Abstract
The present study tested the hypotheses that 1) nitric oxide (NO) is involved in attenuated responses to ANG II in female mice, and 2) there is differential expression of neuronal NO synthase (nNOS) in the subfornical organ (SFO) and paraventricular nucleus (PVN) in response to systemic infusions of ANG II in males vs. females. Aortic blood pressure (BP) was measured in conscious mice with telemetry implants. NG-nitro-l-arginine methyl ester (l-NAME; 100 μg·kg·−1day−1), an inhibitor of NOS, was administrated into the lateral cerebral ventricle for 14 days before and during ANG II pump implantation. Central infusion of l-NAME augmented the pressor effects of systemic ANG II in females (Δ21.5 ± 2.2 vs. Δ9.2 ± 1.5 mmHg) but not in males (Δ29.4 ± 2.5 vs. Δ30.1 ± 2.5 mmHg). Central administration of N5-(1-imino-3-butenyl)-l-ornithine (l-VNIO), a selective nNOS inhibitor, also significantly potentiated the increase in BP induced by ANG II in females (Δ17.5 ± 3.2 vs. Δ9.2 ± 1.5 mmHg). In gonadectomized mice, central l-NAME infusion did not affect the pressor response to ANG II in either males or females. Ganglionic blockade after ANG II infusion resulted in a greater reduction in BP in central l-NAME- or l-VNIO-treated females compared with control females. Western blot analysis of nNOS protein expression indicated that levels were ∼12-fold higher in both the SFO and PVN of intact females compared with those in intact males. Seven days of ANG II treatment resulted in a further increase in nNOS protein expression only in intact females (PVN, to ∼51-fold). Immunohistochemical studies revealed colocalization of nNOS and estrogen receptors in the SFO and PVN. These results suggest that NO attenuates the increase in BP induced by ANG II through reduced sympathetic outflow in females and that increased nNOS protein expression associated with the presence of female sex hormones plays a protective role against ANG II-induced hypertension in female mice.
Keywords: sex hormone, nitric oxide/nitric oxide synthase, blood pressure
nitric oxide (NO) and angiotensin II (ANG II) are important agents that regulate arterial blood pressure (BP). Vasoconstriction produced by sympathoexcitatory effects contributes to ANG II-induced pressor responses (13, 17). Conversely, NO has a hypotensive action via vasodilation and sympathoinhibition (36, 39). It has been shown that interactions between ANG II and NO occur in a variety of tissues, including the central nervous system (CNS) (40, 58). For example, microinjection of either an NO synthase (NOS) inhibitor or ANG II into the lateral ventricle or the paraventricular nucleus (PVN) increases the discharge of renal sympathetic nerves and elevates arterial BP and heart rate (HR) (25, 28, 52). Central or peripheral blockade of NOS potentiates or prolongs the pressor response to ANG II (9, 31). Conversely, overexpression of neuronal NOS (nNOS) within the PVN by adenoviral gene transfer significantly attenuates ANG II pressor responses (28).
There is evidence from human and animal studies that hypertension is delayed and attenuated in females compared with males (11). Previous studies from our laboratory (54) have shown that systemic infusions of ANG II are less effective in inducing hypertension in female mice compared with males. Central estrogen acts to inhibit the generation of reactive oxygen species and to attenuate increases in intracellular Ca2+ concentration induced by ANG II (29, 34, 55, 56). Although these actions of estrogen may contribute to a reduced BP response to ANG II in females, additional downstream signaling mechanisms underlying the sex differences found in ANG II-induced hypertension are not well understood.
Recent studies performed in both peripheral and brain tissues indicate that estrogen affects NO (15, 35, 43). García-Durán et al. (14) reported using a Western blot analysis of protein isolated from neutrophils to show that levels of nNOS were greater in cells acquired from premenopausal women during the ovulatory phase of the menstrual cycle, when estrogen is high, compared with the follicular phase, when circulating levels of estrogen fall. Also, estrogen upregulates the expression of nNOS protein in neutrophils derived from men (14). Positive correlations between circulating estrogen and levels of both plasma and brain NO also have been found in humans and experimental animals (7, 8, 21). These studies suggest that changes in NO production might be one of the cellular biochemical pathways that is affected by estrogen and that this plays a mechanistic role in producing the sex differences seen in experimental hypertension (43).
Circulating ANG II has access to brain sensory circumventricular organs (CVOs), such as the subfornical organ (SFO), organum vasculosum of the lamina terminalis (OVLT), and area postrema (AP), sites where the blood-brain barrier is lacking (24). The PVN lies within the blood-brain barrier and is a key integrative nucleus in the diencephalon that plays an important role in the control of sympathetic nerve activity. This hypothalamic nucleus receives direct monosynaptic input from the SFO and projects directly to areas in the medulla and spinal cord controlling the neural activity of preganglionic sympathetic neurons (51). Of particular interest regarding the role of estrogen and NO in the regulation of ANG II-induced hypertension is the finding that estrogen receptors (ER), NOS and its mRNA, and angiotensin type 1 receptors (AT1) are present in the SFO and PVN (10, 26, 32, 47). With this in mind, we hypothesized that important interactions among ANG II, estrogen, and NO in the SFO and PVN influence sympathetic tone and arterial BP. Corollaries of this hypothesis are that 1) the expression and availability of nNOS in the SFO and PVN of male and female mice is responsible for the differential response to systemic infusions of ANG II, and 2) a greater availability of central NO at these sites is responsible for the attenuated pressor response to ANG II seen in females. To test these hypotheses, we first used telemetry to study BP responses to systemic ANG II infusions in central NG-nitro-l-arginine methyl ester (l-NAME)-treated intact and gonadectomized male and female mice. Second, we analyzed protein isolated from the SFO and PVN by performing Western blotting studies to determine the effects of the presence of sex hormones on nNOS protein expression in intact and gonadectomized male and female mice following control or ANG II treatments. Third, we used immunocytochemistry to examine the distribution of ER and nNOS in the SFO and PVN to determine whether both proteins are present in the same cells.
