Abstract
Adenylyl cyclase (AC) types 5 and 6 (AC5 and AC6) are the two major AC isoforms expressed in the mammalian heart that mediate signals from β-adrenergic receptor stimulation. Because of the unavailability of isoform-specific antibodies, it is difficult to ascertain the expression levels of AC5 protein in the heart. Here we demonstrated the successful generation of an AC5 isoform-specific mouse monoclonal antibody and studied the expression of AC5 protein during cardiac development in different mammalian species. The specificity of the antibody was confirmed using heart and brain tissues from AC5 knockout mice and from transgenic mice overexpressing AC5. In mice, the AC5 protein was highest in the brain but was also detectable in all organs studied, including the heart, brain, lung, liver, stomach, kidney, skeletal muscle, and vascular tissues. Western blot analysis showed that AC5 was most abundant in the neonatal heart and declined to basal levels in the adult heart. AC5 protein increased in the heart with pressure-overload left ventricular hypertrophy. Thus this new AC5 antibody demonstrated that this AC isoform behaves similarly to fetal type genes, such as atrial natriuretic peptide; i.e., it declines with development and increases with pressure-overload hypertrophy.
Keywords: adenylyl cyclase isoforms, monoclonal antibody, pressure overload, hypertrophy
adenylyl cyclase (AC) is an enzyme that catalyzes the conversion of ATP to cAMP. The complexity in understanding the signaling pathway of cAMP can be attributed, in part, to 10 isoforms of AC: nine membrane bound and one soluble, which have been cloned and characterized in mammals (4, 6, 9, 10, 29). Each of these membrane-bound isoforms consists of two hydrophobic domains (with 6 transmembrane spans) and two cytoplasmic domains. The cytoplasmic domains constitute the catalytic site, which is subject to intracellular regulation (6). The AC isoforms have high amino acid homology in their cytoplasmic domains but differ in the sequence of the transmembrane region. The specificity of tissue distribution, the functional properties, and the chromosomal location of the corresponding genes also differentiate these isoforms (4, 10, 12). The major AC isoforms expressed in the heart are type 5 (AC5) and type 6 (AC6) (7, 11, 30). Tissue distribution and developmental expression of AC5 and AC6 mRNA have been previously studied in rats, chicks, and humans (7, 30, 31). However, in the absence of an AC5-specific antibody, it has not been possible to determine the levels of protein expression. It is very important to study the differential regulation of AC5 and AC6 in the heart since AC5 and AC6 behave differently in the pathogenesis of heart failure. We demonstrated that the AC5 knockout (KO) mouse model lives longer and is resistant to stress (32), including chronic pressure overload (21) and chronic catecholamine (22) stress. However, studies by others have suggested that the overexpression of AC6 is beneficial and might be considered for heart failure therapy (23). In addition, based on mRNA measurements, conflicting data have been presented regarding the ontogeny of AC5 (7, 26, 30). All of the important studies related to AC5 in the heart are limited by the absence of a specific antibody. Accordingly, the first goal of this investigation was to develop an AC5-specific monoclonal antibody and then to determine the expression levels of AC5 protein during a physiological process and cardiac development and in response to pathophysiological stress induced by chronic pressure overload.
MATERIALS AND METHODS
Antibody preparation and purification.
The peptide sequence NH2-GNQVSKEMKRMGFEDPKDKN-COOH, which is specific to the cytoplasmic domain (C1b portion) of the AC5 protein and has the least homology with the other AC isoforms, was used to generate the mouse monoclonal antibody. The synthetic peptide (Rockland Immunochemicals, Gilbertville, PA) was conjugated to keyhole limpet hemocyanin to impart immunogenicity to the small peptide and then mixed with adjuvant (Titermax Gold for the first injection and Freund's incomplete adjuvant for subsequent booster doses) and used to immunize Balb/C mice. The fusion of mouse spleen with mouse myeloma cells, Sp2/0, was done using standard hybridoma techniques (17). The selection of the antibody was done by screening the supernatant by ELISA and using the synthetic peptide as an antigen. The CELLine device CL-1000 (BD Biosciences) was used for producing the monoclonal antibody in high yield. Cells were harvested by centrifugation at 3,500 g for 1 h at 4°C. The monoclonal antibody in the supernatant fraction was precipitated with ice-cold ammonium sulfate solution (pH 7.4). The antibody pellet was dissolved in PBS and dialyzed against the same buffer. The dialysate was centrifuged at 10,000 g for 30 min at 4°C to remove aggregates, if any. The supernatant fraction was filtered through a 0.2-mm filter and further purified by immunoaffinity chromatography using a protein G column (Pierce Biotechnology) following the manufacturer's protocol.
