptake of prorenin leads to increased local renin activity and elevated local tissue angiotensin levels (18). To test whether the transgenic mouse ren-2 prorenin would stimulate the rat (pro)renin receptor, we measured the effect of mouse prorenin on Erk phosphorylation in vascular smooth muscle cells expressing the rat (pro)renin receptor. It has been shown by others that (pro)renin increases Erk phosphorylation (14) and TGF-β expression (8) in vitro in an angiotensin-independent manner, i.e., via the (pro)renin receptor. Our data show that mouse ren-2 prorenin was similarly effective in causing Erk phosphorylation as rat prorenin. Furthermore, measurements of cardiac (pro)renin receptor mRNA abundance in I3C-treated TGR yielded no evidence of receptor downregulation. Together these data clearly argue against the above-mentioned alternative hypotheses, thereby suggesting that prorenin may not play an important role in the development of cardiac fibrosis in cyp1a1ren-2 TGR.
Despite inconspicuous histological results, there were some changes in cardiac gene expression that reached statistical significance. Untreated cyp1a1ren-2 TGR and nontransgenic F344 controls differed only with respect to cardiac ET-1 mRNA content, which was lower in transgenic than in nontransgenic rats, whereas all other mRNA species investigated showed similar cardiac abundances in both strains. Cardiac hypertrophy in I3C-treated cyp1a1ren-2 TGR was associated with increased abundances of ET-1, NOX-2, ICAM-1, and TGF-β mRNA. These genes have been commonly used as markers for hypertrophy, oxidative stress, and inflammation. In agreement with the histological results, Col-3 mRNA as a marker for fibrosis was not significantly altered. On the other hand, Col-1 mRNA abundance was slightly increased in I3C-treated cyp1a1ren-2 TGR, possibly suggesting an early stage of imminent fibrosis. The cardiac contents of ET-1 and NOX-2 mRNA were lower in I3C-treated nontransgenic rats than in untreated F344 controls, indicating that I3C may have altered transcript abundances independently of transgene induction. It is noteworthy that I3C treatment lowered cardiac ET-1 and NOX-2 mRNA contents in nontransgenic rats, whereas it increased the abundances of these mRNA species in cyp1a1ren-2 TGR with cardiac hypertrophy. Overall, the changes in cardiac gene expression in this high-prorenin model of arterial hypertension and cardiac hypertrophy were modest and apparently insufficient to cause any discernible effects on cardiac histology other than hypertrophy, despite an increase in Col1 mRNA levels.
Renin has been shown to increase TGF-β and Col-1 mRNA abundance in an angiotensin-independent manner by stimulating the (pro)renin receptor (8). We cannot exclude at this point that prorenin has contributed via this mechanism to the increases in cardiac TGF-β and Col-1 mRNA abundances in the present study. Nevertheless, large chronic elevations of circulating prorenin levels failed to induce cardiac fibrosis as shown here, nor did high circulating prorenin cause significant renal end-organ damage as shown in a previous study (16).
Prorenin and aldosterone production.
In agreement with previous studies (10, 16), we found high circulating prorenin and aldosterone levels in the presence of suppressed plasma active renin. Blood samples were obtained under light ether anesthesia, which is known to stimulate the release of active renin from the juxtaglomerular apparatus and the release of aldosterone from the adrenal cortex (19). Because renin expression in the juxtaglomerular apparatus of cyp1a1ren-2 TGR on I3C is reduced (10), leaving less intracellular renin to be released by the anesthetic, our data may overestimate the extent to which plasma active renin is suppressed in TGR on I3C. In contrast, the high plasma prorenin concentrations in cyp1a1ren-2 TGR on I3C are derived from the liver, where prorenin expression is exclusively controlled by the inductor and remains unaffected by short-lasting anesthesia.