METHODS
Animals
Wild-type (C57BL/6) male and female mice (12–16 wk old) were obtained from Harlan (Indianapolis, IN). They were housed in temperature- and light-controlled animal quarters and provided with mouse chow (7013 NIH-31 modified mouse diet, 0.25% NaCl) ad libitum. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee.
Control and Experimental Groups
In in vivo experiments, mice were divided into 11 groups: intact males with central vehicle or l-NAME infusions, castrated males with central vehicle or l-NAME infusions, intact females with central vehicle, l-NAME, or N5-(1-imino-3-butenyl)-l-ornithine (l-VNIO) infusions, and ovariectomized (OVX) females with central vehicle or l-NAME infusions. Except for two groups of intact male and female mice receiving central l-NAME infusions alone, all of the groups were infused subcutaneously with ANG II. For experiments measuring nNOS protein expression, mice were divided into eight groups: intact and castrated males with or without subcutaneous ANG II infusions, and intact and OVX females with or without subcutaneous infusions.
Surgical Procedures
Gonadectomy.
Ten days before implantation of telemetry probes, bilateral gonadectomies were performed in female and male mice anesthetized with a mixture of ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively). In the females, a single 1- to 2-cm dorsal midline incision was made in the skin and underlying muscles. The ovaries were isolated, tied off with sterile suture, and removed, and the incisions were closed. In the males, a single incision was made in the skin covering the scrotum. The testicles were exteriorized, tied off, and removed. The skin of the scrotum was then sutured.
Telemetry probe implantation.
Implantable mouse BP transmitters (TA11PA-C10; Data Sciences International, St. Paul, MN) were used to chronically measure arterial BP. Mice were anesthetized with a ketamine-xylazine mixture. Through a ventral incision, the left carotid artery was accessed and isolated, and the catheter of a telemetry probe was inserted into the carotid and advanced into the aorta. Through the same ventral incision, a subcutaneous tunnel was formed that passed across the right pectoral area and extended into the right flank, where it was enlarged to form a pocket. The body of the transmitter was slipped into the pocket and secured with tissue adhesive. The ventral incision was then closed with suture.
Chronic intracerebroventricular cannula implantation.
After baseline BP and HR recordings were obtained, the mice were again anesthetized with a ketamine-xylazine mixture, and an intracerebroventricular (ICV) cannula with an ALZET osmotic pump (Durect, Cupertino, CA) was implanted into the right lateral ventricle (coordinates 0.3 mm caudal, 1.0 mm lateral to bregma, and 3.0 mm below the skull surface) for chronic infusions of l-NAME (a nonselective NOS inhibitor, 100 μg·kg−1·day−1; Sigma-Aldrich, St. Louis, MO) or l-VNIO (a selective nNOS inhibitor, 100 μg·kg−1·day−1; Alexis Biomedicals, San Diego, CA). At the end of the experiment, the animals were euthanized and perfused transcardially with saline followed by fixative. The location of the lateral ventricle cannula implantation was verified histologically.
Osmotic pump implantation.
Mice were anesthetized with inhalational isoflurane for implantation of osmotic pumps. Osmotic pumps (ALZET model 1002) containing ANG II (Sigma-Aldrich) at a concentration sufficient to allow an infusion rate of 800 ng·kg−1·min−1 were implanted subcutaneously on the left side of the back.
Western blotting analysis.
Total cellular protein was isolated using a lysis buffer (Cell Signaling, Boston, MA). Protein (30–60 μg) was separated on 10% PAGE, blotted, and probed with anti-nNOS rabbit polyclonal antibody (Cell Signaling) at 1:1,000 (vol/vol) and anti-β-actin rabbit monoclonal antibody (Sigma-Aldrich) at 1:2,500 (vol/vol), respectively. This was followed by incubation in Alexa Fluor 800 anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA) at 1:10,000 (vol/vol). The secondary antibody was detected directly using an Odyssey infrared imaging system in accordance with the manufacturer's instructions (LI-COR Biosciences, Lincoln, NE). Relative nNOS protein expression in the SFO and PVN was normalized to β-actin in each sample. After normalization with β-actin, relative nNOS protein expression was plotted in relation to that of intact males. The linear range of detection for the assay was defined by quantifying serial dilutions of tissue homogenates (45).
Fluorescent immunohistochemistry.
Immunohistochemical studies were performed to assess colocalization of nNOS and ER in the SFO and PVN of female mice. Brain sections were incubated with a rabbit polyclonal anti-nNOS antibody (Cell Signaling) at 1:100 and a mouse monoclonal anti-ER antibody (Invitrogen) at 1:100 in 5% donkey normal serum with 0.2% Triton X-100 for 72 h at 4°C. After being washed thoroughly with PBS, sections were incubated with Rhodamine red-X-conjugated AffiniPure donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at 1:100 and Cy2-conjugated AffiniPure donkey anti-rabbit IgG (Jackson ImmunoResearch) at 1:100 in PBS for 24 h at 4°C. Fluorescence was then identified using confocal microscopy.
Experimental Protocol
Measurement of BP and HR.
All mice were allowed 7 days to recover from transmitter implantation surgery before any measurements were made. Thereafter, BP and HR were telemetrically recorded and stored with the Dataquest ART data acquisition system (Data Sciences International).
To determine the effects of central blockade of NO on ANG II-induced hypertension in male and female mice, ICV cannulas with osmotic pumps were implanted into the right lateral ventricle for chronic infusions of vehicle, l-NAME, or l-VNIO for 14 days. On day 7, osmotic pumps filled with ANG II were implanted subcutaneously.
Determination of BP fall after autonomic blockade.