Animal models.
The transgenic (TG) mouse with cardiac overexpression of AC5 was generated by the insertion of the coding region of the canine AC5 gene (4.3 kb, gene bank accession no. M88649; cloned by Dr. Ishikawa) to a vector containing the mouse α-myosin heavy chain gene promoter region (gene bank accession no. U71441) in a pBlueScript vector followed by poly(A) sequence of the human growth hormone gene. The AC6 TG construct was done similarly by inserting the coding region of the canine AC6 gene (4 kb, gene bank accession no. M94968; cloned by Dr. Ishikawa) into the same vector. AC5 KO (20) and wild-type (WT) mice and 129SVJ mice were also used for ontogenic studies. Commercially available Sprague-Dawley rats and mixed-breed pigs (n = 4 per age group) were used for ontogenic studies. FVB mice were used for transverse aortic banding (25) to induce left ventricular hypertrophy (LVH). At 4 to 5 mo of age, the mice were anesthetized with a mixture of ketamine (65 mg/kg), xylazine (2 mg/kg), and acepromazine (13 mg/kg). A thoracotomy was performed and the transverse aorta was constricted by placing a suture around a 28-gauge needle. The needle was removed and the chest closed. A similar procedure was performed on sham-operated mice without the placement of the suture. After 4 wk of aortic banding, the mouse hearts were harvested and studied. These studies were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School.
AC5 and AC6 transfection.
COS-7 cells were infected with 2 μg of AC5 or AC6 cDNA plasmid, respectively, using 6 μl of Fugene 6 transfection reagent (Roche Applied Science). After 48 h, the cells were harvested, washed twice with PBS, and lysed for 30 min with lysis buffer consisting of 50 mM Tris·HCl, 50 mM NaCl, and 1% Tergitol-type nonyl phenoxylpolyethoxylethanol-40 (NP-40) with protease inhibitors. After centrifugation at 4°C, the lysate was stored in aliquots at −80°C and 15 μg of protein were used for Western blot analysis.
Western blot analysis.
The frozen heart and brain tissues from mice, rats, and pigs were homogenized on ice in buffer containing (in mM) 50 Tris·HCl, 6 MgCl2, 75 sucrose, 1 dithiothreitol, and 1 EDTA (pH 7.6) (TMSDE buffer) and 1 phenylmethylsulphonyl fluoride. The homogenate was centrifuged at 600 g for 8 min at 4°C, and the supernatant was centrifuged again at 69,000 g for 60 min at 4°C to collect the membrane proteins. The membrane pellet was resuspended in TMSDE buffer containing 1% NP-40 and briefly sonicated. The protein concentration was determined with the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL). The membrane sample was solubilized in loading buffer, containing 62.5 mM Tris·HCl (pH 6.8), 25% glycerol, 2% SDS, and 0.1% bromophenol blue, and was separated on a 6% SDS polyacrylamide gel, as previously described (16). The proteins were then transferred to a nitrocellulose membrane and blocked for 1 h with 5% milk in buffer containing 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween-20 (TBST). The membranes were incubated with our affinity-purified, AC5 mouse monoclonal antibody (AC5MAb, 1:500 dilution) or the commercial AC5/6 antibody (C-17) (1:200 dilution; Cat. No. sc-590; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. After incubation with the primary antibody, the blots were then washed with TBST at room temperature and incubated with goat anti-mouse IgG [heavy and light chains (H+L)] (for AC5 detection) or goat anti-rabbit IgG (H+L) (for AC6 detection)-horseradish peroxidase-conjugated secondary antibody for 30 min. Immunoreactive bands were detected with Western Lightning Chemiluminescence Reagent (Perkin Elmer Life Sciences, Boston, MA). All Western blot exposures were in the linear range of detection, and the intensities of the resulting bands were quantified by Quantity One software on GS-800 densitometer (Bio-Rad, Hercules, CA). The plasma membrane marker, pan-cadherin antibody, was used as a loading control.
Northern blot analysis.
Total RNA was isolated from heart tissue that was previously dissected and frozen in liquid nitrogen using the TriReagent isolation method according to the manufacturer's instructions (Sigma, St. Louis, MO). A total of 20 μg of total RNA was separated on a 1.2% agarose gel containing 1× 3-[N-morpholino]propanesulfonic acid and 2% formaldehyde. RNA was then transferred onto a nylon membrane. The membranes were hybridized overnight in PerfectHyb solution (Sigma) containing α-P32-dCTP-labeled AC5-specific probe (a fragment corresponding to nucleotides 1929 to 2129 relative to the initiation of methionine codon). The membranes were washed with 2× saline-sodium citrate (0.3 M sodium chloride and 30 mM sodium citrate), 0.1% SDS buffer for 2× 5 min and 0.1× saline-sodium citrate, and 0.1% SDS buffer for 2× 10 min before exposure for autoradiography.