Although we cannot exclude that ether anesthesia may have influenced our results on aldosterone, the finding of elevated plasma aldosterone levels in the present study together with the finding that aldosterone synthase mRNA abundance was increased in the zona glomerulosa in a previous study (10) strongly indicate that adrenal aldosterone production is stimulated in I3C-treated cyp1a1ren-2 TGR. It is likely that the increased aldosterone production in I3C-treated cyp1a1ren-2 TGR is a consequence of the high plasma prorenin concentrations in these rats. The mechanisms by which prorenin stimulates aldosterone production are, however, not well understood. It is conceivable that prorenin is locally activated by binding to the (pro)renin receptor (14) in the adrenal gland. Nonproteolytic activation of prorenin would lead to ANG formation, which in turn would stimulate aldosterone production. This explanation is supported by the finding that mRNA abundance of aldosterone synthase, the key enzyme in aldosterone biosynthesis that is known to be regulated by ANG II, was increased in the zona glomerulosa of I3C-treated cyp1a1ren-2 TGR. Alternatively, (pro)renin may stimulate the prorenin receptor, causing intracellular signaling (14, 30) that in turn may stimulate aldosterone production. It has recently been shown that prorenin and renin are equally potent in (pro)renin receptor activation (31). In keeping with this notion, TGR overexpressing the (pro)renin receptor in vascular smooth muscle cells showed increased circulating aldosterone levels in the presence of normal plasma renin activity (3).
Aldosterone and cardiac fibrosis.
Experimental evidence for an involvement of aldosterone in cardiac fibrosis comes mainly from studies in rats with ANG II-dependent hypertension or with sodium load (2, 5, 6, 23, 24, 33, 34). In these models, inhibition of aldosterone synthesis (5, 24) or aldosterone receptor binding (2, 23, 24) attenuated cardiac damage. On the other hand, aldosterone did not induce fibrosis in the absence of sodium load (2, 29, 34). In our study, increases in plasma aldosterone levels and arterial blood pressure similar to those seen in ANG II-dependent rat models of cardiac damage (5, 24) were not associated with appreciable histological signs of cardiac fibrosis, neither was Col-3 mRNA content in the heart as an established marker for cardiac fibrosis significantly elevated. Our results support the notion that aldosterone alone does not induce cardiac fibrosis and that additional factors such as sodium load or increased angiotensin levels are needed to produce this type of end-organ damage, as previously suggested (2, 29, 34).
Taken together, our data demonstrate that greatly elevated circulating prorenin levels and moderately elevated circulating aldosterone levels together with moderate hypertension are not sufficient to elicit cardiac fibrosis in rats with circulating angiotensin levels that were lower than those needed to induce pathological phenotypes (i.e., cardiac fibrosis, infarctions, vasculitis, renal disease) (33). Under the conditions applied in this study, the cyp1a1ren-2 transgenic rat model shows many similarities with human primary hyperaldosteronism, including 1) three- to fourfold increases in circulating aldosterone levels, 2) moderate arterial hypertension, 3) low circulating active renin levels, and 4) aldosterone production independent of renal renin secretion. It may therefore be used as a model of primary hyperaldosteronism in future studies.
GRANTS
J. J. Mullins is the recipient of a Wellcome Trust Principal Research Fellowship. The study was supported by grants from the German Federal Ministry of Education and Research to J. Peters and R. Rettig (Grant nos. 01 ZZ 0403 and 0314107).
ACKNOWLEDGMENTS
We thank Doreen Nierath, Brigitte Sturm, Dorothea Albrecht, and Norina Heller for excellent technical assistance.
REFERENCES
- 1.Beggah AT, Escoubet B, Puttini S, Cailmail S, Delage V, Ouvrard-Pascaud A, Bocchi B, Peuchmaur M, Delcayre C, Farman N, Jaisser F. Reversible cardiac fibrosis and heart failure induced by conditional expression of an antisense mRNA of the mineralocorticoid receptor in cardiomyocytes. Proc Natl Acad Sci USA 99: 7160–7165, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brilla CG, Weber KT. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc Res 26: 671–677, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Burckle CA, Jan-Danser AH, Muller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47: 552–556, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Cerutti C, Kurdi M, Bricca G, Hodroj W, Paultre C, Randon J, Gustin MP, Cerutti C, Kurdi M, Bricca G, Hodroj W, Paultre C, Randon J, Gustin MP. Transcriptional alterations in the left ventricle of three hypertensive rat models. Physiol Genom 27: 295–308, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al Saadi N, Dechend R, Wellner M, Meiners S, Maser-Gluth C, Jeng AY, Webb RL, Luft FC, Muller DN. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation 111: 3087–3094, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Fiebeler A, Schmidt F, Muller DN, Park JK, Dechend R, Bieringer M, Shagdarsuren E, Breu V, Haller H, Luft FC. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension 37: 787–793, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Howard LL, Patterson ME, Mullins JJ, Mitchell KD. Salt-sensitive hypertension develops after transient induction of ANG II-dependent hypertension in Cyp1a1-Ren2 transgenic rats. Am J Physiol Renal Physiol 288: F810–F815, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Nishiyama A, Inagami T, Hayashi M. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension 47: 894–900, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, McGrath I, Kotelevtsev Y, Mullins JJ. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem 276: 36727–36733, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Ang Aldo Syst 7: 74–86, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Mitchell KD, Mullins JJ. Enhanced tubuloglomerular feedback in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 289: F1210–F1216, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Muller DN, Klanke B, Feldt S, Cordasic N, Hartner A, Schmieder RE, Luft FC, Hilgers KF. (Pro)renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension 51: 676–681, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson ME, Mouton CR, Mullins JJ, Mitchell KD. Interactive effects of superoxide anion and nitric oxide on blood pressure and renal hemodynamics in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol 289: F754–F759, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Peters B, Grisk O, Becher B, Wanka H, Kuttler B, Lüdemann J, Lorenz G, Rettig R, Mullins JJ, Peters J. Dose-dependent titration of prorenin and blood pressure in Cyp1a1ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosis. J Hypertens 26: 102–109, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Peters BS, Kuttler B, Beineke A, Lorenz G, Thiele A, Nicolai O, Rettig R, Mullins JJ, Peters J. The renin-angiotensin system as a primary cause of polyarteritis nodosa in rats. J Cell Mol Med , 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters J, Farrenkopf R, Clausmeyer S, Zimmer J, Kantachuvesiri S, Sharp MG, Mullins JJ. Functional significance of prorenin internalization in the rat heart. Circ Res 90: 1135–1141, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Peters J, Hilgers KF, Maser-Gluth C, Kreutz R. Role of the circulating renin-angiotensin system in the pathogenesis of hypertension in transgenic rats TGR(mREN2)27. Clin Exp Hypertens 18: 933–948, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Peters J, Münter K, Bader M, Hackenthal E, Mullins JJ, Ganten D. Increased adrenal renin in transgenic hypertensive rats, TGR(mREN2)27, and its regulation by cAMP, angiotensin II, and calcium. J Clin Invest 91: 742–747, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters J, Obermuller N, Woyth A, Peters B, Maser-Gluth C, Kranzlin B, Gretz N. Losartan and angiotensin II inhibit aldosterone production in anephric rats via different actions on the intraadrenal renin-angiotensin system. Endocrinology 140: 675–682, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology 143: 4828–4836, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Rocha R, Stier CTJ, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 141: 3871–3878, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Rossi GP, Di Bello V, Ganzaroli C, Sacchetto A, Cesari M, Bertini A, Giorgi D, Scognamiglio R, Mariani M, Pessina AC. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 40: 23–27, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Rossi GP, Sacchetto A, Pavan E, Palatini P, Graniero GR, Canali C, Pessina AC. Remodeling of the left ventricle in primary aldosteronism due to Conn's adenoma. Circulation 95: 1471–1478, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Saris JJ, 't-Hoen PA, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan-Danser AH. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension 48: 564–571, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Saris JJ, van den Eijnden MM, Lamers JM, Saxena PR, Schalekamp MA, Danser AH. Prorenin-induced myocyte proliferation: no role for intracellular angiotensin II. Hypertension 39: 573–577, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Sato A, Saruta T. Aldosterone-induced organ damage: plasma aldosterone level and inappropriate salt status. Hypertens Res 27: 303–310, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res 99: 1355–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Schefe JH, Neumann C, Goebel M, Danser J, Kirsch S, Gust R, Kintscher U, Unger T, Funke-Kaiser H. Prorenin engages the (pro)renin receptor like renin and both ligand activities are unopposed by aliskiren. J Hypertens 26: 1787–1794, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Susic D, Zhou X, Frohlich ED, Lippton H, Knight M. Cardiovascular effects of prorenin blockade in genetically spontaneously hypertensive rats on normal and high-salt diet. Am J Physiol Heart Circ Physiol 295: H1117–H1121, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Clement S, Gabbiani G, Horisberger JD, Burnier M, Rossier BC, Hummler E. Chronic hyperaldosteronism in a transgenic mouse model fails to induce cardiac remodeling and fibrosis under a normal-salt diet. Am J Physiol Renal Physiol 286: F1178–F1184, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Young M, Fullerton M, Dilley R, Funder J. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest 93: 2578–2583, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]