BP was measured in the presence of the ganglionic blocker hexamethonium (30 mg/kg ip). Ganglionic blockade was repeated in each animal, once during baseline and once after 7 days of ANG II infusion. On experimental days to determine the effects of ganglionic blockade, mice were allowed to adapt for at least 60 min, after which time BP was recorded for 20 min before and 20 min after hexamethonium injection.
nNOS protein expression measurements.
Both intact and gonadectomized male and female mice were either treated with ANG II or vehicle. After 7 days of ANG II infusion, mice were deeply anesthetized with isoflurane. The brains were removed and stored at −80°C until assay. Brains were cut into consecutive 100-μm sections in a cryostat at −20°C, and micropunches were made to collect the SFO and the PVN. Total cellular protein was isolated from tissue punches of the PVN and SFO and analyzed for the protein expression of nNOS by Western blotting.
Data Analysis
Mean arterial blood pressure (MAP) and HR were collected and plotted as mean values for 5 and 14 consecutive days before and during NOS inhibition and ANG II pump implantation, respectively. All data are means ± SE. Statistical analyses of the effects of central administration of l-NAME or l-VNIO on BP before and after ANG II infusion were performed with two-way ANOVAs for repeated measures (Sigma Stat version 2.06). Post hoc analysis was performed with Fisher's least significant difference multiple comparison test where appropriate. A one-way ANOVA was used for comparing changes in BP and nNOS protein expression. Statistical significance was accepted at P < 0.05.
RESULTS
Effects of ICV Infusion of l-NAME on ANG II-Induced Hypertension in Female Mice
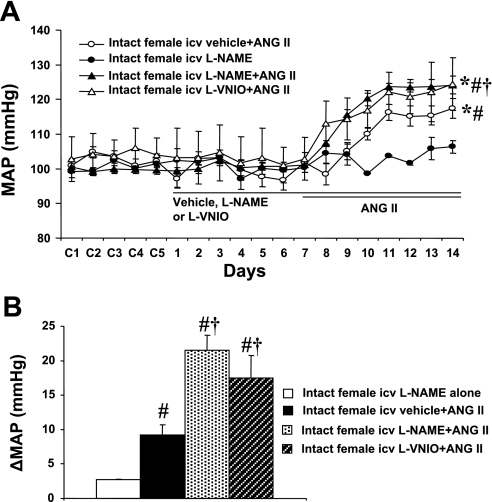
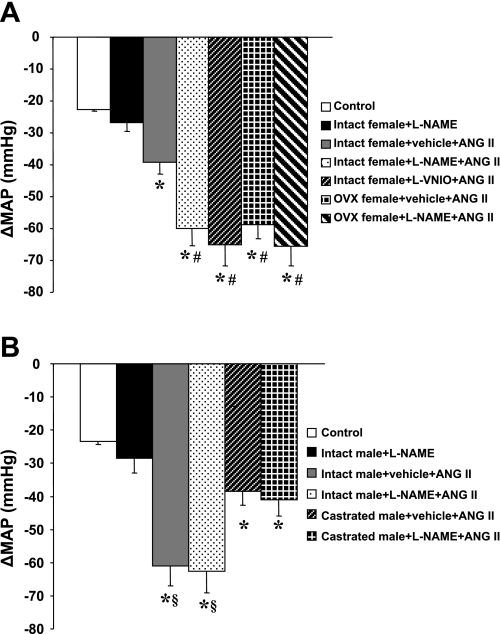
In intact females, baseline MAP was unaltered during central infusion of vehicle, l-NAME (Δ2.7 ± 0.2 mmHg, n = 5; Fig. 1A) or l-VNIO (Fig. 1A). Central l-NAME (n = 6; Fig. 1, A and B) or l-VNIO (n = 6, Fig. 1, A and B) significantly augmented the increase in MAP induced by ANG II (Δ21.5 ± 2.2 and Δ17.5 ± 3.2 mmHg, respectively; P < 0.05) compared with that seen in mice with central vehicle plus systemic ANG II (Δ9.2 ± 1.5 mmHg, n = 6).
Fig. 1.
Central neuronal nitric oxide synthase (nNOS) inhibition in intact female mice. Central blockade of nNOS augments angiotensin II (ANG II)-induced hypertension in intact female mice. A: daily mean arterial pressure (MAP) before and during systemic infusion of ANG II in central vehicle-, NG-nitro-l-arginine methyl ester (l-NAME), or N5-(1-imino-3-butenyl)-l-ornithine (l-VNIO)-treated females. B: average changes in MAP (ΔMAP) induced by ANG II infusion in central vehicle-, l-NAME-, or l-VNIO-treated females. Control days are denoted as C1–C5, followed by 14 days of central vehicle, l-NAME, or l-VNIO and 7 days of ANG II infusion. *P < 0.05 compared with baseline. #P < 0.05 compared with central l-NAME alone. †P < 0.05 compared with vehicle + ANG II.
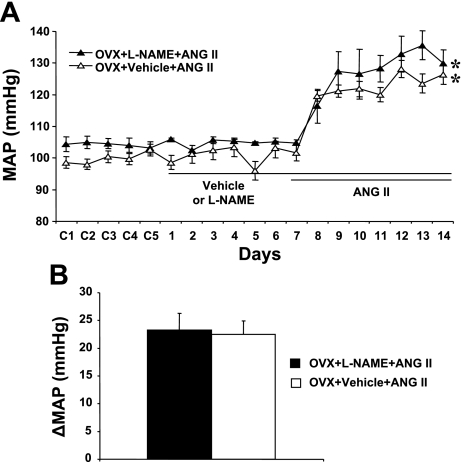
In OVX females, central l-NAME did not significantly potentiate the increase in MAP induced by ANG II (Δ23.2 ± 3.1 mmHg, n = 5; Fig. 2, A and B) compared with that seen in OVX females with central vehicle plus systemic ANG II (Δ22.5 ± 2.5 mmHg, n = 5; Fig. 2, A and B) or in intact females with central l-NAME plus systemic ANG II (Δ21.5 ± 2.2 mmHg, n = 6; Fig. 1, A and B). ANG II infusion did not significantly change HR in any group (data not shown).