Quantitative real-time polymerase chain reaction.
The expression of AC5 mRNA levels was quantified using real-time PCR (ABI Prism 7300). From each sample, 50 ng of total RNA were reverse transcribed (TaqMan, Applied Biosystems) at 42°C for 30 min. The absolute quantification of AC5 and AC6 transcript was measured by TaqMan Probe real-time PCR assays with mouse-specific AC5 forward primer: 5′-CACAATCCGCCTCACTGG-3′, reverse primer: 5′-TCCTCAAAGCCCATCCTCTT-3′, and AC5-specific probe: 5′-/56-FAM/AGCCGAGCGCCCCTTCTACAA/36-TAMTSp/-3′. Real-time PCR was performed in two steps: a 1-min step at 95°C, followed by 40 cycles of a 12-s step at 95°C and a 1-min step at 60°C. Internal standards were prepared for each transcript from their PCR-amplified cDNA (5). Data normalization was performed by quantitative PCR amplification of cyclophilin gene or 18S rRNA.
Statistical analysis.
Statistical analysis was performed using StatView 5.0 statistical software (SAS Institute). The data are reported as means ± SE. Comparisons between two groups were conducted with Student's t-test. Multiple group comparisons were performed by ANOVA. Significance was recorded for P < 0.05.
RESULTS
Characterization of AC5-specific mouse monoclonal antibody.
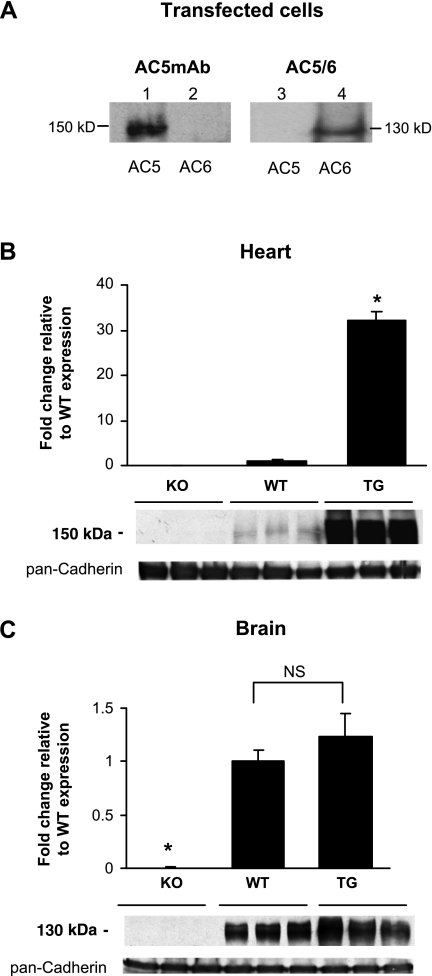
First, we determined the specificity of the AC5 monoclonal antibody (AC5MAb) by Western blot analysis using AC5 or AC6 recombinant proteins expressed in COS-7 cells. As seen in Fig. 1A (lane 1), AC5MAb cross-reacted specifically with the AC5 recombinant protein at a molecular mass of ∼150 kDa. However, the AC5MAb did not cross-react with lysate from AC6-transfected COS-7 cells (Fig. 1A, lane 2). The AC5/6 commercial antibody (Cat. No. sc-590; Santa Cruz Biotechnology) only cross-reacted with lysate from AC6-transfected COS-7 cells (Fig. 1A, lane 4), not from AC5-transfected COS-7 cells (Fig. 1A, lane 3). To further validate the antibody specificity, Western blot analyses were carried out using membrane preparations from the heart and brain tissues of AC5 KO, WT, and TG mice with cardiac-specific overexpression of AC5. AC5MAb did not cross-react with proteins isolated from AC5 KO heart (Fig. 1B) and brain (Fig. 1C). The expression of AC5 increased significantly in AC5 TG hearts compared with the WT hearts (Fig. 1B), whereas the expression in the brain remained comparable with the WT brains (Fig. 1C). Different from the theoretical molecular mass of AC5 (139 kDa), which was calculated using the reported sequence and the ExPasy Server (http://www.expasy.org), AC5MAb cross-reacted with proteins at ∼150 kDa in AC5-transfected cells, in the heart and brain, which could be due to differences in 1) posttranslational modification in AC5 versus AC6 and 2) apparent molecular mass versus calculated molecular mass. For example, the reported molecular mass of Gsα is 46 kDa; however, the apparent molecular mass of Gsα is 45 and 52 kDa (24). In addition, the sizing accuracy for SDS-PAGE depends on the protein characteristics such as amino acid sequence, isoelectric point (pI), structure, and hydrophobicity (1). Since these specific protein characteristics can affect migration, it is possible that not all proteins migrate according to their expected molecular masses (3, 14, 18, 28). In fact, pI is quite different for AC5 (pI 6.65) and AC6 (pI 8.16).