Fig. 2.
Central nNOS inhibition in ovariectomized (OVX) mice. The effects of OVX on ANG II-induced hypertension in central l-NAME-treated female mice were determined. A: daily MAP before and during systemic infusion of ANG II in central vehicle- or l-NAME-treated OVX females. B: average changes in MAP induced by ANG II infusion in central vehicle- or l-NAME-treated OVX females. Control days are denoted as C1–C5, followed by 14 days of central vehicle or l-NAME and 7 days of ANG II infusion. *P < 0.05 compared with baseline.
Effects of ICV Infusion of l-NAME on ANG II-Induced Hypertension in Male Mice
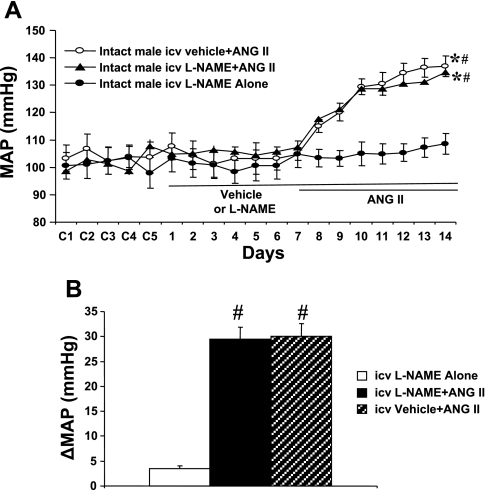
In intact males, baseline MAP was unaltered during central infusion of either vehicle or l-NAME (Δ3.5 ± 0.6 mmHg, n = 5; Fig. 3A). Central l-NAME (n = 6; Fig. 3, A and B) did not result in a further increase in MAP induced by ANG II (Δ29.4 ± 2.5; P > 0.05) compared with that in mice with central vehicle plus systemic ANG II (Δ30.1 ± 2.5 mmHg, n = 6).
Fig. 3.
Central nNOS Inhibition in intact males. Central blockade of nNOS had no effect on the pressor effect of ANG II in female mice. A: daily MAP before and during systemic infusion of ANG II in central vehicle- or l-NAME-treated males. B: average changes in MAP induced by ANG II infusion in central vehicle- or l-NAME-treated males. Control days are denoted C1–C5, followed by 14 days of central vehicle or l-NAME and 7 days of ANG II infusion. *P < 0.05 compared with baseline. #P < 0.05 compared with central l-NAME alone.
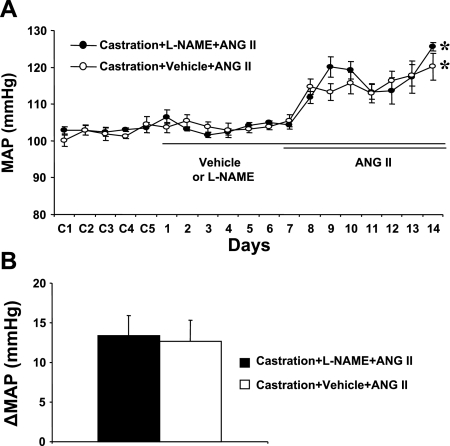
Although castration attenuated ANG II-induced hypertension in males (intact: Δ30.1 ± 2.5 mmHg vs. castrated: Δ12.6 ± 2.7 mmHg; see Fig. 3A vs. Fig. 4A), neither central l-NAME (n = 5; Fig. 4, A and B) nor vehicle treatment (n = 5; Fig. 4, A and B) affected the pressor effect of ANG II in castrated males (Δ13.4 ± 2.6 and Δ12.6 ± 2.7 mmHg, respectively). ANG II infusion did not significantly change HR in any group (data not shown).
Fig. 4.
Central nNOS Inhibition in castrated mice. The effects of castration on ANG II-induced hypertension in central l-NAME-treated male mice were determined. A: daily MAP before and during systemic infusion of ANG II in central vehicle- or l-NAME-treated castrated males. B: average changes in MAP induced by ANG II infusion in central vehicle- or l-NAME-treated castrated males. Control days are denoted C1–C5, followed by 14 days of central vehicle or l-NAME and 7 days of ANG II infusion. *P < 0.05 compared with baseline.
Effects of Autonomic Blockade on BP
Figure 5 shows the decreases in BP with acute ganglionic blockade in all groups of males and females. The averaged reduction in the BP response to hexamethonium injection before infusion of l-NAME and ANG II was −22.6 ± 1.2 mmHg in females and −23.4 ± 0.9 mmHg in males (Fig. 5, A and B). Fourteen days of central l-NAME alone tended to induce a greater reduction in BP, but this did not reach statistical significance for either females (−26.9 ± 2.5 mmHg; Fig. 5A) or males (−27.5 ± 4.5 mmHg; Fig. 5B) compared with control mice.
Fig. 5.
Sympathetic contribution to blood pressure. MAP was measured in response to ganglionic blockade with hexamethonium before l-NAME and ANG II access began (control) and on day 7 of ANG II infusion in all female (A) and male (B) groups. *P < 0.05 compared with control or central l-NAME alone. #P < 0.05 compared with intact females with central vehicle + ANG II. §P < 0.05 compared with castrated males with central vehicle or l-NAME + ANG II.
In female mice (Fig. 5A), after 7 days of ANG II infusion, acute hexamethonium injection resulted in a greater reduction in BP in central l-NAME-treated (−60.1 ± 5.2 mmHg) and central l-VNIO-treated females (−65.1 ± 6.6 mmHg) compared with central vehicle-treated females (−39.3 ± 3.6 mmHg; P < 0.05). Central blockade of NO production in intact females augmented reductions in the BP response to acute hexamethonium, which were similar to that seen in OVX females with or without central l-NAME treatment (−65.6 ± 6.2 and −58.8 ± 4.6 mmHg, respectively).