Fig. 1.
Specificity of the adenylyl cyclase type 5 (AC5) mouse monoclonal antibody (AC5MAb). A: equal amounts of lysates from COS-7 cells transfected with AC5 cDNA (lane 1) and AC6 cDNA (lane 2) were immunoblotted with AC5MAb. The lysates from COS-7 cells transfected with AC5 cDNA (lane 3) and AC6 cDNA (lane 4) were immunoblotted with AC5/6 commercial antibody from Santa Cruz. Membrane preparations from the whole heart (B) and brain (C) of AC5 knockout (KO), wild-type (WT), and cardiac-specific AC5 transgenic (TG) mouse models (n = 7 to 8) were resolved on a 6% SDS-PAGE and immunoprobed with AC5MAb. The data are normalized to the value of WT samples and expressed as means ± SE. The loading control is shown using pan-cadherin antibody. *P < 0.05 vs. all other groups. NS, not significantly different.
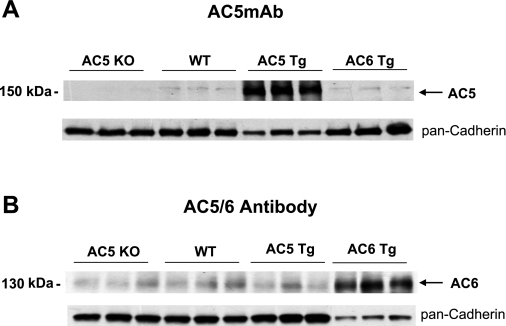
The specificity of the AC5MAb was also tested with the AC6 TG mouse heart. The level of AC5 was similar to WT in the AC6 TG mouse heart (Fig. 2A). We compared these results with those obtained when using a commercial AC5/6 antibody (Cat. No. sc-590; Santa Cruz Biotechnology). AC protein levels detected by this antibody were not different among WT, AC5 KO, and AC5 TG mouse hearts but significantly increased in the AC6 TG hearts (Fig. 2B). These results, together with those in Fig. 1A, indicate that this AC5/6 antibody is unlikely to cross-react with the AC5 isoform and reports mainly AC6. We also tested several other commercially available AC5 antibodies; these antibodies were not able to distinguish AC5 expression level in AC5 TG, AC5 KO, and WT mice.
Fig. 2.
Western blot analysis of AC5MAb with mouse heart. A: AC5 protein is detected by immunoblotting and is absent in AC5 KO and increased in AC5 TG hearts. The level of AC5 was similar to WT in the AC6 TG mouse heart. The amount of protein loaded from AC5 TG heart is 50% of the amount from AC5 KO, WT, and AC6 TG hearts. B: immunoblot using an AC5/6 commercial antibody. The AC levels were not different in hearts of AC5 KO, WT, and AC5 TG but increased in AC6 TG hearts, indicating that this antibody may primarily detect only AC6 protein. The amount of protein loaded from AC6 TG heart is 30% of the amount from AC5 KO, WT, and AC5 TG hearts. The loading control is shown using pan-cadherin antibody.
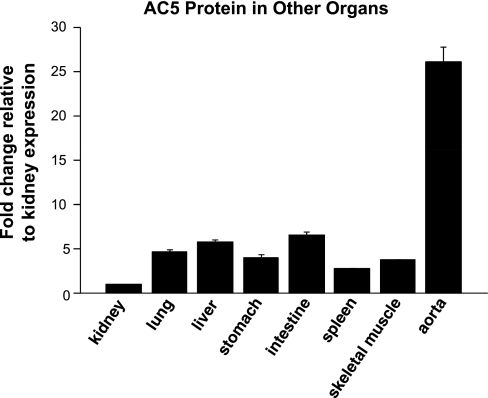
Using the AC5-specific antibody, we examined the tissue distribution of AC5 in organs other than heart and brain. AC5 protein was also found to be expressed in the kidney, lung, liver, stomach, intestine, spleen, skeletal muscle, and vascular tissues (Fig. 3).