In male mice (Fig. 5B), after 7 days of ANG II infusion, acute hexamethonium injection resulted in a greater reduction in BP in central l-NAME-treated (−62.5 ± 6.5 mmHg) and central vehicle-treated intact males (−60.0 ± 5.9 mmHg) compared with central vehicle-treated (−38.5 ± 4.1 mmHg; P < 0.05) and central l-NAME-treated castrated males (−41.1 ± 4.9 mmHg; P < 0.05). Central l-NAME treatment did not potentiate the depressor response to acute hexamethonium in both intact and castrated males after ANG II infusion.
nNOS Protein Expression in the SFO and PVN
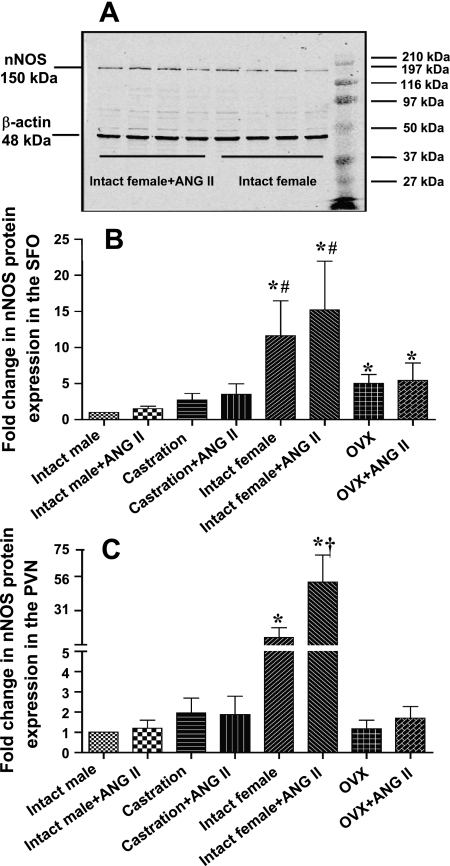
Figure 6 A presents a set of representative Western blots showing nNOS protein expression and β-actin in intact females with or without ANG II treatment. The Western blot analysis data are expressed as the changes in relative nNOS protein expression, normalized to intact males. Basal nNOS protein expression indicated that levels were ∼12-fold higher in both the SFO (Fig. 6B) and the PVN (Fig. 6C) in intact females (n = 8) compared with intact males (n = 8; P < 0.05). nNOS protein was reduced in females (to ∼5-fold in the SFO; to ∼1.2-fold in the PVN, n = 8; P < 0.05) by gonadectomy. Seven days of ANG II treatment resulted in a further increase in nNOS protein expression only in intact females (to ∼51 fold in the PVN, n = 8; P < 0.05).
Fig. 6.
nNOS Western blot analyses. A: a set of representative Western blots showing a well-defined band of immunoreactive nNOS protein expression and β-actin in intact females with or without ANG II treatment. The set of bands at the right are molecular mass markers. The results of Western blot analysis represent the change in nNOS protein expression, which was normalized to that of intact males in the subfornical organ (SFO; B) and paraventricular nucleus (PVN; C) of both sexes with or without ANG II treatment. *P < 0.05 compared with intact male. #P < 0.05 compared with OVX females. †P < 0.05 compared with intact females without ANG II treatment.
Colocalization of ER and nNOS in the SFO and PVN
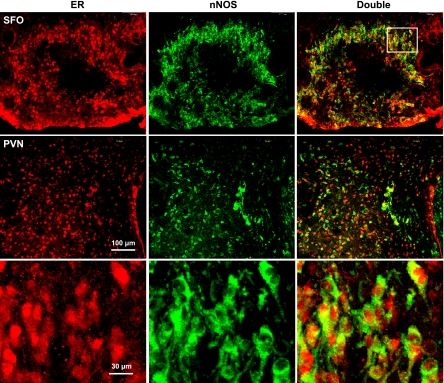
Fluorescence immunohistochemical studies indicated that the SFO and PVN contained high levels of ER and nNOS immunoreactivity in female mice. Approximately 70% of the nNOS-positive cells showed ER immunocytochemical staining (Fig. 7).
Fig. 7.
Immunocytochemistry for nNOS and estrogen receptors (ER). Colocalization of nNOS and ER in the SFO and PVN is shown in intact, untreated female mice. Representative sections show ER (red), nNOS (green), and double labeling (yellow) in SFO and PVN neurons. Magnification is the same for the top 2 rows; bottom row is at higher magnification of the sector indicated by the boxed area.
DISCUSSION
The main findings of this study are 1) central nonselective blockade of NOS or, more specifically, nNOS, augments the pressor effects of systemic ANG II in female but not in male mice; 2) at least a portion of the enhanced BP effect on ANG II-induced hypertension in intact females with central NOS blockade is a result of increased sympathetic outflow; 3) nNOS protein expression in both the SFO and PVN is higher in females with or without ANG II treatment compared with males; and 4) gonadectomy eliminates the augmented effects of NOS blockade on the pressor effect of ANG II in females. Together, these results implicate NO-related mechanisms in the attenuated hypertensive response to ANG II in females and suggest that increased nNOS protein expression associated with the presence of female sex hormones plays a protective role against sympathetically mediated ANG II-induced hypertension in female mice.
Recent studies provide convincing evidence that central ANG II and NO are important components of cellular transduction pathways in neural systems that regulate BP and sympathetic outflow (4, 5). The inhibition of the NO synthesis results in an imbalance between the renin-angiotensin system (RAS) and the NO system in favor of the RAS; thus the effects of ANG II may prevail (20, 30). A substantial body of work has shown that central or peripheral blockade of NO synthesis potentiates or prolongs the pressor response to ANG II (9, 28, 31, 33), upregulates AT1 expression, and activates cardiovascular angiotensin-converting enzyme (49). On the other hand, ANG II has been found to regulate NOS activity and NO release (27, 57). Li et al. (28) reported that push-pull administration of ANG II into the PVN induced an increase in NO release. Gene expression of NOS is also increased in autonomic centers, particularly the PVN, in animals with increased sympathetic activity (37). These findings indicate that during episodes of increased ANG II and sympathetic activity, NO production is increased to inhibit ANG II-mediated effects. This negative feedback mechanism within the CNS may play an important role in maintaining the overall balance and tone of sympathetic outflow.