Fig. 3.
Tissue distribution of AC5 protein expression in young pigs (2 to 3 mo, n = 4). With the use of Western blot analysis, the level of AC5 protein expression was measured in kidney, lung, liver, stomach, intestine, spleen, skeletal muscle, and vascular tissues. The number of samples exceeded that which could be run on one gel, and, accordingly, samples were run on 3 distinct gels; each gel contained the same kidney sample as a control. All data were quantitated with densitometry and normalized to the value of kidney samples. Data are expressed as means ± SE.
Expression of AC5 protein during cardiac development.
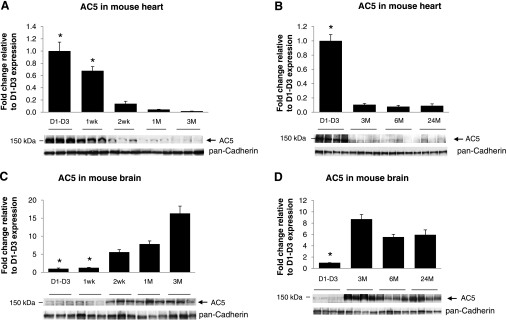
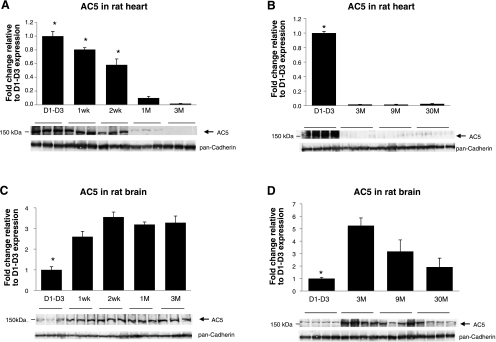
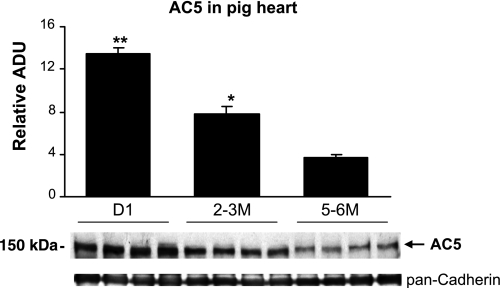
We next examined the ontogenic expression of AC5 protein during heart development. In the mouse hearts, AC5 protein abundance was highest before 1 wk and then declined to lower levels (Fig. 4A), which were indistinguishable from 3 to 24 mo of age (Fig. 4B). In contrast, the AC5 protein abundance was relatively low before 1 wk in mouse brain, but it increased significantly after 2 wk (Fig. 4C) and remained high from 3 to 24 mo of age in adult brains (Fig. 4D). We also analyzed AC5 protein expression in rat heart and brain (Fig. 5) with the similar age patterns of mouse shown in Fig. 4. AC5 protein level was highest before 2 wk and then declined to lower levels in rat heart (Fig. 5A), but in rat brain, the AC5 level was low before 1 wk and then increased significantly (Fig. 5C). In both old-mouse (24 mo old) and old-rat (30 mo old) brain (Fig. 5D), the AC5 level declined compared with the young (3 mo old) brain. These results demonstrated that the mouse and rat heart and brain have the similar AC5 expression patterns. We further analyzed the AC5 protein levels in pig hearts during development. As shown in Fig. 6, the cardiac AC5 protein expression was the highest in 1-day-old pig heart and decreased with age (2 to 3 mo; P < 0.05), with the lowest abundance detected at 5 to 6 mo of age (P < 0.05 vs. young, and P < 0.01 vs. neonates).
Fig. 4.
AC5 protein levels in WT mouse heart and brain tissues. Immunoblotting was performed with AC5MAb using mouse heart and brain homogenates. Western blots were run individually for each age group: neonatal day 1–day 3 (D1–D3), 1 wk, 2 wk, 1 mo (1M), and 3 mo (3M), from mouse heart (n = 3/age group) (A); D1–D3, 3 mo, 6 mo (6M), and 24 mo (24M) from mouse heart (n = 4/age group) (B); and D1–D3, 1 wk, 2 wk, 1 mo, and 3 mo from mouse brain (n = 3/age group) (C); and D1–D3, 3 mo, 6 mo, and 24 mo from mouse brain (n = 4/age group) (D). AC5 protein was highest in mouse neonatal heart and lowest in mouse neonatal brain (before 1 wk) compared with adult (*P < 0.05 vs. other groups). Data are normalized to mouse D1–D3 samples and expressed as means ± SE.
Fig. 5.
AC5 protein levels in rat heart and brain tissues. Immunoblotting was performed with AC5MAb using rat heart and brain homogenates. Western blots were run individually for each age group: D1–D3, 1 wk, 2 wk, 1 mo, and 3 mo from rat heart (n = 3/age group) (A); D1–D3, 3 mo, 9 mo, and 30 mo from rat heart (n = 4/age group) (B); D1–D3, 1 wk, 2 wk, 1 mo, and 3 mo from rat brain (n = 3/age group) (C); and D1–D3, 3 mo, 9 mo, and 30 mo from rat brain (n = 4/age group) (D). AC5 protein was highest in rat neonatal heart (before 2 wk) and lowest in rat neonatal brain (before 1 wk) compared to adult (*P < 0.05 vs. other groups). Data are normalized to rat D1–D3 samples and expressed as means ± SE.
Fig. 6.
AC5 expression varies by age in pig hearts. Western blot analysis of AC5 expression in pig hearts showed highest levels in neonatal animals. Equal amounts of membrane preparations (25 μg) were compared between neonatal (day 1), young (2 to 3 mo), and adult (5 to 6 mo) pig hearts (n = 4). Pan-cadherin was used as a loading control. The level of AC5 protein expression significantly decreased with age. *P < 0.05 compared with the adult; **P < 0.01. Data are expressed as means ± SE. ADU, arbitrary densitometric units.
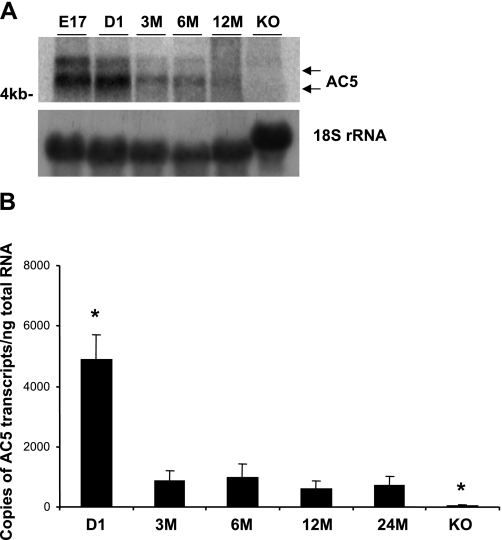
To determine whether the AC5 protein expression parallels the mRNA expression during mouse heart development, Northern blot and quantitative PCR analysis were carried out. Northern blot analysis showed that the AC5 mRNA abundance was high in the embryonic (gestational day 17) and neonatal hearts, whereas it decreased in the adult (Fig. 7A). Quantification of the AC5 transcript confirmed an expression of approximately fivefold higher in the 1-day-old mouse heart compared with the adult heart (Fig. 7B).
Fig. 7.
AC5 mRNA expression in the mouse heart. A: Northern blot analysis with total RNA (20 μg) purified from whole WT mouse heart at 6 different stages: day 17 embryos (E17), day 1 neonates (D1), 3 mo, 6 mo, and AC5 KO as a negative control. B: quantitative real-time RT-PCR with mouse AC5-specific primers and probe (TaqMan). Data are expressed as means ± SE. *P < 0.05, significant difference compared with 6-mo mouse hearts. Again, levels were highest in neonatal hearts.
Expression of AC5 protein in LVH.
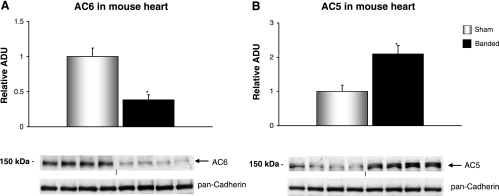
To evaluate the effect of stress on AC isoforms (AC6 and AC5) in the heart, 4- to 5-mo-old WT mice had transverse aortic banding surgically applied for a 4-wk period. The effects of LVH on AC6 (Fig. 8A) and AC5 (Fig. 8B) protein content in the heart are shown, with a significant (P < 0.05) increase in AC5, as opposed to a significant decrease in AC6 protein levels with LVH.
Fig. 8.
AC isoform levels in mouse hearts with 4 wk of aortic banding. AC isoform protein levels were detected with immunoblotting using membrane preparations from sham-operated (n = 7) and 4-wk aortic-banded mice (n = 8). AC6 protein levels fell (A), and AC5 protein levels rose (B). The loading control is shown using pan-cadherin antibody. Data are expressed as means ± SE. *P < 0.05.