In the present study it is interesting that central blockade of NOS by l-NAME did not augment basal BP, ANG II-induced increases in BP, and sympathetic activity in either intact or gonadectomized males. This could be because, in males, the increase in BP induced by ANG II is greater compared with that in females, and this already potentiated increase in BP could not be augmented further by inhibiting NOS activity. Another possibility is that NO production in the male might not be increased during ANG II infusions so that NO has little effect against the ANG II-induced increase in BP. This notion is supported by showing that nNOS protein expression was lower in the basal condition and not increased after ANG II treatment in males in the present study. It has been shown that central ANG II infusion reduces nNOS mRNA and NO release in the posterior hypothalamic nuclei and the PVN and that this decrease in NO expression caused by ANG II results in greater sympathetic activity (4, 5). Finally, it is possible that the prohypertensive effect of testosterone in ANG II-induced hypertension may not involve the NO system in the CNS of males. It has previously been shown that castration increases nNOS activity and that androgen treatment decreases nNOS activity, nNOS mRNA expression, and the number of nNOS-positive neurons in the brain (46). However, in the present study after castration, nNOS protein expression did not appear to increase in parallel with the reduced pressor response to ANG II. The discrepancy between results of the present study and previous findings could be due to different experimental interventions and parameters, chronic vs. acute administration of ANG II, nNOS protein expression vs. nNOS mRNA expression, or nNOS activity.
In the present study, in contrast to males, the pressor effects of systemic ANG II in female mice were augmented by central blockade of NOS. Previous studies from our laboratory (54–56) have demonstrated that sex differences in ANG II-induced hypertension can be at least partially attributed to the protective effects of estrogen in the brain. The present investigation provides further insight into the central actions of estrogen in BP regulation. In humans, estrogen enhances basal NO release in the forearm vasculature in perimenopausal women (48). Likewise, hormone replacement therapy increases plasma levels of NO in postmenopausal women (8, 21). In animals, estrogen replacement in OVX rats reduces BP responses to psychological stress and increases NO levels in the hypothalamus and brain stem (7). Inhibition of NO production in the brain augments the response in restrained OVX/estrogen-treated rats but not in OVX/vehicle-treated animals (7). Although data, including our own, were generated in different species using different experimental paradigms, they all support the hypothesis that the antihypertensive effects of estrogen are mediated, at least in part, by NO.
Since l-NAME is an inhibitor of all isoforms of NOS, it is not clear that the enhancement of BP elicited by l-NAME can be attributed to blockade of the specific neuronal isoform of NOS. Therefore, we performed an experiment to determine whether we could implicate one form of NOS in the CNS in the protective action of estrogen. We used a more selective nNOS inhibitor, l-VNIO. l-VNIO is an irreversible and potent nNOS inhibitor exhibiting more than 100-fold greater affinity for the nNOS isoform over either the endothelial NOS or the inducible NOS isoforms (1). Similar to l-NAME, central administration of l-VNIO significantly augmented the increase in BP induced by ANG II in female mice. These results suggest that brain nNOS probably contributes to an attenuated BP increase to ANG II in mice with available estrogen.
It has been shown that estrogen acting on ER upregulates nNOS in the brain and that this effect appears to be regionally and/or receptor specific (6, 18, 22, 23). To confirm the important role of estrogen, Sica et al. (44) observed a significant decrease of NOS immunoreactive cells in the medial preoptic area of aromatase knockout male mice. A moderate decrease in immunoreactivity was also detected in the PVN. In male mice with ERα knocked out, the nNOS-expressing population of cells was markedly reduced in specific brain regions, including the PVN (35). By using a double mutant mouse in which males lacked functional ERα, androgen receptors, or both, Scordalakes et al. (43) demonstrated that the presence of functional ERα is correlated with more nNOS-immunoreactive cells after testosterone treatment and greater immunoreactive staining in the preoptic area under both testosterone and 17β-estradiol (E2) treatment conditions.
Reports of the effects of estrogen on NOS expression in the PVN are conflicting. Accounts of no changes in nNOS mRNA (6), increases in the numbers of NADPH-diaphorase neurons (42), and decreases in nNOS-positive neurons in the PVN after estrogen treatment (15) have all been described. Although Gingerich and Krukoff (15) demonstrated a decrease in nNOS-positive neurons in cultured PVN slices after estrogen treatment that is ERβ dependent, they showed more recently in cultured hypothalamic neurons that activation of ERβ rapidly increases phosphorylation of nNOS and increases NO production (16). They interpret this as suggesting that estrogen increases NO production by a posttranslational modification of nNOS.
A recent study from García-Durán et al. (14) using a Western blot analysis of protein showed that estrogen induced significant increases in nNOS protein expression in a time- and dose-dependent manner in neutrophils from male and female subjects. In the present study, using a Western blot analysis for relative nNOS protein expression, we found that relative nNOS protein expression in both the SFO and PVN was higher in females under basal conditions and that it was further enhanced by ANG II treatment in females compared with males. OVX results in a significant decrease in relative nNOS expression in females with or without ANG II treatment. These results provide new evidence that there are differences in NO availability and expression in key brain cardiovascular control areas between males and females. Increased expression of nNOS within the SFO and PVN in intact females with or without ANG II treatment is related to the presence of estrogen, and increased production of NO in the brain may be responsible for the reduced pressor effect and sympathetic outflow produced by ANG II.
Compared with the large number of studies that have examined the effects of estrogen on nNOS expression, relatively few have focused on the role of progesterone. Fadel et al. (12) reported that skeletal muscle nNOS is reduced in estrogen-deficient female rats and is restored by E2, but not progesterone, replacement. Skeletal muscle nNOS expression is highly correlated with plasma E2, suggesting that progesterone is unlikely to be a key factor mediating increased nNOS expression.
There also are conflicting reports regarding the expression of nNOS and nNOS activity in near-term pregnant rats, which is a state where estrogen and progesterone are high. Plasma progesterone levels increase early in pregnancy and remain elevated until ∼24 h before parturition, at which time they decrease to nonpregnant levels (3). Heesch et al. (19) reported that nNOS activity and expression are decreased in the PVN of pregnant rats. In their study, brain tissue was harvested on the morning of day 21, which would be 1.5–2 days before parturition. At this time, plasma progesterone levels would likely be elevated above that of nonpregnant rats. In contrast, in pregnant rats on the day of parturition after progesterone falls and is low or in rats where progesterone is withdrawn after hormone treatment to mimic the time of parturition, nNOS activity in both the SON and PVN is increased (38, 53). Together, these data suggest that decreased nNOS expression in the PVN during pregnancy may be attributed to high progesterone levels rather than high estrogen levels.
It has been established that sensory CVOs are primary sites of the central action of circulating ANG II and that the PVN is an important site of integration for the neural control of sympathetic outflow. Immunohistochemistry from others (41) and in the present studies indicate a high degree of the colocalization of ER, nNOS, and AT1 in the PVN and SFO (41). In view of the well-established projection from the SFO to the PVN, such colocalization provides an anatomical basis for the interaction between NO and estrogen in ANG II-induced hypertension. Bains and Ferguson (2) have reported that the pressor response to stimulation of the SFO is enhanced following administration of l-NAME, which is mediated via an angiotensin-NO interaction in the PVN. Another recent study also showed that estrogen attenuates the drinking response induced by ANG II activation of the SFO projections to the PVN (50). These results, combined with ours, suggest that ANG II activates PVN neurons to increase sympathetic outflow and BP via projections from the SFO and that the neuronal activities in the SFO and/or PVN are attenuated by NO production enhanced by estrogen.
In summary, the central actions of estrogen on NO production play an important protective role in the development of ANG II-induced hypertension. This protective effect, at least in part, seems to involve actions to decrease sympathetic outflow.
GRANTS
This work was supported by National Institutes of Health Grants HL-62261, HL-59676, HL-14388, and DK-66086.
REFERENCES
- 1.Babu BR, Griffith OW. N5-imino-3-butenyl-l-ornithine. A neuronal isoform selective mechanism-based inactivator of nitric oxide synthase. J Biol Chem 273: 8882–8889, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Bains JS, Ferguson AV. Angiotensin II neurotransmitter actions in paraventricular nucleus are potentiated by a nitric oxide synthase inhibitor. Regul Pept 50: 53–59, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology 114: 930–940, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 287: H695–H703, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin-1beta mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 39: 519–524, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Ceccatelli S, Grandison L, Scott RE, Pfaff DW, Kow LM. Estradiol regulation of nitric oxide synthase mRNAs in rat hypothalamus. Neuroendocrinology 64: 357–363, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Cherney A, Edgell H, Krukoff TL. NO mediates effects of estrogen on central regulation of blood pressure in restrained, ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 285: R842–R849, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cicinelli E, Ignarro LJ, Schonauer LM, Matteo MG, Galantino P, Balzano G. Effects of short-term transdermal estradiol administration on plasma levels of nitric oxide in postmenopausal women. Fertil Steril 69: 58–61, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Conrad KP, Whittemore SL. NG-monomethyl-l-arginine and nitroarginine potentiate pressor responsiveness of vasoconstrictors in conscious rats. Am J Physiol Regul Integr Comp Physiol 262: R1137–R1144, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA 88: 7797–7801, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 53: 688–708, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Fadel PJ, Zhao W, Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol 549: 243–253, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med 264: 224–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Durán M, de Frutos T, Díaz-Recasens J, García-Gálvez G, Jiménez A, Montón M, Farré J, Sánchez de Miguel L, González-Fernández F, Arriero MD, Rico L, García R, Casado S, López-Farré A. Estrogen stimulates neuronal nitric oxide synthase protein expression in human neutrophils. Circ Res 85: 1020–1026, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Gingerich S, Krukoff TL. Estrogen modulates endothelial and neuronal nitric oxide synthase expression via an estrogen receptor beta-dependent mechanism in hypothalamic slice cultures. Endocrinology 146: 2933–2941, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Gingerich S, Krukoff TL. Activation of ERbeta increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology 55: 878–885, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Gorbea-Oppliger VJ, Fink GD. Cerebroventricular injection of angiotensin II antagonist: effects on blood pressure responses to central and systemic angiotensin II. J Pharmacol Exp Ther 273: 611–616, 1995 [PubMed] [Google Scholar]