DISCUSSION
In this study, we characterized the AC5 protein expression during heart development in mice, rats, and pigs using an AC isoform-specific mouse monoclonal antibody. Our AC5MAb antibody specifically recognizes AC5 protein (Fig. 1A, lane 1) and not AC6 protein in the transfected cells or AC6 TG mouse heart. The fact that no AC protein was detected by AC5MAb in the AC5 KO heart and brain tissues (Fig. 1, B and C) further confirms that our monoclonal antibody is specific for AC5 protein, which is detected at ∼150 kDa. One of the antibodies [AC5/6 (c-17)] commonly used to detect AC5/6 changes in AC research could not demonstrate specific changes in AC5 protein expression between AC5 KO, WT, and AC5 TG mouse hearts (Fig. 2B). In our study, this commercial antibody did not detect recombinant AC5 protein in the transfected cells (Fig. 1A, lane 3), indicating that the AC5/6 antibody favorably cross-reacts with the AC6 protein alone. A recent study (27) also showed that the commercial antibody failed to detect AC5 protein in the AC6 KO hearts, further corroborating our results.
Using the AC5MAb, we were able to show the AC5 protein expression profile among various tissues (Fig. 3). Previously, the AC5 transcript was found in the brain, heart, kidney, liver, lung, and testis (4). We also found a significant amount of AC5 expression in skeletal muscle and vessels. Higher expression levels of AC5 in the brain were detected in thalamus, consistent with previous findings in the rat brain where thalamus expression of AC5 mRNA was high throughout the various stages of brain development (19). AC5 mRNA levels were found to be dramatically increased in the adult rat brain, including the striatum.
The AC5 mRNA and protein expression were higher in neonatal compared with adult hearts in mice, rats, and pigs. Interestingly, this pattern for AC5 expression during development differed from that found in the brain, which was lower in neonates (Figs. 4C and 5C), which was confirmed at both the transcript and the protein levels. Previous studies of AC5 mRNA expression during rat heart development have yielded conflicting information (7, 26, 30). Some studies showed that the expression of the AC5 transcript in the heart increases after birth until cardiac maturation (7, 30), whereas another study (26) showed that the AC5 mRNA decreases from 6 to 24 mo of age in the rat heart. In our study, the expression profile of AC5 during heart development was comparable among mice, rats, and pigs, showing that the neonatal and young hearts have a higher expression than the mature adult heart.
The finding that AC5 protein levels in the heart are highest at birth and decline with age is similar to what is observed with the fetal gene program (13), where increases are observed with LVH. Accordingly, we examined the regulation of AC isoforms in pressure-overload LVH. It would be predicted that AC protein levels would decline with chronic pressure overload, as part of a desensitization program. Indeed, this is what we observed with AC6. Surprisingly, AC5 protein paradoxically increased, further supporting the concept that AC5 behaves like other fetal genes, e.g., atrial natriuretic peptide (2, 8, 15).
In summary, the new AC5 antibody developed in the present investigation represents a novel tool for a proper understanding of the tissue distribution of AC5 and of the ontogenic regulation of AC5 expression, which may be useful for further studies of AC5 signaling. In addition, the availability of a novel type 5 AC antibody will provide new insight into the pathogenesis of disease states. One example, from the current investigation, demonstrating a paradoxical upregulation of AC5 with pressure-overload LVH and its decline with ontogenic development is similar to that of fetal type genes, commonly used markers of cardiac hypertrophy.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-069020, AG-023137, AG-028854, AG-014121, HL-095888, HL-033107, HL-059139, HL-069752, HL-093481, and GM-69861.
ACKNOWLEDGMENTS
We thank Lauren Danridge for patience and help with the manuscript.
REFERENCES
- 1.Agilent Technologies Differences and similarities between the Protein 200 assay and SDS-PAGE: Technical Note, 2001 (http://www.chem.agilent.com/Library/technicaloverviews/Public/59883160.pdf). [Google Scholar]
- 2.Bloch KD, Seidman JG, Naftilan JD, Fallon JT, Seidman CE. Neonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathways. Cell 47: 695–702, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Carlotti F, Zaldumbide A, Charif H, de Koning EJ, Luider TM, Hoeben RC. The 45-kDa form of Pdx-1 does not result from post-translational modifications. Biochem Biophys Res Commun 370: 225–229, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 279: F400–F416, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med 4: 1269–1275, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Dessauer CW, Gilman AG. The catalytic mechanism of mammalian adenylyl cyclase. Equilibrium binding and kinetic analysis of P-site inhibition. J Biol Chem 272: 27787–27795, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Espinasse I, Iourgenko V, Defer N, Samson F, Hanoune J, Mercadier JJ. Type V, but not type VI, adenylyl cyclase mRNA accumulates in the rat heart during ontogenic development. Correlation with increased global adenylyl cyclase activity. J Mol Cell Cardiol 27: 1789–1795, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Gu J, D'Andrea M, Seethapathy M. Atrial natriuretic peptide and its messenger ribonucleic acid in overloaded and overload-released ventricles of rat. Endocrinology 125: 2066–2074, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Huang C, Hepler JR, Chen LT, Gilman AG, Anderson RG, Mumby SM. Organization of G proteins and adenylyl cyclase at the plasma membrane. Mol Biol Cell 8: 2365–2378, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa Y, Homcy CJ. The adenylyl cyclases as integrators of transmembrane signal transduction. Circ Res 80: 297–304, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa Y, Katsushika S, Chen L, Halnon NJ, Kawabe J, Homcy CJ. Isolation and characterization of a novel cardiac adenylylcyclase cDNA. J Biol Chem 267: 13553–13557, 1992 [PubMed] [Google Scholar]
- 12.Iyengar R. Molecular and functional diversity of mammalian Gs-stimulated adenylyl cyclases. FASEB J 7: 768–775, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci USA 85: 339–343, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jassen AK, Brown JM, Panas HN, Miller GM, Xiao D, Madras BK. Variants of the primate vesicular monoamine transporter-2. Brain Res Mol Brain Res 139: 251–257, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Knowlton KU, Rockman HA, Itani M, Vovan A, Seidman CE, Chien KR. Divergent pathways mediate the induction of ANF transgenes in neonatal and hypertrophic ventricular myocardium. J Clin Invest 96: 1311–1318, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 17.Lane RD, Crissman RS, Ginn S. High efficiency fusion procedure for producing monoclonal antibodies against weak immunogens. Methods Enzymol 121: 183–192, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem 279: 21012–21020, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka I, Suzuki Y, Defer N, Nakanishi H, Hanoune J. Differential expression of type I, II, and V adenylyl cyclase gene in the postnatal developing rat brain. J Neurochem 68: 498–506, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Okumura S, Kawabe J, Yatani A, Takagi G, Lee MC, Hong C, Liu J, Takagi I, Sadoshima J, Vatner DE, Vatner SF, Ishikawa Y. Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circ Res 93: 364–371, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci USA 100: 9986–9990, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okumura S, Vatner DE, Kurotani R, Bai Y, Gao S, Yuan Z, Iwatsubo K, Ulucan C, Kawabe J, Ghosh K, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase enhances desensitization of cyclic adenosine monophosphate signal and increases Akt signal with chronic catecholamine stress. Circulation 116: 1776–1783, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Phan HM, Gao MH, Lai NC, Tang T, Hammond HK. New signaling pathways associated with increased cardiac adenylyl cyclase 6 expression: implications for possible congestive heart failure therapy. Trends Cardiovasc Med 17: 215–221, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robishaw JD, Smigel MD, Gilman AG. Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem 261: 9587–9590, 1986 [PubMed] [Google Scholar]
- 25.Sadoshima J, Montagne O, Wang Q, Yang G, Warden J, Liu J, Takagi G, Karoor V, Hong C, Johnson GL, Vatner DE, Vatner SF. The MEKK1-JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J Clin Invest 110: 271–279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarpace PJ, Matheny M, Tumer N. Myocardial adenylyl cyclase type V and VI mRNA: differential regulation with age. J Cardiovasc Pharmacol 27: 86–90, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117: 61–69, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Tate CG, Blakely RD. The effect of N-linked glycosylation on activity of the Na+- and Cl−-dependent serotonin transporter expressed using recombinant baculovirus in insect cells. J Biol Chem 269: 26303–26310, 1994 [PubMed] [Google Scholar]
- 29.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha. GTPgammaS. Science 278: 1907–1916, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Tobise K, Ishikawa Y, Holmer SR, Im MJ, Newell JB, Yoshie H, Fujita M, Susannie EE, Homcy CJ. Changes in type VI adenylyl cyclase isoform expression correlate with a decreased capacity for cAMP generation in the aging ventricle. Circ Res 74: 596–603, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Brown MJ. Differential expression of adenylyl cyclase subtypes in human cardiovascular system. Mol Cell Endocrinol 223: 55–62, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130: 247–258, 2007 [DOI] [PubMed] [Google Scholar]