- 18.Grohe C, Kann S, Fink L, Djoufack PC, Paehr M, van Eickels M, Vetter H, Meyer R, Fink KB. 17β-Estradiol regulates nNOS and eNOS activity in the hippocampus. Neuroreport 15: 89–93, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Heesch CM, Zheng H, Foley CM, Mueller PJ, Hasser EM, Patel KP. Nitric oxide synthase activity and expression are decreased in the paraventricular nucleus of pregnant rats. Brain Res 1251: 140–150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hropot M, Langer KH, Wiemer G, Grötsch H, Linz W. Angiotensin II subtype AT1 receptor blockade prevents hypertension and renal insufficiency induced by chronic NO-synthase inhibition in rats. Naunyn Schmiedebergs Arch Pharmacol 367: 312–317, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Imthrun B, Rosselli M, Jaeger AW, Keller JP, Dubey RK. Differential effects of hormone-replacement therapy on endogenous nitric oxide (nitrite/nitrate) levels in postmenopausal women substituted with 15 beta-estradiol valerate and cyproterone acetate or medroxyprogesterone acetate. J Clin Endocrinol Metab 82: 388–394, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Ishihara T, Araki T, Sakuma Y. Two distinct populations of neurons expressing nitric oxide synthase mRNA in the female rat preoptic area: site specific changes induced by sex steroids. J Nippon Med Sch 68: 328–334, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Ishihara T, Orikasa C, Araki T, Sakuma Y. Sex difference in the expression and regulation of nitric oxide synthase gene in the rat preoptic area. Neurosci Res 43: 147–154, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Kadekaro M, Summy-Long JY. Centrally produced nitric oxide and the regulation of body fluid and blood pressure homeostasis. Clin Exp Pharmacol Physiol 27: 450–459, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptor (ERα and ERβ) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 36: 357–378, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23: 5041–5049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R1035–R1043, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Hay M. 17-beta-estradiol modulation of area postrema potassium currents. J Neurophysiol 84: 1385–1391, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Linz W, Wohlfart P, Schölkens BA, Malinski T, Wiemer G. Interactions among ACE, kinins and NO. Cardiovasc Res 43: 549–561, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Terrell ML, Bui V, Summy-Long JY, Kadekaro M. NO and angiotensin II effects on blood pressure and fluid homeostasis. J Neuroendocrinol 9: 545–552, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144: 2055–2067, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Navar LG, Ichihara A, Chin SY, Imig JD. Nitric oxide-angiotensin II interactions in angiotensin II-dependent hypertension. Acta Physiol Scand 168: 139–147, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Pamidimukkala J, Hay M. 17β-Estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol 285: H1515–H1520, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Panzica GC, Viglietti-Panzica C, Sica M, Gotti S, Martini M, Pinos H, Carrillo B, Collado P. Effects of gonadal hormones on central nitric oxide producing systems. Neuroscience 138: 987–995, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med (Maywood) 226: 814–824, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Plochocka-Zulinska D, Krukoff TL. Increased gene expression of neuronal nitric oxide synthase in brain of adult spontaneously hypertensive rats. Brain Res Mol Brain Res 48: 291–297, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Popeski N, Amir S, Woodside B. Changes in NADPH-d staining in the paraventricular and supraoptic nuclei during pregnancy and lactation in rats: role of ovarian steroids and oxytocin. J Neuroendocrinol 11: 53–61, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Ramchandra R, Barrett CJ, Malpas SC. Nitric oxide and sympathetic nerve activity in the control of blood pressure. Clin Exp Pharmacol Physiol 32: 440–446, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Reis WL, Giusti-Paiva A, Ventura RR, Margatho LO, Gomes DA, Elias LL, Antunes-Rodrigues J. Central nitric oxide blocks vasopressin, oxytocin and atrial natriuretic peptide release and antidiuretic and natriuretic responses induced by central angiotensin II in conscious rats. Exp Physiol 92: 903–911, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Res 837: 254–262, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Sanchez F, Martinez ME, Rubio M, Carretero J, Moreno MN, Vazquez R. Reduced nicotinamide adenine dinucleotide phosphate-diaphorase activity in the paraventricular nucleus of the rat hypothalamus is modulated by estradiol. Neurosci Lett 253: 75–78, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol 453: 336–344, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Sica M, Plumari L, Honda S, Harada N, Absil P, Viglietti-Panzica C, Balthazart J, Panzica GC. Changes in the neuronal nitric oxide synthase immunoreactive system in male mice lacking a functional aromatase gene (Abstract). Horm Behav 41: 490, 2002 [Google Scholar]
- 45.Singh M, Kesterson RA, Jacobs MM, Joers JM, Gore JC, Emeson RB. Hyperphagia-mediated obesity in transgenic mice misexpressing the RNA-editing enzyme ADAR2. J Biol Chem 282: 22448– 22459, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Singh R, Pervin S, Shryne J, Gorski R, Chaudhuri G. Castration increases and androgens decrease nitric oxide synthase activity in the brain: physiologic implications. Proc Natl Acad Sci USA 97: 3672–3677, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song K, Allen AM, Paxinos G, Mendelsohn FAO. Mapping of angiotensin II receptor heterogeneity in rat brain. J Comp Neurol 316: 467–484, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension 28: 330–334, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Takemoto M, Egashira K, Usui M, Numaguchi K, Tomita H, Tsutsui H, Shimokawa H, Sueishi K, Takeshita A. Important role of tissue angiotensin-converting enzyme activity in the pathogenesis of coronary vascular and myocardial structural changes induced by long-term blockade of nitric oxide synthesis in rats. J Clin Invest 99: 278–287, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka J, Kariya K, Miyakubo H, Sakamaki K, Nomura M. Attenuated drinking response induced by angiotensinergic activation of subfornical organ projections to the paraventricular nucleus in estrogen-treated rats. Neurosci Lett 324: 242–246, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev 41: 153–202, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Togashi H, Sakuma I, Yoshioka M, Kobayashi T, Yasuda H, Kitabatake A, Saito H, Gross SS, Levi R. A central nervous system action of nitric oxide in blood pressure regulation. J Pharmacol Exp Ther 262: 343–347, 1992 [PubMed] [Google Scholar]
- 53.Woodside B, Amir S. Reproductive state changes NADPH-diaphorase staining in the paraventricular and supraoptic nuclei of female rats. Brain Res 739: 339–342, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol 295: H1025–H1032, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye S, Nosrati S, Campese VM. Nitric oxide (NO) modulates the neurogenic control of blood pressure in rats with chronic renal failure (CRF). J Clin Invest 99: 540–548, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure: the roles of angiotensin II, nitric oxide, and exercise training. Ann NY Acad Sci 940: 431–443, 2001 [PubMed] [Google Scholar]